Simple Summary
In the natural environment, organisms exhibit intricate interconnections in various forms; one notable interaction among plant species is hemiparasitism. A range of physiological processes and morphological alterations occurs within the species engaged in this relationship. To investigate certain facets of this phenomenon, an in vitro co-culture of the hemiparasitic plant Castilleja tenuiflora and its host species, Baccharis conferta, was employed, resulting in the successful induction of haustoria. This study examined haustoria formation, alterations in root biomass, and the production of chemically significant compounds. In vitro co-cultivation of C. tenuiflora and B. conferta highlights the significance of developing systems that enhance our comprehension of plant–plant interactions.
Abstract
In this study, an in vitro co-culture system of Castilleja tenuiflora and its host, Baccharis conferta, was used, and the impact of their interaction on specialized metabolite content was analyzed. After 4 weeks of co-culture, haustoria formation was verified through environmental scanning electron and confocal microscopy, confirming the successful establishment of the plant–plant interaction. Shoot height and biomass of the aerial part of the hemiparasite were not affected significantly by co-culture. However, root biomass increased by 53% compared to individually grown plants. Co-culture significantly reduced the host’s root length without negatively affecting its overall growth or survival. Phytochemical profile alterations were observed in both species. For C. tenuiflora, the lignans sesamin and eudesmin are proposed as differentially accumulated metabolites, while in B. conferta, the caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, and the flavonoid acacetin were expressed differently. The development and chemical profiles of B. conferta and C. tenuiflora change when they grow in a co-culture because of the host–parasite interaction. Here, we report the feasibility of using a hemiparasite–host system to investigate more profound research questions. Future biotechnological applications of this system include elucidating the genetic regulators involved in haustorium formation, as well as optimizing environmental and physiological conditions to enhance its biosynthetic capacity for the production of specialized metabolites with therapeutic value.
1. Introduction
Parasitism is a highly specialized survival strategy in plants that enables them to obtain resources from their hosts. Parasitic plants are classified into holoparasites and hemiparasites based on their nutrient dependency on hosts. Hemiparasites can photosynthesize but acquire inorganic nutrients from their hosts to gain a competitive advantage [1]. This interaction necessitates physical attachment between the host and the parasite, either aboveground (shoot-to-shoot) or belowground (root-to-root), through a unique organ known as the haustorium. This multifunctional organ facilitates attachment, penetration, and connection with the host, creating a continuum for exchanging water, nutrients, hormones, proteins, and nucleotides [2]. Haustoria formation is a highly regulated process triggered by recognizing host-derived chemical signals known as haustorium-inducing factors (HIFs). Cytokinins, phenolics, quinones, and ROS have been identified as HIFs [2,3].
Although parasitic plants that negatively impact agriculture, such as Striga spp. and Phelipanche spp., have been extensively studied to develop management and control strategies, the hemiparasitic interactions among species with potential therapeutic uses, like medicinal plants, have received limited attention [4,5].
Castilleja tenuiflora Benth. (Orobanchaceae) is a generalist facultative root hemiparasite with medicinal uses [6]. Research on its chemical composition has shown that it accumulates terpenes, including iridoid glycosides, phenylethanoid glycosides (PhGs), flavonoids, and lignans [7,8]. Pharmacological studies have confirmed its efficacy as an antioxidant, anti-inflammatory, cytotoxic, gastroprotective, and antidepressant agent [8,9]. Given its therapeutic potential, we are interested in developing biotechnological strategies to ensure a sufficient supply of plant material as a source of specialized metabolites. However, its hemiparasitic nature imposes certain limitations. In a previous study, we described C. tenuiflora as a facultative parasite of Baccharis conferta Kunth (Asteraceae), detailing the anatomy of the haustorium and how carbon levels in both plants are affected by their parasitic interaction [10]. To induce C. tenuiflora haustoria under laboratory conditions, we applied HIFs to in vitro plantlets, including H2O2 and phenolics [11]. Developing a hemiparasite–host system is essential to deepen our understanding of host–parasite interactions.
Baccharis conferta is a shrub that serves as a nurse plant for other species growing beneath its canopy [12]. It has medicinal properties, and its phytochemistry and pharmacology have been investigated. It synthesizes flavonoids such as cirsimaritin and terpenes like bacchofertin, exhibiting anti-inflammatory and anti-arthritic activities [13]. In previous studies, we reported the establishment of protocols for in vitro propagation, including co-culture with C. tenuiflora, along with the production of caffeoylquinic acids in callus cultures [14,15].
Research has primarily focused on applying HIFs to the parasitic plant to initiate, characterize, and understand haustorium formation [3,4]. Studies on the effects of plant–plant interactions in a (hemi) parasite–host system on physiology and specialized metabolism are limited. For instance, the chemical profile of Helicanthes elasticus was influenced by the hosts on which it grew [5]. Cuscuta campestris induced metabolic changes, such as a reduction in sesquiterpene and monoterpenoid levels in its host Artemisia campestris [16]. This study investigated plant–plant interactions on specialized metabolite content using a co-culture system of B. conferta and C. tenuiflora. The study findings will enhance the understanding of hemiparasites with biotechnological and therapeutic significance.
2. Materials and Methods
2.1. Plant Material
Castilleja tenuiflora plants were grown from shoots that were twenty-one days old (3 cm) in 50 cm test tubes containing 10 mL of Schenk and Hilderbrandt (SH) culture medium, supplemented with 30 g L−1 of sucrose, 2.2 g L−1 of phytagel® (Sigma-Aldrich, St. Louis, MO, USA), and 10 µM of 3-indole acetic acid to obtain rooted plantlets. The culture medium was solidified at a 45° angle, and the tubes were covered with cotton [11]. The cultures were maintained for 21 days in a culture chamber at 25 ± 2 °C under cool white light (LED lamps) with an irradiance of 26 μmol m−2 s−1 and a photoperiod of 16 h light/8 h dark.
Baccharis conferta shoots that were twenty-one days old (3 cm) were grown in Magenta vessels containing semisolid Murashige and Skoog (MS) culture medium with 30 g L−1 sucrose and 2.2 g L−1 phytagel® without any plant growth regulators [14]. These cultures were maintained under the same conditions as previously described.
2.2. Co-Culture Experiments
Co-culture system: Twenty-one days old plants of C. tenuiflora and B. conferta were placed in Magenta vessels with semisolid MS medium, 30 g L−1 sucrose, and 2.2 g L−1 phytagel® without any plant growth regulators. One plantlet of each species was positioned 1 cm apart from the other [15].
Three experimental groups were tested and are as follows: (1) C. tenuiflora co-cultured with B. conferta (Ct-Bc); (2) C. tenuiflora growing axenically without a host (Ct), with one plantlet placed in each Magenta vessel; and (3) B. conferta growing axenically without a parasite (Bc), also with one plantlet placed in each Magenta vessel. These cultures were maintained under the same conditions as described above. All analyses were conducted after 4 weeks.
Plant growth analysis: Morphological indices were evaluated using 11 plants from each group. The following variables were measured: plant height (cm), root length (cm), root-to-shoot ratio, and fresh weight (g) of both the aerial part and the root.
2.3. Environmental Scanning Electron Microscopy (ESEM)
Root sections of approximately 1 cm were cut and fixed in a solution (formaldehyde (10 mL), glacial acetic acid (5 mL), ethanol (50 mL), milli-Q water (35 mL), and sucrose (0.8 g)) for 24 h. Subsequently, three washes were performed with a phosphate buffer at pH 7.2 (0.2 M). Then, the dehydration process was conducted through successive immersions in ethanol at increasing concentrations (30%, 50%, 70%, 80%, and 90%). Between each ethanol immersion, the samples were allowed to stand for one hour. Finally, they were stored in 90% ethanol. Samples were placed on aluminum stubs with double-sided carbon adhesive tape and observed under an Environmental Scanning Electron Microscope (EVO LS10, Zeiss, Oberkochen, Germany). All observations were conducted under constant operating conditions: a beam acceleration voltage of 20 kV, a backscattered electron detector, and a water vapor pressure of 80 Pa. Images were captured at 2048 × 1536 pixels and stored in TIFF format. At least 10 root sections for each treatment were analyzed.
2.4. Confocal Laser Scanning Microscopy (CLSM)
Samples were observed using a Confocal Laser Scanning Microscope (LSM 800, Zeiss, Oberkochen, Germany). Root tips were fixed as described previously, and then the samples were mounted on glass slides and examined in lambda mode (a sequence of images collected at different wavelengths) at laser wavelengths of 405 nm and 488 nm with a 5% capacity. Figure 1F,G were captured in Z-stack mode, resulting in 36 slices with a 1.7 µm separation. Figure 1E was created using two channels (confocal and ESID). The images were produced using the ZEN 2.3 Blue edition software, and all micrographs were taken using Carl Zeiss’s EC Plan-Neofluar 20×/0.50 M27 objective.
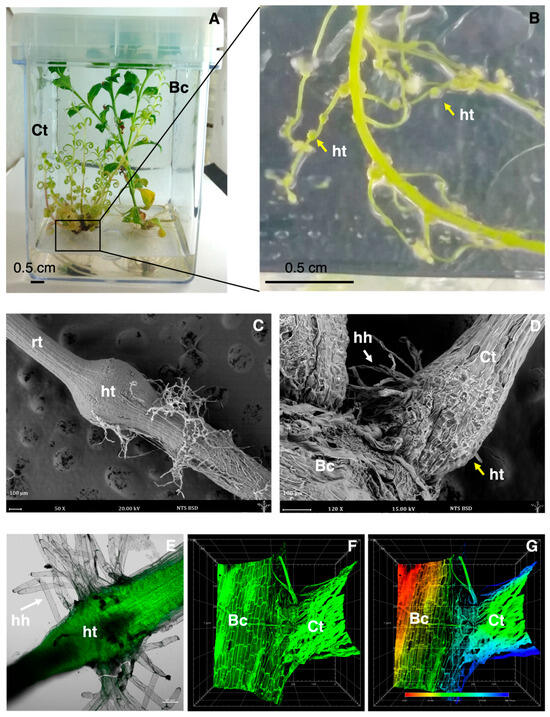
Figure 1.
Representative images of the in vitro co-culture of Castilleja tenuiflora and Baccharis conferta (A) and plants of C. tenuiflora grown in co-culture showing the development of haustoria in the roots (B). Environmental Scanning Electron Microscopy micrographs of the roots of C. tenuiflora showing the development of haustorium (C) and the attachment to the roots of B. conferta (D). Laser scanning confocal microscopy micrographs of the haustoria of C. tenuiflora (E) developed in co-culture with B. conferta; (F,G) roots of C. tenuiflora and B. conferta attached in co-culture by haustorial bridge. Bc: Baccharis conferta; Ct: C. tenuiflora; ht: haustorium; hh: haustorial hairs; rt: roots. The yellow arrows indicate haustoria (ht), and the white arrows indicate haustorial hairs (hh).
2.5. Metabolite Analysis Through HPLC and LC-MS
Plant material (aerial parts and roots) was collected from each treatment, oven-dried at 40 °C for 48 h, and ground using a mortar and pestle. Subsequently, a microextraction of the whole plant material (1 g of dry matter (DM) in 50 milliliters of methanol) was conducted through sonication in methanol for 30 min and vacuum-filtered using Millipore® membranes (0.20 μm). The filtrate was concentrated under reduced pressure with a rotary evaporator (V-250, Buchi, Flawil, Switzerland) operating at 210 mbar at 40 °C and 50 rpm. Finally, the samples were frozen at −20 °C for 24 h and freeze-dried for 3 h. For the HPLC and LC-MS analyses, the extracts were dissolved in HPLC-grade methanol (MeOH) at a concentration of 6 mg mL−1, sonicated for 30 min, filtered through a nylon acro disk (0.45 μm), and placed in a transparent glass vial.
LC-MS analysis was conducted using a Shimadzu LCMS 2020 system (Shimadzu, Tokyo, Japan) equipped with an electrospray ionization (ESI) source and LC-MS Labsolutions software, version 5.0.
For the analysis of B. conferta, we used an RP-18 column (Lichrospher 100. 250 × 4 mm, RP 18, 5 μm) (Merck, Darmstadt, Germany) connected to a column guard at 36 °C. The mobile phase consisted of 0.5% acetic acid aqueous solution as solvent A and acetonitrile as solvent B at a flow rate of 0.9 mL min−1. Gradient elution was conducted as follows: 0–1 min, 100–0% A–B; 1–3 min, 95–5% A–B; 3–20, 70–30%, A–B; 20–23 min, 50–50% A–B; 23–25 min, 20–80% A–B; 25–27 min, 0–100%·A–B; 27–30 min, 100–0% A–B [14].
C. tenuiflora was analyzed at 40 °C using a reverse-phase Chromolith® High-Resolution RP-18 column (100 mm × 4 mm, 5 μm) (Merck, Darmstadt, Germany). The mobile phase consisted of Milli-Q water as solvent A and acetonitrile as solvent B. The gradient system was as follows: 0–2 min, 100–0% A–B; 2–5 min, 90–10% A–B; 5–10, 85–15%, A–B; 10–14 min, 80–20% A–B; 14–18 min, 75–25% A–B; 18–23 min, 70–30%·A–B; 23–27 min, 0–100% A–B; 27–30 min, 100–0% A–B. The injection volume was set to 20 μL, and the flow rate was 1 mL min−1.
Detection occurred within the 200–400 nm wavelength range. Mass spectra were recorded in the negative ion mode under the following operating conditions: 50–1000 m/z scan; N2 drying gas at 10 L min−1; nebulizer gas flow at 1.54 L min−1; interface voltage at 4.5 kV; and detector voltage at 1.2 kV.
2.6. Analysis of Differential Abundance of Metabolites
The metabolites analyzed in this study were selected based on their pharmacological significance. The fingerprints analyzed for B. conferta included phenolics (3-O-caffeoylquinic acid, caffeic acid, and 4,5-di-O-caffeoylquinic acid) and flavonoids (hispidulin, cirsimaritin, acacetin, pectolinarigenin, and 6-methoxykaempferide) [13]. In contrast, for C. tenuiflora, they comprised iridoids (aucubin and bartsioside), phenylethanoid glycosides (verbascoside and isoverbascoside), and lignans (tenuifloroside, sesamin, eudesmin, magnolin, and kobusin) [7]. Compounds were identified based on the determination of their retention time, ion m/z [M+H]-, and UV–Vis spectra, according to the literature reports, and according to compounds previously detected or isolated from B. conferta or C. tenuiflora (Tables S1 and S2). The identification was additionally confirmed for most compounds using available reference chemical standards.
The relative abundance of compounds was estimated based on the area of each peak and its contribution to the total signal area, as indicated by the chromatogram. The chromatogram’s raw data files were processed manually. The peak picking of each metabolite was performed with a 0.2 min retention time tolerance. The absorbance measurement was performed at 205 nm for iridoids [7], 280 nm for flavonoids and lignans [7,13], 325 nm for caffeic acid and caffeoylquinic acids [17], and 330 nm for PhGs [7]. Normalized peak intensity was logarithmically (Log10) transformed for further analysis. Differences in metabolite levels between axenic and co-culture were identified using a t-test and p-value as univariate statistical analysis. Metabolites with a p-value < 0.05 and a fold change (FC) ≥ 1.5 or FC ≤ 0.5 were considered differentially accumulating metabolites (DAMs) [18].
2.7. Statistical Analysis
Data is presented as the mean ± standard error of the mean (SEM). The normal distribution was confirmed using the Shapiro–Wilk test. Unpaired Student’s t-test and p-value were used to examine significant differences between two conditions (axenic vs. co-culture). p-values less than 0.05 (p < 0.05) were considered statistically significant. All statistical analyses were performed using GraphPad Prism version 10.4.2 for macOS (GraphPad Software, Boston, MA, USA).
3. Results
3.1. Formation of Haustorium in Co-Culture
An in vitro co-culture system was used to investigate the effects of host–parasite interaction between B. conferta and C. tenuiflora (Figure 1A). C. tenuiflora, when co-cultured with B. conferta, formed stronger roots (Figure 1B) with lateral protuberances (Figure 1B), which, based on previous experience, should correspond to haustoria [10,11].
The analysis conducted using ESEM (Figure 1C,D) and confocal microscopy (Figure 1E–G) confirmed the induction of C. tenuiflora haustoria under co-cultivation conditions. According to the ESEM analysis, haustoria measured between 500 and 900 µm in diameter (Figure 1C), and haustorial hairs were also formed (Figure 1D), which confirmed the plant’s attachment.
Figure 1E shows the roots from C. tenuiflora plants in co-cultivation conditions and the formation of globular haustoria with haustorial hairs using confocal microscopy, and the establishment of vascular connections under co-cultivation conditions (Figure 1F). In Figure 1G, using the “Depth Coding” tool (red, green, and blue), it was demonstrated the physical contact between the roots of C. tenuiflora and B. conferta by differentiating them with distinct color tones: reddish hues indicating proximity to the base of the stage (0 μm), and bluish hues corresponding to a higher position of the first root. This confirmed the presence of a zone where both roots interact in the same plane (indicated by green).
3.2. Growth in Co-Culture
The height of C. tenuiflora plants was similar (t = 0.1171, p = 0.9079) in both axenic culture and co-culture with B. conferta (Figure 2A). Although the roots were a little shorter (t = 2.077, p = 0.0509) in the co-culture (Figure 2B), their biomass was higher (53%), which aligns with the significant difference (t = 3.296, p = 0.0036) in the root-to-shoot ratio observed in a co-culture compared to that for axenic conditions (Figure 2C). However, the total fresh biomass of C. tenuiflora only increased by 18.7% in a co-culture relative to axenic plants.
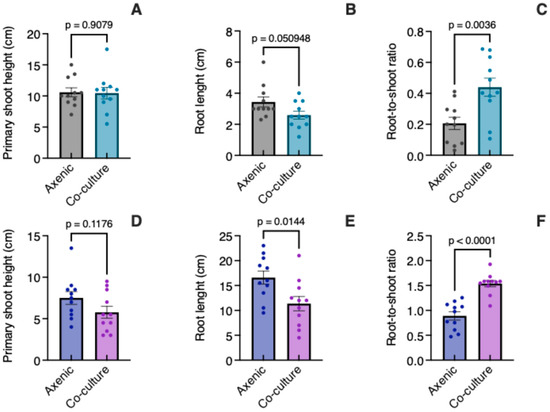
Figure 2.
Castilleja tenuiflora (A–C) and Baccharis conferta (D–F) growth in axenic culture and co-culture. The root-to-shoot ratio is the ratio of root fresh weight to shoot fresh weight. Data are expressed as mean ± standard error (n = 11). Significant differences between axenic and co-culture plants were determined using an unpaired Student’s t-test (p < 0.05).
The plants of B. conferta were similar in height in a co-culture to those grown axenically (t = 1.636, p = 0.1176) (Figure 2D). In co-culture, their roots were significantly shorter (t = 2.680, p = 0.0144) (Figure 2E) and still exhibited greater biomass (26%), highlighting a significant difference in the root-to-shoot ratio (t = 6.202, p < 0.0001) (Figure 2F). The proportion of root biomass in C. tenuiflora (14%) was half in axenic conditions than in co-culture conditions (30%) (Figure 3A). For B. conferta, the proportion of root biomass was also higher when in a co-culture with its host, reaching almost 60% (Figure 3B). No symptoms of adverse effects such as wilting, chlorosis, or necrosis were observed in B. conferta plants grown under co-culture.
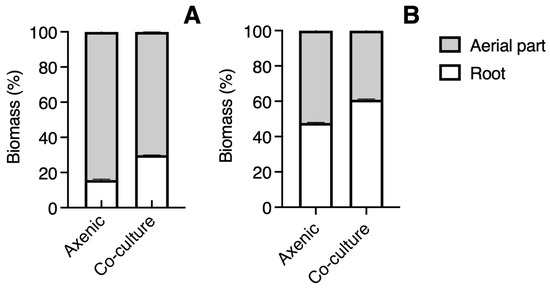
Figure 3.
Proportion of total fresh biomass from aerial part and roots of Castilleja tenuiflora (A) and Baccharis conferta (B) in axenic culture and co-culture conditions.
3.3. Specialized Metabolites in Co-Culture
The LC-MS analysis was performed to relatively quantify specialized metabolites in C. tenuiflora and B. conferta grown under axenic and co-culture conditions. This analysis resulted in semi-quantitative profiling of 17 selected chemical compounds. The aim was to evaluate the impact of plant–plant interaction on the specialized metabolism of C. tenuiflora and its host, B. conferta.
PhGs were the most abundant metabolites in C. tenuiflora, followed by iridoids and lignans (Table S3). Two phenylethanoid glycosides (PhGs)—isoverbascoside and verbascoside—were identified (peaks 3 and 4, Figure S3). The two iridoids included aucubin (peak 1, Figure S1) and bartsioside (peak 2, Figure S1), where aucubin was the most abundant. For lignans, magnolin (peak 6, Figure S2) and tenuifloroside (peak 5, Figure S1) predominated over eudesmin (peak 7, Figure S2), kobusin (peak 8, Figure S1), and sesamin (peak 9, Figure S1).
In B. conferta, caffeoylquinic acids predominated over caffeic acid (Table S4); 4,5-di-O-caffeoylquinic acid (peak 3, Figure S7) constituted the most abundant metabolite, followed by chlorogenic acid (peak 1, Figure S7). For flavonoids, cirsimaritin (peak 5, Figure S7) and 6-methoxykaempferide (peak 8, Figure S7) predominated over hispidulin (peak 4, Figure S7), acacetin (peak 6, Figure S7), or pectolinaringenin (peak 7, Figure S7). In both species, the overall profiles were consistent across the two culture conditions (axenic and co-culture), with variations observed only in their relative abundance levels.
Figure 4 presents the relative abundance (log10 transformed) of the nine metabolites produced by C. tenuiflora when grown axenically and in co-culture with its host. As mentioned before, aucubin is the most abundant iridoid in C. tenuiflora, and its relative abundance was unaffected by co-culture conditions (t = 0.1209, p = 0.9053) (Figure 4A, Table S3). In contrast, bartsioside (Figure 4B, Table S3) decreased significantly in co-culture (t = 2.782, p = 0.0133) compared to axenic culture. Both PhGs changed significantly, with isoverbascoside decreasing (t = 3.866, p = 0.0014) while verbascoside increased (t = 22.39, p < 0.0001) (Figure 4C,D, Table S3).
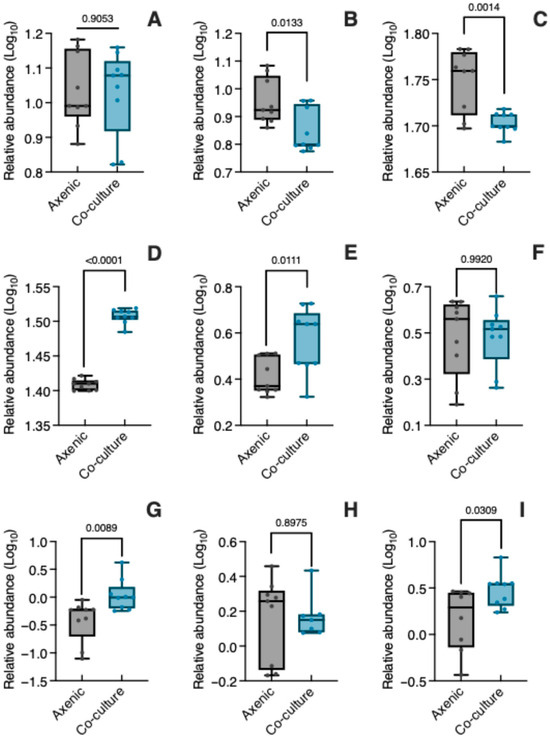
Figure 4.
Relative abundance of specialized metabolites in Castilleja tenuiflora when growing axenically and in co-culture. (A) aucubin; (B) bartsioside; (C) isoverbascoside; (D) verbascoside; (E) tenuifloroside; (F) magnolin; (G) edudesmin; (H) kosubin; (I) sesamin. Relative abundance was log10 transformed. Significant differences between axenic and co-culture plants were determined using an unpaired Student’s t-test (p < 0.05).
Like iridoids and PhGs, lignans changed differentially in axenic and co-culture conditions (Figure 4E–I, Table S3). Tenuifloroside (t = 2.871, p = 0.0111), eudesmin (t = 2.974, p = 0.0089) and sesamin (t = 2.382, p = 0.0309) increased significantly under co-culture, with magnolin (t = 0.0101, p = 0.9920) and kobusin (t = 0.1311, p = 0.8975) having similar relative abundances in both culture conditions.
Among DAMs, the analysis indicated that the lignans eudesmin (2.38-fold change) and sesamin (1.78-fold change) were expressed differentially in C. tenuiflora when parasitizing B. conferta.
The relative abundance (log10 transformed) of the eight metabolites found in B. conferta is presented in Figure 5. The two caffeoylquinic acids, namely chlorogenic acid (t = 2.606, p = 0.0191) (Figure 5A, Table S4) and 4,5-di-O-caffeoylquinic acid (t = 6.005, p < 0.0001) (Figure 5C, Table S4), were significantly more abundant in B. conferta when co-cultured with C. tenuiflora compared to growing axenically. In contrast, caffeic acid was more abundant in the axenic condition (t = 3.822, p = 0.0015) (Figure 5B, Table S4). Given that caffeic acid is a precursor of phenylethanoid glycosides, its potential translocation to C. tenuiflora was investigated by assessing its presence in both the root and aerial parts, using LC-MS. However, it was not possible to determine its relative abundance due to coelution with phenylethanoid glycosides (found at a retention time of 7.01 min, Figure S5).
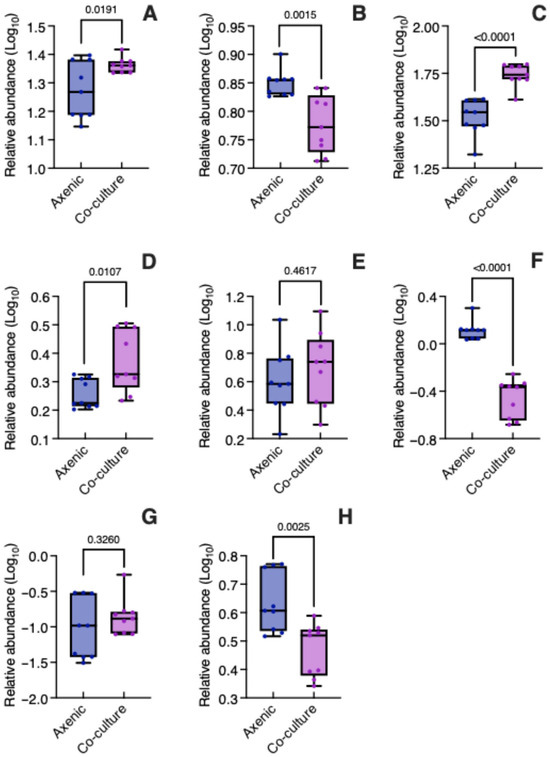
Figure 5.
Relative abundance of specialized metabolites in Baccharis conferta when grown axenically and in co-culture. (A) chlorogenic acid; (B) caffeic acid; (C) 4,5 di-O-caffeoylquinic acid; (D) hispidulin; (E) cirsimaritin; (F) acacetin; (G) pectolinarigenin; (H) 6-methoykaempferide. Relative abundance was log10 transformed. Significant differences between axenic and co-culture plants were determined using an unpaired Student’s t-test (p < 0.05).
The relative abundance of hispidulin (t = 2.888, p = 0.0107) (Figure 5D, Table S4) increased significantly in co-culture, while acacetin (t = 9.517, p < 0.0001) (Figure 5F, Table S4) and 6-methoxykaempferide (t = 3.588, p = 0.0025) (Figure 5H, Table S4) significantly decreased. The relative abundance of cirsimaritin (t = 0.7542, p = 0.4617) (Figure 5E, Table S4) and pectolinaringenin (t = 1.013, p = 0.3260) (Figure 5G, Table S4) was similar in both axenic and co-culture conditions.
Among DAMs, the analysis indicated that 4,5-di-O-caffeoylquinic acid (1.56-fold change) and acacetin (0.26-fold change) were expressed differentially in B. conferta when parasitized by C. tenuiflora.
4. Discussion
Our results indicate that C. tenuiflora develops lateral haustoria when co-cultured in vitro with B. conferta. The haustoria resemble those observed in wild plants [10] and those produced in vitro after applying HIFs such as H2O2 or vanillin [11]. The formation of haustorial hairs was also observed, which is essential for efficient parasitism [19].
The formation of haustoria involves four distinct phases: recognition, attachment, invasion, and connection [2]. In the rhizosphere, host plants release molecules that work as host recognition signals. Among these molecules, strigolactones (SLs) have received special attention as signaling compounds for plant–plant interaction [20,21]. So far, the composition of the root exudates of B. conferta or other species of this genus has not been elucidated. The characterization of the root exudates of several species in the family Asteraceae (which includes Baccharis) revealed the presence of SLs, with orobanchyl acetate and 5-deoxystrigol as the significant components [22]. Recently, using the SL analog GR24 5DO, it has been proposed that Castilleja foliolosa possesses a large clade of KAI2d proteins, which are receptors for exogenous SLs and are related to the formation of haustorium-like structures [23], as noted for other facultative hemiparasites. It is known that the parasite recognizes the host with high specificity, and that these proteins exhibit high sensitivity and specificity in recognizing specific mixtures of SLs, which enables them to discriminate between host and non-host plants, resulting in the formation of haustoria [24]. Therefore, it can be hypothesized that strigolactones have a signaling role in the establishment of the host–parasite interaction in B. conferta-C. tenuiflora co-culture.
To establish the co-culture system, it was essential to promote root development in both species, which, based on previous experience, was achieved using SH medium for C. tenuiflora and MS medium for B. conferta. For the co-culture experiments, MS medium was selected, as it provides a higher nitrogen content compared to SH medium (60 mM vs. 27.4 mM) [25], thus helping to prevent a possible negative effect of C. tenuiflora on B. conferta survival. Previous studies have shown that parasitism tends to increase under nitrogen-deficient conditions; however, such conditions may also compromise host viability [26]. Environmental factors—including water availability, light, and nutrient supply, particularly nitrogen and phosphorus, along with their interactions—have been proposed to influence the dynamics between host and parasite [27,28,29]. Consequently, the performance of both species is expected to differ under culture conditions other than MS. The specific effects of nutrient supply on this interaction remain an open question and should be addressed in future studies.
Plant height of B. conferta under co-culture and axenic conditions was statistically similar, and no symptoms of adverse effects were observed. In contrast, root biomass yield was higher for both the host and the parasite in a co-culture, corresponding to a significant difference in the root-to-shoot ratio. As observed in the root hemiparasite Rhinanthus alectorolophus (Orobanchaceae), environmental conditions affect both the parasitic and autotrophic components, and the hemiparasite’s photosynthesis plays a role in the assimilation of resources from the host [30].
Host type, host’s growth rate, and resistance to the parasite all influence the parasite’s growth [31,32]. A field study observed that the physiology of C. applegatei was affected by the host type; however, this did not impact the community due to the presence of N-fixing hosts [32]. Furthermore, apparent competition for resources was observed in the co-culture, which may explain why the total biomass of both species was similar. In a parasitic–host system, the mass is typically lower due to its reduction by the parasite compared to that by the host when grown alone [31].
In the parasitic relationship between Struthanthus flexicalulis (Loranthaceae) and Baccharis dracunculifolia, parasitism caused significant physiological imbalances that adversely affected the host’s development [33]. The resistance of potential hosts to infection does not necessarily prevent the hemiparasite from forming haustoria and establishing a connection with its host. This resistance may be reflected as a form of relative tolerance, allowing the host to experience minimal impact on its growth and development, while simultaneously enabling the hemiparasite to develop properly [34]. In this context, variations in the parasitized B. conferta are not significant, suggesting that it has developed a certain degree of resistance that allows it to tolerate infection by C. tenuiflora, which may be associated with potential non-nitrogen limitation conditions (as provided by the MS culture medium), already discussed. This result is consistent with observations made in B. conferta natural populations, where no significant variations in height and chlorophyll content between parasitized and non-parasitized plants were found [10]. In this regard, it has been proposed that, through co-evolution, native hosts have developed tolerance to native parasites, as seen in the cases of B. conferta and C. tenuiflora. It is hypothesized that the mechanisms of tolerance include a poor haustorial connection [35].
Castilleja tenuiflora, growing on B. conferta, enhances its accumulation of PhGs and lignans, which share a common origin in the shikimate pathway, while reducing the accumulation of iridoids synthesized via the terpenes pathway. Among the molecules identified as signals of plant–plant interactions, H2O2 is recognized as crucial for haustorial induction and, consequently, the establishment of parasitic interactions [36]. Our research group has previously reported that haustorium formation in C. tenuiflora correlates with the accumulation of H2O2 [11]. Additionally, we observed that eliciting C. tenuiflora plantlets with H2O2 increased the total content of phenolic compounds, particularly promoting the synthesis of lignans. We discovered that H2O2 upregulates key enzyme-encoding genes (PAL, TyrDC, GOT2, AO3, ADD, and CHS) involved in the phenolic pathway [37]. The connection between signaling molecules of plant–plant interactions and specialized metabolism represents an interesting research topic for further exploration.
The in vitro co-culture of B. conferta with its host or parasite differentially influenced the synthesis of specialized metabolites. Specifically, the relative abundance of the caffeoylquinic acids, chlorogenic acid and 4,5-diQ, increased while that of caffeic acid decreased. Similarly, the relative abundance of the flavonoid acacetin diminished. The decrease in caffeic acid concentration is of particular interest, given its role as a biosynthetic precursor of the PhGs found in C. tenuiflora. Although its potential translocation was investigated, quantification was not feasible due to coelution with verbascoside, which was markedly more abundant. Moreover, the experimental design did not account for the possibility of interspecific metabolite transfer, suggesting the need for further investigation.
The translocation of specialized metabolites between interacting plants has been documented, such as the case of flavonoid transfer from Mangifera indica to its hemiparasite Dendropthoe falcata [38], the translocation of isoquinoline alkaloids to the hemiparasite Tristerix verticillatus from its host Berberis montana [39], or the uptake of quinolizidine alkaloids by C. miniata and C. indivisa from Lupinus [40]. Parasitism of S. flexicaulis on B. dracunculifolia affected flavonoid balance, as the parasitized individuals exhibited lower phenolic content than their non-parasitized counterparts [33]. Similarly, the production of flavonoids in the leaves of Theobroma cacao L. decreased due to the hemiparasitic species Acanthosyris paulo-alvinii [41]. In the hemiparasite Psittacanthus schiedeanus (Loranthaceae), RNA-seq methodology identified differentially expressed genes related to synthesis, signaling, homeostasis, and response to auxin and jasmonic acid synthesis, showing the complexity of the molecular events involved in the host–parasite relationship [42]. Besides other roles, jasmonates have been implicated in signaling the biosynthesis of flavonoids [43].
Overall, co-culture conditions predominantly enhanced differentially the production of phenolic-type compounds in both species: caffeoylquinic acids in B. conferta and lignans in C. tenuiflora. Chlorogenic acids (CGAs), a subclass of caffeoylquinic acids, play a role in chemical defense mechanisms due to their strong antioxidant capacity. They also serve as intermediates in the lignin biosynthetic pathway, as they can be remobilized for monolignol synthesis and contribute to restricting pathogen invasion [44,45]. Considering that parasitism imposes stress on the host plant, the observed increase in CGAs in B. conferta may reflect a defensive response to parasitization, which is consistent with its resistance to C. tenuiflora.
In S. hermonthica, an obligate parasitic plant, it has been reported that exogenous quinones, acting as HIFs, transcriptionally activate genes involved in monolignol biosynthesis. Moreover, external monolignols, such as coniferyl alcohol, are incorporated into the pre-haustorial cell wall. These findings supported the hypothesis that monolignols play a dual role, acting both as signaling molecules for haustorium induction and as structural precursors for lignin deposition during haustorium development [46]. Coniferyl alcohol, a key monolignol derived from the phenylpropanoid pathway, is a common precursor for both lignin and lignan biosynthesis [47]. Therefore, the observed increase in lignans, such as eudesmin and sesamin, in C. tenuiflora grown in co-culture with its host may reflect an upregulation of monolignol biosynthesis associated with the parasitic process.
The co-culture system offers distinct advantages over field-based approaches, particularly given the inherent fragility of parasitic roots and the reversible nature of haustoria formation. This in vitro model provides a controlled environment that enables detailed investigation of the complex physiological and molecular interactions between hosts and parasites. Elucidating the regulatory mechanisms governing haustorium formation may significantly advance our understanding of plant parasitism and its underlying biology. Moreover, this system could facilitate biotechnological efforts aimed at domestication and cultivation of C. tenuiflora, while enabling the optimization of physiological parameters required to maximize its production of therapeutically relevant specialized metabolites.
5. Conclusions
Induction of haustoria in C. tenuiflora is feasible under an in vitro co-culture with B. conferta. The development and chemical profiles of B. conferta and C. tenuiflora change during the co-culture due to the host–parasite interaction. Research currently being performed in our laboratory explores the genetic basis of haustorium formation, among other questions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology14080990/s1, Table S1: Chemical compounds identified in C. tenuiflora by LC-PDA-ESI-MS; Table S2: Chemical compounds identified in Baccharis conferta by LC-PDA-ESI-MS; Table S3: Comparative relative area under the curve (AUC) values of detected compounds in C. tenuiflora grown under axenic and co-culture; Table S4: Comparative relative area under the curve (AUC) values of detected compounds in B. conferta grown under axenic and co-culture; Figure S1: Representative chromatogram of C. tenuiflora at 205 nm; Figure S2: Representative chromatogram of C. tenuiflora at 280 nm; Figure S3: Representative chromatogram of C. tenuiflora at 330 nm; Figure S4: Representative MS Spectra of the compounds identified in C. tenuiflora; Figure S5: HPLC chromatogram (A) of C. tenuiflora root extract at 325nm, UV spectra (B) and mass spectra (C) of peak at Rt = 7.01 min; Figure S6: Representative chromatogram of B. conferta at 280 nm; Figure S7: Representative chromatogram of B. conferta at 325 nm; Figure S8: Representative MS Spectra of the compounds identified in B. conferta.
Author Contributions
Conceptualization, J.L.T.-E. and G.T.-T.; methodology, A.L.L.-P., G.S.-M. and D.T.-M.; formal analysis, A.L.L.-P. and V.M.-P.; investigation, A.L.L.-P., A.R.L.-L. and V.M.-P.; resources, J.L.T.-E. and G.T.-T.; writing—original draft preparation, A.L.L.-P. and G.T.-T.; writing—review and editing, J.L.T.-E. and G.T.-T.; funding acquisition, G.T.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACyT, grant number: CB-01-2013-220007) and Secretaría de Investigación y Posgrado, Instituto Politécnico Nacional (SIP-IPN; grant numbers: 1776—20170469/20170569, and 2360-20250853). ALLP was awarded a fellowship from CONACYT (grant number: 702942) and Programa Institucional de Formación de Investigadores (BEIFI-IPN, 20170496).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The experimental data used to support the results are included in the article and Supplementary Material; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Natural Products Laboratory, the Chromatography Laboratory, and the Advanced Microscopy Laboratory at CEPROBI-IPN for providing the necessary facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 4,5diQ | 4,5-di-O-caffeoylquinic acid |
| HIFs | haustorium-inducing factors |
| PhGs | phenylethanoid glycosides |
References
- Tesitel, J.; Plavcova, L.; Cameron, D.D. Heterotrophic carbon gain by the root hemiparasites, Rhinanthus minor and Euphrasia rostkoviana (Orobanchaceae). Planta 2010, 231, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef] [PubMed]
- Goyet, V.; Wada, S.; Cui, S.; Wakatake, T.; Shirasu, K.; Montiel, G.; Simier, P.; Yoshida, S. Haustorium Inducing Factors for Parasitic Orobanchaceae. Front. Plant Sci. 2019, 10, 1056. [Google Scholar] [CrossRef]
- Brun, G.; Leman, J.K.H.; Wicke, S. Comparative gene expression analysis of differentiated terminal and lateral haustoria of the obligate root parasitic plant Phelipanche ramosa (Orobanchaceae). Plants People Planet 2023, 7, 360–366. [Google Scholar] [CrossRef]
- Ajithkumar, T.G.; Thomas, S.; Mathew, L. Influence of hosts on the production of bioactive compounds in the hemiparasitic plant Helicanthes elasticus. Environ. Exp. Biol. 2021, 19, 161–171. [Google Scholar] [CrossRef]
- Béjar, E.; Reyes-Chilpa, R.; Jiménez-Estrada, M. Bioactive Compounds from Selected Plants Used in the XVI Century Mexican Traditional Medicine. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2000; Volume 24, Part E; pp. 799–844. [Google Scholar] [CrossRef]
- Arango-De la Pava, L.D.; Zamilpa, A.; Trejo-Espino, J.L.; Domínguez-Mendoza, B.E.; Jiménez-Ferrer, E.; Pérez-Martínez, L.; Trejo-Tapia, G. Synergism and Subadditivity of Verbascoside-Lignans and -Iridoids Binary Mixtures Isolated from Castilleja tenuiflora Benth. on NF-κB/AP-1 Inhibition Activity. Molecules 2021, 26, 547. [Google Scholar] [CrossRef]
- Ramirez-Cisneros, M.A.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; Rao, P.P.; Aburto-Amar, R.; Rodriguez-Lopez, V. In vitro COX-1 and COX-2 enzyme inhibitory activities of iridoids from Penstemon barbatus, Castilleja tenuiflora, Cresentia alata and Vitex mollis. Bioorg. Med. Chem. Lett. 2015, 25, 4505–4508. [Google Scholar] [CrossRef]
- Moreno-Escobar, J.A.; Bazaldúa, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; Rodríguez-López, V. Cytotoxic and antioxidant activities of selected Lamiales species from Mexico. Pharm. Biol. 2011, 49, 1243–1248. [Google Scholar] [CrossRef]
- Montes-Hernández, E.; Sandoval-Zapotitla, E.; Bermúdez-Torres, K.; Trejo-Espino, J.L.; Trejo-Tapia, G. Hemiparasitic interaction between Castilleja tenuiflora (Orobanchaceae) and Baccharis conferta (Asteraceae): Haustorium anatomy and C- and N-fluxes. Bot. Sci. 2019, 97, 192–201. [Google Scholar] [CrossRef]
- Salcedo-Morales, G.; Jiménez-Aparicio, A.R.; Cruz-Sosa, F.; Trejo-Tapia, G. Anatomical and histochemical characterization of in vitro haustorium from roots of Castilleja tenuiflora. Biol. Plant. 2014, 58, 164–168. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, L.R.; Domínguez-Hernández, D.; Pineda-López, M.R.; Lara-González, R. Does Baccharis conferta Shrub Act as a Nurse Plant to the Abies religiosa Seedling? Open For. Sci. J. 2011, 4, 67–70. [Google Scholar] [CrossRef]
- Gutierrez-Roman, A.S.; Trejo-Tapia, G.; Gonzalez-Cortazar, M.; Jimenez-Ferrer, E.; Trejo-Espino, J.L.; Zamilpa, A.; Ble-Gonzalez, E.A.; Camacho-Diaz, B.H.; Herrera-Ruiz, M. Anti-arthritic and anti-inflammatory effects of Baccharis conferta Kunth in a kaolin/carrageenan-induced monoarthritis model. J. Ethnopharmacol. 2022, 288, 114996. [Google Scholar] [CrossRef]
- Leyva-Peralta, A.L.; Salcedo-Morales, G.; Medina-Pérez, V.; López-Laredo, A.R.; Trejo-Espino, J.L.; Trejo-Tapia, G. Morphogenesis and in vitro production of caffeoylquinic and caffeic acids in Baccharis conferta Kunth. In Vitro Cell. Dev. Biol.—Plant 2019, 55, 581–589. [Google Scholar] [CrossRef]
- Leyva, A.L. Baccharis conferta Kunth: Cultivo In Vitro, Interacción con Castilleja tenuiflora Benth. Master’s Thesis, Instituto Politécnico Nacional, Morelos, Mexico, 2018. [Google Scholar]
- Landi, M.; Misra, B.B.; Nocito, F.F.; Lucchini, G.; Bruno, L.; Malara, A.; Abenavoli, M.R.; Araniti, F. Metabolic changes induced by Cuscuta campestris Yunck in the host species Artemisia campestris subsp. variabilis (Ten.) Greuter as a strategy for successful parasitisation. Planta 2022, 256, 118. [Google Scholar] [CrossRef]
- Clifford, M.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Tong, L.; Jiang, Y.; Zhang, X.; Zhang, X.; Zhang, W.; Ren, G.; Chen, Z.; Zhao, Y.; Guo, S.; Yan, H.; et al. Metabolic and molecular basis of flavonoid biosynthesis in Lycii fructus: An integration of metabolomic and transcriptomic analysis. J. Pharm. Biomed. Anal. 2025, 255, 116653. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wakatake, T.; Hashimoto, K.; Saucet, S.B.; Toyooka, K.; Yoshida, S.; Shirasu, K. Haustorial Hairs Are Specialized Root Hairs That Support Parasitism in the Facultative Parasitic Plant Phtheirospermum japonicum. Plant Physiol. 2016, 170, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Kyozuka, J.; Nomura, T.; Shimamura, M. Origins and evolution of the dual functions of strigolactones as rhizosphere signaling molecules and plant hormones. Curr. Opin. Plant Biol. 2022, 65, 102154. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Cui, S.; White, A.R.F.; Nelson, D.C.; Yoshida, S.; Shirasu, K. Strigolactones are chemoattractants for host tropism in Orobanchaceae parasitic plants. Nat. Commun. 2022, 13, 4653. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. Characterization of strigolactones exuded by Asteraceae plants. Plant Growth Regul. 2011, 65, 495–504. [Google Scholar] [CrossRef]
- Bürger, M.; Peterson, D.; Chory, J. Strigolactones initiate the formation of haustorium-like structures in Castilleja. iScience 2024, 27, 111491. [Google Scholar] [CrossRef] [PubMed]
- Huizinga SBouwmeester, H. KAI2 Can Do: Karrikin Receptor Function in Plant Development and Response to Abiotic and Biotic Factors. Plant Cell Physiol. 2023, 64, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Avilez-Montalvo, R.; Loyola-Vargas, V.M. Appendix A: The Components of the Culture Media. In Plant Cell Culture Protocols, Methods in Molecular Biology; Loyola-Vargas, V.M., Ocho-Alejo, N., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1815, pp. 493–502. [Google Scholar] [CrossRef]
- Irving, L.J.; Kim, D.; Schwier, N.; Vaughan, J.K.E.; Ong, G.; Hama, T. Host nutrient supply affects the interaction between the hemiparasite Phtheirospermum japonicum and its host Medicago sativa. Environ. Exp. Bot. 2019, 162, 125–132. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Watling, J.R.; Facelli, J.M. The combined effects of water and nitrogen on the relationship between a native hemiparasite and its invasive host. New Phytol. 2021, 229, 1728–1739. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Facelli, E.; Delean, S.; Facelli, J.M. Does phosphorus influence performance of a native hemiparasite and its impact on a native legume? Physiol. Plant 2021, 173, 1889–1900. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Facelli, J.M.; Watling, J.R. Does nitrogen affect the interaction between a native hemiparasite and its native or introduced leguminous hosts? New Phytol. 2017, 213, 812–821. [Google Scholar] [CrossRef]
- Tesitel, J.; Tesitelova, T.; Fisher, J.P.; Leps, J.; Cameron, D.D. Integrating ecology and physiology of root-hemiparasitic interaction: Interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytol. 2015, 205, 350–360. [Google Scholar] [CrossRef]
- Hautier, Y.; Hector, A.; Vojtech, E.; Purves, D.; Turnbull, L.A. Modelling the growth of parasitic plants. J. Ecol. 2010, 98, 857–866. [Google Scholar] [CrossRef]
- Haynes, A.F. Presence of N-fixing neighbors increases leaf N and δ13C in Castilleja applegatei, a root hemiparasite. Plant Ecol. 2021, 223, 213–228. [Google Scholar] [CrossRef]
- Monteiro, G.F.; Boanares, D.; Novais, S.; França, M.G.C.; Antonini, Y.; Barbosa, M.; Oki, Y.; Fernandes, G.W. Imbalance of water potential and photosynthetic efficiency in the parasitic relationship between Struthanthus flexicaulis and Baccharis dracunculifolia. Fol. Geob. 2022, 57, 71–82. [Google Scholar] [CrossRef]
- Mwangangi, I.M.; Buchi, L.; Haefele, S.M.; Bastiaans, L.; Runo, S.; Rodenburg, J. Combining host plant defence with targeted nutrition: Key to durable control of hemiparasitic Striga in cereals in sub-Saharan Africa? New Phytol. 2021, 230, 2164–2178. [Google Scholar] [CrossRef] [PubMed]
- Facelli, E.; Wynn, N.; Tsang, H.T.; Watling, J.R.; Facelli, J.M. Defence responses of native and invasive plants to the native generalist vine parasite Cassytha pubescens—anatomical and functional studies. Aust. J. Bot. 2020, 68, 300–309. [Google Scholar] [CrossRef]
- Keyes, W.J.; Taylor, J.V.; Apkarian, R.P.; Lynn, D.G. Dancing together. Social controls in parasitic plant development. Plant Physiol. 2001, 127, 1508–1512. [Google Scholar] [CrossRef]
- Rubio-Rodriguez, E.; Vera-Reyes, I.; Rodriguez-Hernandez, A.A.; Lopez-Laredo, A.R.; Ramos-Valdivia, A.C.; Trejo-Tapia, G. Mixed elicitation with salicylic acid and hydrogen peroxide modulates the phenolic and iridoid pathways in Castilleja tenuiflora plants. Planta 2023, 258, 20. [Google Scholar] [CrossRef]
- Jadhav, R.B.; Anarthe, S.J.; Surana, S.J.; Gokhale, S.B. Host-hemiparasite transfer of the C-glucosyl xanthone mangiferin between Mangifera indica and Dendrophthoe falcata. J. Plant Int. 2005, 1, 171–177. [Google Scholar] [CrossRef]
- Cabezas, N.J.; Urzúa, A.M.; Niemeyer, H.M. Translocation of isoquinoline alkaloids to the hemiparasite, Tristerix verticillatus from its host, Berberis montana. Biochem. Syst. Ecol. 2009, 37, 225–227. [Google Scholar] [CrossRef]
- Adler, L.S.; Wink, M. Transfer of quinolizidine alkaloids from hosts to hemiparasites in two Castilleja-Lupinus associations: Analysis of floral and vegetative tissues. Biochem. Syst. Ecol. 2001, 29, 551–561. [Google Scholar] [CrossRef]
- da Silva, N.M.; Oliveira, B.R.M.; Santos Silva, J.V.d.; de Carvalho Neto, C.H.; dos Santos, M.L.S.; de Oliveira, F.F.; de Almeida, A.-A.F. Hemiparasitism of Acanthosyris paulo-alvinii on Theobroma cacao, via root system through of haustorium, promotes anatomical, physiological, biochemical, and molecular changes and induce the host plant death. New For. 2024, 56, 1. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Venancio-Rodriguez, C.A.; Vasquez-Aguilar, A.A.; Alonso-Sanchez, A.G.; Perez-Torres, C.A.; Villafan, E.; Ramirez-Barahona, S.; Galicia, S.; Sosa, V.; Rebollar, E.A.; et al. Transcriptional Basis for Haustorium Formation and Host Establishment in Hemiparasitic Psittacanthus schiedeanus Mistletoes. Front. Genet. 2022, 13, 929490. [Google Scholar] [CrossRef]
- Li, C.; Gong, Q.; Liu, P.; Xu, Z.; Yu, Q.; Dai, H.; Shi, Y.; Si, J.; Zhang, X.; Chen, D.; et al. Co-expressed network analysis based on 289 transcriptome samples reveals methyl jasmonate-mediated gene regulatory mechanism of flavonoid compounds in Dendrobium catenatum. Plant Physiol. Biochem. 2024, 206, 108226. [Google Scholar] [CrossRef]
- Volpi, E.S.N.; Mazzafera, P.; Cesarino, I. Should I stay or should I go: Are chlorogenic acids mobilized towards lignin biosynthesis? Phytochemistry 2019, 166, 112063. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Goswami, S.K.; Gujjar, R.S.; Kumar, R.; Kumar, R.; Lal, M.K.; Kumari, M. Mechanistic insights on lignin-mediated plant defense against pathogen infection. Plant Physiol. Biochem. 2025, 228, 110224. [Google Scholar] [CrossRef]
- Cui, S.; Takeda-Kimura, Y.; Wakatake, T.; Luo, J.; Tobimatsu, Y.; Yoshida, S. Striga hermonthica induces lignin deposition at the root tip to facilitate prehaustorium formation and obligate parasitism. Plant Commun. 2025, 6, 101294. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Guo, C.; Peng, J.; Shao, F.; Sheng, S.; Wang, S. Transcriptomic Insights and Cytochrome P450 Gene Analysis in Kadsura coccinea for Lignan Biosynthesis. Genes 2024, 15, 270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).