Formation and Biological Characteristics Analysis of Artificial Gynogenetic WuLi Carp Induced by Inactivated Sperm of Megalobrama Amblycephala
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Technical Method for Inducing Gynogenesis in WuLi Carp
2.3. Measurement of Countable Traits
2.4. Study on the Ploidy of Gynogenetic Offspring
2.5. Study on the Fertility of Gynogenetic Offspring
2.6. 5S rDNA Detection Experiment
2.7. Fluorescence in Situ Hybridization (FISH) Experiment
2.8. Microsatellite Experiment
2.9. Growth Performance Experiment
2.10. Nutrient Composition Analysis of GWB
3. Results
3.1. Results of Gynogenetic Black Carp Production
3.2. The Measurement Results of Countable Traits
3.3. Ploidy Detection Results
3.4. Histological Analysis Results of Gonadal Tissues
3.5. 5S rDNA Sequencing Results
3.6. FISH Experimental Results
3.7. Microsatellite Experimental Results
3.8. Growth Performance Experiment Results
3.9. Nutrient Composition Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, C.; Walsh, G.S.; Moens, C. Making Gynogenetic Diploid Zebrafish by Early Pressure. J. Vis. Exp. 2009, 28, e1396. [Google Scholar] [CrossRef] [PubMed]
- Manan, H.; Hidayati, A.N.; Lyana, N.A.; Amin-Safwan, A.; Ma, H.; Kasan, N.A.; Ikhwanuddin, M. A review of gynogenesis manipulation in aquatic animals. Aquac. Fish. 2022, 7, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Zou, C.; Lu, Y.; Yue, X.; Song, Z.; Yang, R.; You, F. Genetic diversity and signatures of selection in the Mito-gynogenetic olive flounder Paralichthys olivaceus revealed by genome-wide SNP markers. Aquaculture 2022, 553, 738062. [Google Scholar] [CrossRef]
- Zhou, L.; Gui, J. Natural and artificial polyploids in aquaculture. Aquac. Fish. 2017, 2, 103–111. [Google Scholar] [CrossRef]
- Chen, S.L.; Tian, Y.S.; Yang, J.F.; Shao, C.W.; Ji, X.S.; Zhai, J.M.; Liao, X.L.; Zhuang, Z.M.; Su, P.Z.; Xu, J.Y.; et al. Artificial gynogenesis and sex determination in half-smooth tongue sole (Cynoglossus semilaevis). Mar. Biotechnol. 2009, 11, 243–251. [Google Scholar] [CrossRef]
- Mao, Z.; Fu, Y.; Wang, S.; Wang, Y.; Luo, K.; Zhang, C.; Tao, M.; Liu, S. Further evidence for paternal DNA transmission in gynogenetic grass carp. Sci. China Life Sci. 2020, 63, 1287–1296. [Google Scholar] [CrossRef]
- Wu, P.; Zeng, Y.; Qin, Q.; Ji, W.; Wu, C.; Zhou, Y.; Zhao, R.; Tao, M.; Zhang, C.; Tang, C.; et al. Formation and identification of artificial gynogenetic mandarin fish (Siniperca chuatsi) induced by inactivated sperm of largemouth bass (Micropterus salmoides). Aquaculture 2023, 577. [Google Scholar] [CrossRef]
- Liang, Z.; Zou, L.; Tian, L.; Liu, M.; Li, C.; Xiao, G.; Cai, J.; Zhang, Y.; Li, S.; An, M.; et al. Genetic origin and differentiation of ten paddy field–farmed Cyprinus carpio strains in China. Aquaculture 2022, 561, 738573. [Google Scholar] [CrossRef]
- Xu, Y.L.; Chen, Z.; Qin, J.Q.; Wen, L.T.; Pan, X.H.; Zhou, K.Q.; Huang, Y.; Lin, Y.; Du, X.S. Estimation of Genetic Parameters for the Main Growth Traits of Quanzhou Hehua Carp. Guangxi Sci. 2022, 29, 801–808. [Google Scholar] [CrossRef]
- Zhou, K.Q.; Pan, X.H.; Lin, Y.; Du, X.S.; Huang, Y.; Pan, Z.Z.; Wen, L.T.; Qin, J.Q.; Zhang, F.; Chen, Z. Analysis of the Relationship between Morphological Traits and Body Mass of Guangxi Hehua Carp. Henan Agric. Sci. 2020, 49, 159–165. [Google Scholar] [CrossRef]
- Wen, J.; Teng, Q.; Shen, J. Reproduction and Cultivation Techniques of Hehua Carp. Guide Fish. Fortune 2023, 11, 37–41. [Google Scholar]
- Zhou, A.; Sun, D.; Chen, Y.; Chen, S.; Li, Q.; Chen, Y.; Zou, J. Analysis on the Aquaculture Technology and Benefits of Lian Shan Paddy Field Hehua Carp. Sci. Fish Farming 2021, 2, 82. [Google Scholar] [CrossRef]
- Wang, T.; Huang, K.; Sun, L.; Zuo, T.; Lin, Y.; Liang, Y. Analysis of Nutritional Components and Safety Evaluation of Hehua Carp Muscle. J. South. Agric. 2019, 50, 1579–1586. [Google Scholar] [CrossRef]
- Fan, J.; Ma, D.; Zhu, H.; Huang, Z.; Huang, J.; Li, H. Effects of Morphological Traits on Body Mass of the F5 Generation of Hehua Carp. Guangdong Agric. Sci. 2021, 48, 124–130. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, Q.; Wang, C.; Cao, L.; Zhou, Y.; Qin, H.; Zhao, C.; Liu, S. Molecular Organization and Chromosomal Localization Analysis of 5S rDNA Clusters in Autotetraploids Derived From Carassius auratus Red Var.(♀) × Megalobrama amblycephala(♂). Front. Genet. 2019, 10, 437. [Google Scholar] [CrossRef]
- Liao, A.; Zhang, S.; Yu, Q.; Wang, Y.; Tan, H.; Wu, P.; Ding, Y.; Hu, B.; Liu, W.; Tao, M.; et al. Formation and characterization of artificial gynogenetic northern snakehead (Channa argus) induced by inactivated sperm of mandarin fish (Siniperca chuatsi). Aquaculture 2025, 595, 741488. [Google Scholar] [CrossRef]
- Qin, Q.; Huo, Y.; Liu, Q.; Wang, C.; Zhou, Y.; Liu, S. Induced gynogenesis in autotetraploids derived from Carassius auratus red var. (♀) × Megalobrama amblycephala (♂). Aquaculture 2018, 495, 710–714. [Google Scholar] [CrossRef]
- Geng, R.J.; Xie, M.H.; Wang, W.M.; Liu, H. Microsatellite Parentage Identification of Megalobrama amblycephala. Freshw. Fish. 2018, 48, 9–15. [Google Scholar] [CrossRef]
- Korkut, A.Y.; Kop, A.; Demirtaş, N.; Cihaner, A. Determination methods of growth performance in fish feeding. Ege J. Fish. Aquat. Sci. 2007, 24, 1. [Google Scholar]
- Kašpar, V.; Hubálek, M.; Pšenička, M.; Arai, K.; Taggart, J.B.; Franěk, R. Cold-shock androgenesis in common carp (Cyprinus carpio). Aquaculture 2022, 548, 737610. [Google Scholar] [CrossRef]
- Zhai, G.; Shu, T.; Chen, K.; Lou, Q.; Jia, J.; Huang, J.; Shi, C.; Jin, X.; He, J.; Jiang, D.; et al. Successful Production of an All-Female Common Carp (Cyprinus carpio L.) Population Using cyp17a1-Deficient Neomale Carp. Engineering 2022, 8, 181–189. [Google Scholar] [CrossRef]
- Martínez, P.; Viñas, A.M.; Sánchez, L.; Díaz, N.; Ribas, L.; Piferrer, F. Genetic architecture of sex determination in fish: Applications to sex ratio control in aquaculture. Front. Genet. 2014, 5, 340. [Google Scholar] [CrossRef]
- Suzuki, R.; Oshiro, T.; Nakanishi, T. Survival, Growth and Fertility of Gynogenetic Diploids Induced in the Cyprinid Loach, Misgurnus anguillicaudatus. Aquaculture 1985, 48, 45–55. [Google Scholar] [CrossRef]
- Zhong, H.; Sun, Y.; Liu, M.; Chen, H.; Yu, P.; Wu, C.; Zhu, X.; Wang, X.; Wu, Y.; Tang, N.; et al. Induction of diploid gynogenesis in Micropterus salmoides using irradiated heterogeneous sperm from Siniperca chuatsi. Aquaculture 2024, 590, 741021. [Google Scholar] [CrossRef]
- Shen, Z.G.; Wang, H.P. Molecular players involved in temperature-dependent sex determination and sex differentiation in Teleost fish. Genet. Sel. Evol. 2014, 46, 26. [Google Scholar] [CrossRef]
- Valdivieso, A.; Wilson, C.A.; Amores, A.; da Silva Rodrigues, M.; Nóbrega, R.H.; Ribas, L.; Postlethwait, J.H.; Piferrer, F. Environmentally-induced sex reversal in fish with chromosomal vs. polygenic sex determination. Environ. Res. 2022, 213, 113549. [Google Scholar] [CrossRef]
- Canosa, L.F.; Bertucci, J.I. The Effect of Environmental Stressors on Growth in Fish and Its Endocrine Control. Front. Endocrinol. 2023, 14, 1109461. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, Z.; Wang, L.; Lu, Y.; Bi, W.; Zhou, D.; Wang, L.; Peng, Z.; You, F. Comparative study on growth performance and morphological characteristics of the meio- and mito-gynogenesis olive flounder (Paralichthys olivaceus). Aquaculture 2021, 535, 736387. [Google Scholar] [CrossRef]
- Luo, K.K.; Xiao, J.; Liu, S.J.; Wang, J.; He, W.G.; Hu, J.; Qin, Q.; Zhang, C.; Tao, M.; Liu, Y. Massive production of all-female diploids and triploids in the crucian carp. Int. J. Biol. Sci. 2011, 7, 487–495. [Google Scholar] [CrossRef]
- Zhi, Y.; Liu, Q.G.; Wu, J.M.; Liu, D. Morphologic and genetic analysis of the artificially induced gynogenesis in Qingtian paddy field carp. J. Shanghai Ocean. Univ. 2022, 31, 839–848. [Google Scholar]
- Liu, S.; Sun, Y.; Zhang, C.; Luo, K.; Liu, Y. Production of gynogenetic progeny from allotetraploid hybrids red crucian carp × common carp. Aquaculture 2004, 236, 193–200. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Xiao, J.; Tao, M.; Zhang, C.; Luo, K.; Liu, Y. Evidence for the evolutionary origin of goldfish derived from the distant crossing of red crucian carp × common carp. BMC Genet. 2014, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiao, N.; Zhao, L.; Zhang, M.; Zhou, P.; Huang, X.; Hu, F.; Yang, C.; Shu, Y.; Li, W.; et al. Evidence for the paternal mitochondrial DNA in the crucian carp-like fish lineage with hybrid origin. Sci. China Life Sci. 2020, 63, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Xu, L.; Wu, C.; Wang, S.; Liu, Q.; Cao, L.; Mao, Z.; Wang, Y.; Hu, F.; Zhou, R.; et al. Two types of gynogenetic blunt snout bream derived from different sperm. Aquaculture 2019, 511, 734250. [Google Scholar] [CrossRef]

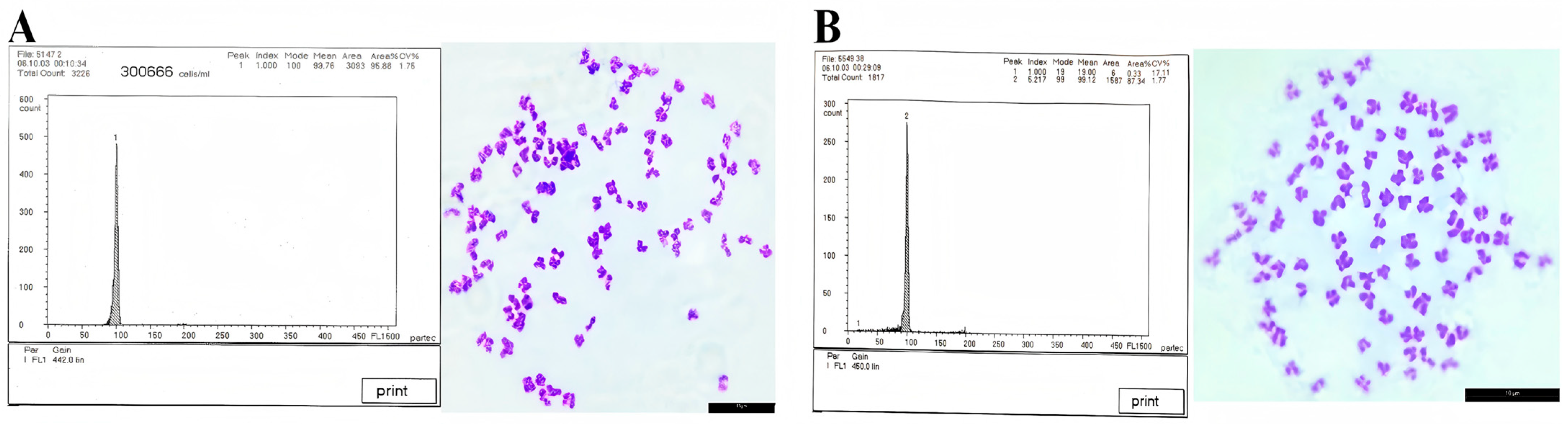

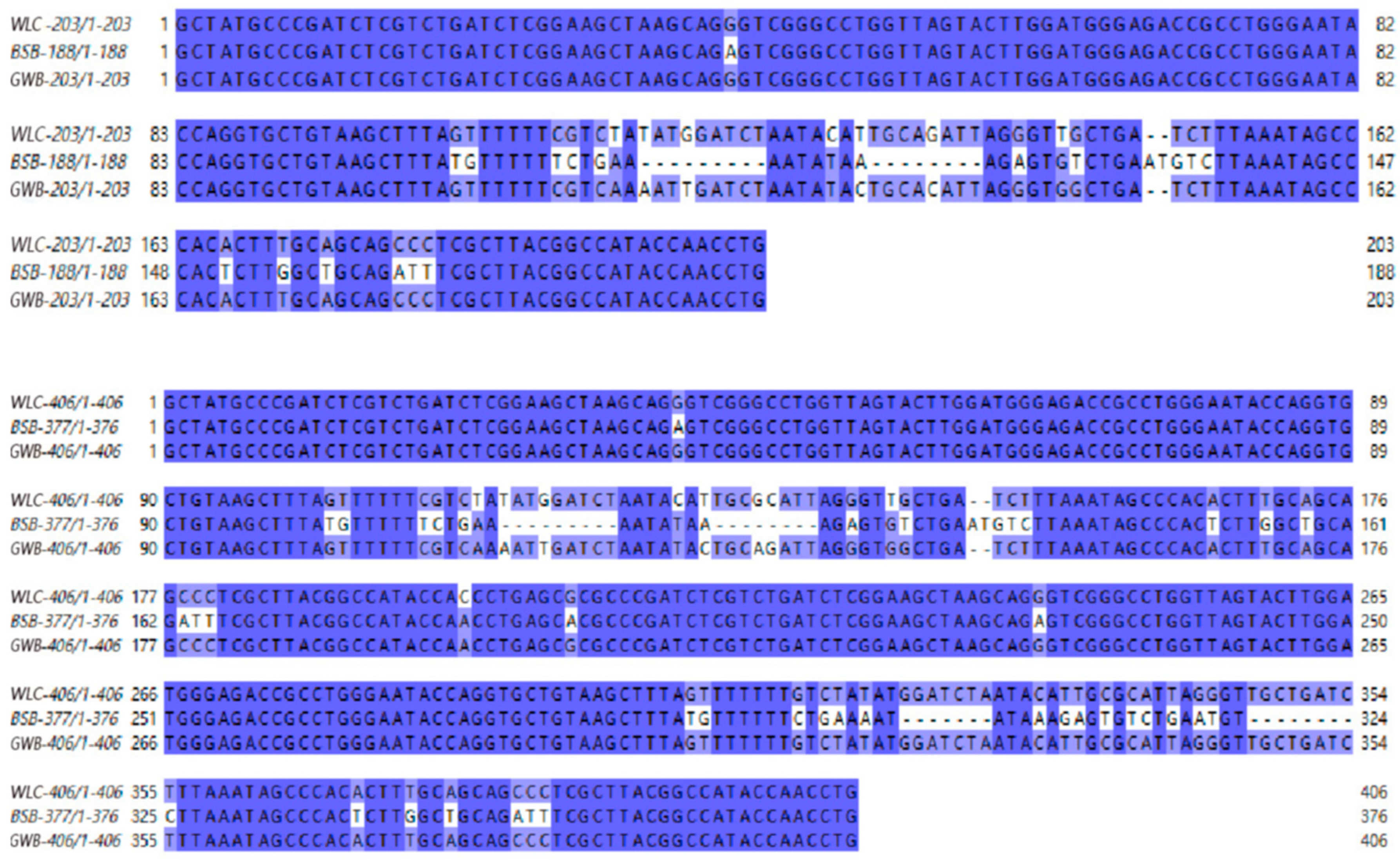
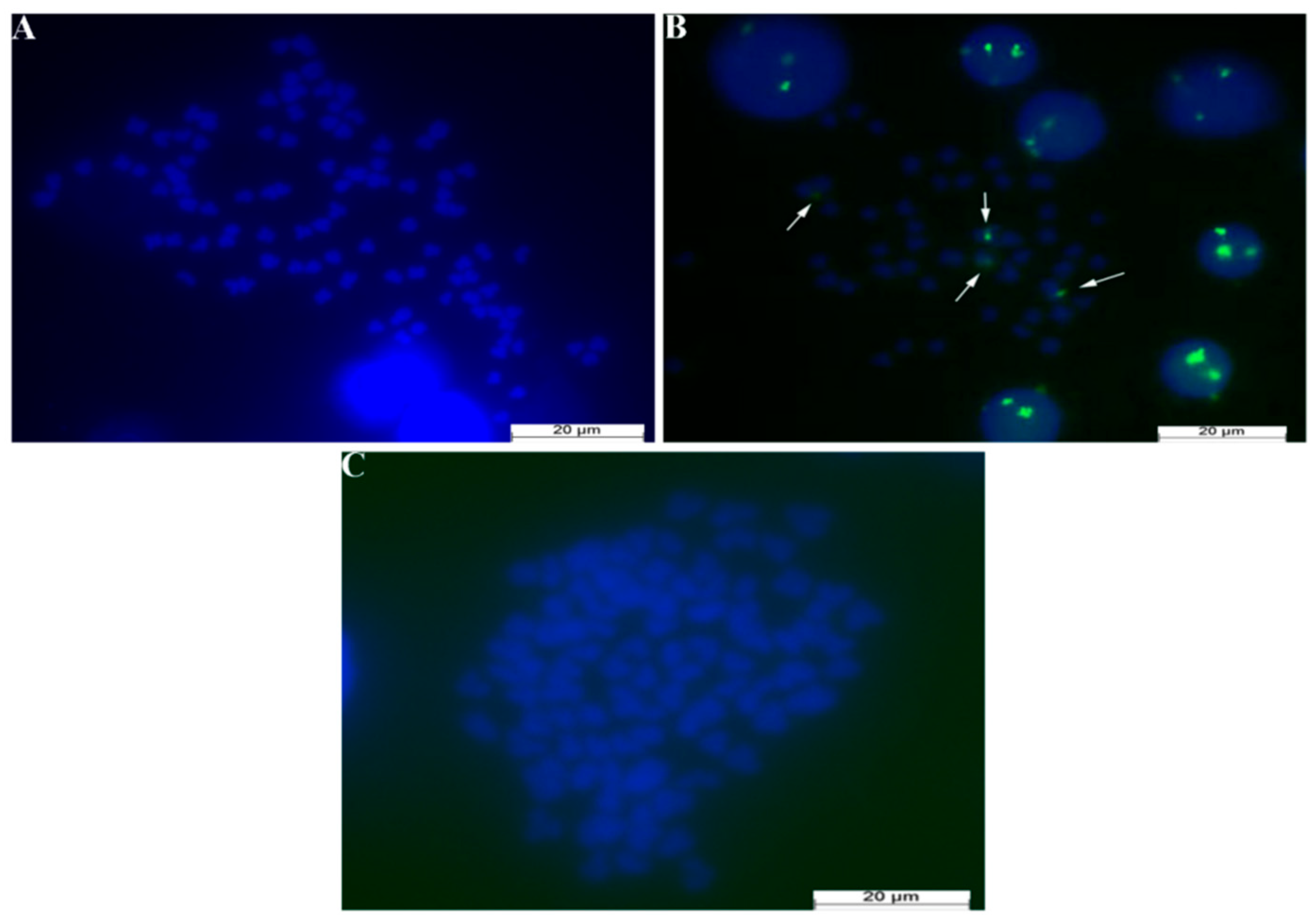

| Sample | Lateral Line Scales | Scales Above the Lateral Line | Scales Below the Lateral Line | Dorsal Fin | Pectoral Fin | Pelvic Fin | Anal Fin |
|---|---|---|---|---|---|---|---|
| WLC | 35.30 ± 1.14 b | 6.00 ± 0.00 a | 5.70 ± 0.30 a | III~16–18 b | 11–14 b | 7–10 b | III~5–7 a |
| BSB | 55.60 ± 1.88 c | 11.30 ± 0.60 b | 9.40 ± 0.98 c | III~7–9 a | 12–15 c | 9.00 c | III~25–28 c |
| GWB | 34.52 ± 0.778 a | 5.80 ± 0.40 a | 6.30 ± 0.40 b | III~15–19 b | 9–12 a | 7–9 a | III~7–9 b |
| Sample | WL/SL | BH/SL | HL/SL | HD/SL | CPL/SL | CPH/SL |
|---|---|---|---|---|---|---|
| WLC | 1.32 ± 0.07 a | 0.35 ± 0.03 a | 0.23 ± 0.01 | 0.18 ± 0.02 b | 0.19 ± 0.02 b | 0.13 ± 0.01 |
| BSB | 1.24 ± 0.03 | 0.41 ± 0.01 c | 0.21 ± 0.01 | 0.15 ± 0.01 a | 0.14 ± 0.01 a | 0.13 ± 0.01 |
| GWB | 1.22 ± 0.05 | 0.38 ± 0.02 b | 0.22 ± 0.03 | 0.20 ± 0.01 c | 0.21 ± 0.02 c | 0.13 ± 0.01 |
| Sample | Average DNA | Proportion | |
|---|---|---|---|
| Observation | Anticipated Value | ||
| WLC | 99.76 | - | - |
| GWB | 99.12 | WLC/GWB = 1.01 a | 1 |
| Sample | Initial Day (g) | 30 Days (g) | 60 Days (g) | 90 Days (g) |
|---|---|---|---|---|
| WLC | 1 ± 0.23 | 14.7 ± 2.25 | 48.5 ± 4.88 | 70.6 ± 4.28 |
| GWB | 1 ± 0.11 | 26.3 ± 3.63 | 51.5 ± 5.72 | 81.7 ± 2.85 |
| (A) | ||
| WLC (g/100 g) | GWB (g/100 g) | |
| Crude Protein | 18.50 ± 0.4582 | 17.96 ± 0.4932 |
| Crude Fat | 3.26 ± 0.1527 a | 4.13 ± 0.1527 b |
| Asp | 1.3800 ± 0.0000 a | 1.2966 ± 0.0057 b |
| Thr @ | 0.6600 ± 0.0000 a | 0.6266 ± 0.0057 b |
| Ser | 0.5600 ± 0.0000 a | 0.5466 ± 0.0057 b |
| Glu | 1.8166 ± 0.0115 a | 1.6666 ± 0.0152 b |
| Gly | 1.1966 ± 0.0057 a | 1.2600 ± 0.1000 b |
| Ala | 1.1966 ± 0.0057 a | 1.1766 ± 0.0057 b |
| Cys | 0.1000 ± 0.0000 | 0.1000 ± 0.0000 |
| Val @ | 0.8033 ± 0.0057 a | 0.7366 ± 0.0057 b |
| Met @ | 0.2433 ± 0.0057 a | 0.2000 ± 0.0000 b |
| IIe @ | 0.6933 ± 0.0152 a | 0.6400 ± 0.1000 b |
| Leu @ | 1.2233 ± 0.0152 a | 1.1433 ± 0.0152 b |
| Tyr @ | 0.5100 ± 0.0000 a | 0.4766 ± 0.0057 b |
| Phe | 0.6566 ± 0.0057 a | 0.6066 ± 0.0057 b |
| Lys @ | 1.4200 ± 0.0000 a | 1.3133 ± 0.0057 b |
| His @ | 0.5000 ± 0.0000 a | 0.3866 ± 0.0057 b |
| Arg @ | 0.9866 ± 0.0057 a | 0.9700 ± 0.0000 b |
| Pro | 0.6366 ± 0.0115 | 0.6366 ± 0.0057 |
| Total Content of Essential Amino Acids | 7.0398 | 6.4930 |
| Total Content of Non-essential Amino Acids | 7.5430 | 7.2896 |
| (B) | ||
| WLC (g/100 g) | GWB (g/100 g) | |
| C14:0 * | 0.0183 ± 0.0008 | 0.0241 ± 0.0027 |
| C15:0 * | 0.007 ± 0.0005 | 0.0171 ± 0.001 |
| C16:0 * | 0.3501 ± 0.0155 | 0.3997 ± 0.0136 |
| C16:1 * | 0.0642 ± 0.0031 | 0.0854 ± 0.0049 |
| C17:0 * | 0.0087 ± 0.0004 | 0.0155 ± 0.0006 |
| C18:0 | 0.1103 ± 0.0012 | 0.1094 ± 0.0062 |
| C20:2 * | 0.0109 ± 0.0007 | 0.0106 ± 0.0007 |
| C20:3n6 * | 0.0193 ± 0.0015 | 0.0133 ± 0.0008 |
| C20:3n3 * | - | 0.0043 ± 0.0005 |
| C22:1n9 | 0.0195 ± 0.0015 | 0.0172 ± 0.0015 |
| C20:4n6 | 0.0474 ± 0.0007 | 0.0457 ± 0.0032 |
| C20:5n3 * | 0.0044 ± 0.0006 | 0.0070 ± 0.0007 |
| C24:0 * | 0.0076 ± 0.0005 | 0.0128 ± 0.0009 |
| C24:1 * | 0.0055 ± 0.0003 | 0.0040 ± 0.0004 |
| C22:6n3 | 0.0409 ± 0.0011 | 0.0403 ± 0.0029 |
| Total Content of Unsaturated Fatty Acids | 0.2121 | 0.2378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Hu, E.; Xiao, Q.; Huang, X.; Wang, C.; Xu, X.; Zhang, K.; Zhou, Y.; Bai, J.; Liu, Z.; et al. Formation and Biological Characteristics Analysis of Artificial Gynogenetic WuLi Carp Induced by Inactivated Sperm of Megalobrama Amblycephala. Biology 2025, 14, 994. https://doi.org/10.3390/biology14080994
Xu X, Hu E, Xiao Q, Huang X, Wang C, Xu X, Zhang K, Zhou Y, Bai J, Liu Z, et al. Formation and Biological Characteristics Analysis of Artificial Gynogenetic WuLi Carp Induced by Inactivated Sperm of Megalobrama Amblycephala. Biology. 2025; 14(8):994. https://doi.org/10.3390/biology14080994
Chicago/Turabian StyleXu, Xiaowei, Enkui Hu, Qian Xiao, Xu Huang, Chongqing Wang, Xidan Xu, Kun Zhang, Yue Zhou, Jinhai Bai, Zhengkun Liu, and et al. 2025. "Formation and Biological Characteristics Analysis of Artificial Gynogenetic WuLi Carp Induced by Inactivated Sperm of Megalobrama Amblycephala" Biology 14, no. 8: 994. https://doi.org/10.3390/biology14080994
APA StyleXu, X., Hu, E., Xiao, Q., Huang, X., Wang, C., Xu, X., Zhang, K., Zhou, Y., Bai, J., Liu, Z., Jiang, Y., Tang, Y., Deng, X., Li, S., Peng, W., Xiong, L., Yang, Y., Li, Z., Ma, M., ... Liu, S. (2025). Formation and Biological Characteristics Analysis of Artificial Gynogenetic WuLi Carp Induced by Inactivated Sperm of Megalobrama Amblycephala. Biology, 14(8), 994. https://doi.org/10.3390/biology14080994






