Ultrastructure and Transcriptomic Analysis Reveal Alternative Pathways of Zona Radiata Formation in Culter alburnus with Different Spawning Habits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Histological Analysis
2.3. Transmission Electron Microscopy (TEM)
2.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.5. DEGs Identify, Trend Analysis, and Pathway Functional Enrichment
3. Results
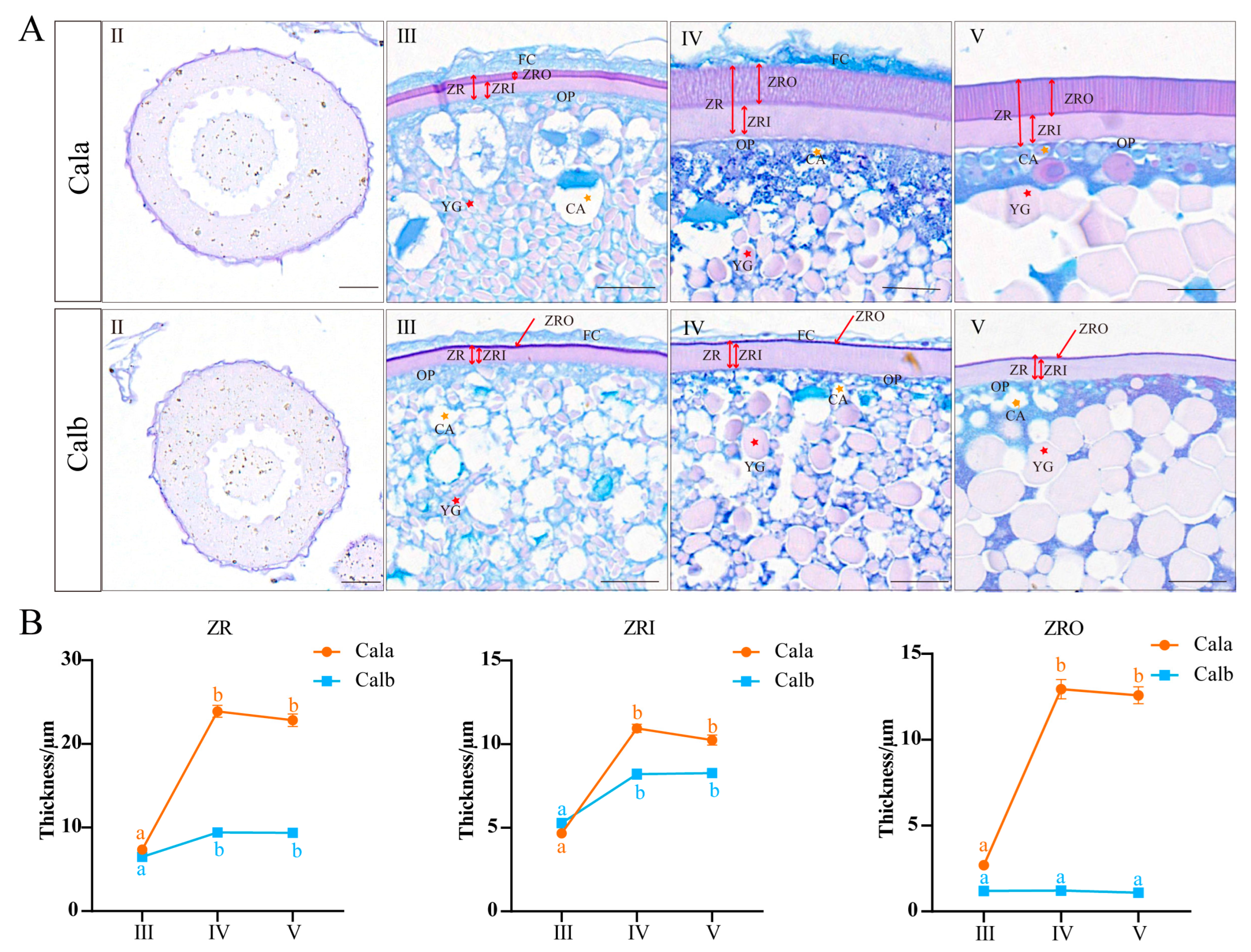
3.1. Histology and Thickness of Zona Radiata in Oocytes at Different Developmental Stages
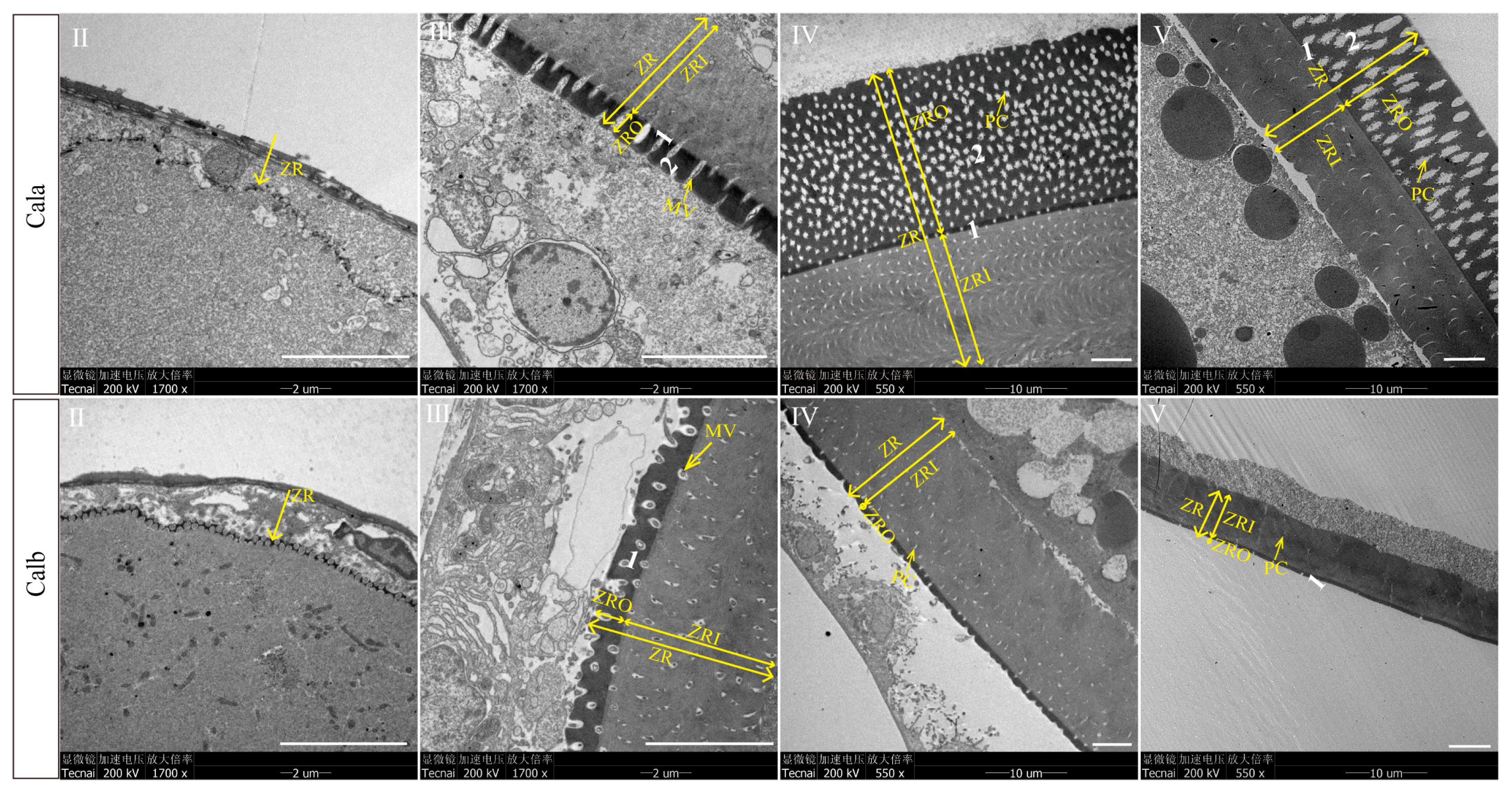
3.2. Ultrastructure of Zona Radiata in Oocytes at Different Developmental Stages
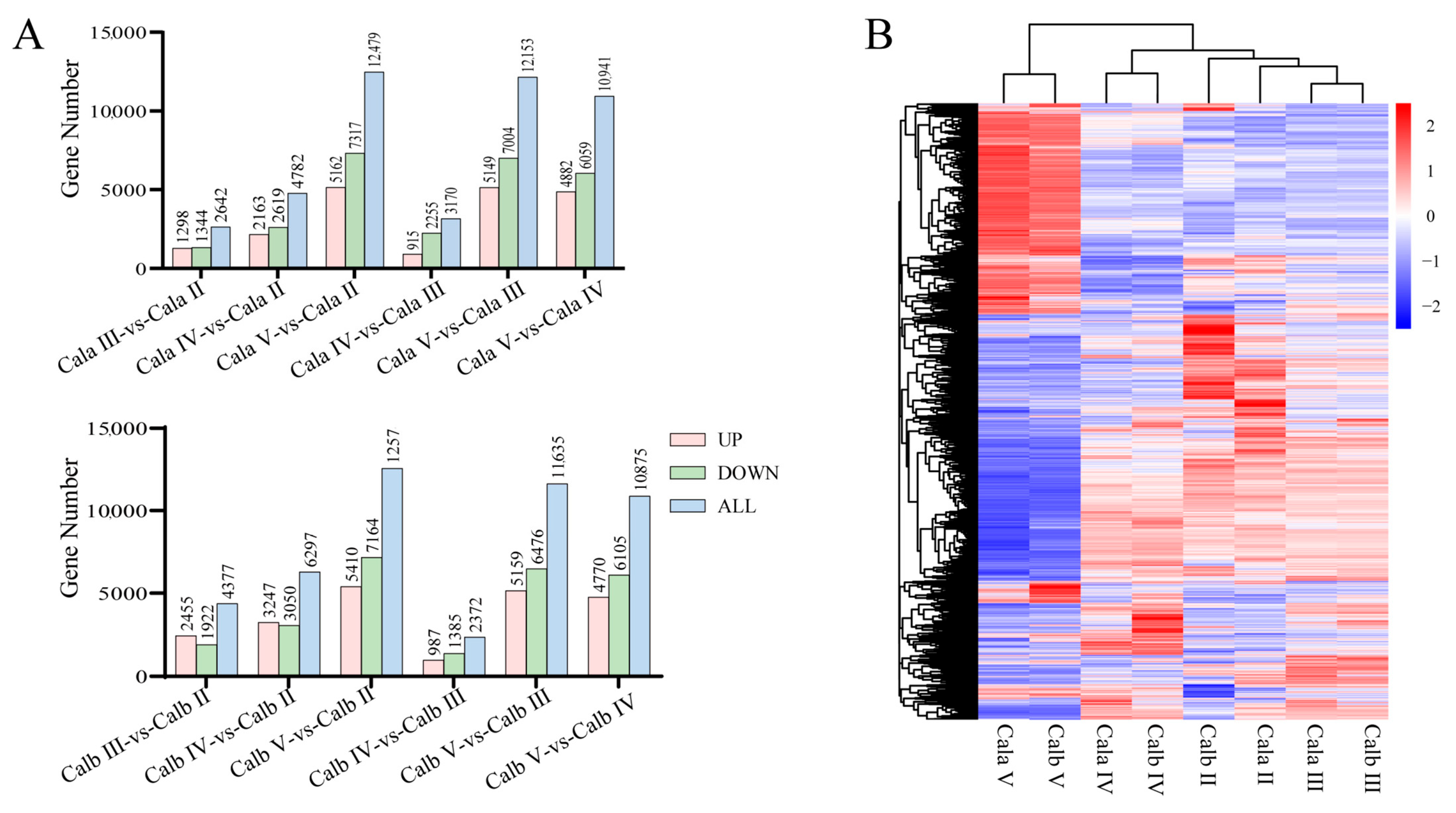
3.3. Transcriptome Assembly and Differentially Expressed Gene (DEG) Profiles in Follicles at Different Developmental Stages
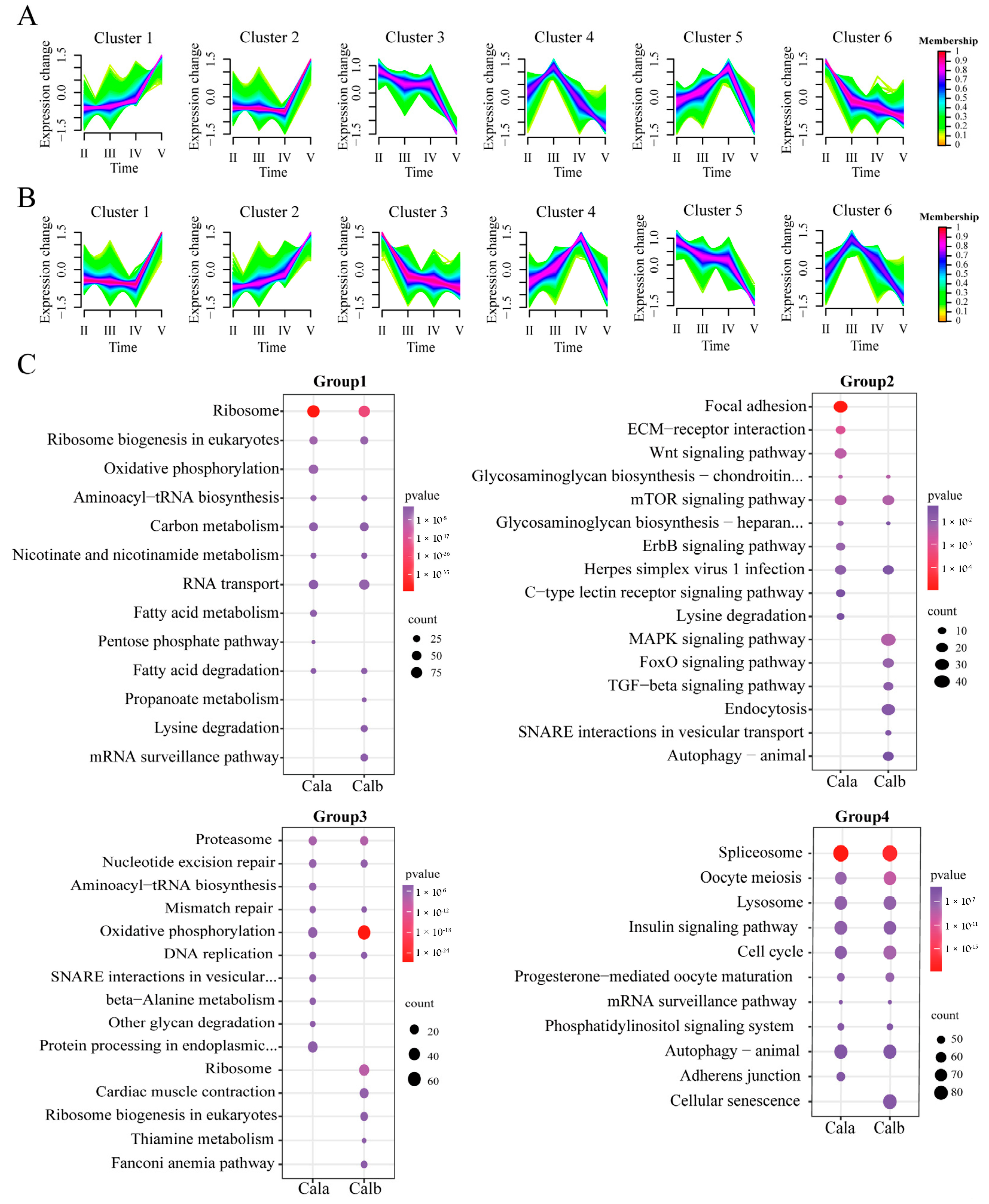
3.4. Trend Analysis of DEGs Across the Developmental Stages of Follicles in Cala and Calb
3.5. Genes Related to the Zona Pellucida (ZP) Proteins and Glycosaminoglycan Biosynthesis
3.6. Comparative Transcriptome Analysis of Cala and Calb Follicles at Stage Ⅲ
3.7. qRT-PCR to Verify DEGs
4. Discussion
4.1. Structure Formation Process and Temporal Gene Expression Patterns Associated with ZR Development Across Different Oogenesis Stages
4.2. Structural Differences in ZR Between Cala and Calb
4.3. Molecular Mechanisms Underlying Differences in ZR Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| wfdc | WAP-type (Whey Acidic Protein) ‘four-disulfide core’ |
| a2ml | alpha-2-macroglobulin-like |
| ZR | zona radiata |
| GAG | glycosaminoglycan |
| ZGA | zygotic genome activation |
| Cala | C. alburnus producing adhesive eggs |
| Calb | C. alburnus producing semi-buoyant eggs |
| ZP | zona pellucida |
| FPKM | Fragments Per Kilobase of transcript per Million mapped reads |
| Mfuzz | a fuzzy c-means clustering algorithm |
References
- Chen, F.; Wang, Y.; He, J.; Chen, L.; Xue, G.; Zhao, Y.; Peng, Y.; Smith, C.; Zhang, J.; Chen, J.; et al. Molecular mechanisms of spawning habits for the adaptive radiation of endemic east asian Cyprinid fishes. Research 2022, 2022, 9827986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yu, D.; Tang, Q.; Yang, J.; Chen, Y.; Liu, H. Macro-evolutionary patterns of East Asian opsariichthyin-xenocyprinin-cultrin fishes related to the formation of river and river-lake environments under monsoon climate. Water Biol. Secur. 2022, 1, 100036. [Google Scholar] [CrossRef]
- Tao, W.; Zou, M.; Wang, X.; Gan, X.; Mayden, R.L.; He, S. Phylogenomic analysis resolves the formerly intractable adaptive diversification of the endemic clade of East Asian Cyprinidae (Cypriniformes). PLoS ONE 2010, 5, e13508. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Grande, T.; Wilson, M. Fishes of the World, 5th ed.; John Wiley &Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Teletchea, F.; Gardeur, J.-N.; Kamler, E.; Fontaine, P. The relationship of oocyte diameter and incubation temperature to incubation time in temperate freshwater fish species. J. Fish Biol. 2009, 74, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Smith, C.; Wang, Y.; He, J.; Xia, W.; Xue, G.; Chen, J.; Xie, P. The evolution of alternative buoyancy mechanisms in freshwater fish eggs. Front. Ecol. Evol. 2021, 9, 2021. [Google Scholar] [CrossRef]
- Chen, F.; Xue, G.; Wang, Y.; Zhang, H.; Clift, P.D.; Xing, Y.; He, J.; Albert, J.S.; Chen, J.; Xie, P. Evolution of the Yangtze River and its biodiversity. Innovation 2023, 4, 100417. [Google Scholar] [CrossRef]
- Shu, L.; Suter, M.J.F.; Raesaenen, K. Evolution of egg coats: Linking molecular biology and ecology. Mol. Ecol. 2015, 24, 4052–4073. [Google Scholar] [CrossRef]
- Stehr, C.M.; Hawkes, J.W. The comparative ultrastructure of the egg membrane and associated pore structures in the starry flounder, Platichthys stellatus (Pallas), and pink salmon, Oncorhynchus gorbuscha (Walbaum). Cell Tissue Res. 1979, 202, 347–356. [Google Scholar] [CrossRef]
- Sano, K.; Kawaguchi, M.; Katano, K.; Tomita, K.; Inokuchi, M.; Nagasawa, T.; Hiroi, J.; Kaneko, T.; Kitagawa, T.; Fujimoto, T.; et al. Comparison of egg envelope thickness in teleosts and its relationship to the sites of ZP potein synthesis. J. Exp. Zool. Part. B Mol. Dev. Evol. 2017, 328, 240–258. [Google Scholar] [CrossRef]
- Mansour, N.; Lahnsteiner, F.; Patzner, R.A. Physiological and biochemical investigations on egg stickiness in common carp. Anim. Reprod. Sci. 2009, 114, 256–268. [Google Scholar] [CrossRef]
- Rizzo, E.; Sato, Y.; Barreto, B.P.; Godinho, H.P. Adhesiveness and surface patterns of eggs in neotropical freshwater teleosts. J. Fish Biol. 2002, 61, 615–632. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; He, J.; Chen, J.; Xue, G.; Zhao, Y.; Peng, Y.; Xie, P. Comparative ultrastructure and proteomics of two economic species (common carp and grass carp) egg envelope. Aquaculture 2022, 546, 737276. [Google Scholar] [CrossRef]
- Murray, D.S.; Bain, M.M.; Adams, C.E. Adhesion mechanisms in European whitefish Coregonus lavaretus eggs: Is this a survival mechanism for high-energy spawning grounds? J. Fish Biol. 2013, 83, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Kim, J.G.; Park, J.Y. Comparative structural study of the zona radiata of the eggs of three Gobiobotia (Cyprinidae, Teleostei), endemic Korean freshwaters. Microscopy 2017, 66, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Wang, Y.-W.; Huang, F.-L. Cross-linking of ZP2 and ZP3 by transglutaminase is required for the formation of the outer layer of fertilization envelope of carp egg. Mol. Reprod. Dev. 2002, 63, 237–244. [Google Scholar] [CrossRef]
- Minin, A.; Ozerova, S. Spontaneous activation of fish eggs is abolished by protease inhibitors. Russ. J. Dev. Biol. 2008, 39, 293–296. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Shibata, Y.; Iwamatsu, T.; Suzuki, N.; Young, G.; Naruse, K.; Nagahama, Y.; Yoshikuni, M. An oocyte-specific astacin family protease, alveolin, is released from cortical granules to trigger egg envelope hardening during fertilization in medaka (Oryzias latipes). Dev. Biol. 2012, 372, 239–248. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Zhang, T.-T.; Yang, W.-X. Formation of zona radiata and ultrastructural analysis of egg envelope during oogenesis of Chinese perch Siniperca chuatsi. Micron 2010, 41, 7–14. [Google Scholar] [CrossRef]
- Kaviani, E.F.; Shabanipour, N.; Mirnategh, S.B. Light and electron microscope structural study of the zona radiata in the oocyte of zebrafish (Danio rerio). Microscopy 2013, 62, 377–382. [Google Scholar] [CrossRef]
- Ma, X.-X.; Zhu, J.-Q.; Zhou, H.; Yang, W.-X. The formation of zona radiata in Pseudosciaena crocea revealed by light and transmission electron microscopy. Micron 2012, 43, 435–444. [Google Scholar] [CrossRef]
- ŻElazowska, M. Formation and structure of egg envelopes in Russian sturgeon Acipenser gueldenstaedtii (Acipenseriformes: Acipenseridae). J. Fish Biol. 2010, 76, 694–706. [Google Scholar] [CrossRef]
- Birk, D.S.; Onose, S.; Kinoshita, M.; Murata, K. Medaka, Oryzias latipes, egg envelopes are created by ovarian-expressed ZP proteins and liver-expressed choriogenins. Zool. Lett. 2022, 8, 11. [Google Scholar] [CrossRef]
- Modig, C.; Raldúa, D.; Cerdà, J.; Olsson, P.E. Analysis of vitelline envelope synthesis and composition during early oocyte development in gilthead seabream (Sparus aurata). Mol. Reprod. Dev. 2008, 75, 1351–1360. [Google Scholar] [CrossRef]
- Murata, K.; Conte, F.; McInnis, E.; Fong, T.; Cherr, G. Identification of the origin and localization of chorion (egg envelope) proteins in an ancient fish, the White sturgeon, Acipenser transmontanus. Biol. Reprod. 2014, 90, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, F.; He, J.; Xue, G.; Chen, J.; Xie, P. Cellular and molecular modification of egg envelope hardening in fertilization. Biochimie 2021, 181, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Vallot, A.; Tachibana, K. The emergence of genome architecture and zygotic genome activation. Curr. Opin. Cell Biol. 2020, 64, 50–57. [Google Scholar] [CrossRef]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J.M. Zygotic genome activation in vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef]
- Qu, J.; Li, R.; Xie, Y.; Liu, Y.; Liu, J. Differential transcriptomic profiling provides new insights into oocyte development and lipid droplet formation in Japanese flounder (Paralichthys olivaceus). Aquaculture 2021, 550, 737843. [Google Scholar] [CrossRef]
- Meng, F.; Sun, S.; Xu, X.; Yu, W.; Gan, R.; Zhang, L.; Zhang, W. Transcriptomic analysis provides insights into the growth and maturation of ovarian follicles in the ricefield eel (Monopterus albus). Aquaculture 2022, 555, 738251. [Google Scholar] [CrossRef]
- Lai, X.J.; Peng, S.; Wang, Y.L. Dynamic transcriptome analysis of ovarian follicles in artificial maturing Japanese eel (Anguilla japonica). Theriogenology 2022, 180, 176–188. [Google Scholar] [CrossRef]
- Mazzeo, I.; Peñaranda, D.S.; Gallego, V.; Hildahl, J.; Nourizadeh-Lillabadi, R.; Asturiano, J.F.; Pérez, L.; Weltzien, F.A. Variations in the gene expression of zona pellucida proteins, zpb and zpc, in female European eel (Anguilla anguilla) during induced sexual maturation. Gen. Comp. Endocrinol. 2012, 178, 338–346. [Google Scholar] [CrossRef]
- Zhu, B.; Pardeshi, L.; Chen, Y.; Ge, W. Transcriptomic Analysis for Differentially Expressed Genes in Ovarian Follicle Activation in the Zebrafish. Front. Endocrinol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, D.-M.; Li, Q.; Wang, G.-Y.; Chen, J.; Feifei, Z.; Li, P.; Sun, Y.-H. Genetic diversity analysis of Topmouth Culter (Culter alburnus) based on microsatellites and D-loop sequences. Environ. Biol. Fishes 2021, 104. [Google Scholar] [CrossRef]
- Jiang, H.; Qian, Y.; Zhang, Z.; Meng, M.; Deng, Y.; Wang, G.; He, S.; Yang, L. Chromosome-level genome assembly and whole-genome resequencing of topmouth culter (Culter alburnus) provide insights into the intraspecific variation of its semi-buoyant and adhesive eggs. Mol. Ecol. Resour. 2023, 23, 1841–1852. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Santos, J.E.; Padilha, G.E.V.; Bomcompagni-Júnior, O.; Santos, G.B.; Rizzo, E.; Bazzoli, N. Ovarian follicle growth in the catfish Iheringichthys labrosus (Siluriformes: Pimelodidae). Tissue Cell 2006, 38, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Shabanipour, N.; Hossayni, S.N. Histological and ultrastructural study of zona radiata in oocyte of common carp Cyprinus carpio (Linnaeus 1758). Micron 2010, 41, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Flegler, C. Electron microscopic studies on the development of the chorion of the viviparous teleost Dermogenys pusillus (Hemirhamphidae). Cell Tissue Res. 1977, 179, 255–270. [Google Scholar] [CrossRef]
- Menn, F.L.; Cerdà, J.; Babin, P.J. Ultrastructural aspects of the ontogeny and differentiation of ray-finned fish ovarian follicles. In the Fish Oocyte: From Basic Studies to Biotechnological Applications; Babin, P.J., Cerdà, J., Lubzens, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–37. [Google Scholar]
- Kanamori, A.; Naruse, K.; Mitani, H.; Shima, A.; Hori, H. Genomic organization of ZP domain containing egg envelope genes in medaka (Oryzias latipes). Gene 2003, 305, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.Y.; Williams, Z.; Wassarman, P.M. Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3. Mol. Biol. Cell 2002, 13, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Monne, M.; Han, L.; Jovine, L. Tracking down the ZP domain:: From the mammalian zona pellucida to the molluscan vitelline envelope. Semin. Reprod. Med. 2006, 24, 204–216. [Google Scholar] [CrossRef]
- Messaddeq, N.; Hergueux, J.; Weickert, J.-L.; Romand, R. Study of the chorion of seasonal and non-seasonal Africa and Neotropical oviparous Cyprinodontiforme fishes. Environ. Biol. Fishes 2018, 101, 287–299. [Google Scholar] [CrossRef]
- Park, C.-W.; Kim, J.-G. A comparative morphological study on the characteristics of egg envelopes of three Cultrinae Fishes (Cyprinidae, Teleostei) in Korea. Life 2024, 14, 840. [Google Scholar] [CrossRef]
- Loureiro, M.; DeSa, R.O. External morphology of the chorion of the annual fishes Cynolebias (Cyprinodontiformes: Rivulidae). Copeia 1996, 1016–1022. [Google Scholar] [CrossRef]
- Thompson, A.W.; Furness, A.I.; Stone, C.; Rade, C.M.; Orti, G. Microanatomical diversification of the zona pellucida in aplochelioid killifishes. J. Fish Biol. 2017, 91, 126–143. [Google Scholar] [CrossRef]
- Cao, Y.-Q.; Wang, Y.-X.; Zhao, Y.; Zhang, J.; He, X.; Xie, P.; Chen, J.; Sun, Y.-H. Transfer of the zp3a gene results in changes in egg adhesiveness and buoyancy in transgenic zebrafish. Zool. Res. 2023, 44, 259–268. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Huang, F.-L. Fibroin-like substance is a major component of the outer layer of fertilization envelope via which carp egg adheres to the substratum. Mol. Reprod. Dev. 2002, 62, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Budde, B.S.; Mizumoto, S.; Kogawa, R.; Becker, C.; Altmüller, J.; Thiele, H.; Rüschendorf, F.; Toliat, M.R.; Kaleschke, G.; Hämmerle, J.M.; et al. Skeletal dysplasia in a consanguineous clan from the island of Nias/Indonesia is caused by a novel mutation in B3GAT3. Hum. Genet. 2015, 134, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Izumikawa, T.; Sato, B.; Kitagawa, H. Identification of phosphatase that dephosphorylates xylose in the glycosaminoglycan-protein linkage region of proteoglycans. J. Biol. Chem. 2014, 289, 6695–6708. [Google Scholar] [CrossRef]
- Habicher, J.; Varshney, G.K.; Waldmann, L.; Snitting, D.; Allalou, A.; Zhang, H.; Ghanem, A.; Magi, C.O.; Dierker, T.; Kjellen, L.; et al. Chondroitin/dermatan sulfate glycosyltransferase genes are essential for craniofacial development. PLoS Genet. 2022, 18, e1010067. [Google Scholar] [CrossRef]
- Shimbo, M.; Suzuki, R.; Fuseya, S.; Sato, T.; Kiyohara, K.; Hagiwara, K.; Okada, R.; Wakui, H.; Tsunakawa, Y.; Watanabe, H.; et al. Postnatal lethality and chondrodysplasia in mice lacking both chondroitin sulfate N-acetylgalactosaminyltransferase-1 and-2. PLoS ONE 2017, 12, e0190333. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S. Presence of a proteinase inhibitor in the vitelline envelope and its role in eggs of the fish Tribolodon hakonensis. J. Exp. Zool. 1998, 281, 626–635. [Google Scholar] [CrossRef]
- Rezanujjaman, M.; Pachoensuk, T.; Hossain, M.F.; Jyoti, M.M.S.; Rana, M.R.; Tsutsumi, E.; Mouri, T.; Susilo, M.B.; Wanlada, K.; Yamamoto, C.; et al. Zebrafish prss59.1 is involved in chorion development. Gen. Comp. Endocrinol. 2024, 349, 114453. [Google Scholar] [CrossRef]
- Hosomi, O.; Ohe, Y.; Takeya, A.; Hosaka, K.; Okubo, T.; Iyobe, S.; Kudo, S. Nucleotide and protein sequences of a proteinase inhibitor from the vitelline envelope of dace (Tribolodon hakonensis) eggs. J. Exp. Zool. Part. A Comp. Exp. Biol. 2004, 301A, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Nukumi, N.; Iwamori, T.; Kano, K.; Naito, K.; Tojo, H. Whey acidic protein (WAP) regulates the proliferation of mammary epithelial cells by preventing serine protease from degrading laminin. J. Cell. Physiol. 2007, 213, 793–800. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, R.; Ferran, B.; Alonso, J.; Marti-Pamies, I.; Aguilo, S.; Calvayrac, O.; Rodriguez, C.; Martinez-Gonzalez, J. NR4A receptors up-regulate the antiproteinase alpha-2 macroglobulin (A2M) and modulate MMP-2 and MMP-9 in vascular smooth muscle cells. Thromb. Haemost. 2015, 113, 1323–1334. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, X.; Browning, S.; Scuderi, G.; Hanna, L.S.; Wei, L. Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Arthritis Res. Ther. 2017, 19, 175. [Google Scholar] [CrossRef]
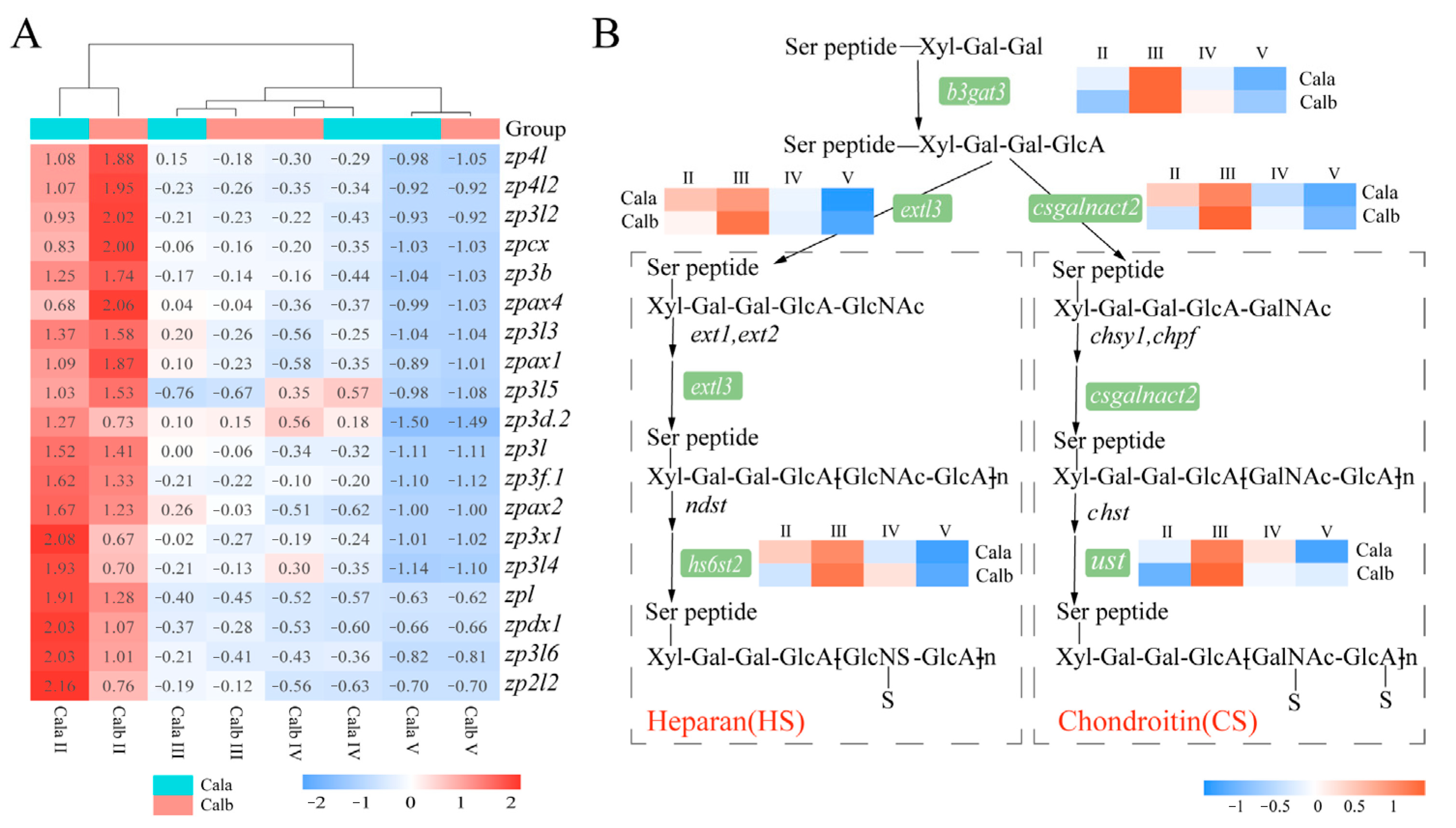
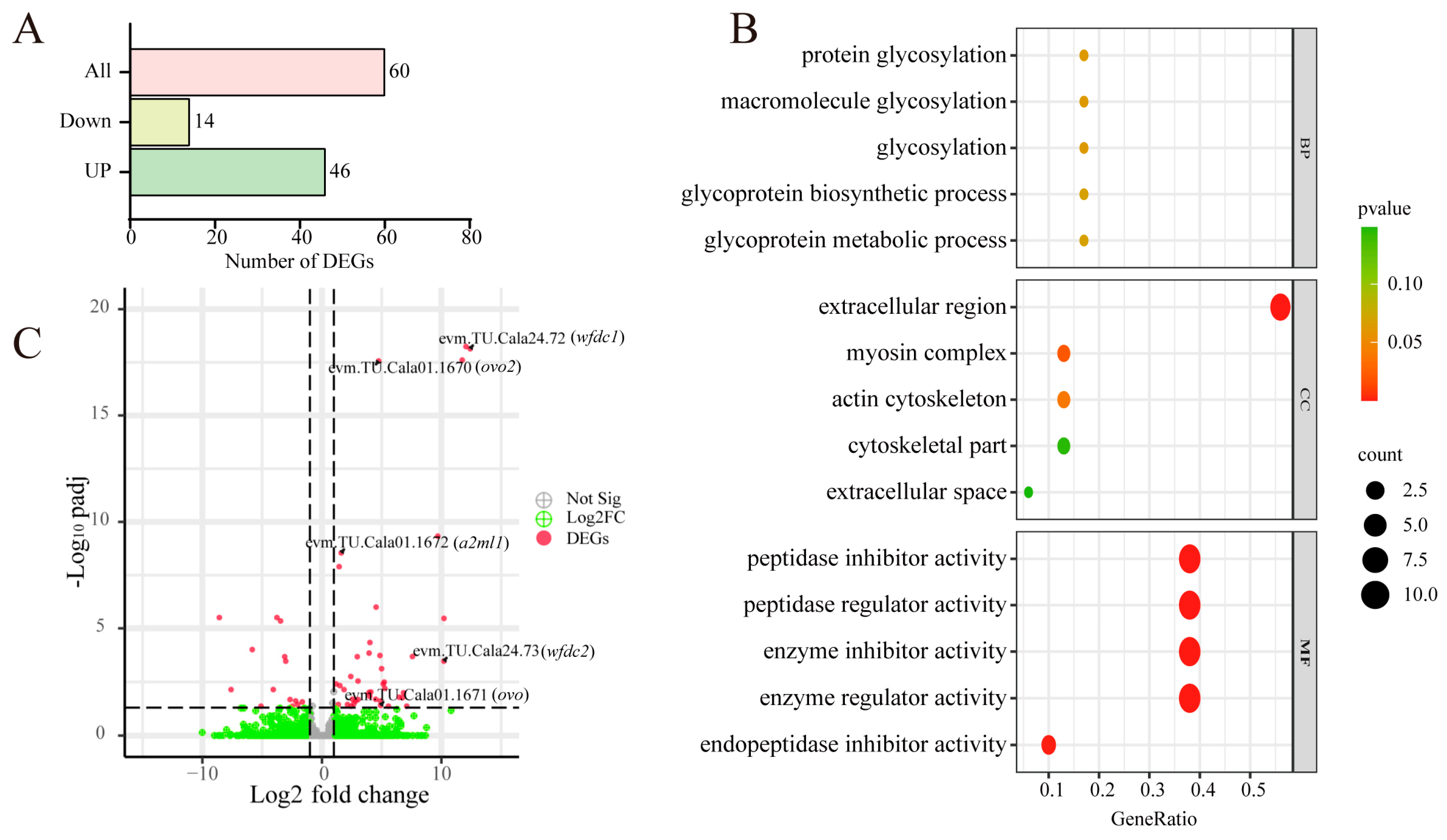
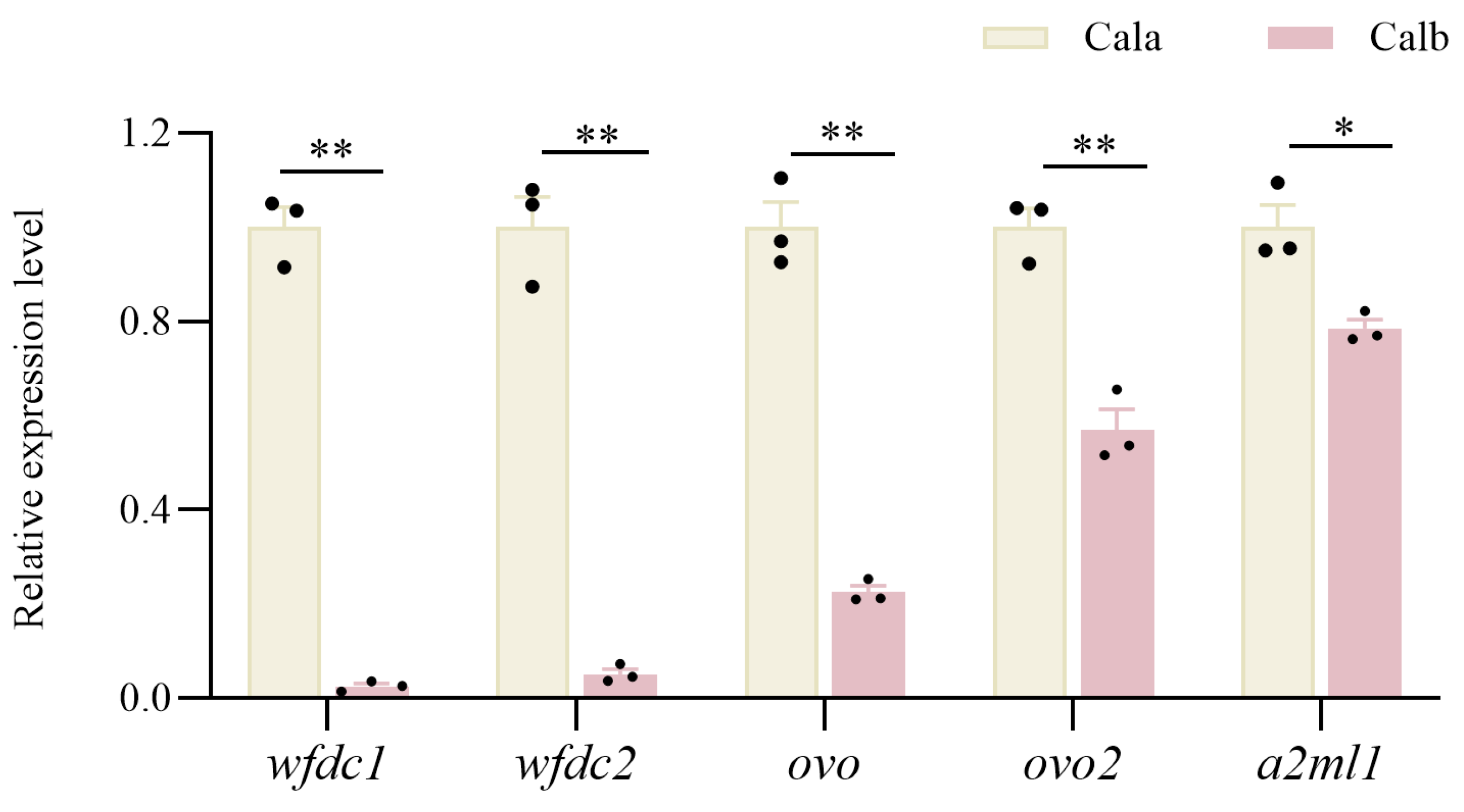
| Gene ID | Description | Log2 (Fold Change 1) | GO Enrichment |
|---|---|---|---|
| novel. 2876 | WAP-type (Whey Acidic Protein) ‘four-disulfide core’ | 15.04 *** | extracellular region/peptidase inhibitor |
| novel. 868 | 15.09 *** | ||
| novel. 3103 | 13.10 *** | ||
| evm. TU. Cala24.72 | 12.42 *** | ||
| novel. 3101 | 11.75 *** | ||
| novel. 3499 | 9.70 *** | ||
| novel. 742 | 10.22 *** | ||
| evm. TU. Cala24.73 | 10.22 *** | ||
| evm. TU.Cala01.1672 | Alpha-2-macroglobulin-like protein 1 | 1.62 *** | |
| evm. TU.Cala01.1670 | Ovostatin homolog 2 | 4.75 *** | peptidase inhibitor |
| evm. TU.Cala01.1671 | Ovostatin | 4.91 * | peptidase inhibitor |
| evm. TU.Cala14.543 | Collagen alpha-1(XIV) chain | 2.14 * | cytoskeleton |
| evm. TU.Cala24.648 | Myosin-7B | 7.10 * | cytoskeleton |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xue, G.; Peng, Y.; Zhang, J.; Chen, F.; Wang, Y.; He, J.; Chen, J.; Xie, P. Ultrastructure and Transcriptomic Analysis Reveal Alternative Pathways of Zona Radiata Formation in Culter alburnus with Different Spawning Habits. Biology 2025, 14, 987. https://doi.org/10.3390/biology14080987
Zhao Y, Xue G, Peng Y, Zhang J, Chen F, Wang Y, He J, Chen J, Xie P. Ultrastructure and Transcriptomic Analysis Reveal Alternative Pathways of Zona Radiata Formation in Culter alburnus with Different Spawning Habits. Biology. 2025; 14(8):987. https://doi.org/10.3390/biology14080987
Chicago/Turabian StyleZhao, Yan, Ge Xue, Yanghui Peng, Jia Zhang, Feng Chen, Yeke Wang, Jun He, Jun Chen, and Ping Xie. 2025. "Ultrastructure and Transcriptomic Analysis Reveal Alternative Pathways of Zona Radiata Formation in Culter alburnus with Different Spawning Habits" Biology 14, no. 8: 987. https://doi.org/10.3390/biology14080987
APA StyleZhao, Y., Xue, G., Peng, Y., Zhang, J., Chen, F., Wang, Y., He, J., Chen, J., & Xie, P. (2025). Ultrastructure and Transcriptomic Analysis Reveal Alternative Pathways of Zona Radiata Formation in Culter alburnus with Different Spawning Habits. Biology, 14(8), 987. https://doi.org/10.3390/biology14080987







