Simple Summary
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis occurring without significant alcohol consumption or other specific liver injury factors. The exact pathophysiological mechanisms underlying NAFLD remain incompletely understood. Autophagy is an intracellular process in eukaryotic cells involving the degradation and recycling of cytoplasmic components via membrane trafficking pathways. Impaired or defective autophagy is closely associated with the development and progression of NAFLD. Restoring autophagic function may represent a key pathway for alleviating hepatocyte injury. This review aims to summarize the association between autophagy and NAFLD, with a specific focus on the role of autophagy as a core mechanism. Recent research advances in dietary and exercise interventions, pharmacological treatments (including modern drug therapies and plant-derived compounds), and other approaches (such as hormones, nanoparticles, gut microbiota, and vitamins) are discussed. Additionally, a brief overview of autophagy-related molecular targets relevant to NAFLD treatment is provided.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is a clinicopathological syndrome characterised by hepatic steatosis in the absence of significant alcohol consumption or other specific causes of liver injury. It has become one of the leading causes of liver dysfunction worldwide. However, the precise pathophysiological mechanisms underlying NAFLD remain unclear, and effective therapeutic strategies are still under investigation. Autophagy, a vital intracellular process in eukaryotic cells, enables the degradation and recycling of cytoplasmic components through a membrane trafficking pathway. Recent studies have demonstrated a strong association between impaired or deficient autophagy and the development and progression of NAFLD. Restoring autophagic function may represent a key approach to mitigating hepatocellular injury. Nevertheless, due to the complexity of autophagy regulation and its context-dependent effects on cellular function, therapeutic strategies targeting autophagy in NAFLD remain limited. This review aims to summarise the relationship between autophagy and NAFLD, focusing on autophagy as a central mechanism. We discuss the latest research advances regarding interventions such as diet and exercise, pharmacological therapies (including modern pharmacological therapy and plant-derived compounds), and other approaches (such as hormones, nanoparticles, gut microbiota, and vitamins). Furthermore, we briefly highlight potential autophagy-related molecular targets that may offer novel therapeutic insights for NAFLD management.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a collective term for liver disorders associated with metabolic dysfunction, and it represents one of the most prevalent chronic liver diseases worldwide, affecting nearly 25% of the adult population. This imposes a significant burden on global healthcare systems. In the absence of excessive alcohol consumption or other liver diseases, NAFLD may progress from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH), with or without hepatic fibrosis, and eventually to cirrhosis and hepatocellular carcinoma [1]. Moreover, as a multisystem disease, NAFLD markedly increases the risk of developing type 2 diabetes, central obesity, dyslipidemia, chronic kidney disease, cardiovascular diseases, and heart failure [2] (Figure 1). Unfortunately, due to its complex pathogenesis, no approved targeted therapies are currently available for NAFLD.
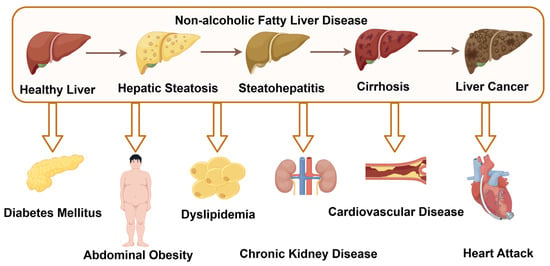
Figure 1.
The progression of NAFLD and its associated complications (by Figdraw 2.0, ID = YRPUYadda8).
Autophagy is a vital process by which eukaryotic cells maintain intracellular homeostasis under stress conditions such as nutrient deprivation, infection, or hypoxia, through the clearance of damaged organelles, proteins, or cellular debris. Based on the pathway through which cellular components are delivered to lysosomes, autophagy is categorised into three main types: macroautophagy (which is the focus of this manuscript), microautophagy, and chaperone-mediated autophagy [3]. Among them, macroautophagy is the most extensively studied and commonly observed form. Briefly, intracellular substrates destined for degradation are sequestered by a double-membraned structure known as the isolation membrane, forming an autophagosome, which subsequently fuses with the lysosome to form an autolysosome where degradation occurs [3]. Autophagy can also be classified based on the nature of its cargo into selective and non-selective autophagy. Non-selective autophagy is typically activated under general stress conditions such as nutrient deprivation, during which it non-specifically engulfs cytoplasmic constituents (including organelles and proteins) for degradation and recycling into energy and metabolic intermediates. In contrast, selective autophagy specifically identifies and removes particular substrates, such as damaged mitochondria (termed mitophagy), lipid droplets (lipophagy) or portions of the endoplasmic reticulum (ER-phagy) [4].
Previous studies have demonstrated that autophagy is implicated in the pathogenesis of various conditions, including neurodegeneration, cancer, myopathies, infections, inflammatory diseases, and lysosomal storage disorders [5]. More recently, increasing evidence has revealed a close relationship between autophagy and NAFLD. Autophagy contributes to hepatic homeostasis by regulating lipid metabolism, improving insulin resistance (IR) and hepatocellular injury, and suppressing inflammation and endoplasmic reticulum stress (ERS) [6]. Clinical studies have shown that autophagosomes are markedly reduced and autophagic activity is impaired in liver biopsy specimens from patients with NAFLD [7]. Furthermore, impaired or defective autophagy may exacerbate hepatic lipid accumulation and worsen steatosis [8]. These findings suggest that restoration or induction of autophagy could represent a promising therapeutic strategy for the treatment of NAFLD.
2. Autophagy
Autophagy is a multistep process orchestrated by a group of evolutionarily conserved autophagy-related genes (ATGs). To date, more than 40 highly homologous ATGs have been identified in both yeast and mammalian cells. Under the regulation of these ATGs, the autophagic process proceeds in a well-coordinated manner. Autophagy involves several key steps: initiation and nucleation of the phagophore, elongation and expansion of the autophagosomal membrane, fusion of the autophagosome with the lysosome, and subsequent degradation and recycling of the sequestered intracellular contents within the autolysosome [9,10].
Under stress conditions such as nutrient deprivation, hypoxia, oxidative stress, infection, inflammation, or exposure to various chemical agents, the ULK1 complex composed of unc-51-like kinases 1 and 2 (ULK1 and ULK2), ATG13, ATG101, and focal adhesion kinase family interacting protein of 200 kDa (FIP200) becomes activated [9]. This complex phosphorylates the class III phosphatidylinositol 3-kinase (PI3K) complex, also known as the Beclin1–Vps34 complex, which includes ATG14, VPS15, VPS34, AMBRA1, UVRAG, and Beclin1. This phosphorylation event initiates the formation of the phagophore, a double-membraned isolation membrane that marks the beginning of autophagosome assembly.
Beclin1 can interact with anti-apoptotic proteins such as BCL-2 and BCL-XL, which inhibit autophagosome formation by suppressing Beclin1 activity. Subsequently, the E1- and E2-like enzymatic activities of ATG7 and ATG10 catalyse the conjugation of ATG5 to ATG12. The resulting complex interacts with ATG16L to form the ATG5–ATG12–ATG16L conjugate. At the same time, ATG7 promotes the processing of LC3 (microtubule-associated protein 1A/1B-light chain 3) via ATG4B, enabling the conjugation of LC3-I with phosphatidyl-ethanolamine (PE) to produce LC3-II, the membrane-bound form of LC3 [3]. These two conjugation systems cooperatively drive the elongation of the autophagosomal membrane. The fusion of the autophagosome with the lysosome is mediated by SNARE proteins such as STX17 and VAMP8. This fusion results in the formation of the autolysosome, where the sequestered intracellular contents are degraded by lysosomal hydrolases. The degradation products, including amino acids and fatty acids, can be recycled by the cell for reuse or further participation in metabolic pathways [11] (Figure 2).
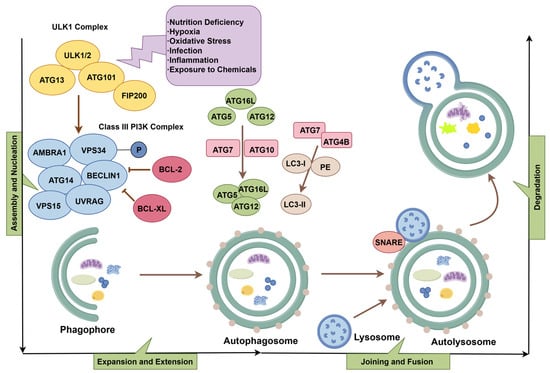
Figure 2.
The process of autophagy (by Figdraw 2.0, ID = UTARU504f4).
3. Impaired Autophagy Aggravates NAFLD
Under physiological conditions, autophagy plays a crucial role in maintaining hepatic metabolic homeostasis. During nutrient deprivation, hepatic autophagy is activated to promote the breakdown of intracellular substrates, thereby supplying energy to cells and organs. Conversely, under nutrient-rich conditions, autophagy is suppressed, favoring anabolic metabolism over catabolism [6]. Autophagy also alleviates hepatocellular damage induced by high-glucose and high-lipid conditions to some extent [12,13,14]. However, when autophagy is impaired or deficient, excessive accumulation of lipid droplets, inflammation, ERS, and apoptosis in the liver can aggravate metabolic dysregulation and contribute to the onset and progression of NAFLD [8] (Figure 3). For example, liver biopsy specimens from NAFLD patients have shown an association between hepatic inflammation and defective autophagic activity [15]. Another study indicated that impaired autophagic flux during NAFLD progression is related to ERS-induced hepatocyte apoptosis [16]. These findings suggest that inflammation and ERS may partly mediate autophagy dysfunction in NAFLD. Additional studies have confirmed that mice with autophagy deficiency exhibit hepatomegaly and parenchymal liver injury, characterised by inflammatory infiltration, hepatocyte apoptosis, pericellular fibrosis, and prominent proliferation of bile duct epithelial cells [17]. In patients with NASH, autophagy deficiency in liver sinusoidal endothelial cells has been shown to promote hepatic inflammation, endothelial-to-mesenchymal transition, apoptosis, and the development of liver fibrosis [18].
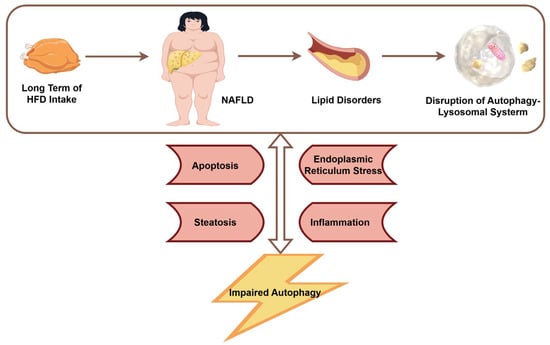
Figure 3.
The relationship between NAFLD and autophagy (by Figdraw 2.0, ID = ITTRP111a0).
4. Restoration of Autophagy Ameliorates NAFLD
Based on the above findings, it can be inferred that autophagy dysfunction plays a critical role in the pathogenesis of NAFLD. Autophagy may represent an integrative therapeutic target for NAFLD, and restoring or inducing autophagic activity may attenuate or even halt disease progression. This provides a novel direction for the development of treatment strategies against NAFLD.
In this context, we summarise recent therapeutic approaches aimed at enhancing autophagy, including lifestyle interventions such as diet and exercise, pharmacological agents including modern drugs and plant-derived compounds, and other interventions such as hormones, nanoparticles, gut microbiota modulation, and vitamins (Figure 4).
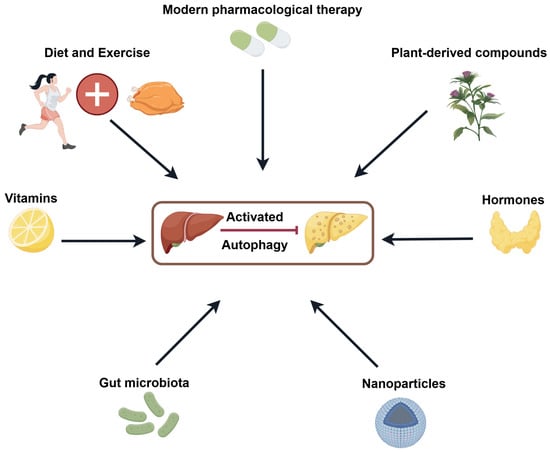
Figure 4.
Strategies for treating NAFLD by inducing autophagy (by Figdraw 2.0, ID = PRUTU5c4a3).
4.1. Diet and Exercise
A healthy diet combined with regular physical activity has long been regarded as the first-line therapeutic approach for NAFLD. Properly structured lifestyle interventions can help reduce body weight, alleviate hepatic inflammation, steatosis, and lipid accumulation, enhance liver function, and ultimately slow disease progression [19]. Mechanistic studies on the preventive and therapeutic effects of diet and exercise in NAFLD have been extensively conducted. These investigations primarily focus on improving hepatic lipid droplet dynamics, reducing inflammation, ERS, and oxidative stress, as well as restoring autophagy, mitochondrial dysfunction, and gut microbiota imbalance [20,21,22]. Among these mechanisms, the restoration of autophagy is considered one of the key contributors to the beneficial effects of dietary and exercise interventions in NAFLD management.
Previous studies have shown that chronic nutrient excess can induce hepatic steatosis and liver injury, potentially through mechanisms involving impaired autophagic activity and ERS [23]. In contrast, caloric restriction enhances hepatic autophagy, mitochondrial biogenesis, and the expression of inflammation-related proteins [24]. Intermittent fasting (IF) is a dietary strategy characterised by alternating periods of fasting and feeding, including protocols such as alternate-day fasting (ADF), time-restricted feeding (TRF), and the fasting-mimicking diet (FMD) [25]. IF can reduce the risk of metabolic disorders in overweight or obese individuals by alleviating oxidative stress, optimising circadian rhythm, and promoting ketogenesis. Strong evidence supports the therapeutic potential of IF in managing NAFLD [26]. Animal experiments have demonstrated that IF can significantly reduce body weight, liver weight, and the homeostasis model assessment of insulin resistance (HOMA-IR) index in NAFLD mouse models [27]. In addition, IF effectively decreases hepatic lipid accumulation and inflammation, thereby attenuating lipotoxicity associated with NAFLD [28,29]. Mechanistic studies have further revealed that IF exerts its protective effects by activating the MIF/AMPK and AMPK/ULK1 signalling pathways, and by inhibiting mTOR phosphorylation, thus regulating autophagy and apoptosis to improve hepatic function in NAFLD [27,29]. Beyond caloric restriction, several dietary supplements have shown promise in alleviating NAFLD by promoting hepatocellular autophagy. These include medium-chain fatty acids [30], corn peptides [31], and γ-linolenic acid [32], all of which reduce hepatic lipid accumulation. Notably, branched-chain amino acids (BCAAs) may activate the mTOR pathway, suppressing the conversion of free fatty acids (FFA) to triglycerides (TG) and inhibiting autophagy, thereby exacerbating hepatic lipotoxicity [33]. However, some studies suggest that moderate BCAA supplementation does not necessarily exacerbate insulin resistance, impair glucose tolerance, or directly induce lipotoxicity [34,35,36]. The observed inconsistencies across studies may be attributed to tissue-specific effects of BCAAs and variations in dietary contexts, which warrant further investigation for conclusive evidence.
In addition to dietary adjustment, exercise can also reduce hepatic lipid accumulation and improve NAFLD by enhancing hepatic autophagy [37,38]. Lysosomes are important organelles for degrading intracytoplasmic lipid droplets (LDs). Lipophagy is a type of selective autophagy that targets lipid droplets for degradation to maintain cellular lipid homeostasis [39]. Exercise can promote lipophagy via regulating lysosome number and function, which further ameliorates hepatic steatosis [40]. Obesity, hepatic steatosis, inflammation and hepatic injury significantly improved in NAFLD mice after 15 weeks of aerobic plate training (60 min/day, 5 days/week). Further studies revealed that exercise regulated hepatic LDs dynamics by inhibiting the expansion of abnormal LDs, promoting lysosomal co-localisation with LDs during lipid phagocytosis, and inducing lysosomal clearance of LDs [41]. Similarly, ET (endurance training) and VPA (voluntary physical activity) improved hepatic mitochondrial biogenesis-related proteins and autophagy signalling. In addition, ET reduced susceptibility to hepatic mitochondrial permeability transition pore (mPTP) and positively regulated factors associated with mitochondrial transcription, fusion and autophagy. It hinted that autophagy/mitochondrial autophagy induction may be an important approach for exercise to protect the liver [42]. In addition, certain proteins that regulate lipids may also serve as important bridges that link motility and autophagy. Fatty acid-binding protein (FABP1) is a hepatic fatty acid binding protein that inhibits TG metabolism, cholesterol uptake and lipid transport [43]. A research team trained NAFLD mice to swim for 12 weeks and discovered that exercise down-regulated FABP1, which subsequently restored lysosomal protease activity and lysosomal acidification, significantly increased autophagic flux, and preserved lipid homeostasis in the liver [44]. In addition, exercise attenuates hepatic steatosis by activating autophagy through AMPK-related pathways. Guarino et al. [45] demonstrated that exercise increased LC3-II/LC3-I and activated the AMPK/mTOR pathway during improving biochemical and histological parameters in NAFLD. Furthermore, Li et al. [46] showed that exercise also ameliorated LDs metabolic disorders in NAFLD by activating the AMPK/Sirtuin1 (SIRT1) pathway and lipophagy. Recently, studies that adopted a combination of diet and exercise strategies have also found that hepatic autophagy played an important role in slowing down the process of NAFLD. The specific mechanism was related to the reduction of inflammation and ERS, activation of the AMPK/ULK1 pathway, and inhibition of the Akt/mTOR/ULK1 pathway [47,48]. Notably, current evidence demonstrates a dose-response relationship between exercise and autophagy activation. Moderate-to-high intensity aerobic exercise with intermediate duration (e.g., 30–60 min at 50–70% VO2max) appears most effective in inducing physiological autophagy [49,50]. However, optimal dosing should be individualized based on factors such as age, health status, and environmental conditions [51,52]. Taken together, the above studies suggested that regulation of diet and/or exercise-related lifestyle can help delay the progression of NAFLD, and one important mechanism may be related to the activation of autophagy (Table 1).

Table 1.
Diet and exercise regimens associated with autophagy induction in treating NAFLD.
4.2. Modern Pharmacological Therapy
For metabolic diseases such as NAFLD, pharmacological intervention should be considered when lifestyle management alone fails to control disease progression. The development and progression of NAFLD are closely associated with lipotoxicity, insulin resistance (IR), oxidative stress, and inflammation. Therefore, drugs that regulate glucose and lipid metabolism, improve IR, and possess anti-inflammatory and antioxidant properties may be beneficial for the treatment of NAFLD. In recent years, emerging evidence has demonstrated that modern pharmacological therapies, including glucose-lowering and lipid-lowering agents, can effectively reduce hepatic lipid accumulation and alleviate liver fibrosis. Notably, these therapeutic effects are, at least in part, mediated through the restoration of autophagic homeostasis.
4.2.1. SGLT-2i
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a new class of oral hypoglycemic agents. At present, several SGLT2 inhibitors, including dapagliflozin, canagliflozin, empagliflozin, and ertugliflozin, have been approved by the United States Food and Drug Administration [53]. These drugs exert their hypoglycemic effects primarily by inhibiting sodium-glucose cotransporters in the renal proximal tubules, thereby preventing glucose reabsorption. In addition to lowering blood glucose levels in patients with diabetes, they have also been shown to protect the kidney and cardiovascular system, reduce visceral and ectopic fat, improve lipid profiles and insulin resistance, and lower body weight, serum uric acid, and blood pressure [54]. In recent years, increasing evidence has demonstrated the potential of SGLT2 inhibitors as promising therapeutic agents for the treatment of NAFLD. The underlying mechanisms may be closely related to the induction of autophagy [55]. For example, empagliflozin enhances autophagy in hepatic macrophages through the AMPK and mTOR signalling pathways. This activation further suppresses inflammation mediated by the interleukin 17 and interleukin 23 axis, thereby reducing liver injury in mouse models of NAFLD combined with type 2 diabetes mellitus [56]. In a recent study, Chun and colleagues reported that SGLT2 expression is elevated in the liver tissues of patients with nonalcoholic steatohepatitis. This finding provides a theoretical basis for the hepatic action of SGLT2 inhibitors [57]. Empagliflozin has also been found to activate the AMPK and TFEB pathway by reducing O-GlcNAcylation levels in the liver. This leads to enhanced autophagic flux and ultimately attenuates hepatic lipid accumulation, inflammation, and fibrosis [57]. Recent studies have also shown that dapagliflozin and canagliflozin improve NAFLD through autophagy regulation [58,59]. Dapagliflozin increases the levels of autophagy-related markers such as LC3B and Beclin1, reduces p62 expression, and induces autophagy through the AMPK and mTOR pathway [59]. Canagliflozin promotes autophagy by increasing the ratio of LC3 II to LC3 I and by upregulating Atg7, thereby regulating hepatic lipid metabolism and suppressing inflammation [58]. Taken together, SGLT2 inhibitors may alleviate hepatic steatosis by activating autophagy. They represent a potentially effective therapeutic approach for NAFLD and offer new perspectives for clinical application.
4.2.2. GLP1-RA
Glucagon-like peptide 1 (GLP-1) is an incretin hormone that stimulates insulin secretion in a glucose-dependent manner upon binding to its receptor. It also inhibits glucagon secretion and exerts additional effects, including anti-inflammatory activity, cardiovascular protection, delayed gastric emptying, and regulation of lipid metabolism. Currently, GLP-1 receptor agonists (GLP-1RAs) are widely used in the clinical management of diabetes and obesity [60,61]. Recent studies have demonstrated that GLP-1RAs can improve liver injury and metabolic disturbances in patients with NAFLD. Similar to the mechanism of action of sodium-glucose cotransporter 2 inhibitors, GLP-1RAs improve NAFLD by suppressing hepatic inflammation and oxidative stress, enhancing lipid metabolism, and promoting autophagy [62]. For instance, liraglutide has been shown to induce autophagy through the SIRT1 and SIRT3-mediated FOXO3a and LC3 pathway, and to enhance mitochondrial structure by upregulating proteins involved in mitochondrial fission, fusion, and the respiratory chain. These effects ultimately reduce oxidative stress and improve hepatic function in NAFLD [63]. Furthermore, liraglutide can induce the expression of autophagy-related proteins such as LC3B, Beclin1, and Atg7, and activate both the AMPK and mTOR signalling pathway and the ROR alpha-mediated autophagy pathway, thereby reducing hepatic lipid deposition. Similarly, Yu and colleagues found that liraglutide alleviates mitochondrial dysfunction and reactive oxygen species generation in nonalcoholic steatohepatitis by promoting mitophagy [64]. Exenatide has also been reported to reduce oxidative stress and inhibit the NLRP3 inflammasome by enhancing hepatic autophagy and mitophagy. These effects contribute to the protection of liver function in mice with NAFLD and type 2 diabetes mellitus [65]. Moreover, exenatide can activate the AKT and mTOR pathway and promote the autophagy lysosome pathway, thereby increasing autophagic flux and reducing lipotoxicity and lipid accumulation in hepatocytes [66,67]. These findings suggest that GLP-1 receptor agonists may represent an important therapeutic strategy against NAFLD by promoting hepatic autophagy, regulating mitochondrial function and structure, and suppressing inflammation and oxidative stress. In the future, GLP-1RAs may become a valuable treatment option for patients with NAFLD. Table S1 provides a comprehensive comparison of the drug profiles between SGLT-2i and GLP-1RAs.
4.2.3. Biguanides
Metformin is the principal member of the biguanide class of glucose-lowering agents. It suppresses hepatic gluconeogenesis, enhances fatty acid oxidation, inhibits lipogenesis, and increases insulin sensitivity. Recent studies have applied metformin to the management of nonalcoholic fatty liver disease and have shown that it can improve the condition by inducing autophagy [68]. Metformin activates autophagy by down-regulating STAT3 and simultaneously reduces the expression of inflammatory cytokines such as IL-1β, IL-6, and TNF-α, thereby exerting therapeutic effects in nonalcoholic steatohepatitis [69]. Song and colleagues [70] further demonstrated that metformin alleviates hepatocellular lipid accumulation by stimulating SIRT1-mediated autophagy through an AMPK-independent mechanism. It can also promote Parkin-mediated mitophagy, improving hepatic lipid metabolism [71]. Zhang et al. [72] confirmed that metformin attenuates hepatic steatosis and insulin resistance in mouse models of nonalcoholic fatty liver disease by enhancing TFEB-mediated autophagy. The most recent research indicates that metformin activates tristetraprolin via the AMPK and SIRT1 pathway; tristetraprolin then suppresses inflammation to reduce hepatocellular necrosis and promotes hepatic lipophagy by inhibiting mTORC1 and increasing TFEB nuclear translocation [73]. Collectively, these findings suggest that metformin mitigates nonalcoholic fatty liver disease by activating hepatocellular autophagy through the modulation of multiple transcription factors.
4.2.4. Lipid-Modifying Drugs
Under normal physiological conditions, triglycerides (TG) stored in LDs can be hydrolysed into free fatty acids (FFA) to supply energy to the body. In patients with NAFLD, the degradation of TG in LDs is impaired, leading to hepatic TG accumulation and the development of insulin resistance (IR). IR, in turn, promotes the influx of additional FFAs into the liver, further exacerbating hepatic lipid accumulation and steatosis [74]. Several lipid-lowering agents have been shown to delay the progression of NAFLD by stabilising lipid profiles, reducing IR, and alleviating oxidative stress and inflammation. Some studies suggest that the induction of autophagy may represent a key mechanism by which these lipid-modulating drugs exert their therapeutic effects on NAFLD. For instance, Yoo and colleagues [75] reported that fenofibrate reduces hepatic lipid accumulation by activating lipophagy and the transcription factors TFEB and TFE3 through PPARα agonism. Another recent study also confirmed that the beneficial effects of fenofibrate on hepatic steatosis, IR, and gut microbiota modulation depend on TFEB-mediated autophagy [76]. Ezetimibe has also been shown to alleviate hepatic steatosis and IR in obese and type 2 diabetic rats by inducing autophagy, which results in reduced serum glucose, insulin, and lipid levels [77]. Its mechanism involves the activation of the Nrf2-Keap1 antioxidant signalling pathway through p62-dependent autophagy, thereby protecting hepatocytes from oxidative injury [78]. In addition to these agents, other drugs from various therapeutic classes have also been reported to improve hepatic steatosis through autophagy activation. These include pioglitazone (the PPAR-γ agonist) [79] and gemigliptin (the DPP-4 inhibitor) [80] from the category of antidiabetic agents, irbesartan [81] among antihypertensive drugs, the nonsteroidal anti-inflammatory drugs celecoxib [82] and valdecoxib [83] and the FXR agonist obeticholic acid [84]. These findings are summarised in Table 2.

Table 2.
Modern pharmacological therapy related to autophagy induction in treating NAFLD.
4.3. Plant-Derived Compounds
In recent years, plant extracts have been increasingly shown to improve NAFLD by modulating autophagy. Given the wide variety of compounds, we have organised the relevant findings based on autophagy-related signalling pathways and their targeted mechanisms involved in NAFLD pathogenesis (Table 3).
4.3.1. Autophagy Involved in the AMPK Signalling Pathway
AMPK is a key regulator of energy metabolism and plays an essential role in hepatic lipid homeostasis. Activation of AMPK and its associated signalling pathways represents an important mechanism by which many bioactive compounds modulate autophagy [88]. Several plant-derived compounds, including icariin [89], Toona sinensis bark and fruit extracts [90], naringenin [91] and psoralen [92], have been shown to directly promote AMPK phosphorylation, thereby inducing autophagy and reducing hepatic lipid accumulation in models of NAFLD. Schisandrin B, a lignan compound isolated from Schisandra chinensis, activates autophagy via the AMPK and mTOR signalling pathway, suppresses hepatic steatosis, and promotes fatty acid oxidation [93]. Similarly, pterostilbene, extracted from Pterocarpus species, has been shown to activate autophagy by upregulating Nrf2 expression and promoting the AMPK and mTOR pathway, thereby alleviating oxidative stress associated with lipid overload in hepatocytes and enhancing fatty acid catabolism [94]. In addition, several traditional Chinese medicines or plant extracts have been confirmed through in vivo and/or in vitro experiments to ameliorate NAFLD via similar pathways. These include mangiferin [95], atractyloside [96], sweroside [97], thymiquinone [98], and red pepper seeds [99]. Sirt1 also participates in the regulation of hepatic lipid metabolism by inducing autophagy, and it is known to act cooperatively with AMPK. Several compounds, such as ginsenoside Rb2 [100], sodium isosteviol [101], and apple polyphenol extract [102], have been reported to restore hepatic autophagy and improve lipid metabolism in NAFLD through the SIRT1 and AMPK signalling pathway. Furthermore, catalpol [103] and aurantio-obtusin [104] have been shown to alleviate hepatic steatosis by activating AMPK and TFEB-dependent autophagy.
4.3.2. Autophagy Involved in the TFEB Signalling Pathway
Defective fusion between autophagosomes and lysosomes impairs autophagic flux, leading to intracellular lipid accumulation and contributing to the development of NAFLD. TFEB is considered a master regulator of autophagy and lysosomal biogenesis. Under stress conditions, TFEB translocates from the cytoplasm and lysosomal surface into the nucleus, where it promotes lysosomal biogenesis, enhances autophagy, and facilitates mitochondrial fatty acid degradation. Phillyrin, a lignan extracted from Forsythia suspensa, has been shown to restore hepatic lipophagy and reduce lipid accumulation and inflammation by stimulating endoplasmic reticulum calcium release in hepatocytes, thereby activating calcineurin and regulating TFEB dephosphorylation and nuclear translocation [105]. A lead compound IA, isolated from Paeonia lactiflora, has been reported to alleviate high-fat diet (HFD)-induced liver injury by activating farnesoid X receptor (FXR) and promoting lipid degradation through TFEB-mediated autophagy induction [106]. Similarly, compounds such as ajugol [107], polydatin [108], nuciferine [109], and formononetin [110] have also been found to restore autophagic flux and alleviate NAFLD by enhancing TFEB-mediated autophagy–lysosome pathways and lipid-specific autophagy. Current research has primarily focused on animal models and cellular experiments, with no direct reports of clinical trials specifically investigating autophagy pathways such as AMPK/mTOR and TFEB in human NAFLD. Future human clinical trials are needed to validate the translational effects of these pathways in NAFLD treatment, particularly regarding the clinical safety and efficacy of TFEB activators or AMPK/mTOR modulators.
4.3.3. Autophagy Involved in Oxidative Stress and Endoplasmic Reticulum Stress (ERS) Pathways
ERS and oxidative stress are major drivers of the onset and progression of NAFLD. Excessive ERS and oxidative stress generate reactive oxygen species that damage mitochondria, and because autophagosome formation usually begins on mitochondrial or endoplasmic reticulum membranes, inappropriate ERS and oxidative stress can impair autophagy to some extent [111]. Quercetin, a flavonoid polyphenol with antioxidant and immunomodulatory activities, reduces hepatic triglyceride content through the IRE1α/XBP1s pathway, increases very-low-density lipoprotein assembly and lipophagy, and thereby mitigates high-fat diet-induced NAFLD [112]. Scutellarin suppresses the IRE1α/XBP1 branch, up-regulates Foxo1-mediated autophagy, and the resulting autophagy activation relieves ERS and ultimately down-regulates SREBP-1c-dependent lipogenesis [113]. Recent evidence indicates that aescin promotes hepatic autophagy by activating the Keap1/Nrf2 antioxidant pathway, improving lipid accumulation in NAFLD [114]. Zhang and colleagues reported that physalin B extracted from Physalis species increases the autophagy markers p62 and LC3 II/I while activating the p62/Keap1/Nrf2 antioxidant pathway, which alleviates hepatic oxidative stress and improves nonalcoholic steatohepatitis [115]. These findings suggest that autophagy, oxidative stress, and ERS regulate one another and together play critical roles in the progression of NAFLD.
4.3.4. Autophagy Involved in Inflammatory Pathways
Inflammation is a hallmark feature of NASH. Lipid overload in hepatocytes causes lipotoxicity, which promotes the release of damage-associated molecular patterns (DAMPs). These DAMPs can bind to pattern recognition receptors (PRRs), thereby activating hepatic immune responses involving resident Kupffer cells and other inflammatory cells, leading to a cascade of inflammatory signalling events [116]. Previous studies have shown that defective autophagy exacerbates hepatic inflammation in NAFLD, whereas activation of autophagy alleviates hepatic steatosis and inflammation [117,118]. Scoparone, a natural bioactive compound isolated from Fritillaria, has been reported to enhance macrophage autophagy and suppress inflammation by modulating the ROS/P38/Nrf2 axis and the PI3K/AKT/mTOR signaling pathway in macrophages [119]. Similarly, glycyrrhetinic acid, extracted from licorice root, alleviates impaired autophagic flux and excessive hepatocyte apoptosis by regulating the STAT3 and HIF-1α pathway in macrophages, resulting in reduced production of inflammatory cytokines [120]. In addition, other plant-derived compounds such as phloretin [121], resveratrol [118], and magnolol [122] have been shown to activate hepatic autophagy, reduce inflammation, and attenuate liver injury associated with NAFLD.
4.3.5. Mitophagy
Mitophagy, the selective degradation of damaged or dysfunctional mitochondria via the autophagic pathway, has emerged as a key cellular process for maintaining mitochondrial quality control [4]. Several compounds have been shown to improve NAFLD by enhancing mitophagy, offering new therapeutic possibilities [123]. Mechanistic studies have revealed that cyanidin-3-O-glucoside (C3G) improves hepatic steatosis and glucose metabolism by upregulating the expression and mitochondrial localisation of PINK1 and Parkin, thereby promoting PINK1-mediated mitophagy [124]. Akebia saponin D (ASD), the most abundant component in the rhizome of Dipsacus asper, has been reported to reduce hepatic lipid accumulation by targeting BNip3-mediated mitophagy [125]. Two independent studies on quercetin have shown that it attenuates liver injury, histopathological changes, and lipid metabolism disturbances in NAFLD by activating mitophagy through both AMPK-dependent and frataxin-regulated PINK1/Parkin-dependent pathways [126,127]. These findings suggest that enhancing selective mitophagy may represent a promising strategy for the treatment of NAFLD.

Table 3.
Herbal medicines or plant extracts associated with autophagy induction for NAFLD treatment.
Table 3.
Herbal medicines or plant extracts associated with autophagy induction for NAFLD treatment.
| Items | Medicines/Plant Extracts | Sources/Properties | NAFLD Models | Mechanisms for Improving NAFLD | Years | Ref |
|---|---|---|---|---|---|---|
| AMPK-related | Icaritin | Herba Epimedii | Huh-7/L02 cells + sodium oleate | Increasing energy expenditure and regulating autophagy by | 2021 | [89] |
| autophagy | activating the AMPK pathway | |||||
| Bark and fruit extracts | - | HepG2 cells + FFAs | Activating the AMPK pathway and upregulating | 2019 | [90] | |
| of Toona sinensis | the autophagic flux | |||||
| Naringenin | Fruits, vegetables and nuts | Sprague-Dawley male rats fed HFD, | Enhancing energy expenditure and regulating autophagy | 2021 | [91] | |
| Huh-7/L02 cells + sodium oleate | via AMPK | |||||
| Psoralen | Buguzhi | L02 cells + sodium oleate | Alleviating IR and promoting autophagy via AMPK | 2022 | [92] | |
| Schisandrin B | Schisandra chinensis | HepG2 cells/MPHs + FFAs | Activation of autophagy through the AMPK/mTOR pathway | 2022 | [93] | |
| Pterostilbene | Pterocarpus, blueberry | C57BL/6 male mice injected with tyloxapol, | Activation of the AMPK/mTOR pathway and autophagy | 2023 | [94] | |
| and grape plants | HepG2 + FFAs | by promoting Nrf2 | ||||
| Mangiferin | Mango | Kunming male mice fed HFD | Regulation of autophagy through the AMPK/mTOR pathway | 2017 | [95] | |
| Atractyloside | A diterpenoid glycoside | ICR male mice fed HFD | Activation of autophagy via the ANT-AMPK-mTORC1 pathway | 2021 | [96] | |
| Sweroside | Alfalfa buds | C57BL/6J male mice fed HFD, MPHs + PA | Activating AMPK/mTOR-mediated autophagy | 2023 | [97] | |
| Thymoquinone | Seeds of Nigella sativa | C57BL/6N mice fed HFD, HepG2 cells + FFAs | Inducing autophagy via AMPK/mTOR/ULK1-dependent | 2023 | [98] | |
| signaling pathway | ||||||
| Red pepper seed extract | - | C57BL/6 male mice fed HFD, HepG2 cells + OA | Downregulation of hepatic lipids via AMPK/mTOR pathway | 2022 | [99] | |
| Ginsenoside Rb2 | Panax ginseng | ob/ob male mice fed NCD, HepG2 cells/MPHs + OA | Restoring autophagy via induction of sirt1 | 2017 | [100] | |
| and activation of AMPK | ||||||
| Isosteviol sodium | Stevia rebaudiana | Sprague-Dawley male rats fed HFD, LO2 cells + FFAs | Initiating autophagy via the Sirt1/AMPK pathway | 2022 | [101] | |
| Apple polyphenol extract | - | HepG2 cells + FFAs | Activation of autophagy mediated by SIRT1/AMPK signalling | 2021 | [102] | |
| Catalpol | Rehmannia | C57BL/6 male mice fed HFD, ob/ob male | Through AMPK/TFEB-dependent autophagy | 2019 | [103] | |
| mice fed NCD, HepG2 cells + PA | ||||||
| Aurantio-obtusin | Cassia semen | C57BL/6J male mice fed HFSW, MPHs + FFAs | Through AMPK/autophagy- and AMPK/TFEB-mediated | 2022 | [104] | |
| suppression of lipid accumulation | ||||||
| TFEB-related | Phillygenin | Forsythia suspense | C57BL/6J male mice fed HFD,AML-12/MPHs + PA | Through regulating the Ca2+-calcineurin-TFEB axis to | 2022 | [105] |
| autophagy | restore lipophagy | |||||
| Isopropylidenyl | Chi-Shao | C57BL/6N male mice fed CDAHFD, Sprague- | Through FXR activation and TFEB-mediated autophagy | 2022 | [106] | |
| anemosapogenin | Dawley rats induced BDL, LX-2 cells+ | |||||
| TGF-β1, Huh7 cells + OA | ||||||
| Ajugol | Rehmannia glutinosa | C57BL/6 male mice fed HFD, AML-12 cells + PA | Through the TFEB-mediated autophagy-lysosomal pathway and lipophagy | 2021 | [107] | |
| Polydatin | A precursor of resveratrol | db/db mice fed MCD, LO2 cells + PA | Restoring lysosomal function and autophagic flux through TFEB | 2019 | [108] | |
| Nuciferine | Lotus leaf | C57BL/6N male mice fed HFD, MPHs/AML12 cells + PA | Activating TFEB-mediated autophagy-lysosomal pathway | 2022 | [109] | |
| Formononetin | A natural isoflavone | C57BL/6J mice fed HFD, HepG2 cells/MPHs + FFAs | Through TFEB-mediated lysosome biogenesis and lipophagy | 2019 | [110] | |
| Oxidative stress | Quercetin | Flavonoid polyphenols | Sprague-Dawley male rats fed HFD, HepG2 cells + FFA | Promoting VLDL assembly and lipophagy via the IRE1a/XBP1s pathway | 2018 | [112] |
| and ERS-related | Scutellarin | Erigeron breviscapus | C57BL/6 male mice fed HFD, HepG2 cells/MPHs + PA | Enhancing autophagy and inhibiting ERS via the IRE1α/XBP1 pathway | 2022 | [113] |
| autophagy | Aescin | Aesculus chinensis Bunge | C57BL/6male mice fed HFD,HepG2 cells + FFAs | Activation of antioxidant and autophagy via the Keap1-Nrf2 pathway | 2023 | [114] |
| Physalin B | Physalis species | C57BL/6J mice fed MCD,LO2 cells + FFA | Stimulating autophagy and P62-KEAP1-NRF2 antioxidative signalling | 2021 | [115] | |
| Inflammation- | Scoparone | Artemisia capillaris | C57BL/6J mice fed MCD,AML-12 cells + PA, | Inhibiting ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway | 2020 | [119] |
| related | RAW264.7 cells + LPS | and enhancing autophagic flux in macrophages | ||||
| autophagy | Glycyrrhetinic acid | Glycyrrhiza uralensis | C57BL/6 male mice fed HFHFr, RAW264.7 cells + PA | Modulating macrophage STAT3-HIF-1α pathway and | 2022 | [120] |
| ameliorating impaired autophagic flux | ||||||
| Phloretin | Apple fruits | C57BL/6Jmale mice fed WD, Huh7 cells + FFA | Mitigating oxidative damage, inflammation, and fibrotic responses | 2022 | [121] | |
| by restoring autophagic fluxes | ||||||
| Resveratrol | A polyphenol | C57BL/6male mice fed MCD, AML12 cells + MCD medium | Lessening hepatic inflammation by modulating autophagy | 2015 | [118] | |
| Magnolol | Magnolia officinalis | Wistar male rats injected with tyloxapol, | Inhibition of NLRP3 inflammasome activation | 2020 | [122] | |
| HepG2 cells + PA | by restoration of autophagy | |||||
| Mitophagy | cyanidin-3-O- | An anthocyanin in | C57BL/6 mice fed HFD, AML-12/HepG2 cells + PA | Promoting PINK1-mediated mitophagy | 2020 | [124] |
| glucoside | flavonoids | |||||
| Akebia Saponin D | Dipsacus asper Wall | BRL cells + OA | Through BNip3-mediated mitophagy | 2018 | [125] | |
| Quercetin | A flavonoid | C57BL/6Jmale mice fed HFD, HepG2 cells + OA/PA | Enhancing frataxin-mediated PINK1/Parkin-dependent mitophagy | 2018 | [127] | |
| C57BL/6J male mice fed MCD, HepG2 cells + OA | Through AMPK-mediated hepatic mitophagy | 2023 | [126] |
4.4. Others
4.4.1. Hormones
Thyroid hormones (THs) play an essential role in organ development, cellular differentiation, and the regulation of protein, carbohydrate, and lipid metabolism. Increasing evidence has indicated that reduced thyroid hormone activity is associated with an elevated risk of NAFLD [128]. However, TH supplementation may exert protective effects against NAFLD, partly through the induction of hepatic autophagy [129]. Recent studies have demonstrated that administration of T3 or T4 in NASH mouse models restores hepatic autophagy and mitochondrial biogenesis, thereby enhancing fatty acid β-oxidation and reducing lipotoxicity, oxidative stress, inflammation, and fibrosis [130]. Another study investigating the metabolic effects of THs on the liver supports their therapeutic potential in improving liver homeostasis [131].
In addition, melatonin has also shown beneficial effects in improving NAFLD to some extent [132]. In a cadmium-induced NAFLD model, melatonin attenuates mitochondrial damage, oxidative stress, and hepatic lipid accumulation by restoring PPARα expression and autophagic flux [133]. Furthermore, melatonin supplementation has been shown to improve mitochondrial and liver function in NAFLD by restoring mitophagy through inhibition of the NR4A1/DNA-PKcs/p53 signalling pathway [134].
4.4.2. Nanoparticles
Nanoparticles (NPs) are particulate materials typically ranging in size from approximately 50 to 200 nanometers. Due to their favourable characteristics, including high drug-loading capacity, variable shapes and sizes, and stable ligand binding, NPs have emerged as promising tools for targeted therapeutic delivery and modulation of cellular processes. They have shown great potential in medical applications [135]. In a study on acid-activated acidifying NPs for targeted therapy of NAFLD in mice, these NPs were able to re-acidify lysosomes, restore autophagy and mitochondrial function, and reverse fasting hyperglycemia and hepatic steatosis in the treated animals [136]. Another investigation demonstrated that lycopene-loaded nanoliposomes significantly attenuated oxidative stress, inflammation, and apoptosis in liver tissue while inducing autophagy, thereby exerting therapeutic effects against NAFLD [137]. Additionally, researchers have developed nifedipine-loaded nanoparticles, which not only enhanced autophagic clearance in hepatocytes but also improved insulin resistance and glucose tolerance in obese mice and mitigated metabolic disturbances associated with NAFLD [138]. More importantly, nanoparticles loaded with the autophagy-inducing peptide Tat-Beclin (T-B) have been engineered. Compared to the soluble peptide alone, the nanoparticle formulation induced more sustained and potent autophagic activity. Both T-B and NP-T-B effectively reduced lipid accumulation in NAFLD cellular models [139]. These findings suggest that nanoparticles may hold broad therapeutic potential in the treatment of NAFLD through autophagy modulation and targeted metabolic regulation.
4.4.3. Gut Microbiota
The gut microbiota, consisting of bacteria, archaea, viruses, and fungi residing in the human gastrointestinal tract, plays a critical role in host physiology. Dysbiosis of the gut microbiota is not only a characteristic feature of NAFLD but also contributes significantly to its pathogenesis [140]. Recent studies have demonstrated that supplementation with selenium-enriched probiotics alleviates liver dysfunction and hepatic steatosis in NAFLD rats by activating autophagy through the AMPK and SIRT1 signalling pathway [141]. Furthermore, it has been reported that Urolithin A, a gut microbiota-derived metabolite, promotes hepatic lipophagy via the AMPK and ULK1 pathway both in vitro and in vivo. This compound suppresses lipogenesis, enhances fatty acid β-oxidation, and reduces lipid overaccumulation in the liver, thereby improving NAFLD [142]. In addition, Lactobacillus bifidus SF has been shown to reduce inflammation and hepatic lipid accumulation by mitigating oxidative stress and regulating autophagy, which together contribute to the improvement of NAFLD [143].
4.4.4. Vitamins
Vitamins are small organic molecules that play essential roles in supporting growth and development, maintaining physiological functions, and regulating metabolic processes [144]. Clinical trials have provided evidence that vitamin supplementation may offer therapeutic benefits in the treatment of NAFLD, and the underlying mechanisms are increasingly thought to involve autophagy induction [145,146]. Vitamin D3, a steroid hormone, exerts its biological activity primarily through binding to the vitamin D receptor (VDR) in the form of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] [144]. Li and colleagues [147] reported that intraperitoneal injection of 1,25(OH)2D3 for 4 weeks in high-fat diet-fed mice alleviated hepatic lipid accumulation, possibly through the upregulation of ATG16L1 to promote autophagy. Lim and colleagues [148] further explored the autophagy-related mechanisms of vitamin D3 in a NAFLD model with type 2 diabetes mellitus. Their findings showed that under high-glucose conditions, vitamin D3 improved hepatic lipid metabolism by activating autophagy through regulation of the AMPK/Akt–mTOR pathway. In addition, another study demonstrated that supplementation with nicotinamide, the amide form of vitamin B3, protected hepatocytes from palmitic acid-induced lipotoxicity via SIRT1-dependent autophagy activation [149]. These findings suggest that the induction of autophagy may represent a key mechanism through which vitamins exert hepatoprotective effects. Further details are summarised in Table 4.

Table 4.
Alternative approaches to treating NAFLD related to autophagy induction.
5. Conclusions and Outlook
NAFLD is a common chronic liver disease caused by multiple etiologies. Histologically, it can evolve from simple hepatic steatosis eventually to hepatocellular carcinoma. Hence, it is particularly important to explore the pathogenesis and treatment of NAFLD. Autophagy, a process by which eukaryotic cells clean up damaged or excess intracellular components under stress, has a crucial role in maintaining metabolic homeostasis in the liver. A growing number of studies have pointed out that impaired autophagy exists in NAFLD, and impaired autophagy can be accompanied by inflammation, ERS, and apoptosis, which further aggravate NAFLD and form a vicious circle. Thus, restoration of autophagy may become an important line of thought in therapy for NAFLD. In the present study, we summarise the latest literature on the treatment of NAFLD from the perspective of restoring or inducing autophagy in terms of diet and exercise, drugs (modern pharmacological therapy and plant-derived compounds), and other measures (hormones, nanoparticles, gut microbes, and vitamins). The potential molecular targets of NAFLD related to autophagy are also briefly described, hoping to provide some reference for future research on autophagy and NAFLD treatment. However, since most of the above findings are derived from cell or animal trials and the elaboration of the degree of autophagy induction is vague, there is still an urgent need for a large amount of clinical evidence to validate their efficacy and safety in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14080989/s1, Table S1: A comprehensive clinical comparison between SGLT-2i and GLP-1RA. Refs. [151,152,153,154,155,156,157,158,159,160,161,162].
Author Contributions
Conceptualization, M.Z. and Y.W.; Methodology, M.Z. and Y.L.; Software, M.Z. and Y.W.; Validation, Y.L. and Y.W.; Formal Analysis, M.Z.; Investigation, M.Z. and Y.L.; Writing—Original Draft Preparation, M.Z.; Writing—Review & Editing, M.Z. and Y.L.; Visualization, M.Z. and Y.W.; Supervision, M.Z. and Y.W.; Project Administration, M.Z. and Y.L.; Funding Acquisition, Y.W. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated by this study will be made available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San, P.J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a molecular target for cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef]
- Qian, H.; Chao, X.; Williams, J.; Fulte, S.; Li, T.; Yang, L.; Ding, W.X. Autophagy in liver diseases: A review. Mol. Aspects Med. 2021, 82, 100973. [Google Scholar] [CrossRef]
- Carotti, S.; Aquilano, K.; Zalfa, F.; Ruggiero, S.; Valentini, F.; Zingariello, M.; Francesconi, M.; Perrone, G.; Alletto, F.; Antonelli-Incalzi, R.; et al. Lipophagy Impairment Is Associated With Disease Progression in NAFLD. Front. Physiol. 2020, 11, 850. [Google Scholar] [CrossRef]
- Jonas, W.; Schwerbel, K.; Zellner, L.; Jahnert, M.; Gottmann, P.; Schurmann, A. Alterations of Lipid Profile in Livers with Impaired Lipophagy. Int. J. Mol. Sci. 2022, 23, 11863. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, K.; Hogstrand, C.; Xu, Y.H.; Chen, G.H.; Wei, C.C.; Luo, Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARgamma pathways. Cell Mol. Life Sci. 2020, 77, 1987–2003. [Google Scholar] [CrossRef]
- Cai, N.; Zhao, X.; Jing, Y.; Sun, K.; Jiao, S.; Chen, X.; Yang, H.; Zhou, Y.; Wei, L. Autophagy protects against palmitate-induced apoptosis in hepatocytes. Cell Biosci. 2014, 4, 28. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Q.; Song, S.; Liu, F.; He, B.; Gao, X. Protective role of autophagy in methionine-choline deficient diet-induced advanced nonalcoholic steatohepatitis in mice. Eur. J. Pharmacol. 2016, 770, 126–133. [Google Scholar] [CrossRef]
- Fukuo, Y.; Yamashina, S.; Sonoue, H.; Arakawa, A.; Nakadera, E.; Aoyama, T.; Uchiyama, A.; Kon, K.; Ikejima, K.; Watanabe, S. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol. Res. 2014, 44, 1026–1036. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, A.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillon, J.; Lo, I.O.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014, 5, e1179. [Google Scholar] [CrossRef]
- Kwanten, W.J.; Vandewynckel, Y.P.; Martinet, W.; De Winter, B.Y.; Michielsen, P.P.; Van Hoof, V.O.; Driessen, A.; Timmermans, J.P.; Bedossa, P.; Van Vlierberghe, H.; et al. Hepatocellular autophagy modulates the unfolded protein response and fasting-induced steatosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G599–G609. [Google Scholar] [CrossRef]
- Hammoutene, A.; Biquard, L.; Lasselin, J.; Kheloufi, M.; Tanguy, M.; Vion, A.C.; Merian, J.; Colnot, N.; Loyer, X.; Tedgui, A.; et al. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J. Hepatol. 2020, 72, 528–538. [Google Scholar] [CrossRef]
- Acosta, A.; Streett, S.; Kroh, M.D.; Cheskin, L.J.; Saunders, K.H.; Kurian, M.; Schofield, M.; Barlow, S.E.; Aronne, L. White Paper AGA: POWER—Practice Guide on Obesity and Weight Management, Education, and Resources. Clin. Gastroenterol. Hepatol. 2017, 15, 631–649.e610. [Google Scholar] [CrossRef]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, J.; Gu, Q. Exercise restores bioavailability of hydrogen sulfide and promotes autophagy influx in livers of mice fed with high-fat diet. Can. J. Physiol. Pharmacol. 2017, 95, 667–674. [Google Scholar] [CrossRef]
- Zhou, X.; Fouda, S.; Li, D.; Zhang, K.; Ye, J.M. Involvement of the Autophagy-ER Stress Axis in High Fat/Carbohydrate Diet-Induced Nonalcoholic Fatty Liver Disease. Nutrients 2020, 12, 2626. [Google Scholar] [CrossRef]
- Kim, K.E.; Jung, Y.; Min, S.; Nam, M.; Heo, R.W.; Jeon, B.T.; Song, D.H.; Yi, C.O.; Jeong, E.A.; Kim, H.; et al. Caloric restriction of db/db mice reverts hepatic steatosis and body weight with divergent hepatic metabolism. Sci. Rep. 2016, 6, 30111. [Google Scholar] [CrossRef]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical application of intermittent fasting for weight loss: Progress and future directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef]
- Rozanski, G.; Pheby, D.; Newton, J.L.; Murovska, M.; Zalewski, P.; Slomko, J. Effect of Different Types of Intermittent Fasting on Biochemical and Anthropometric Parameters among Patients with Metabolic-Associated Fatty Liver Disease (MAFLD)-A Systematic Review. Nutrients 2021, 14, 91. [Google Scholar] [CrossRef]
- Li, D.; Dun, Y.; Qi, D.; Ripley-Gonzalez, J.W.; Dong, J.; Zhou, N.; Qiu, L.; Zhang, J.; Zeng, T.; You, B.; et al. Intermittent fasting activates macrophage migration inhibitory factor and alleviates high-fat diet-induced nonalcoholic fatty liver disease. Sci. Rep. 2023, 13, 13068. [Google Scholar] [CrossRef]
- Elsayed, H.R.H.; El-Nablaway, M.; Khattab, B.A.; Sherif, R.N.; Elkashef, W.F.; Abdalla, A.M.; El, N.E.M.; Abd-Elmonem, M.M.; El-Gamal, R. Independent of Calorie Intake, Short-term Alternate-day Fasting Alleviates NASH, With Modulation of Markers of Lipogenesis, Autophagy, Apoptosis, and Inflammation in Rats. J. Histochem. Cytochem. 2021, 69, 575–596. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Wang, L.; Shi, R.; Chu, C.; Shi, Z.; Liu, P.; Li, Y.; Liu, X.; Liu, Z. Alternate-day fasting prevents non-alcoholic fatty liver disease and working memory impairment in diet-induced obese mice. J. Nutr. Biochem. 2022, 110, 109146. [Google Scholar] [CrossRef]
- Wang, M.E.; Singh, B.K.; Hsu, M.C.; Huang, C.; Yen, P.M.; Wu, L.S.; Jong, D.S.; Chiu, C.H. Increasing Dietary Medium-Chain Fatty Acid Ratio Mitigates High-fat Diet-Induced Non-Alcoholic Steatohepatitis by Regulating Autophagy. Sci. Rep. 2017, 7, 13999. [Google Scholar] [CrossRef]
- Yao, Z.; Li, X.; Wang, W.; Ren, P.; Song, S.; Wang, H.; Xie, Y.; Li, X.; Li, Z. Corn peptides attenuate non-alcoholic fatty liver disease via PINK1/Parkin-mediated mitochondrial autophagy. Food Nutr. Res. 2023, 67, 9547. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Z.; Tu, J.; Wang, Z.; Gao, X.; Deng, K.; El-Samahy, M.A.; You, P.; Fan, Y.; Wang, F. gamma-Linolenic Acid Prevents Lipid Metabolism Disorder in Palmitic Acid-Treated Alpha Mouse Liver-12 Cells by Balancing Autophagy and Apoptosis via the LKB1-AMPK-mTOR Pathway. J. Agric. Food Chem. 2021, 69, 8257–8267. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef]
- Lee, J.; Vijayakumar, A.; White, P.J.; Xu, Y.; Ilkayeva, O.; Lynch, C.J.; Newgard, C.B.; Kahn, B.B. BCAA Supplementation in Mice with Diet-induced Obesity Alters the Metabolome Without Impairing Glucose Homeostasis. Endocrinology 2021, 162, bqab062. [Google Scholar] [CrossRef]
- Komorowski, J.R.; Ojalvo, S.P.; Sylla, S.; Tastan, H.; Orhan, C.; Tuzcu, M.; Sahin, N.; Sahin, K. The addition of an amylopectin/chromium complex to branched-chain amino acids enhances muscle protein synthesis in rat skeletal muscle. J. Int. Soc. Sports Nutr. 2020, 17, 26. [Google Scholar] [CrossRef]
- Blair, M.C.; Neinast, M.D.; Jang, C.; Chu, Q.; Jung, J.W.; Axsom, J.; Bornstein, M.R.; Thorsheim, C.; Li, K.; Hoshino, A.; et al. Branched-chain amino acid catabolism in muscle affects systemic BCAA levels but not insulin resistance. Nat. Metab. 2023, 5, 589–606. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Lee, D.E.; Brown, J.L.; Brown, L.A.; Perry, R.A., Jr.; Greene, E.S.; Carvallo, C.F.R.; Washington, T.A.; Greene, N.P. Moderate physical activity promotes basal hepatic autophagy in diet-induced obese mice. Appl. Physiol. Nutr. Metab. 2017, 42, 148–156. [Google Scholar] [CrossRef]
- Cook, J.J.; Wei, M.; Segovia, B.; Cosio-Lima, L.; Simpson, J.; Taylor, S.; Koh, Y.; Kim, S.; Lee, Y. Endurance exercise-mediated metabolic reshuffle attenuates high-caloric diet-induced non-alcoholic fatty liver disease. Ann. Hepatol. 2022, 27, 100709. [Google Scholar] [CrossRef]
- Laval, T.; Ouimet, M. A role for lipophagy in atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 431–432. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liu, Z.; Ruan, X.; Wang, H.; Zhang, Q.; Cao, L.; Song, L.; Chen, Y.; Sun, Y. Moderate Treadmill Exercise Alleviates NAFLD by Regulating the Biogenesis and Autophagy of Lipid Droplet. Nutrients 2022, 14, 4910. [Google Scholar] [CrossRef]
- Goncalves, I.O.; Passos, E.; Diogo, C.V.; Rocha-Rodrigues, S.; Santos-Alves, E.; Oliveira, P.J.; Ascensao, A.; Magalhaes, J. Exercise mitigates mitochondrial permeability transition pore and quality control mechanisms alterations in nonalcoholic steatohepatitis. Appl. Physiol. Nutr. Metab. 2016, 41, 298–306. [Google Scholar] [CrossRef]
- Su, P.; Chen, J.G.; Tang, D.H. Exercise against nonalcoholic fatty liver disease: Possible role and mechanism of lipophagy. Life Sci. 2023, 327, 121837. [Google Scholar] [CrossRef]
- Pi, H.; Liu, M.; Xi, Y.; Chen, M.; Tian, L.; Xie, J.; Chen, M.; Wang, Z.; Yang, M.; Yu, Z.; et al. Long-term exercise prevents hepatic steatosis: A novel role of FABP1 in regulation of autophagy-lysosomal machinery. FASEB J. 2019, 33, 11870–11883. [Google Scholar] [CrossRef]
- Guarino, M.; Kumar, P.; Felser, A.; Terracciano, L.M.; Guixe-Muntet, S.; Humar, B.; Foti, M.; Nuoffer, J.M.; St-Pierre, M.V.; Dufour, J.F. Exercise Attenuates the Transition from Fatty Liver to Steatohepatitis and Reduces Tumor Formation in Mice. Cancers 2020, 12, 1407. [Google Scholar] [CrossRef]
- Li, H.; Dun, Y.; Zhang, W.; You, B.; Liu, Y.; Fu, S.; Qiu, L.; Cheng, J.; Ripley-Gonzalez, J.W.; Liu, S. Exercise improves lipid droplet metabolism disorder through activation of AMPK-mediated lipophagy in NAFLD. Life Sci. 2021, 273, 119314. [Google Scholar] [CrossRef]
- Yang, J.; Sainz, N.; Felix-Soriano, E.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Fernandez-Galilea, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Effects of Long-Term DHA Supplementation and Physical Exercise on Non-Alcoholic Fatty Liver Development in Obese Aged Female Mice. Nutrients 2021, 13, 501. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Zeng, L.Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.Y.; Fang, C.W.; Wang, F.; Qin, X.J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef]
- Wang, C.; Liang, J.; Ren, Y.; Huang, J.; Jin, B.; Wang, G.; Chen, N. A Preclinical Systematic Review of the Effects of Chronic Exercise on Autophagy-Related Proteins in Aging Skeletal Muscle. Front. Physiol. 2022, 13, 930185. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Morales-Alamo, D.; Calbet, J.A.L. Exercise-mediated modulation of autophagy in skeletal muscle. Scand. J. Med. Sci. Sports 2018, 28, 772–781. [Google Scholar] [CrossRef]
- McCormick, J.J.; King, K.E.; Goulet, N.; Carrillo, A.E.; Fujii, N.; Amano, T.; Boulay, P.; Kenny, G.P. The effect of an exercise- and passive-induced heat stress on autophagy in young and older males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2025, 328, R289–R299. [Google Scholar] [CrossRef]
- McCormick, J.J.; McManus, M.K.; King, K.E.; Goulet, N.; Kenny, G.P. The intensity-dependent effects of exercise and superimposing environmental heat stress on autophagy in peripheral blood mononuclear cells from older men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 326, R29–R42. [Google Scholar] [CrossRef]
- Yabiku, K. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Concurrent Type 2 Diabetes Mellitus and Non-Alcoholic Steatohepatitis: A Review of the Evidence. Front. Endocrinol. 2021, 12, 768850. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef]
- Androutsakos, T.; Nasiri-Ansari, N.; Bakasis, A.D.; Kyrou, I.; Efstathopoulos, E.; Randeva, H.S.; Kassi, E. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. Int. J. Mol. Sci. 2022, 23, 3107. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, X.; Li, T.; Fang, T.; Cheng, Y.; Han, L.; Sun, B.; Chen, L. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int. Immunopharmacol. 2021, 94, 107492. [Google Scholar] [CrossRef]
- Chun, H.J.; Kim, E.R.; Lee, M.; Choi, D.H.; Kim, S.H.; Shin, E.; Kim, J.H.; Cho, J.W.; Han, D.H.; Cha, B.S.; et al. Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis. Metabolism 2023, 145, 155612. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, W.; Wang, B.; Xu, T.; Wang, J.; Wei, D. Canagliflozin Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Lipid Metabolism and Inhibiting Inflammation through Induction of Autophagy. Yonsei Med. J. 2022, 63, 619–631. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-mTOR Pathway. Front. Pharmacol. 2021, 12, 589273. [Google Scholar] [CrossRef]
- Papamargaritis, D.; le Roux, C.W.; Holst, J.J.; Davies, M.J. New therapies for obesity. Cardiovasc. Res. 2022, 119, 2825–2842. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Dai, Y.; He, H.; Li, S.; Yang, L.; Wang, X.; Liu, Z.; An, Z. Comparison of the Efficacy of Glucagon-Like Peptide-1 Receptor Agonists in Patients With Metabolic Associated Fatty Liver Disease: Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 622589. [Google Scholar] [CrossRef]
- Tong, W.; Ju, L.; Qiu, M.; Xie, Q.; Chen, Y.; Shen, W.; Sun, W.; Wang, W.; Tian, J. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol. Res. 2016, 46, 933–943. [Google Scholar] [CrossRef]
- Yu, X.; Hao, M.; Liu, Y.; Ma, X.; Lin, W.; Xu, Q.; Zhou, H.; Shao, N.; Kuang, H. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur. J. Pharmacol. 2019, 864, 172715. [Google Scholar] [CrossRef]
- Shao, N.; Yu, X.Y.; Ma, X.F.; Lin, W.J.; Hao, M.; Kuang, H.Y. Exenatide Delays the Progression of Nonalcoholic Fatty Liver Disease in C57BL/6 Mice, Which May Involve Inhibition of the NLRP3 Inflammasome through the Mitophagy Pathway. Gastroenterol. Res. Pract. 2018, 2018, 1864307. [Google Scholar] [CrossRef]
- Yu, H.H.; Wang, H.C.; Hsieh, M.C.; Lee, M.C.; Su, B.C.; Shan, Y.S. Exendin-4 Attenuates Hepatic Steatosis by Promoting the Autophagy-Lysosomal Pathway. Biomed. Res. Int. 2022, 2022, 4246086. [Google Scholar] [CrossRef]
- Lin, C.; Fang, J.; Xiang, Q.; Zhou, R.; Yang, L. Exendin-4 promotes autophagy to relieve lipid deposition in a NAFLD cell model by activating AKT/mTOR signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 1073–1078. [Google Scholar]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef]
- Li, Y.L.; Li, X.Q.; Wang, Y.D.; Shen, C.; Zhao, C.Y. Metformin alleviates inflammatory response in non-alcoholic steatohepatitis by restraining signal transducer and activator of transcription 3-mediated autophagy inhibition in vitro and in vivo. Biochem. Biophys. Res. Commun. 2019, 513, 64–72. [Google Scholar] [CrossRef]
- Song, Y.M.; Lee, Y.H.; Kim, J.W.; Ham, D.S.; Kang, E.S.; Cha, B.S.; Lee, H.C.; Lee, B.W. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy 2015, 11, 46–59. [Google Scholar] [CrossRef]
- Song, Y.M.; Lee, W.K.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. Metformin Restores Parkin-Mediated Mitophagy, Suppressed by Cytosolic p53. Int. J. Mol. Sci. 2016, 17, 122. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Y.; Liu, J.; Deng, Y.; Zhou, B.; Wen, Y.; Li, M.; Wen, D.; Ying, Y.; Luo, S.; et al. Metformin Alleviates Hepatic Steatosis and Insulin Resistance in a Mouse Model of High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease by Promoting Transcription Factor EB-Dependent Autophagy. Front. Pharmacol. 2021, 12, 689111. [Google Scholar] [CrossRef]
- Park, J.; Rah, S.Y.; An, H.S.; Lee, J.Y.; Roh, G.S.; Ryter, S.W.; Park, J.W.; Yang, C.H.; Surh, Y.J.; Kim, U.H.; et al. Metformin-induced TTP mediates communication between Kupffer cells and hepatocytes to alleviate hepatic steatosis by regulating lipophagy and necroptosis. Metabolism 2023, 141, 155516. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, I.K.; Ahn, K.J.; Chung, H.Y.; Hwang, Y.C. Fenofibrate, a PPARalpha agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy. Metabolism 2021, 120, 154798. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Y.; Liu, J.; Wang, D.; Geng, Z.; Wen, D.; Chen, H.; Wang, H.; Li, L.; Zhu, X.; et al. Fenofibrate improves hepatic steatosis, insulin resistance, and shapes the gut microbiome via TFEB-autophagy in NAFLD mice. Eur. J. Pharmacol. 2023, 960, 176159. [Google Scholar] [CrossRef]
- Chang, E.; Kim, L.; Park, S.E.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Park, C.Y. Ezetimibe improves hepatic steatosis in relation to autophagy in obese and diabetic rats. World J. Gastroenterol. 2015, 21, 7754–7763. [Google Scholar] [CrossRef]
- Lee, D.H.; Han, D.H.; Nam, K.T.; Park, J.S.; Kim, S.H.; Lee, M.; Kim, G.; Min, B.S.; Cha, B.S.; Lee, Y.S.; et al. Ezetimibe, an NPC1L1 inhibitor, is a potent Nrf2 activator that protects mice from diet-induced nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2016, 99, 520–532. [Google Scholar] [CrossRef]
- Hsiao, P.J.; Chiou, H.C.; Jiang, H.J.; Lee, M.Y.; Hsieh, T.J.; Kuo, K.K. Pioglitazone Enhances Cytosolic Lipolysis, beta-oxidation and Autophagy to Ameliorate Hepatic Steatosis. Sci. Rep. 2017, 7, 9030. [Google Scholar] [CrossRef]
- Song, Y.; Yang, H.; Kim, J.; Lee, Y.; Kim, S.H.; Do, I.G.; Park, C.Y. Gemigliptin, a DPP4 inhibitor, ameliorates nonalcoholic steatohepatitis through AMP-activated protein kinase-independent and ULK1-mediated autophagy. Mol. Metab. 2023, 78, 101806. [Google Scholar] [CrossRef]
- He, J.; Ding, J.; Lai, Q.; Wang, X.; Li, A.; Liu, S. Irbesartan Ameliorates Lipid Deposition by Enhancing Autophagy via PKC/AMPK/ULK1 Axis in Free Fatty Acid Induced Hepatocytes. Front. Physiol. 2019, 10, 681. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Zhu, H.D.; Sheng, J.Q.; Wu, X.L.; He, X.X.; Tian, D.A.; Liao, J.Z.; Li, P.Y. Celecoxib alleviates nonalcoholic fatty liver disease by restoring autophagic flux. Sci. Rep. 2018, 8, 4108. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, W.; Abd, E.-A.A.M.; Hacimuftuoglu, A.; Jeong, J.H.; Jung, T.W. Valdecoxib attenuates lipid-induced hepatic steatosis through autophagy-mediated suppression of endoplasmic reticulum stress. Biochem. Pharmacol. 2022, 199, 115022. [Google Scholar] [CrossRef]
- Tawfiq, R.A.; Nassar, N.N.; Hammam, O.A.; Allam, R.M.; Elmazar, M.M.; Abdallah, D.M.; Attia, Y.M. Obeticholic acid orchestrates the crosstalk between ileal autophagy and tight junctions in non-alcoholic steatohepatitis: Role of TLR4/TGF-β1 axis. Chem. Biol. Interact. 2022, 361, 109953. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE((-/-)) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef]
- He, Q.; Sha, S.; Sun, L.; Zhang, J.; Dong, M. GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem. Biophys. Res. Commun. 2016, 476, 196–203. [Google Scholar] [CrossRef]
- Yu, X.; Bian, X.; Zhang, H.; Yang, S.; Cui, D.; Su, Z. Liraglutide ameliorates hepatic steatosis via retinoic acid receptor-related orphan receptor alpha-mediated autophagy pathway. IUBMB Life 2023, 75, 856–867. [Google Scholar] [CrossRef]
- Trefts, E.; Shaw, R.J. AMPK: Restoring metabolic homeostasis over space and time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Li, F.; Zou, J.; Wang, Y.H.; Xu, M.X.; Wang, Y.L.; Li, R.X.; Sun, Y.T.; Lu, S.; et al. Icaritin Attenuates Lipid Accumulation by Increasing Energy Expenditure and Autophagy Regulated by Phosphorylating AMPK. J. Clin. Transl. Hepatol. 2021, 9, 373–383. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, H.J.; Huang, B.M.; Chen, Y.C.; Chang, C.F. Polyphenol-Rich Extracts from Toona sinensis Bark and Fruit Ameliorate Free Fatty Acid-Induced Lipogenesis through AMPK and LC3 Pathways. J. Clin. Med. 2019, 8, 1664. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Zou, J.; Wang, Y.H.; Xu, M.X.; Huang, W.; Yu, D.J.; Zhang, L.; Zhang, Y.Y.; Sun, X.D. Naringenin Attenuates Non-Alcoholic Fatty Liver Disease by Enhancing Energy Expenditure and Regulating Autophagy via AMPK. Front. Pharmacol. 2021, 12, 687095. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, F.; Zou, J.; Li, X.; Xu, M.; Yu, D.; Ma, Y.; Huang, W.; Sun, X.; et al. Psoralen Suppresses Lipid Deposition by Alleviating Insulin Resistance and Promoting Autophagy in Oleate-Induced L02 Cells. Cells 2022, 11, 1067. [Google Scholar] [CrossRef]
- Yan, L.S.; Zhang, S.F.; Luo, G.; Cheng, B.C.; Zhang, C.; Wang, Y.W.; Qiu, X.Y.; Zhou, X.H.; Wang, Q.G.; Song, X.L.; et al. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway. Metabolism 2022, 131, 155200. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Y.; Cheng, J.; Peng, Y.; Zhang, Q.; Li, Z.; Zhao, L.; Deng, X.; Feng, H. Pterostilbene alleviated NAFLD via AMPK/mTOR signaling pathways and autophagy by promoting Nrf2. Phytomedicine 2023, 109, 154561. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.Y.; Wang, L.; Teng, T.; Zhou, M.; Wang, S.G.; Tian, Y.Z.; Du, L.; Yin, X.X.; Sun, Y. Mangiferin ameliorates fatty liver via modulation of autophagy and inflammation in high-fat-diet induced mice. Biomed. Pharmacother. 2017, 96, 328–335. [Google Scholar] [CrossRef]
- Zhang, P.; Cheng, X.; Sun, H.; Li, Y.; Mei, W.; Zeng, C. Atractyloside Protect Mice Against Liver Steatosis by Activation of Autophagy via ANT-AMPK-mTORC1 Signaling Pathway. Front. Pharmacol. 2021, 12, 736655. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Hu, K.; Yang, Q.; Li, Y.; Huang, M. Sweroside alleviates hepatic steatosis in part by activating AMPK/mTOR-mediated autophagy in mice. J. Cell Biochem. 2023, 124, 1012–1022. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Wang, Z.; Lei, L. Thymoquinone attenuates hepatic lipid accumulation by inducing autophagy via AMPK/mTOR/ULK1-dependent pathway in nonalcoholic fatty liver disease. Phytother. Res. 2023, 37, 781–797. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, H.J.; You, M.; Kim, H.A. Red Pepper Seeds Inhibit Hepatic Lipid Accumulation by Inducing Autophagy via AMPK Activation. Nutrients 2022, 14, 4247. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, T.; Yang, L.; Wang, H.Y. Ginsenoside Rb2 Alleviates Hepatic Lipid Accumulation by Restoring Autophagy via Induction of Sirt1 and Activation of AMPK. Int. J. Mol. Sci. 2017, 18, 1063. [Google Scholar] [CrossRef]
- Mei, Y.; Hu, H.; Deng, L.; Sun, X.; Tan, W. Therapeutic effects of isosteviol sodium on non-alcoholic fatty liver disease by regulating autophagy via Sirt1/AMPK pathway. Sci. Rep. 2022, 12, 12857. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple polyphenol extract alleviates lipid accumulation in free-fatty-acid-exposed HepG2 cells via activating autophagy mediated by SIRT1/AMPK signaling. Phytother. Res. 2021, 35, 1416–1431. [Google Scholar] [CrossRef]
- Ren, H.; Wang, D.; Zhang, L.; Kang, X.; Li, Y.; Zhou, X.; Yuan, G. Catalpol induces autophagy and attenuates liver steatosis in ob/ob and high-fat diet-induced obese mice. Aging 2019, 11, 9461–9477. [Google Scholar] [CrossRef]
- Zhou, F.; Ding, M.; Gu, Y.; Fan, G.; Liu, C.; Li, Y.; Sun, R.; Wu, J.; Li, J.; Xue, X.; et al. Aurantio-Obtusin Attenuates Non-Alcoholic Fatty Liver Disease Through AMPK-Mediated Autophagy and Fatty Acid Oxidation Pathways. Front. Pharmacol. 2021, 12, 826628. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, X.; Zhai, Y.; Liu, H.; Guan, L.; Qiao, Y.; Jiang, J.; Peng, L. Phillygenin ameliorates nonalcoholic fatty liver disease via TFEB-mediated lysosome biogenesis and lipophagy. Phytomedicine 2022, 103, 154235. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.; Zhong, W.; Xia, G.; Xia, H.; Wang, L.; Wei, X.; Li, Y.; Shang, H.; He, H.; et al. Multiple anti-non-alcoholic steatohepatitis (NASH) efficacies of isopropylidenyl anemosapogenin via farnesoid X receptor activation and TFEB-mediated autophagy. Phytomedicine 2022, 102, 154148. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Liu, H.; Guan, L.; Xu, S.; Wang, Z.; Qiu, Y.; Liu, H.; Peng, L.; Men, X. Ajugol enhances TFEB-mediated lysosome biogenesis and lipophagy to alleviate non-alcoholic fatty liver disease. Pharmacol. Res. 2021, 174, 105964. [Google Scholar] [CrossRef]
- Chen, X.; Chan, H.; Zhang, L.; Liu, X.; Ho, I.H.T.; Zhang, X.; Ho, J.; Hu, W.; Tian, Y.; Kou, S.; et al. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and autophagic flux. J. Cell Mol. Med. 2019, 23, 4290–4300. [Google Scholar] [CrossRef]
- Du, X.; Di Malta, C.; Fang, Z.; Shen, T.; Niu, X.; Chen, M.; Jin, B.; Yu, H.; Lei, L.; Gao, W.; et al. Nuciferine protects against high-fat diet-induced hepatic steatosis and insulin resistance via activating TFEB-mediated autophagy-lysosomal pathway. Acta Pharm. Sin. B 2022, 12, 2869–2886. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Li, X.; Wang, Q.; Yan, M.; Zhang, H.; Zhao, T.; Zhang, N.; Zhang, P.; Peng, L.; et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J. Nutr. Biochem. 2019, 73, 108214. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Zhu, X.; Xiong, T.; Liu, P.; Guo, X.; Xiao, L.; Zhou, F.; Tang, Y.; Yao, P. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem. Toxicol. 2018, 114, 52–60. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, Z.; Luan, H.; Huang, Y.; Shen, Y.; Sheng, L.; Liang, J.; Wu, F. Scutellarin ameliorates hepatic lipid accumulation by enhancing autophagy and suppressing IRE1alpha/XBP1 pathway. Phytother. Res. 2022, 36, 433–447. [Google Scholar] [CrossRef]
- Yu, H.; Yan, S.; Jin, M.; Wei, Y.; Zhao, L.; Cheng, J.; Ding, L.; Feng, H. Aescin can alleviate NAFLD through Keap1-Nrf2 by activating antioxidant and autophagy. Phytomedicine 2023, 113, 154746. [Google Scholar] [CrossRef]
- Zhang, M.H.; Li, J.; Zhu, X.Y.; Zhang, Y.Q.; Ye, S.T.; Leng, Y.R.; Yang, T.; Zhang, H.; Kong, L.Y. Physalin B ameliorates nonalcoholic steatohepatitis by stimulating autophagy and NRF2 activation mediated improvement in oxidative stress. Free Radic. Biol. Med. 2021, 164, 1–12. [Google Scholar] [CrossRef]
- Arrese, M.; Cabrera, D.; Kalergis, A.M.; Feldstein, A.E. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 2016, 61, 1294–1303. [Google Scholar] [CrossRef]
- Fukada, H.; Yamashina, S.; Izumi, K.; Komatsu, M.; Tanaka, K.; Ikejima, K.; Watanabe, S. Suppression of autophagy sensitizes Kupffer cells to endotoxin. Hepatol. Res. 2012, 42, 1112–1118. [Google Scholar] [CrossRef]
- Ji, G.; Wang, Y.; Deng, Y.; Li, X.; Jiang, Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015, 14, 134. [Google Scholar] [CrossRef]
- Liu, B.; Deng, X.; Jiang, Q.; Li, G.; Zhang, J.; Zhang, N.; Xin, S.; Xu, K. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. Biomed. Pharmacother. 2020, 125, 109895. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, W.; Wang, Y.; Zhu, S.; Chai, R.; Xu, Z.; Zhang, X.; Yan, Y.; Yang, L.; Bian, Y. Glycyrrhetinic acid regulates impaired macrophage autophagic flux in the treatment of non-alcoholic fatty liver disease. Front. Immunol. 2022, 13, 959495. [Google Scholar] [CrossRef]
- Chhimwal, J.; Goel, A.; Sukapaka, M.; Patial, V.; Padwad, Y. Phloretin mitigates oxidative injury, inflammation, and fibrogenic responses via restoration of autophagic flux in in vitro and preclinical models of NAFLD. J. Nutr. Biochem. 2022, 107, 109062. [Google Scholar] [CrossRef]
- Kuo, N.C.; Huang, S.Y.; Yang, C.Y.; Shen, H.H.; Lee, Y.M. Involvement of HO-1 and Autophagy in the Protective Effect of Magnolol in Hepatic Steatosis-Induced NLRP3 Inflammasome Activation In Vivo and In Vitro. Antioxidants 2020, 9, 924. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, X.; Liao, R. Mechanism and regulation of mitophagy in nonalcoholic fatty liver disease (NAFLD): A mini-review. Life Sci. 2023, 312, 121162. [Google Scholar] [CrossRef]
- Li, X.; Shi, Z.; Zhu, Y.; Shen, T.; Wang, H.; Shui, G.; Loor, J.J.; Fang, Z.; Chen, M.; Wang, X.; et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice. Br. J. Pharmacol. 2020, 177, 3591–3607. [Google Scholar] [CrossRef]
- Gong, L.L.; Yang, S.; Zhang, W.; Han, F.F.; Lv, Y.L.; Wan, Z.R.; Liu, H.; Jia, Y.J.; Xuan, L.L.; Liu, L.H. Akebia saponin D alleviates hepatic steatosis through BNip3 induced mitophagy. J. Pharmacol. Sci. 2018, 136, 189–195. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef]
- Liu, P.; Lin, H.; Xu, Y.; Zhou, F.; Wang, J.; Liu, J.; Zhu, X.; Guo, X.; Tang, Y.; Yao, P. Frataxin-Mediated PINK1-Parkin-Dependent Mitophagy in Hepatic Steatosis: The Protective Effects of Quercetin. Mol. Nutr. Food Res. 2018, 62, e1800164. [Google Scholar] [CrossRef]
- van den Berg, E.H.; van Tienhoven-Wind, L.J.; Amini, M.; Schreuder, T.C.; Faber, K.N.; Blokzijl, H.; Dullaart, R.P. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: The Lifelines Cohort Study. Metabolism 2017, 67, 62–71. [Google Scholar] [CrossRef]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef]
- Zhou, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Camat, M.D.; James, A.; Wu, Y.; Ching, J.; Kovalik, J.P.; et al. Thyroid Hormone Decreases Hepatic Steatosis, Inflammation, and Fibrosis in a Dietary Mouse Model of Nonalcoholic Steatohepatitis. Thyroid 2022, 32, 725–738. [Google Scholar] [CrossRef]
- Iannucci, L.F.; Cioffi, F.; Senese, R.; Goglia, F.; Lanni, A.; Yen, P.M.; Sinha, R.A. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci. Rep. 2017, 7, 2023. [Google Scholar] [CrossRef]
- Joshi, A.; Upadhyay, K.K.; Vohra, A.; Shirsath, K.; Devkar, R. Melatonin induces Nrf2-HO-1 reprogramming and corrections in hepatic core clock oscillations in Non-alcoholic fatty liver disease. FASEB J. 2021, 35, e21803. [Google Scholar] [CrossRef]
- Sun, J.; Bian, Y.; Ma, Y.; Ali, W.; Wang, T.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z.; Zou, H. Melatonin alleviates cadmium-induced nonalcoholic fatty liver disease in ducks by alleviating autophagic flow arrest via PPAR-alpha and reducing oxidative stress. Poult. Sci. 2023, 102, 102835. [Google Scholar] [CrossRef]
- Zhou, H.; Du, W.; Li, Y.; Shi, C.; Hu, N.; Ma, S.; Wang, W.; Ren, J. Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018, 64, e12450. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Zeng, J.; Acin-Perez, R.; Assali, E.A.; Martin, A.; Brownstein, A.J.; Petcherski, A.; Fernandez-Del-Rio, L.; Xiao, R.; Lo, C.H.; Shum, M.; et al. Restoration of lysosomal acidification rescues autophagy and metabolic dysfunction in non-alcoholic fatty liver disease. Nat. Commun. 2023, 14, 2573. [Google Scholar] [CrossRef]
- Salem, G.A.; Mohamed, A.A.; Khater, S.I.; Noreldin, A.E.; Alosaimi, M.; Alansari, W.S.; Shamlan, G.; Eskandrani, A.A.; Awad, M.M.; El-Shaer, R.A.A.; et al. Enhancement of biochemical and genomic pathways through lycopene-loaded nano-liposomes: Alleviating insulin resistance, hepatic steatosis, and autophagy in obese rats with non-alcoholic fatty liver disease: Involvement of SMO, GLI-1, and PTCH-1 genes. Gene 2023, 883, 147670. [Google Scholar] [CrossRef]
- Lee, S.; Han, D.; Kang, H.G.; Jeong, S.J.; Jo, J.E.; Shin, J.; Kim, D.K.; Park, H.W. Intravenous sustained-release nifedipine ameliorates nonalcoholic fatty liver disease by restoring autophagic clearance. Biomaterials 2019, 197, 1–11. [Google Scholar] [CrossRef]
- Zagkou, S.; Marais, V.; Zeghoudi, N.; Guillou, E.L.; Eskelinen, E.L.; Panasyuk, G.; Verrier, B.; Primard, C. Design and Evaluation of Autophagy-Inducing Particles for the Treatment of Abnormal Lipid Accumulation. Pharmaceutics 2022, 14, 1379. [Google Scholar] [CrossRef]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158. [Google Scholar] [CrossRef]
- Pant, R.; Sharma, N.; Kabeer, S.W.; Sharma, S.; Tikoo, K. Selenium-Enriched Probiotic Alleviates Western Diet-Induced Non-alcoholic Fatty Liver Disease in Rats via Modulation of Autophagy Through AMPK/SIRT-1 Pathway. Biol. Trace Elem. Res. 2023, 201, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Song, Y.; Yuan, M.; Chen, L.; Zhang, Q.; Hu, J.; Meng, Y.; Li, S.; Zheng, G.; Qiu, Z. Ellagitannins-Derived Intestinal Microbial Metabolite Urolithin A Ameliorates Fructose-Driven Hepatosteatosis by Suppressing Hepatic Lipid Metabolic Reprogramming and Inducing Lipophagy. J. Agric. Food Chem. 2023, 71, 3967–3980. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Tao, F.; Peng, L.; Chen, S.; Ren, Z.; Chen, J.; Yu, B.; Wei, H.; Wan, C. In Vitro Probiotic Properties of Bifidobacterium animalis subsp. lactis SF and Its Alleviating Effect on Non-Alcoholic Fatty Liver Disease. Nutrients 2023, 15, 1355. [Google Scholar] [CrossRef] [PubMed]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D and Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): An Update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef]
- Nagashimada, M.; Ota, T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life 2019, 71, 516–522. [Google Scholar] [CrossRef]
- Li, R.; Guo, E.; Yang, J.; Li, A.; Yang, Y.; Liu, S.; Liu, A.; Jiang, X. 1,25(OH)2 D3 attenuates hepatic steatosis by inducing autophagy in mice. Obesity 2017, 25, 561–571. [Google Scholar] [CrossRef]
- Lim, H.; Lee, H.; Lim, Y. Effect of vitamin D3 supplementation on hepatic lipid dysregulation associated with autophagy regulatory AMPK/Akt-mTOR signaling in type 2 diabetic mice. Exp. Biol. Med. 2021, 246, 1139–1147. [Google Scholar] [CrossRef]
- Shen, C.; Dou, X.; Ma, Y.; Ma, W.; Li, S.; Song, Z. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr. Res. 2017, 40, 40–47. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Grossi, I.; Garcia-Gomez, R.; Patel, G.A.; Salvi, A.; Lavazza, A.; De Petro, G.; Monsalve, M.; Rezzani, R. Melatonin Effects on Non-Alcoholic Fatty Liver Disease Are Related to MicroRNA-34a-5p/Sirt1 Axis and Autophagy. Cells 2019, 8, 1053. [Google Scholar] [CrossRef]
- Palecek, E.J.; Kimzey, M.M.; Zhang, J.; Marsden, J.; Bays, C.; Moran, W.P.; Mauldin, P.D.; Schreiner, A.D. Glucagon-like peptide-1 receptor agonist therapy effects on glycemic control and weight in a primary care clinic population. J. Investig. Med. 2024, 72, 911–919. [Google Scholar] [CrossRef]
- Suzuki, A.; Hayashi, A.; Oda, S.; Fujishima, R.; Shimizu, N.; Matoba, K.; Taguchi, T.; Toki, T.; Miyatsuka, T. Prolonged impacts of sodium glucose cotransporter-2 inhibitors on metabolic dysfunction-associated steatotic liver disease in type 2 diabetes: A retrospective analysis through magnetic resonance imaging. Endocr. J. 2024, 71, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; D’Alessio, D.A. Slow and Steady Wins the Race: 25 Years Developing the GLP-1 Receptor as an Effective Target for Weight Loss. J. Clin. Endocrinol. Metab. 2022, 107, 2148–2153. [Google Scholar] [CrossRef]
- Pereira, M.J.; Eriksson, J.W. Emerging Role of SGLT-2 Inhibitors for the Treatment of Obesity. Drugs. 2019, 79, 219–230. [Google Scholar] [CrossRef] [PubMed]
- van Ruiten, C.C.; Hesp, A.C.; van Raalte, D.H. Sodium glucose cotransporter-2 inhibitors protect the cardiorenal axis: Update on recent mechanistic insights related to kidney physiology. Eur. J. Intern. Med. 2022, 100, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Huelgas, R.; Sanz-Cánovas, J.; Cobos-Palacios, L.; López-Sampalo, A.; Pérez-Belmonte, L.M. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors for cardiovascular and renal protection: A treatment approach far beyond their glucose-lowering effect. Eur. J. Intern. Med. 2022, 96, 26–33. [Google Scholar] [CrossRef]
- Xu, R.; Liu, B.; Zhou, X. Comparison of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter Protein-2 Inhibitors on Treating Metabolic Dysfunction-Associated Steatotic Liver Disease or Metabolic Dysfunction-Associated Steatohepatitis: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Endocr. Pract. 2025, 31, 521–535. [Google Scholar]
- Wiegley, N.; So, P.N. Sodium-Glucose Cotransporter 2 Inhibitors and Urinary Tract Infection: Is There Room for Real Concern? Kidney360 2022, 3, 1991–1993. [Google Scholar] [CrossRef]
- Gorgojo-Martínez, J.J.; Mezquita-Raya, P.; Carretero-Gómez, J.; Castro, A.; Cebrián-Cuenca, A.; de Torres-Sánchez, A.; García-de-Lucas, M.D.; Núñez, J.; Obaya, J.C.; Soler, M.J.; et al. Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus. J. Clin. Med. 2022, 12, 145. [Google Scholar] [CrossRef]
- D’Marco, L.; Morillo, V.; Gorriz, J.L.; Suarez, M.K.; Nava, M.; Ortega, Á.; Parra, H.; Villasmil, N.; Rojas-Quintero, J.; Bermúdez, V. SGLT2i and GLP-1RA in Cardiometabolic and Renal Diseases: From Glycemic Control to Adipose Tissue Inflammation and Senescence. J. Diabetes Res. 2021, 2021, 9032378. [Google Scholar] [CrossRef]
- Wilbon, S.S.; Kolonin, M.G. GLP1 Receptor Agonists-Effects beyond Obesity and Diabetes. Cells 2023, 13, 65. [Google Scholar] [CrossRef]
- Choi, J.G.; Winn, A.N.; Skandari, M.R.; Franco, M.I.; Staab, E.M.; Alexander, J.; Wan, W.; Zhu, M.; Huang, E.S.; Philipson, L.; et al. First-Line Therapy for Type 2 Diabetes With Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Cost-Effectiveness Study. Ann. Intern. Med. 2022, 175, 1392–1400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).