Physiological Changes and Transcriptomics of Elodea nuttallii in Response to High-Temperature Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Growth Measurements
2.3. Antioxidant Enzyme Activity Assay
2.4. Determination of Malondialdehyde (MDA), Pro, SP, and SS Contents
2.5. Chlorophyll Content Measurements
2.6. RNA Sequencing
2.7. Statistical Analysis
3. Results
3.1. Effects of High-Temperature Stress on Growth Parameters of E. nuttallii
3.2. Changes in Antioxidant Enzyme Activities of E. nuttallii
3.3. Changes in Malondialdehyde (MDA) Content of E. nuttallii
3.4. Changes in the Contents of Osmoregulatory Compounds in E. nuttallii
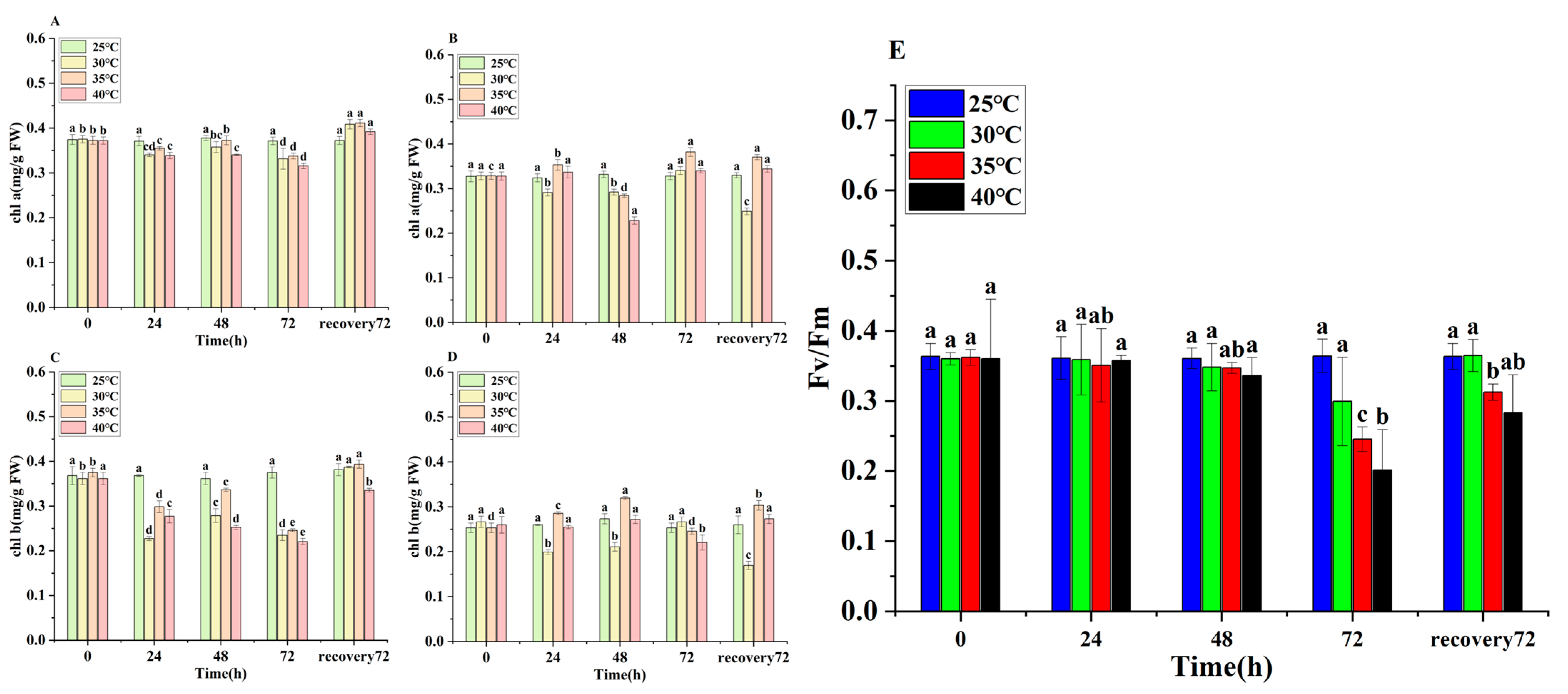
3.5. Changes in Chlorophyll Contents and Chlorophyll Fluorescence of E. nuttallii
3.6. Transcriptome Analysis of Gene Expression in E. nuttallii Under HTS
3.7. Analysis of Differentially Expressed Genes Related to Photosynthesis
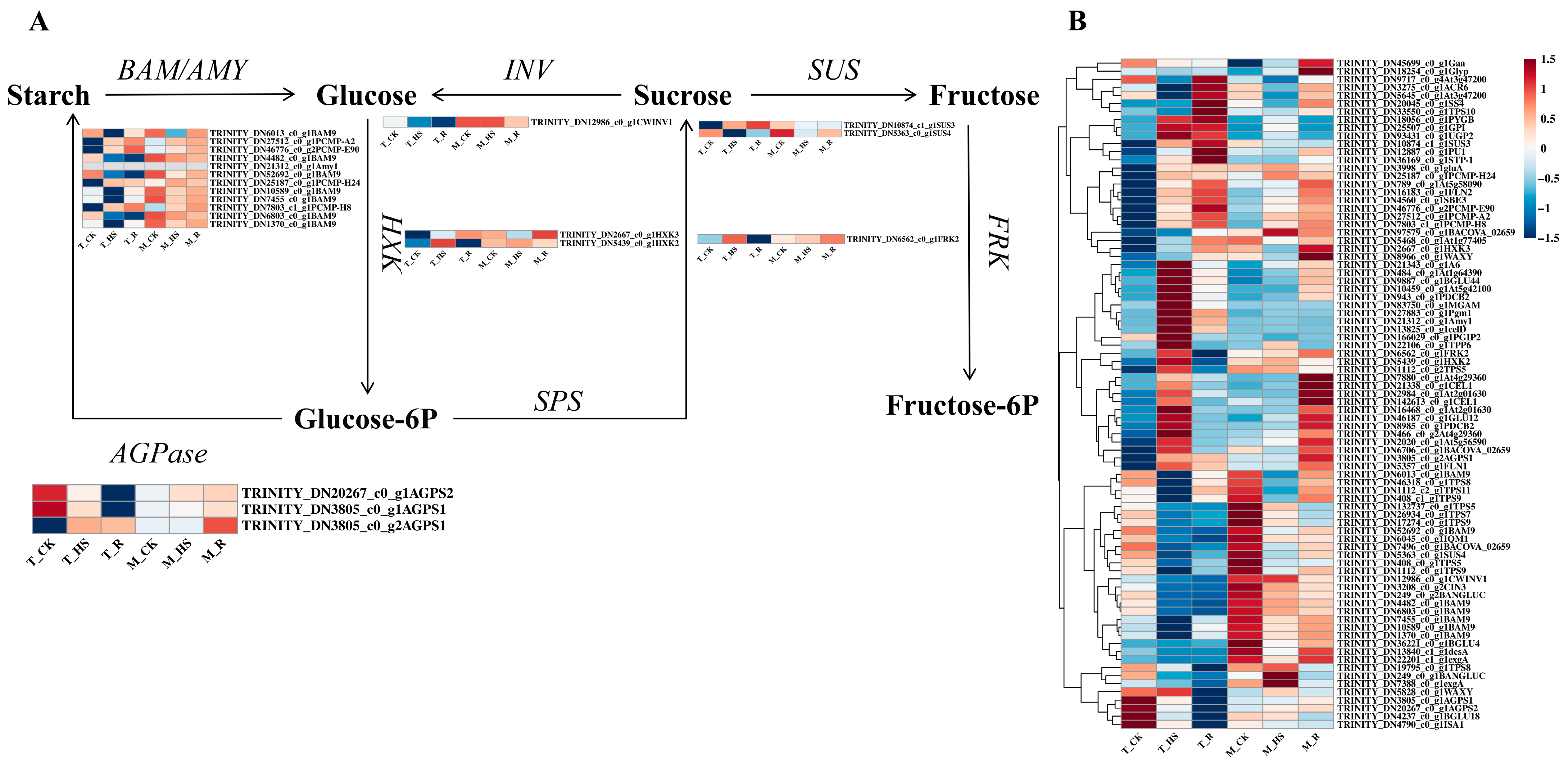
3.8. Analysis of Differentially Expressed Genes Related to Sugar Metabolic Pathways
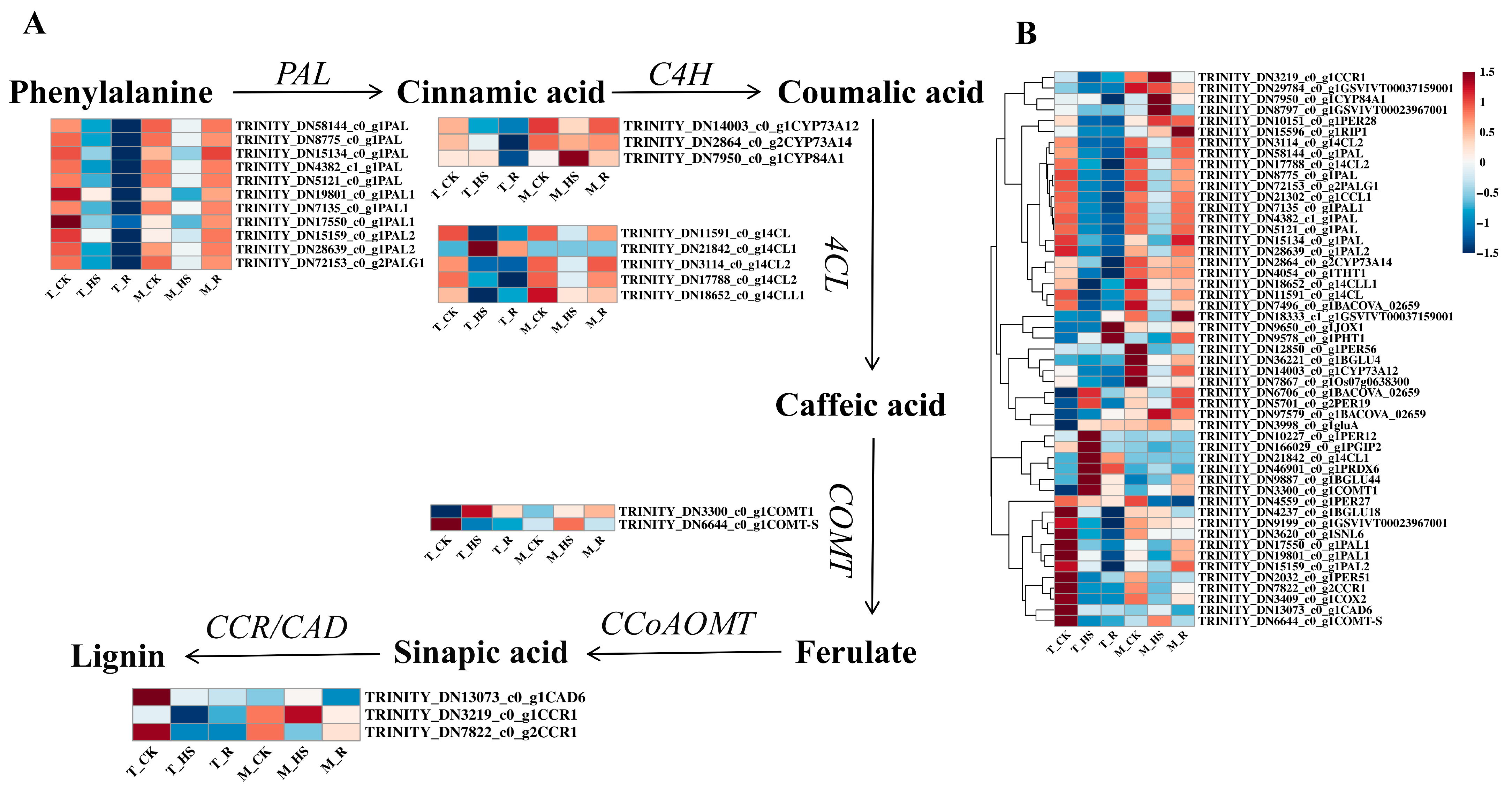
3.9. Analysis of Differentially Expressed Genes Related to Phenylpropanoid Biosynthesis Pathway
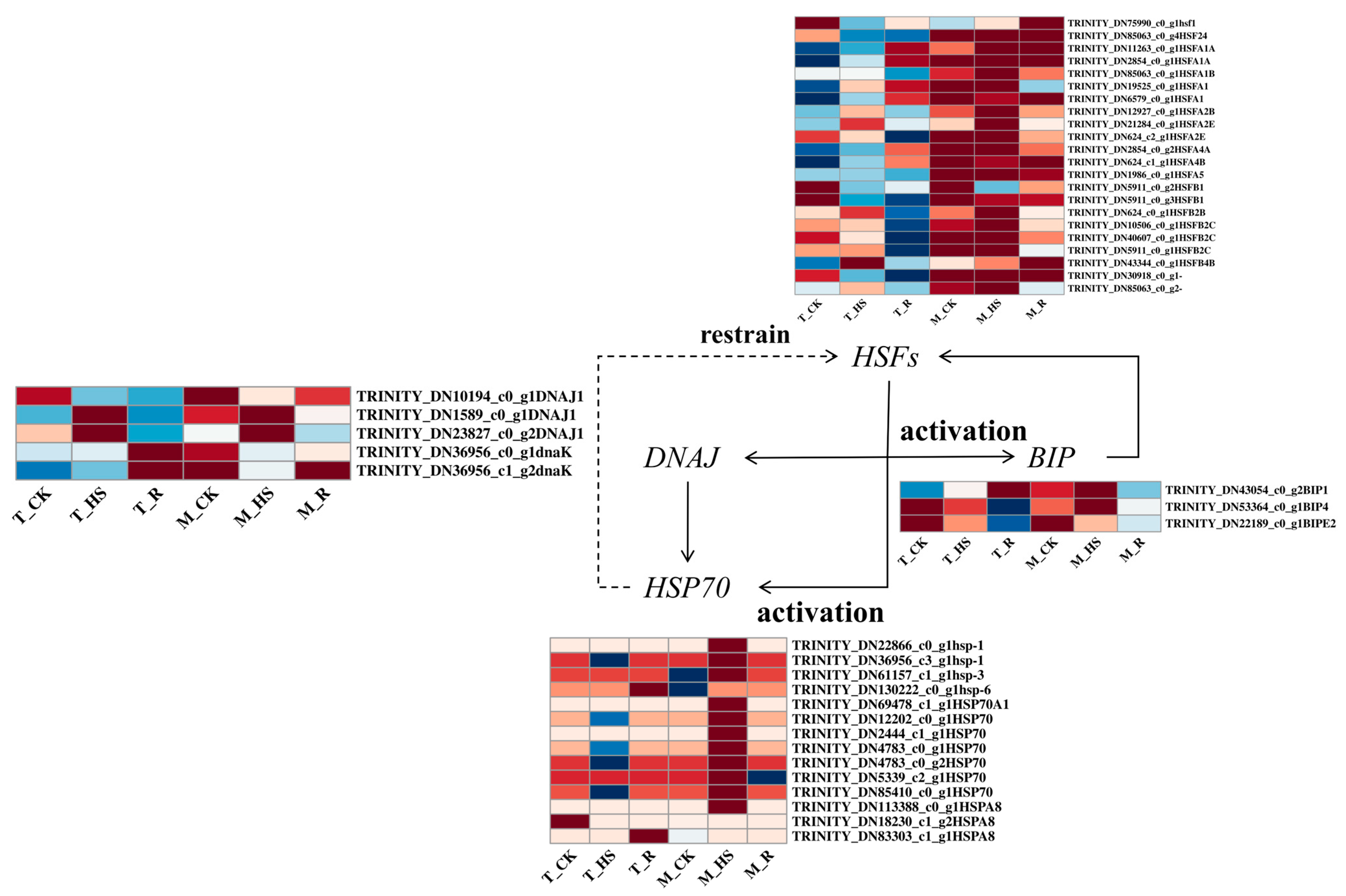
3.10. Analysis of Differentially Expressed Genes Related to Heat-Shock Proteins (HSPs) and Heat-Shock Transcription Factors (HSFs)
3.11. Response of E. nuttallii to HTS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, S.Q.; Wang, X.C.; Tao, N.P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Shi, L.L.; Jin, M.J.; Shen, M.X.; Lu, C.Y.; Wang, H.H.; Zhou, X.W.; Mei, L.J.; Yin, S.X. Using Ipomoea aquatic as an environmental-friendly alternative to Elodea nuttallii for the aquaculture of Chinese mitten crab. PeerJ 2019, 7, e6785. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Bao, M.M.; Li, X.G.; Huang, H.B.; Kong, Y. Preliminary analysis on maintenance and management for weed in river crab pond during high-temperature period. Sci. Fish Farming 2020, 8, 36–38. [Google Scholar] [CrossRef]
- Wang, Q.D.; Liu, J.S.; Zhang, S.Y.; Lian, Y.X.; Ding, H.Y.; Du, X.; Li, Z.J.; De Silva, S.S. Sustainable farming practices of the Chinese mitten crab (Eriocheir sinensis) around Hongze Lake, lower Yangtze River Basin, China. Ambio 2015, 45, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Song, X.H.; Chen, G.J.; Feng, Y.Q.; Wu, L.K.; Shen, Z.H. Utilization of natural feed resources in a macrophyte-dominated lake for aquaculture of Chinese mitten crab (Eriocheir sinensis) and its purification effects on water environment. J. Lake Sci. 2013, 25, 723–728. [Google Scholar] [CrossRef][Green Version]
- Zhong, S.Q. Cultivation and management technique for //Elodea in rice field. Sci. Fish Farming 2018, 17–18. [Google Scholar] [CrossRef]
- Wang, M.H.; Shen, Q.H.; Tang, S.K.; Qin, Q.; Cai, Y.X. Purification Effect of Elodea nuttallii on Water in Pond Stocked Pelteobagrus fulvidraco. J. Hydroecology 2009, 30, 48–51. Available online: https://kns.cnki.net/kcms2/article/abstract?v=__pNPjlwk1qQI5WpyyCIaJvXpVbX2vkDB8aOFbSgTB13DMG259wWl7cH0qA4ldRnEtRGrLX51Xmv_97l_aWksF19pd9b-HlRMiFybLmiCexyiGcN0XbG_U7QAr-xCWoe_6rBbq0VPY4xaSrmgv1vJb8ezOCRYXcqED0zTTOQ77JFaO96gV0SNA==&uniplatform=NZKPT&language=CHS (accessed on 9 May 2025).
- Draga, M.; Szczęśniak, E.; Rosadziński, S.; Bryl, Ł.; Lisek, D.; Gąbka, M. Alien aquatic plants in Poland: Temporal and spatial distribution patterns and the effects of climate change. Glob. Ecol. Conserv. 2024, 55, e03247. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Y.; Xue, J.; Zhang, Z.; Cao, J.; Yang, N.; Wan, F.; Xian, X.; Liu, W. Future Climate Change Increases the Risk of Suitable Habitats for the Invasive Macrophyte Elodea nuttallii. Biology 2025, 14, 504. [Google Scholar] [CrossRef]
- Ke, W.; Wu, D.; Pan, Y.; Qian, Y.; Wu, J.; Xia, Y. Influence of water quality factors on the ecological cultivation of Chinese mitten crabs in Yangcheng Lake. Hubei Agric. Sci. 2024, 63, 123–126. [Google Scholar] [CrossRef]
- Wu, K.; Ma, X.Z.; Wang, Y.C.; Wang, W.; Lang, Y.L. Effect of three water plants decomposition on water quality. J. Shanghai Ocean Univ. 2016, 25, 726–734. Available online: https://kns.cnki.net/kcms2/article/abstract?v=__pNPjlwk1p7rZP-U1TkCylNm8miyvjBVtAAnWcqJSryqtRBwNIwo9uCCGSJNn-byLdiW8xNmO6Oug8RoV1ehG2EE8TyLNrfCkL2K7VpTqdKDnyY-wYoCzJ_FhiazPfePDPadqs3aFbR__BRjA7OlYAUl_6OfLFHPpRkALTnx7u9cQIUTiypfCA==&uniplatform=NZKPT&language=CHS (accessed on 9 May 2025).
- Zhang, G.B.; Jiang, X.D.; Chen, W.B.; Zhou, W.Q.; Luo, M.; Wu, X.G. Effect of submerged macrophytes planting mode on performance and economic profit of all-male adult Eriocheir sinensis culture. S. China Fish. Sci. 2023, 19, 107–115. Available online: https://link.cnki.net/urlid/44.1683.S.20221216.1546.001 (accessed on 9 May 2025).
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Berry, J.A.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 2003, 31, 491–543. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Ahamed, K.U.; Hakeem, K.R.; Ozturk, M.; Fujita, M. Plant Responses and Tolerance to High Temperature Stress: Role of Exogenous Phytoprotectants. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 385–435. [Google Scholar] [CrossRef]
- Li, X.Q.; Hu, X.Y.; Zuo, Y.Q.; Zhang, B.L.; Mo, J.Y.; Cai, X.H.; Fei, S.M. Research Advances in Physiological Effect of Heat Stress on Plant. J. Southwest For. Univ. (Nat. Sci.) 2009, 29, 72–76. Available online: https://kns.cnki.net/kcms2/article/abstract?v=__pNPjlwk1okRSYnze3mE1oqVyUl2IPnwSj5yDNKCU9mWOZkyfAqwApdeI_zji9ZXfwoqSmYEFp-rDSWfwBftPSVI1xmGEf5syhGUBfgQWFBXOFhB5_C89nxEFQeZAdC5-JdFaysTNVnR-qkmMrcEZTZ6LBqy9XtnrW4gGW0JtZoggMonhuGuA==&uniplatform=NZKPT&language=CHS (accessed on 9 May 2025).
- Wang, L.J.; Li, H.H.; Zhao, C.Z.; Li, S.F.; Kong, L.Y.; Wu, W.W.; Kong, W.S.; Liu, Y.; Wei, Y.Y.; Zhu, J.K.; et al. The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant Cell Environ. 2016, 40, 56–68. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the Temperature-Stress Metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2016, 90, 856–867. [Google Scholar] [CrossRef]
- Vidya, M.K.; Kumar, V.G.; Sejian, V.; Bagath, M.; Krishnan, G.; Bhatta, R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2017, 37, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Ahmed, J.U.; Mollah, M.M.I.; Taewan, K. Physiological Mechanism of Thermotolerance in Wheat (Triticum aestivum Lin.) Seedlings. Am. J. Plant Sci. 2018, 9, 2719–2727. [Google Scholar] [CrossRef]
- Smith, E.J.; Tsurutani, B.T.; Feldman, W.C.; Slavin, J.A.; Bame, S.J. Slow mode shocks in the earth’s magnetotail: ISEE-3. Geophys. Res. Lett. 2013, 11, 1054–1057. [Google Scholar] [CrossRef]
- Chetti, M.B.; Nobel, P.S. High-Temperature Sensitivity and Its Acclimation for Photosynthetic Electron Transport Reactions of Desert Succulents. Plant Physiol. 1987, 84, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H. Photosynthesis of Ivy Leaves (Hedera helix) after Heat Stress I. CO2-Gas Exchange and Diffusion Resistances. Physiol. Plant. 1978, 44, 400–406. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Yang, F.; Liu, C.Y.; Zhao, Y.Q.; Li, M.Y.; Lu, X.J.; Ge, J.; Zhang, B.W.; Li, M.Q.; Yang, Y.; et al. Transcriptome sequencing of garlic reveals key genes related to the heat stress response. Sci. Rep. 2024, 14, 15956. [Google Scholar] [CrossRef]
- Mangelsen, E.; Kilian, J.; Harter, K.; Jansson, C.; Wanke, D.; Sundberg, E. Transcriptome Analysis of High-Temperature Stress in Developing Barley Caryopses: Early Stress Responses and Effects on Storage Compound Biosynthesis. Mol. Plant 2011, 4, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Atapaththu, K.S.S.; Asaeda, T. Growth and stress responses of Nuttall’s waterweed Elodea nuttallii (Planch) St. John to water movements. Hydrobiologia 2014, 747, 217–233. [Google Scholar] [CrossRef]
- Chalanika De Silva, H.C.; Asaeda, T. Stress response and tolerance of the submerged macrophyte Elodea nuttallii (Planch) St. John to heat stress: A comparative study of shock heat stress and gradual heat stress. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2017, 152, 787–794. [Google Scholar] [CrossRef]
- Ei, H.H.; Zheng, T.D.; Farooq, M.U.; Zeng, R.; Su, Y.; Zhang, Y.J.; Liang, Y.K.; Tang, Z.C.; Ye, X.Y.; Jia, X.M.; et al. Impact of selenium, zinc and their interaction on key enzymes, grain yield, selenium, zinc concentrations, and seedling vigor of biofortified rice. Environ. Sci. Pollut. Res. 2020, 27, 16940–16949. [Google Scholar] [CrossRef]
- Yang, L.; Lai, L.; Zhou, J.; Yi, S.; Sun, Q.; Li, H.; Jiang, L.; Zheng, Y. Enzyme and osmotic adjustment compounds of key species can help explain shrub encroachment in a semiarid sandy grassland. Ecol. Indic. 2019, 101, 541–551. [Google Scholar] [CrossRef]
- Xue, H.L.; Sun, Y.X.; Li, L.; Bi, Y.; Hussain, R.; Zhang, R.; Long, H.T.; Nan, M.; Pu, L.M. Acetylsalicylic acid (ASA) induced fusarium rot resistance and suppressed neosolaniol production by elevation of ROS metabolism in muskmelon fruit. Sci. Hortic. 2020, 265, 109264. [Google Scholar] [CrossRef]
- Wan, H.L.; Kong, X.B.; Liu, Y.H.; Jin, F.; Han, L.X.; Xu, M.; Li, X.M.; Li, L.; Yang, J.; Lai, D.N.; et al. Residue analysis and effect of preharvest forchlorfenuron (CPPU) application on quality formation of kiwifruit. Postharvest Biol. Technol. 2023, 195, 112144. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, J.; Jiang, C.; Li, W. Effects of low temperature stress on SOD activity, soluble protein content and soluble sugar content in Camellia sinensis leaves. J. Anhui Agric. Univ. 2011, 38, 24–26. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Int. Sci. Congr. Assoc. 2014, 4, 63–69. Available online: https://www.researchgate.net/publication/269699354_Spectrophotometric_Analysis_of_Chlorophylls_and_Carotenoids_from_Commonly_Grown_Fern_Species_by_Using_Various_Extracting_Solvents (accessed on 12 May 2025).
- Pan, C.L.; Lu, H.L.; Yang, C.Y.; Wang, L.; Chen, J.M.; Yan, C.L. Comparative transcriptome analysis reveals different functions of Kandelia obovata superoxide dismutases in regulation of cadmium translocation. Sci. Total Environ. 2021, 771, 144922. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Xia, X.Z.; Ji, W.; Li, T.Y.; Xu, X.Y.; Chen, J.R.; Chen, X.; Zhu, X.T. Effects of High Temperature Stress on the Physiological and Biochemical Characteristics of Paeonia ostii. Int. J. Mol. Sci. 2023, 24, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Luo, H.; Song, Y.; Hong, E.; Li, Z.; Lin, B.; Fan, C.; Wang, H.; Song, X.; Jin, S.; et al. Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress. Agronomy 2022, 12, 1203. [Google Scholar] [CrossRef]
- Gupta, D.; Eldakak, M.; Rohila, J.S.; Basu, C. Biochemical analysis of ‘kerosene tree’ Hymenaea courbaril L. under heat stress. Plant Signal. Behav. 2014, 9, e972851. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Murata, N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- da Silva, F.L.; Lucho, S.R.; Klumb, E.K.; Egewarth, J.; Bianchi, V.J. Changes in Sugar Metabolism and Gene Transcriptional Responses in Flood-Stressed Grafted Prunus persica Plants. Plant Mol. Biol. Rep. 2025. [Google Scholar] [CrossRef]
- Shang, S.; Zhang, Z.; Liu, X.; Liu, L.; Tang, X. Genome-wide characterization of aquaporins in Spirodela polyrhiza and expression of aquaporin gene family under high-temperature stress. Nord. J. Bot. 2022, 2022, e03567. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for their Antioxidant Activity. Free Radic. Res. 2009, 36, 1199–1208. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Ortega, A.; Garrido, I.; Casimiro, I.; Espinosa, F. Effects of antimony on redox activities and antioxidant defence systems in sunflower (Helianthus annuus L.) plants. PLoS ONE 2017, 12, e0183991. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Liu, P.; Liu, S.; Luo, T.; Jin, S.; Xu, Q.; Xu, J.; Cheng, Y.; Deng, X. Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. BMC Plant Biol. 2013, 13, 44. [Google Scholar] [CrossRef]
- Shihan, A.; Httenschwiler, S.; Milcu, A.; Joly, F.X.; Santonja, M.; Fromin, N. Changes in soil microbial substrate utilization in response to altered litter diversity and precipitation in a Mediterranean shrubland. Biol. Fertil. Soils 2017, 53, 171–185. [Google Scholar] [CrossRef]
- Ding, R.; Missaoui, A.M. Phenotyping Summer Dormancy in Tall Fescue: Establishment of a Surrogate Phenotype and a Dormancy Rating System in Humid Environments. Crop Sci. 2016, 56, 2579–2593. [Google Scholar] [CrossRef]
- Norton, M.R.; Volaire, F.; Lelievre, F. Summer dormancy in Festuca arundinacea Schreb.; the influence of season of sowing and a simulated mid-summer storm on two contrasting cultivars. Aust. J. Agric. Res. 2006, 57, 1267–1277. [Google Scholar] [CrossRef]
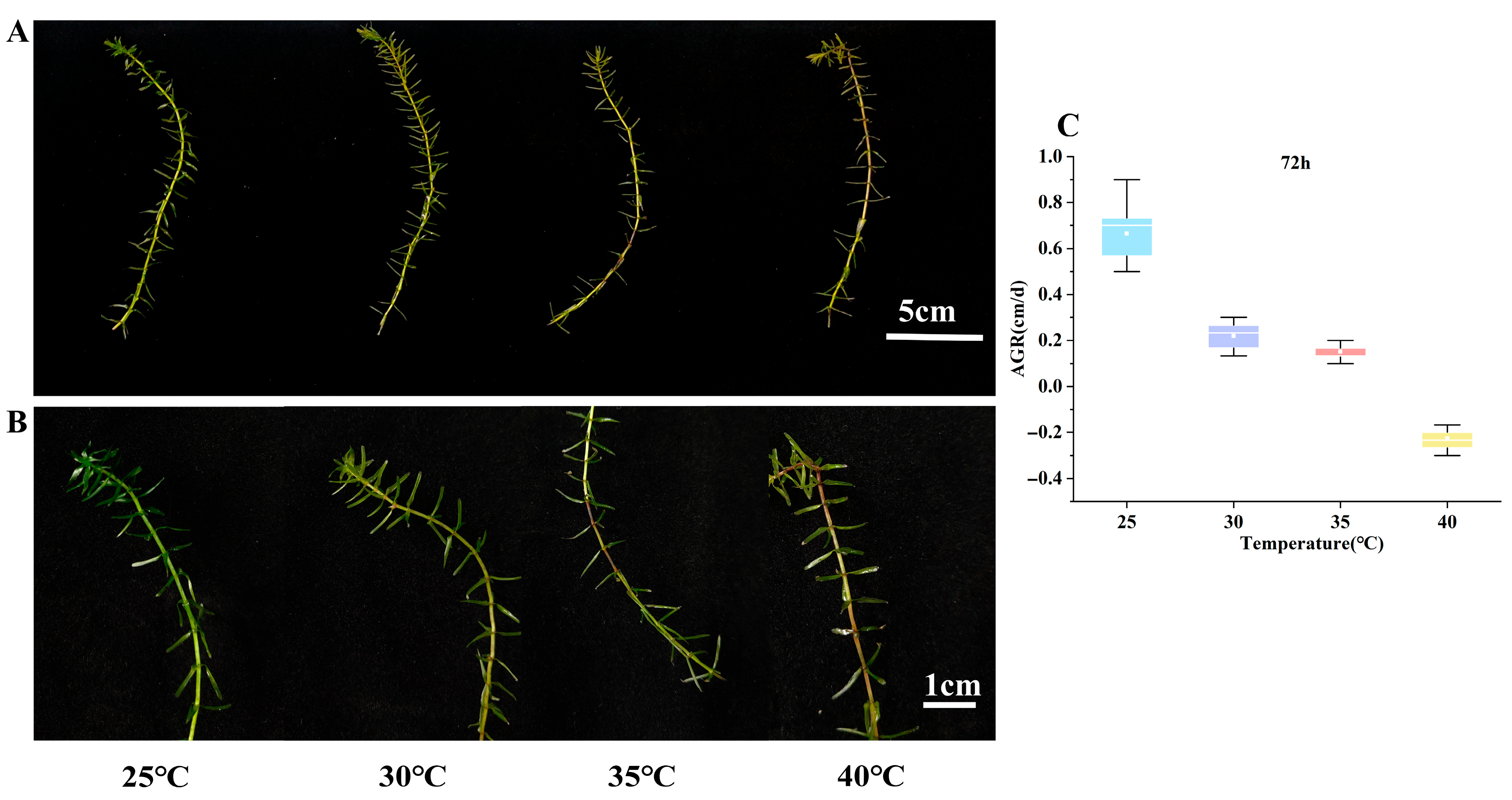





| Time | 25 °C | 30 °C | 35 °C | 40 °C |
|---|---|---|---|---|
| 24 h | 0.49 ± 0.13 a | 0.35 ± 0.07 b | 0.21 ± 0.08 b | −0.04 ± 0.07 c |
| 48 h | 0.54 ± 0.12 a | 0.22 ± 0.07 b | 0.17 ± 0.05 b | −0.17 ± 0.05 c |
| 72 h | 0.66 ± 0.12 a | 0.22 ± 0.05 b | 0.15 ± 0.04 b | −0.23 ± 0.04 c |
| Recovery 25 °C 72 h | 0.49 ± 0.06 a | 0.2 ± 0.04 b | 0.11 ± 0.02 c | −0.15 ± 0.02 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Jin, Y.; Zha, M.; Mao, Y.; Ren, W.; Guo, Z.; Zhang, Y.; Zhou, B.; Zhang, T.; He, Q.; et al. Physiological Changes and Transcriptomics of Elodea nuttallii in Response to High-Temperature Stress. Biology 2025, 14, 993. https://doi.org/10.3390/biology14080993
Xu Y, Jin Y, Zha M, Mao Y, Ren W, Guo Z, Zhang Y, Zhou B, Zhang T, He Q, et al. Physiological Changes and Transcriptomics of Elodea nuttallii in Response to High-Temperature Stress. Biology. 2025; 14(8):993. https://doi.org/10.3390/biology14080993
Chicago/Turabian StyleXu, Yanling, Yuanyuan Jin, Manrong Zha, Yuhan Mao, Wenqiang Ren, Zirao Guo, Yufei Zhang, Beier Zhou, Tao Zhang, Qi He, and et al. 2025. "Physiological Changes and Transcriptomics of Elodea nuttallii in Response to High-Temperature Stress" Biology 14, no. 8: 993. https://doi.org/10.3390/biology14080993
APA StyleXu, Y., Jin, Y., Zha, M., Mao, Y., Ren, W., Guo, Z., Zhang, Y., Zhou, B., Zhang, T., He, Q., Liu, S., & Jiang, B. (2025). Physiological Changes and Transcriptomics of Elodea nuttallii in Response to High-Temperature Stress. Biology, 14(8), 993. https://doi.org/10.3390/biology14080993





