Haustorium Formation and Specialized Metabolites Biosynthesis Using Co-Culture of Castilleja tenuiflora Benth. and Baccharis conferta Kunth
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Co-Culture Experiments
2.3. Environmental Scanning Electron Microscopy (ESEM)
2.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. Metabolite Analysis Through HPLC and LC-MS
2.6. Analysis of Differential Abundance of Metabolites
2.7. Statistical Analysis
3. Results
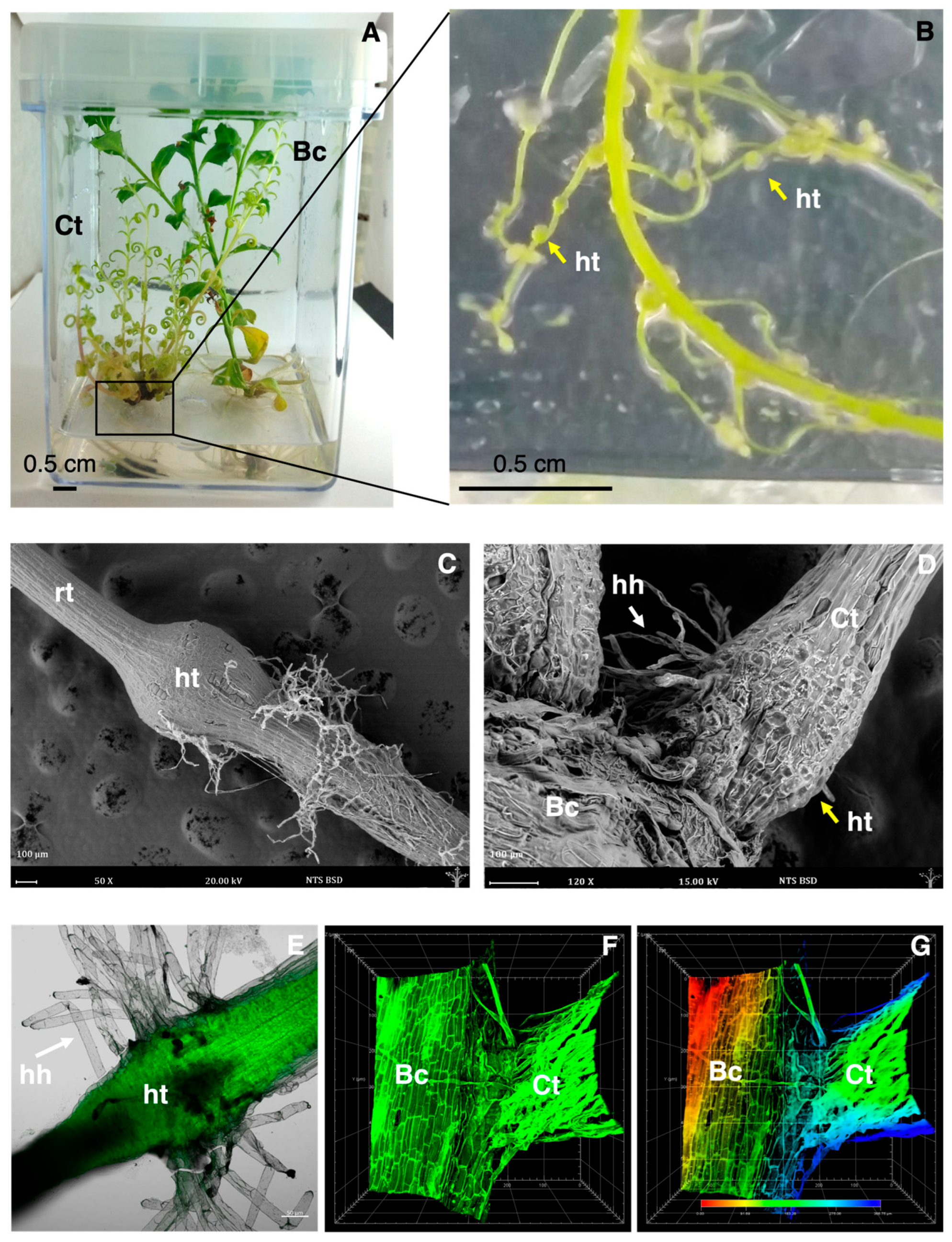
3.1. Formation of Haustorium in Co-Culture
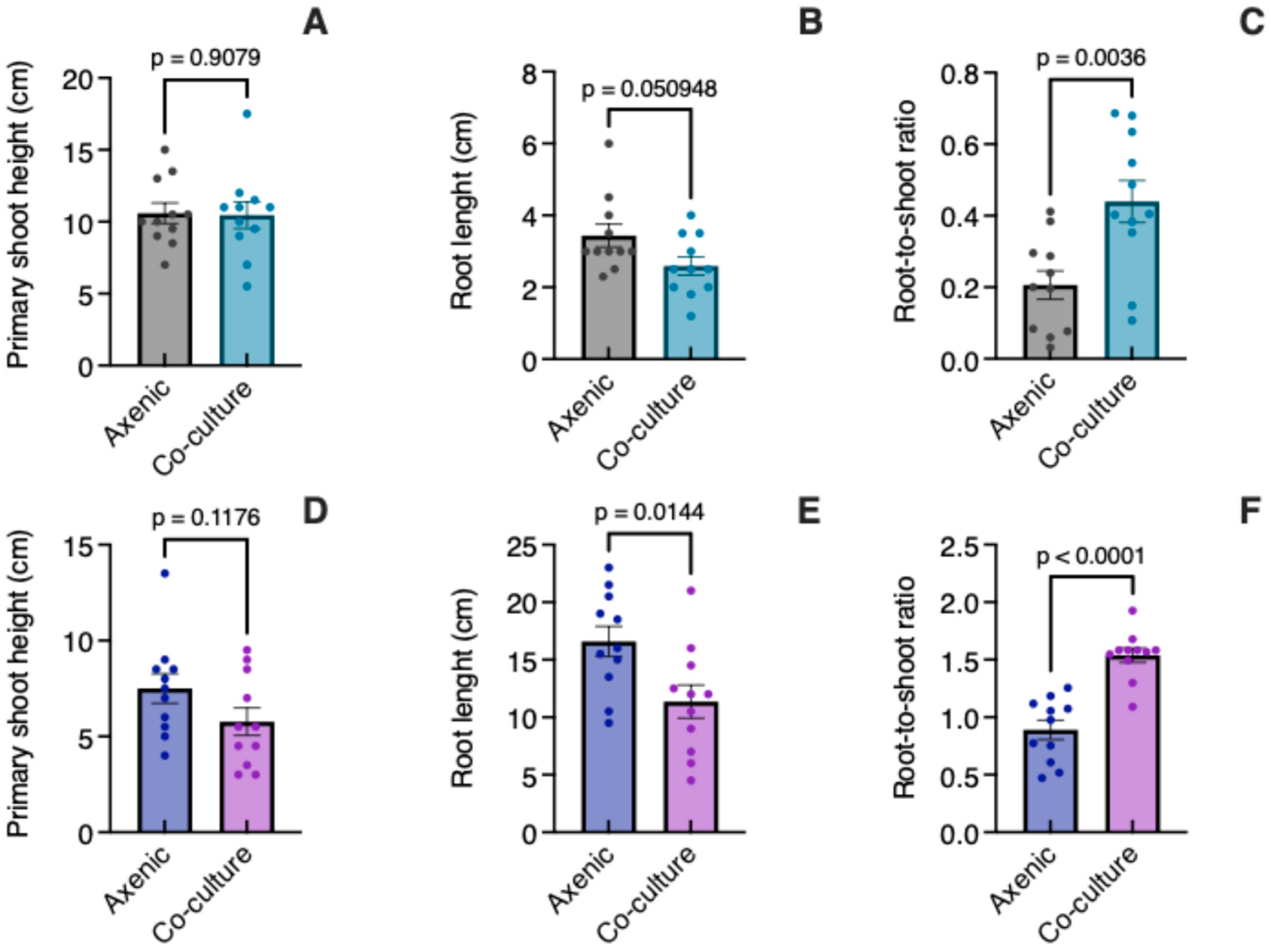
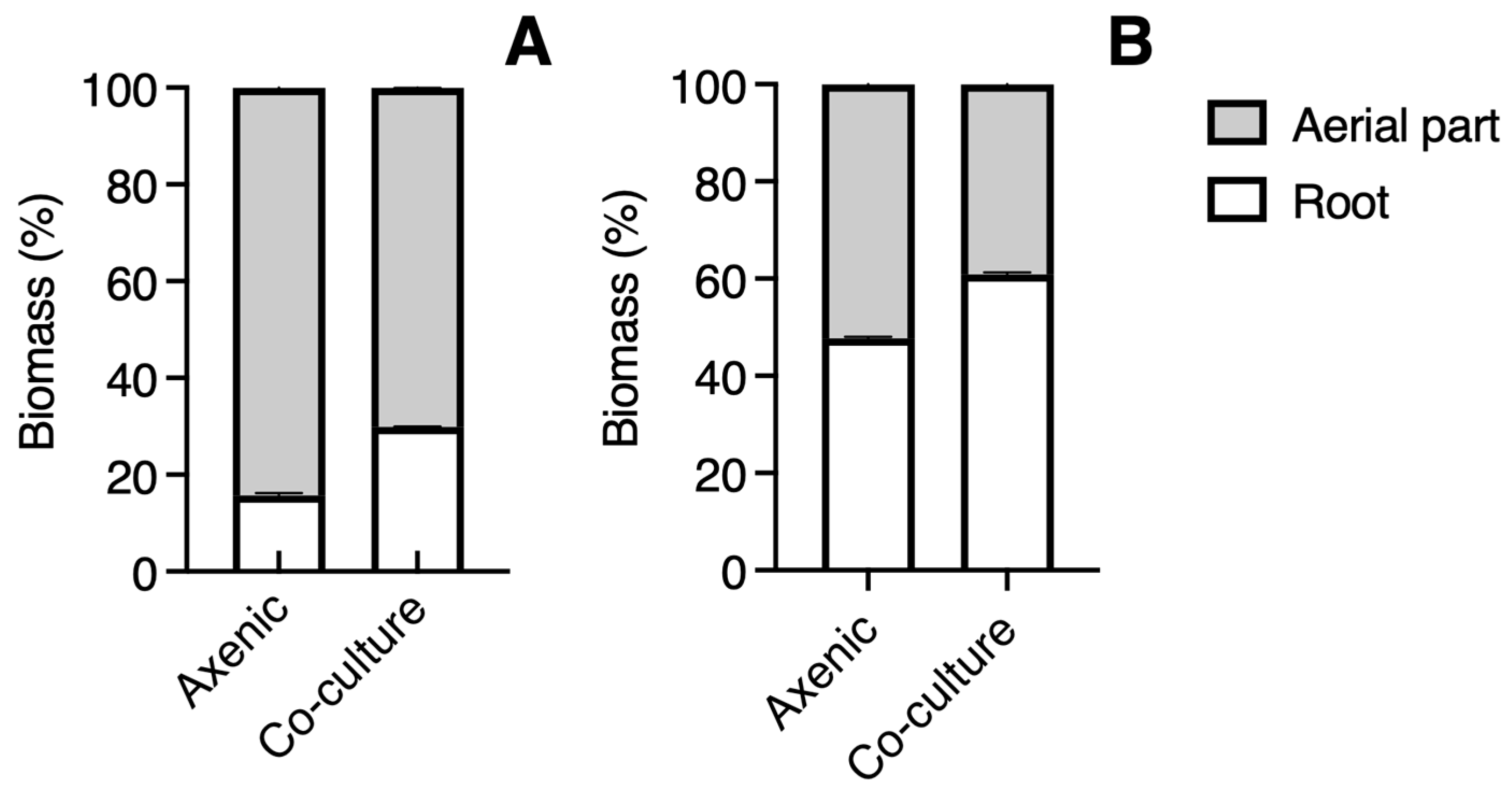
3.2. Growth in Co-Culture
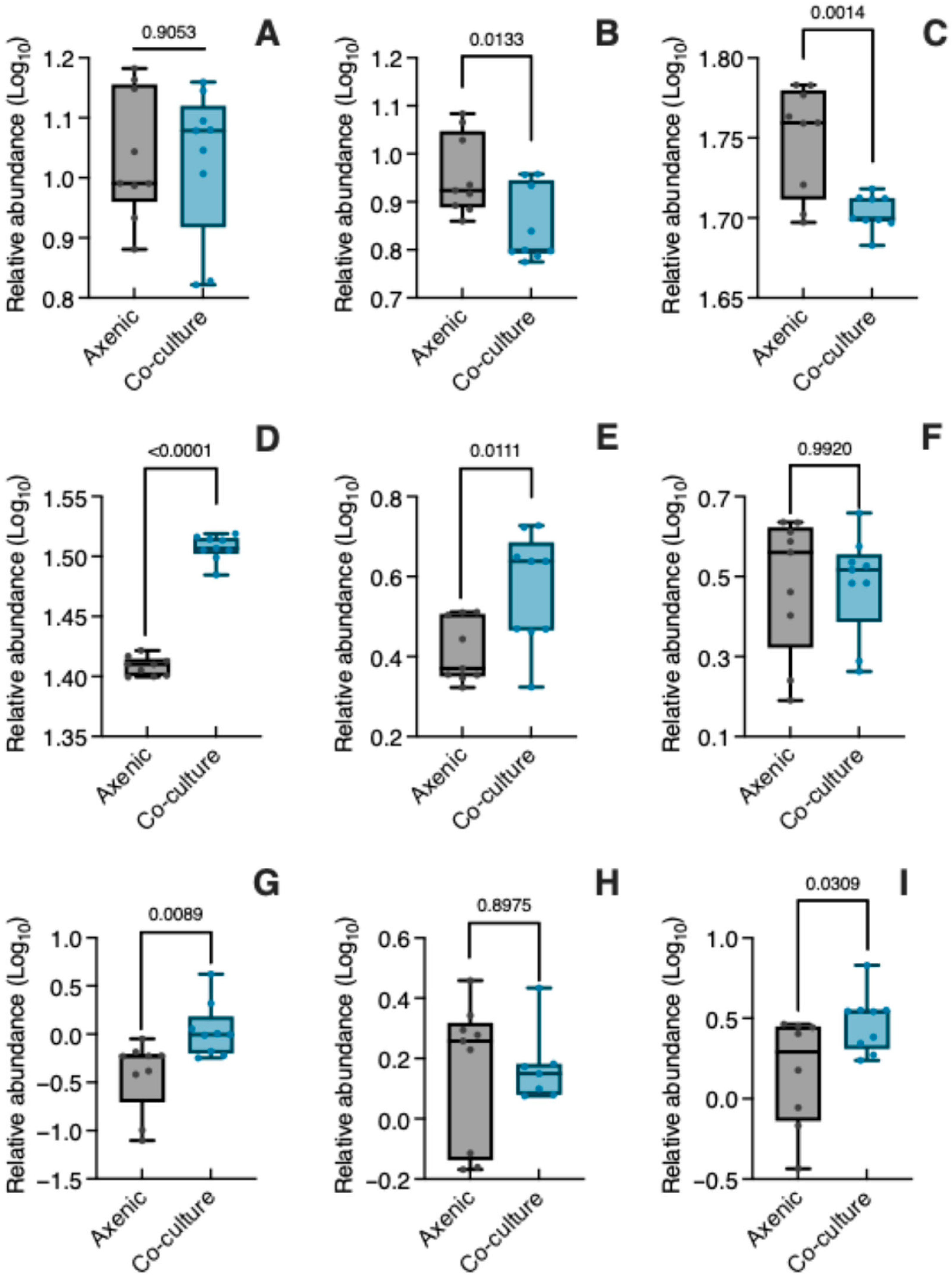
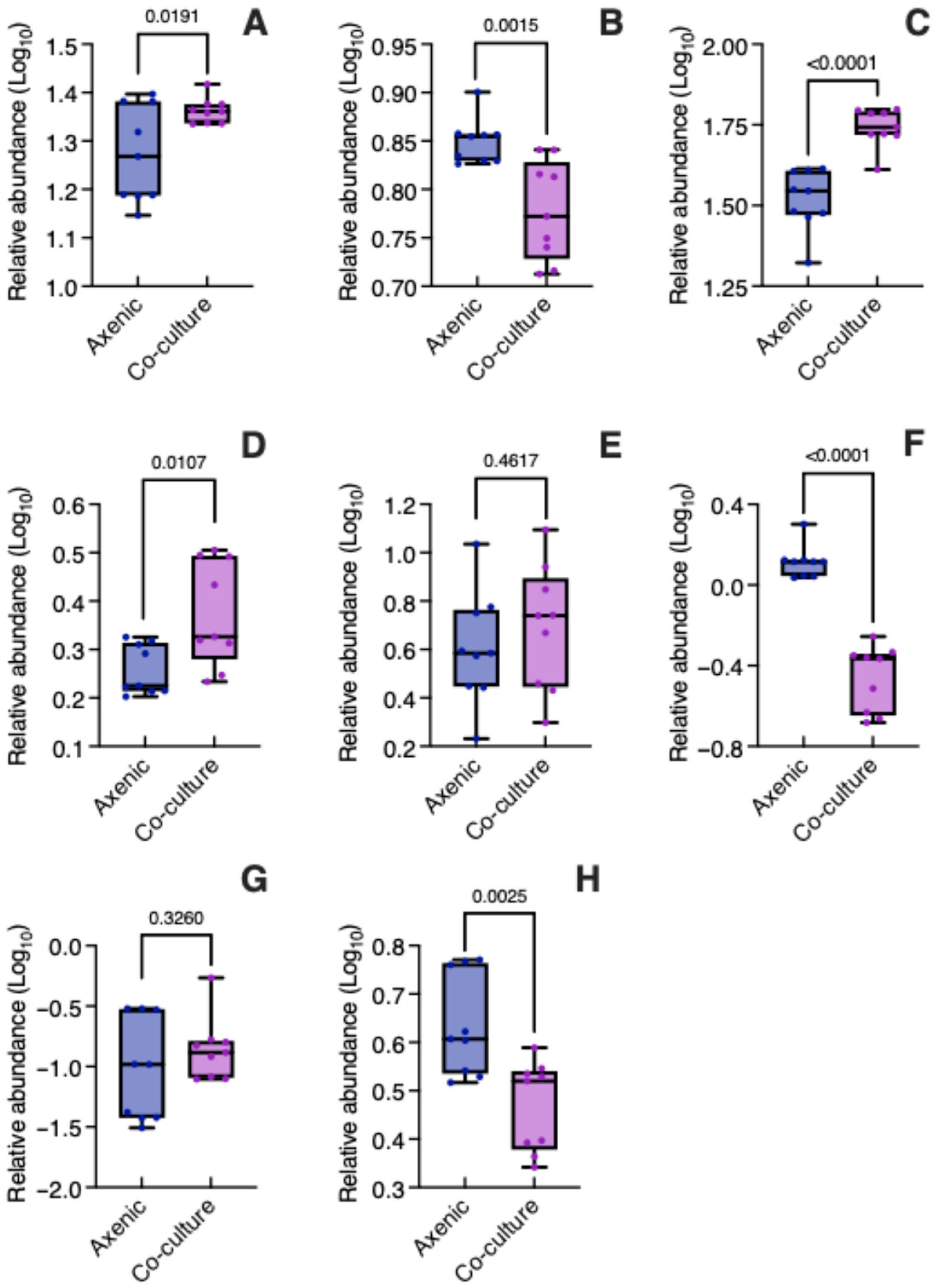
3.3. Specialized Metabolites in Co-Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4,5diQ | 4,5-di-O-caffeoylquinic acid |
| HIFs | haustorium-inducing factors |
| PhGs | phenylethanoid glycosides |
References
- Tesitel, J.; Plavcova, L.; Cameron, D.D. Heterotrophic carbon gain by the root hemiparasites, Rhinanthus minor and Euphrasia rostkoviana (Orobanchaceae). Planta 2010, 231, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef] [PubMed]
- Goyet, V.; Wada, S.; Cui, S.; Wakatake, T.; Shirasu, K.; Montiel, G.; Simier, P.; Yoshida, S. Haustorium Inducing Factors for Parasitic Orobanchaceae. Front. Plant Sci. 2019, 10, 1056. [Google Scholar] [CrossRef]
- Brun, G.; Leman, J.K.H.; Wicke, S. Comparative gene expression analysis of differentiated terminal and lateral haustoria of the obligate root parasitic plant Phelipanche ramosa (Orobanchaceae). Plants People Planet 2023, 7, 360–366. [Google Scholar] [CrossRef]
- Ajithkumar, T.G.; Thomas, S.; Mathew, L. Influence of hosts on the production of bioactive compounds in the hemiparasitic plant Helicanthes elasticus. Environ. Exp. Biol. 2021, 19, 161–171. [Google Scholar] [CrossRef]
- Béjar, E.; Reyes-Chilpa, R.; Jiménez-Estrada, M. Bioactive Compounds from Selected Plants Used in the XVI Century Mexican Traditional Medicine. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2000; Volume 24, Part E; pp. 799–844. [Google Scholar] [CrossRef]
- Arango-De la Pava, L.D.; Zamilpa, A.; Trejo-Espino, J.L.; Domínguez-Mendoza, B.E.; Jiménez-Ferrer, E.; Pérez-Martínez, L.; Trejo-Tapia, G. Synergism and Subadditivity of Verbascoside-Lignans and -Iridoids Binary Mixtures Isolated from Castilleja tenuiflora Benth. on NF-κB/AP-1 Inhibition Activity. Molecules 2021, 26, 547. [Google Scholar] [CrossRef]
- Ramirez-Cisneros, M.A.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; Rao, P.P.; Aburto-Amar, R.; Rodriguez-Lopez, V. In vitro COX-1 and COX-2 enzyme inhibitory activities of iridoids from Penstemon barbatus, Castilleja tenuiflora, Cresentia alata and Vitex mollis. Bioorg. Med. Chem. Lett. 2015, 25, 4505–4508. [Google Scholar] [CrossRef]
- Moreno-Escobar, J.A.; Bazaldúa, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; Rodríguez-López, V. Cytotoxic and antioxidant activities of selected Lamiales species from Mexico. Pharm. Biol. 2011, 49, 1243–1248. [Google Scholar] [CrossRef]
- Montes-Hernández, E.; Sandoval-Zapotitla, E.; Bermúdez-Torres, K.; Trejo-Espino, J.L.; Trejo-Tapia, G. Hemiparasitic interaction between Castilleja tenuiflora (Orobanchaceae) and Baccharis conferta (Asteraceae): Haustorium anatomy and C- and N-fluxes. Bot. Sci. 2019, 97, 192–201. [Google Scholar] [CrossRef]
- Salcedo-Morales, G.; Jiménez-Aparicio, A.R.; Cruz-Sosa, F.; Trejo-Tapia, G. Anatomical and histochemical characterization of in vitro haustorium from roots of Castilleja tenuiflora. Biol. Plant. 2014, 58, 164–168. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, L.R.; Domínguez-Hernández, D.; Pineda-López, M.R.; Lara-González, R. Does Baccharis conferta Shrub Act as a Nurse Plant to the Abies religiosa Seedling? Open For. Sci. J. 2011, 4, 67–70. [Google Scholar] [CrossRef]
- Gutierrez-Roman, A.S.; Trejo-Tapia, G.; Gonzalez-Cortazar, M.; Jimenez-Ferrer, E.; Trejo-Espino, J.L.; Zamilpa, A.; Ble-Gonzalez, E.A.; Camacho-Diaz, B.H.; Herrera-Ruiz, M. Anti-arthritic and anti-inflammatory effects of Baccharis conferta Kunth in a kaolin/carrageenan-induced monoarthritis model. J. Ethnopharmacol. 2022, 288, 114996. [Google Scholar] [CrossRef]
- Leyva-Peralta, A.L.; Salcedo-Morales, G.; Medina-Pérez, V.; López-Laredo, A.R.; Trejo-Espino, J.L.; Trejo-Tapia, G. Morphogenesis and in vitro production of caffeoylquinic and caffeic acids in Baccharis conferta Kunth. In Vitro Cell. Dev. Biol.—Plant 2019, 55, 581–589. [Google Scholar] [CrossRef]
- Leyva, A.L. Baccharis conferta Kunth: Cultivo In Vitro, Interacción con Castilleja tenuiflora Benth. Master’s Thesis, Instituto Politécnico Nacional, Morelos, Mexico, 2018. [Google Scholar]
- Landi, M.; Misra, B.B.; Nocito, F.F.; Lucchini, G.; Bruno, L.; Malara, A.; Abenavoli, M.R.; Araniti, F. Metabolic changes induced by Cuscuta campestris Yunck in the host species Artemisia campestris subsp. variabilis (Ten.) Greuter as a strategy for successful parasitisation. Planta 2022, 256, 118. [Google Scholar] [CrossRef]
- Clifford, M.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Tong, L.; Jiang, Y.; Zhang, X.; Zhang, X.; Zhang, W.; Ren, G.; Chen, Z.; Zhao, Y.; Guo, S.; Yan, H.; et al. Metabolic and molecular basis of flavonoid biosynthesis in Lycii fructus: An integration of metabolomic and transcriptomic analysis. J. Pharm. Biomed. Anal. 2025, 255, 116653. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wakatake, T.; Hashimoto, K.; Saucet, S.B.; Toyooka, K.; Yoshida, S.; Shirasu, K. Haustorial Hairs Are Specialized Root Hairs That Support Parasitism in the Facultative Parasitic Plant Phtheirospermum japonicum. Plant Physiol. 2016, 170, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Kyozuka, J.; Nomura, T.; Shimamura, M. Origins and evolution of the dual functions of strigolactones as rhizosphere signaling molecules and plant hormones. Curr. Opin. Plant Biol. 2022, 65, 102154. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Cui, S.; White, A.R.F.; Nelson, D.C.; Yoshida, S.; Shirasu, K. Strigolactones are chemoattractants for host tropism in Orobanchaceae parasitic plants. Nat. Commun. 2022, 13, 4653. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. Characterization of strigolactones exuded by Asteraceae plants. Plant Growth Regul. 2011, 65, 495–504. [Google Scholar] [CrossRef]
- Bürger, M.; Peterson, D.; Chory, J. Strigolactones initiate the formation of haustorium-like structures in Castilleja. iScience 2024, 27, 111491. [Google Scholar] [CrossRef] [PubMed]
- Huizinga SBouwmeester, H. KAI2 Can Do: Karrikin Receptor Function in Plant Development and Response to Abiotic and Biotic Factors. Plant Cell Physiol. 2023, 64, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Avilez-Montalvo, R.; Loyola-Vargas, V.M. Appendix A: The Components of the Culture Media. In Plant Cell Culture Protocols, Methods in Molecular Biology; Loyola-Vargas, V.M., Ocho-Alejo, N., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1815, pp. 493–502. [Google Scholar] [CrossRef]
- Irving, L.J.; Kim, D.; Schwier, N.; Vaughan, J.K.E.; Ong, G.; Hama, T. Host nutrient supply affects the interaction between the hemiparasite Phtheirospermum japonicum and its host Medicago sativa. Environ. Exp. Bot. 2019, 162, 125–132. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Watling, J.R.; Facelli, J.M. The combined effects of water and nitrogen on the relationship between a native hemiparasite and its invasive host. New Phytol. 2021, 229, 1728–1739. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Facelli, E.; Delean, S.; Facelli, J.M. Does phosphorus influence performance of a native hemiparasite and its impact on a native legume? Physiol. Plant 2021, 173, 1889–1900. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Facelli, J.M.; Watling, J.R. Does nitrogen affect the interaction between a native hemiparasite and its native or introduced leguminous hosts? New Phytol. 2017, 213, 812–821. [Google Scholar] [CrossRef]
- Tesitel, J.; Tesitelova, T.; Fisher, J.P.; Leps, J.; Cameron, D.D. Integrating ecology and physiology of root-hemiparasitic interaction: Interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytol. 2015, 205, 350–360. [Google Scholar] [CrossRef]
- Hautier, Y.; Hector, A.; Vojtech, E.; Purves, D.; Turnbull, L.A. Modelling the growth of parasitic plants. J. Ecol. 2010, 98, 857–866. [Google Scholar] [CrossRef]
- Haynes, A.F. Presence of N-fixing neighbors increases leaf N and δ13C in Castilleja applegatei, a root hemiparasite. Plant Ecol. 2021, 223, 213–228. [Google Scholar] [CrossRef]
- Monteiro, G.F.; Boanares, D.; Novais, S.; França, M.G.C.; Antonini, Y.; Barbosa, M.; Oki, Y.; Fernandes, G.W. Imbalance of water potential and photosynthetic efficiency in the parasitic relationship between Struthanthus flexicaulis and Baccharis dracunculifolia. Fol. Geob. 2022, 57, 71–82. [Google Scholar] [CrossRef]
- Mwangangi, I.M.; Buchi, L.; Haefele, S.M.; Bastiaans, L.; Runo, S.; Rodenburg, J. Combining host plant defence with targeted nutrition: Key to durable control of hemiparasitic Striga in cereals in sub-Saharan Africa? New Phytol. 2021, 230, 2164–2178. [Google Scholar] [CrossRef] [PubMed]
- Facelli, E.; Wynn, N.; Tsang, H.T.; Watling, J.R.; Facelli, J.M. Defence responses of native and invasive plants to the native generalist vine parasite Cassytha pubescens—anatomical and functional studies. Aust. J. Bot. 2020, 68, 300–309. [Google Scholar] [CrossRef]
- Keyes, W.J.; Taylor, J.V.; Apkarian, R.P.; Lynn, D.G. Dancing together. Social controls in parasitic plant development. Plant Physiol. 2001, 127, 1508–1512. [Google Scholar] [CrossRef]
- Rubio-Rodriguez, E.; Vera-Reyes, I.; Rodriguez-Hernandez, A.A.; Lopez-Laredo, A.R.; Ramos-Valdivia, A.C.; Trejo-Tapia, G. Mixed elicitation with salicylic acid and hydrogen peroxide modulates the phenolic and iridoid pathways in Castilleja tenuiflora plants. Planta 2023, 258, 20. [Google Scholar] [CrossRef]
- Jadhav, R.B.; Anarthe, S.J.; Surana, S.J.; Gokhale, S.B. Host-hemiparasite transfer of the C-glucosyl xanthone mangiferin between Mangifera indica and Dendrophthoe falcata. J. Plant Int. 2005, 1, 171–177. [Google Scholar] [CrossRef]
- Cabezas, N.J.; Urzúa, A.M.; Niemeyer, H.M. Translocation of isoquinoline alkaloids to the hemiparasite, Tristerix verticillatus from its host, Berberis montana. Biochem. Syst. Ecol. 2009, 37, 225–227. [Google Scholar] [CrossRef]
- Adler, L.S.; Wink, M. Transfer of quinolizidine alkaloids from hosts to hemiparasites in two Castilleja-Lupinus associations: Analysis of floral and vegetative tissues. Biochem. Syst. Ecol. 2001, 29, 551–561. [Google Scholar] [CrossRef]
- da Silva, N.M.; Oliveira, B.R.M.; Santos Silva, J.V.d.; de Carvalho Neto, C.H.; dos Santos, M.L.S.; de Oliveira, F.F.; de Almeida, A.-A.F. Hemiparasitism of Acanthosyris paulo-alvinii on Theobroma cacao, via root system through of haustorium, promotes anatomical, physiological, biochemical, and molecular changes and induce the host plant death. New For. 2024, 56, 1. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Venancio-Rodriguez, C.A.; Vasquez-Aguilar, A.A.; Alonso-Sanchez, A.G.; Perez-Torres, C.A.; Villafan, E.; Ramirez-Barahona, S.; Galicia, S.; Sosa, V.; Rebollar, E.A.; et al. Transcriptional Basis for Haustorium Formation and Host Establishment in Hemiparasitic Psittacanthus schiedeanus Mistletoes. Front. Genet. 2022, 13, 929490. [Google Scholar] [CrossRef]
- Li, C.; Gong, Q.; Liu, P.; Xu, Z.; Yu, Q.; Dai, H.; Shi, Y.; Si, J.; Zhang, X.; Chen, D.; et al. Co-expressed network analysis based on 289 transcriptome samples reveals methyl jasmonate-mediated gene regulatory mechanism of flavonoid compounds in Dendrobium catenatum. Plant Physiol. Biochem. 2024, 206, 108226. [Google Scholar] [CrossRef]
- Volpi, E.S.N.; Mazzafera, P.; Cesarino, I. Should I stay or should I go: Are chlorogenic acids mobilized towards lignin biosynthesis? Phytochemistry 2019, 166, 112063. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Goswami, S.K.; Gujjar, R.S.; Kumar, R.; Kumar, R.; Lal, M.K.; Kumari, M. Mechanistic insights on lignin-mediated plant defense against pathogen infection. Plant Physiol. Biochem. 2025, 228, 110224. [Google Scholar] [CrossRef]
- Cui, S.; Takeda-Kimura, Y.; Wakatake, T.; Luo, J.; Tobimatsu, Y.; Yoshida, S. Striga hermonthica induces lignin deposition at the root tip to facilitate prehaustorium formation and obligate parasitism. Plant Commun. 2025, 6, 101294. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Guo, C.; Peng, J.; Shao, F.; Sheng, S.; Wang, S. Transcriptomic Insights and Cytochrome P450 Gene Analysis in Kadsura coccinea for Lignan Biosynthesis. Genes 2024, 15, 270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Peralta, A.L.; Trejo-Espino, J.L.; Salcedo-Morales, G.; Tapia-Maruri, D.; Medina-Pérez, V.; López-Laredo, A.R.; Trejo-Tapia, G. Haustorium Formation and Specialized Metabolites Biosynthesis Using Co-Culture of Castilleja tenuiflora Benth. and Baccharis conferta Kunth. Biology 2025, 14, 990. https://doi.org/10.3390/biology14080990
Leyva-Peralta AL, Trejo-Espino JL, Salcedo-Morales G, Tapia-Maruri D, Medina-Pérez V, López-Laredo AR, Trejo-Tapia G. Haustorium Formation and Specialized Metabolites Biosynthesis Using Co-Culture of Castilleja tenuiflora Benth. and Baccharis conferta Kunth. Biology. 2025; 14(8):990. https://doi.org/10.3390/biology14080990
Chicago/Turabian StyleLeyva-Peralta, Annel Lizeth, José Luis Trejo-Espino, Guadalupe Salcedo-Morales, Daniel Tapia-Maruri, Virginia Medina-Pérez, Alma Rosa López-Laredo, and Gabriela Trejo-Tapia. 2025. "Haustorium Formation and Specialized Metabolites Biosynthesis Using Co-Culture of Castilleja tenuiflora Benth. and Baccharis conferta Kunth" Biology 14, no. 8: 990. https://doi.org/10.3390/biology14080990
APA StyleLeyva-Peralta, A. L., Trejo-Espino, J. L., Salcedo-Morales, G., Tapia-Maruri, D., Medina-Pérez, V., López-Laredo, A. R., & Trejo-Tapia, G. (2025). Haustorium Formation and Specialized Metabolites Biosynthesis Using Co-Culture of Castilleja tenuiflora Benth. and Baccharis conferta Kunth. Biology, 14(8), 990. https://doi.org/10.3390/biology14080990







