Transcriptome Analysis Reveals Candidate Pathways and Genes Involved in Wheat (Triticum aestivum L.) Response to Zinc Deficiency
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Zinc Concentration in Wheat Grain
2.2. RNA-Seq Data Quality Assessment
2.3. Differentially Expressed Gene (DEG) Analysis
2.4. GO Enrichment of DEGs
2.5. Analysis of DEGs Related to Zinc Ion Transport
2.6. KEGG Pathway Enrichment of DEGs
2.7. Downregulation of Lignin Biosynthesis-Related DEGs Under Zinc Deficiency
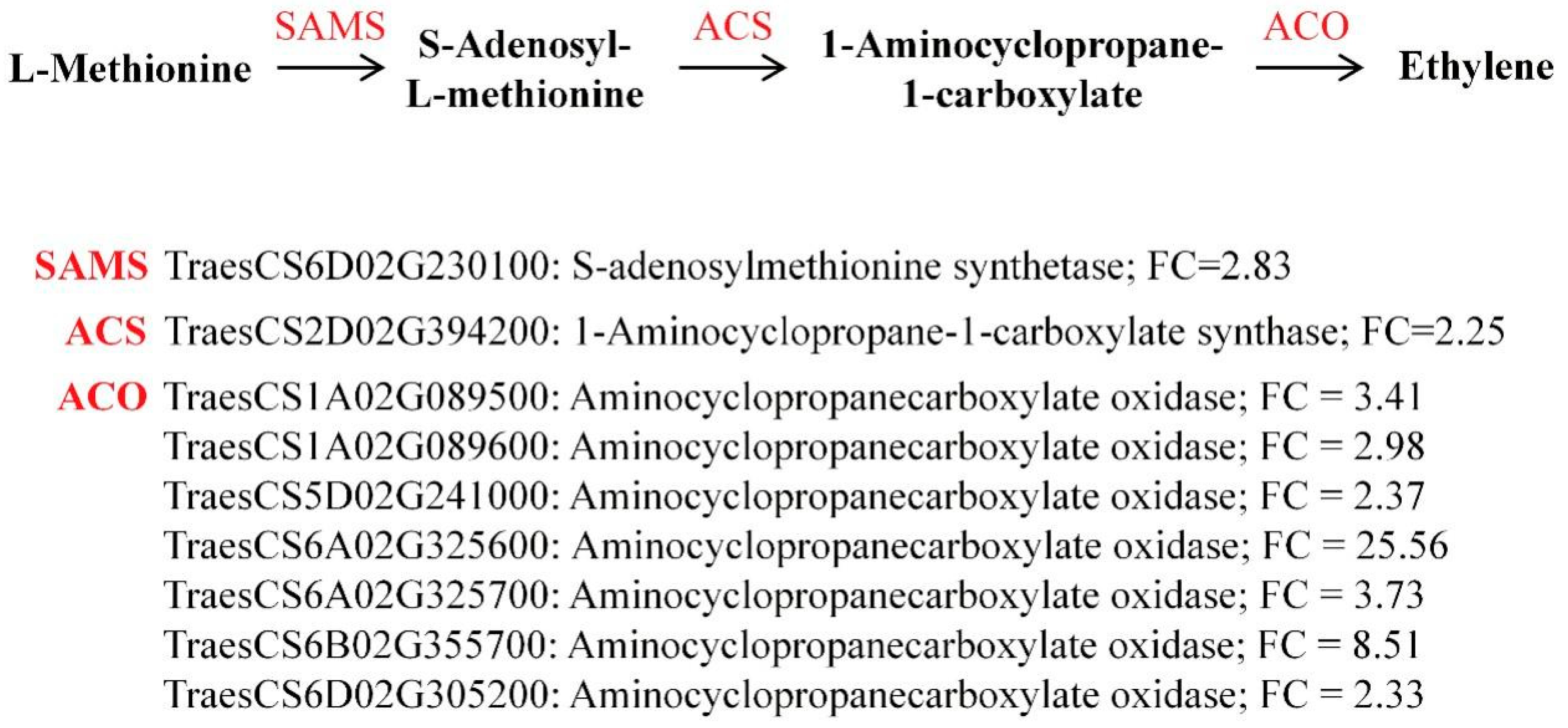
2.8. Upregulation of DEGs Involved in S-Adenosylmethionine and Ethylene Biosynthesis Under Zinc Deficiency
2.9. qPCR Validation of RNA-Seq-Identified DEGs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Root Morphological Scanning
4.3. Determination of Organic Acids in Root Exudates
4.4. RNA-Sequencing
4.5. Differential Gene Expression Analysis
4.6. qRT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Zn | Zinc |
| Fe | Iron |
| ZIP | ZRT, IRT-like protein |
| DMA | Deoxymugineic acid |
| HMA | Heavy metal ATPase |
| MAs | Mugineic acids |
| VIT | Vacuolar iron transporter |
| NAS | Nicotianamine synthase |
| DMAS | Deoxymugineic acid synthase |
| tDT | Tonoplast dicarboxylate transporter |
| NAAT | Nicotianamine aminotransferase |
| FC | Fold change |
| CCR | Cinnamoyl-CoA reductase |
| CAD | Cinnamyl alcohol dehydrogenase |
| COMT | Caffeic acid-O-methyltransferase |
| LAC | Laccases |
| EIN3 | Ethylene-insensitive protein 3 |
| ETR | Ethylene receptor |
| ACO | 1-aminocyclopropane-1-carboxylate oxidase |
| ACS | 1-aminocyclopropane-1-carboxylate |
| SAMS | S-adenosylmethionine synthase |
| NA | Nicotianamine |
| PSs | Phytosiderophores |
References
- Verma, P.K.; Verma, S.; Chakrabarty, D.; Pandey, N. Biotechnological approaches to enhance Zinc uptake and utilization efficiency in cereal crops. J. Plant Nutr. Soil Sci. 2021, 21, 2412–2424. [Google Scholar] [CrossRef]
- Hotz, C.; Braun, K.H. Assessment of the risk of Zinc deficiency in populations and options for its control. J. Food Nutr. Bull. 2004, 2, 94–204. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2017, 69, 172–180. [Google Scholar] [CrossRef]
- Amiri, R.; Bahraminejad, S.; Cheghamirza, K. Estimating genetic variation and genetic parameters for grain iron, zinc and protein concentrations in bread wheat genotypes grown in Iran. J. Cereal. Sci. 2018, 80, 16–23. [Google Scholar] [CrossRef]
- Fan, M.-S.; Zhao, F.-J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.; McClafferty, B. Review: Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Chen, X.-P.; Zhang, Y.-Q.; Tong, Y.-P.; Xue, Y.-F.; Liu, D.-Y.; Zhang, W.; Deng, Y.; Meng, Q.-F.; Yue, S.-C.; Yan, P.; et al. Harvesting more grain zinc of wheat for human health. Sci. Rep. 2017, 7, 7016. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2007, 302, 1–17. [Google Scholar] [CrossRef]
- Tingmiao, H.; Huang, Q.; She, X.; Ma, X.; Huang, M.; Cao, H.; He, G.; Liu, J.; Liang, D.; Malhi, S.; et al. Grain zinc concentration and its relation to soil nutrient availability in different wheat cropping regions of China. Soil Till Res. 2019, 191, 57–65. [Google Scholar] [CrossRef]
- Erenoglu, E.B.; Kutman, U.B.; Ceylan, Y.; Yildiz, B.; Cakmak, I. Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol. 2011, 189, 438–448. [Google Scholar] [CrossRef]
- Xue, Y.F.; Yue, S.C.; Zhang, Y.Q.; Cui, Z.L.; Chen, X.P.; Yang, F.C.; Cakmak, I.; McGrath, S.; Zhang, F.S.; Zou, C.Q. Grain and shoot zinc accumulation in winter wheat affected by nitrogen management. Plant Soil 2012, 361, 153–163. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Borrill, P.; Connorton, J.M.; Balk, J.; Miller, A.J.; Sanders, D.; Uauy, C. Biofortification of wheat grain with iron and zinc: Integrating novel genomic resources and knowledge from model crops. Front. Plant Sci. 2014, 5, 53. [Google Scholar] [CrossRef]
- Evens, N.P.; Buchner, P.; Williams, L.E.; Hawkesford, M.J. The role of ZIP transporters and group F bZIP transcription factors in the Zn-deficiency response of wheat (Triticum aestivum). Plant J. Cell Mol. Biol. 2017, 92, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.I.; Palmgren, M.G. Many rivers to cross: The journey of zinc from soil to seed. Front. Plant Sci. 2014, 5, 30. [Google Scholar] [CrossRef]
- Vatansever, R.; Filiz, E.; Eroglu, S. Genome-wide exploration of metal tolerance protein (MTP) genes in common wheat (Triticum aestivum): Insights into metal homeostasis and biofortification. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2017, 30, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Peng, K.; Lou, G.; Ren, Z.; Sun, X.; Wang, Z.; Xing, J.; Song, C.; Cang, J. Transcriptome analysis of the winter wheat Dn1 in response to cold stress. BMC Plant Biol. 2022, 22, 277. [Google Scholar] [CrossRef]
- Xi, W.; Hao, C.; Li, T.; Wang, H.; Zhang, X. Transcriptome analysis of roots from wheat (Triticum aestivum L.) varieties in response to drought stress. Int. J. Mol. Sci. 2023, 24, 7245. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Zhu, Y.; Shang, Y.; Wu, Z.; Tao, Y.; Wang, H.; Li, D.; Zhang, C. Transcriptome Profiling Reveals the Gene Network Responding to Low Nitrogen Stress in Wheat. Plants 2024, 13, 371. [Google Scholar] [CrossRef]
- Kaur, G.; Shukla, V.; Kumar, A.; Kaur, M.; Goel, P.; Singh, P.; Shukla, A.; Meena, V.; Kaur, J.; Singh, J.; et al. Integrative analysis of hexaploid wheat roots identifies signature components during iron starvation. J. Exp. Bot. 2019, 70, 6141–6161. [Google Scholar] [CrossRef] [PubMed]
- Liedschulte, V.; Duncan Battey, J.N.; Laparra, H.; Kleinhans, S.; Bovet, L.; Goepfert, S. Zinc uptake and HMA4 activity are required for micro-and macroelement balance in tobacco (Nicotiana tabacum). Phytochemistry 2021, 191, 112911. [Google Scholar] [CrossRef]
- Kaznina, N.M.; Dubovets, N.I.; Repkina, N.S.; Batova, Y.V.; Ignatenko, A.A.; Orlovskaya, O.A.; Titov, A.F. The HMA2 gene expression in leaves of introgressive wheat lines under Zn optimum and deficiency content in root environment. Doklady. Biochem. Biophys. 2022, 505, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Takahashi, M.; Tsukamoto, T.; Watanabe, S.; Matsuhashi, S.; Yazaki, J.; Kishimoto, N.; Kikuchi, S.; Nakanishi, H.; Mori, S.; et al. Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J. Cell Mol. Biol. 2006, 48, 85–97. [Google Scholar] [CrossRef]
- Dinkelaker, B.; RÖMheld, V.; Marschner, H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Env. 1989, 12, 285–292. [Google Scholar] [CrossRef]
- White, M.C.; Decker, A.M.; Chaney, R.L. Metal complexation in xylem fluid. I: Chemical composition of tomato and soybean stem exudate. Plant Physiol. 1981, 67, 292–300. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Nielsen, F.H. History of zinc in agriculture. Adv. Nutr. 2012, 3, 783–789. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Wheat genotypes differ in Zn efficiency when grown in chelate-buffered nutrient solution. Plant Soil 1995, 176, 317–324. [Google Scholar] [CrossRef]
- Pélissier, P.M.; Motte, H.; Beeckman, T. Lateral root formation and nutrients: Nitrogen in the spotlight. Plant Physiol. 2021, 187, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Genc, Y.; Huang, C.Y.; Langridge, P. A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J. Exp. Bot. 2007, 58, 2775–2784. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, F.; Fu, C.X.; Zhang, H.Y.; Wang, Y.A. Effects of zinc deficiency stress on the root architecture and zinc accumulation of the different apple rootstocks. Acta Hortic. Sin. 2012, 39, 613–620. [Google Scholar]
- Chen, W.; He, Z.; Yang, X.; Feng, Y. Zinc efficiency is correlated with root morphology, ultrastructure, and antioxidative enzymes in rice. J. Plant Nutr. 2009, 32, 287–305. [Google Scholar] [CrossRef]
- Kong, D.; Khan, S.A.; Wu, H.; Liu, Y.; Ling, H.Q. Biofortification of iron and zinc in rice and wheat. J. Integr. Plant Biol. 2022, 64, 1157–1167. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Mahmood, A.; Chattha, M.U.; Nawaz, M.; Subhani, M.N.; Kharal, M.; Khan, S. Biofortification of wheat cultivars to combat zinc deficiency. Front. Plant Sci. 2017, 8, 281. [Google Scholar] [CrossRef]
- Lu, X.; Liu, S.; Zhi, S.; Chen, J.; Ye, G. Comparative transcriptome profile analysis of rice varieties with different tolerance to zinc deficiency. Plant Biol. 2021, 23, 375–390. [Google Scholar] [CrossRef]
- Tantriani; Cheng, W.; Oikawa, A.; Tawaraya, K. Phosphorus deficiency alters root length, acid phosphatase activity, organic acids, and metabolites in root exudates of soybean cultivars. Physiol. Plant. 2023, 175, e14107. [Google Scholar] [CrossRef]
- Qin, L.; Li, Z.; Li, B.; Wang, J.; Zu, Y.; Jiang, M.; Li, Y. Organic acid excretion in root exudates as a mechanism of cadmium uptake in a Sonchus asper-Zea mays intercropping system. Bull. Environ. Contam. Toxicol. 2021, 107, 1059–1064. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Li, Y.; Zhang, J.; Zhong, L.; Li, L.; Zhong, S.; Gu, R. Phlomoides rotata adapts to low-nitrogen environments by promoting root growth and increasing root organic acid exudate. BMC Plant Biol. 2024, 24, 1234. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.; Rammig, A.; Fuchslueger, L.; Lugli, L.F.; Quesada, C.A.; Fleischer, K. Plant phosphorus-use and -acquisition strategies in Amazonia. New Phytol. 2022, 234, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.R.; Li, J.Y.; Li, Z.S. Adapted responses in the rhizosphere of P-efficient wheat genotype to stress of phosphorus deficiency. Acta Bot. Boreali-Occident. Sin. 2000, 20, 1–7. [Google Scholar]
- Tolrà, R.P.; Charlotte, P.; Barceló, J. Zinc hyperaccumulation in Thlaspi caerulescens. II. Influence on organic acids. J. Plant Nutr. 1996, 19, 1541–1550. [Google Scholar] [CrossRef]
- Hoffland, E.; Wei, C.; Wissuwa, M. Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil. 2006, 283, 155–162. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ismail, A.M.; Yanagihara, S. Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol. 2006, 142, 731–741. [Google Scholar] [CrossRef]
- Hurth, M.A.; Suh, S.J.; Kretzschmar, T.; Geis, T.; Bregante, M.; Gambale, F.; Martinoia, E.; Neuhaus, H.E. Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol. 2005, 137, 901–910. [Google Scholar] [CrossRef]
- Hall, J.L.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Guo, L.; Li, H.; Nie, X.; Chai, S.; Zheng, W. Genome-wide identification of wheat zip gene family and functional characterization of the TaZIP13-B in Plants. Front. Plant Sci. 2021, 12, 748146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.H.; Yi, H.Y.; Gong, J.M. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. Cell Mol. Biol. 2012, 72, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Romheld, V.; Marschner, H. Release of Zn mobilizing root exudates in different plant species as affected by Zn nutritional status. J Plant Nutr. 1991, 14, 675–686. [Google Scholar] [CrossRef]
- Walter, A.; Römheld, V.; Marschner, H.; Mori, S. Is the release of phytosiderophores in zinc-deficient wheat plants a response to impaired iron utilization? Physiol. Plant. 2006, 92, 493–500. [Google Scholar] [CrossRef]
- Corso, M.; An, X.; Jones, C.Y.; Gonzalez-Doblas, V.; Schvartzman, M.S.; Malkowski, E.; Willats, W.G.T.; Hanikenne, M.; Verbruggen, N. Adaptation of Arabidopsis halleri to extreme metal pollution through limited metal accumulation involves changes in cell wall composition and metal homeostasis. New Phytol. 2021, 230, 669–682. [Google Scholar] [CrossRef]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, H.Q.; Chen, J.; Chang, J.D.; Zhao, F.J. Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J. Integr. Plant Biol. 2023, 65, 570–593. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, S.-Q.; Yang, C.; Cao, D.; Fan, S.; Zhang, X.J.B. Comparative transcriptome analysis reveals candidate genes and pathways for potential branch growth in elm (Ulmus pumila) cultivars. Biology 2022, 11, 711. [Google Scholar] [CrossRef]
- Geldner, N. The endodermis. Annu. Rev. Plant Biol. 2013, 64, 531–558. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Cui, D.; Ye, Z.H. Secondary cell wall biosynthesis. New Phytol. 2019, 221, 1703–1723. [Google Scholar] [CrossRef] [PubMed]
- Ricachenevsky, F.K.; de Araújo Junior, A.T.; Fett, J.P.; Sperotto, R.A. You shall not pass: Root vacuoles as a symplastic checkpoint for metal translocation to shoots and possible application to grain nutritional quality. Front. Plant Sci. 2018, 9, 412. [Google Scholar] [CrossRef]
- Aleamotu’a, M.; McCurdy, D.W.; Collings, D.A. Phi thickenings in roots: Novel secondary wall structures responsive to biotic and abiotic stresses. J. Exp. Bot. 2019, 70, 4631–4642. [Google Scholar] [CrossRef]
- Mao, C.; Yi, K.; Yang, L.; Zheng, B.; Wu, Y.; Liu, F.; Wu, P. Identification of aluminium-regulated genes by cDNA-AFLP in rice (Oryza sativa L.): Aluminium-regulated genes for the metabolism of cell wall components. J. Exp. Bot. 2004, 55, 137–143. [Google Scholar] [CrossRef]
- Yoon, J.; Choi, H.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Jiang, C.; Wei, S.; Jiao, H.; Ran, K.; Dong, R.; Wang, S. The regulation of plant lignin biosynthesis under boron deficiency conditions. Physiol. Plant. 2022, 174, e13815. [Google Scholar] [CrossRef]
- Chabannes, M.; Barakate, A.; Lapierre, C.; Marita, J.M.; Ralph, J.; Pean, M.; Danoun, S.; Halpin, C.; Grima-Pettenati, J.; Boudet, A.M. Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J. 2001, 28, 257–270. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- z Kiani, C.; Ahmad, A.; Hamid Reza, S. Growth promotion, increase of iron, potassium and cell wall components following silicon application in rice under iron deficiency. Pizhūhishhā-Yi Zirā̒ī-I Īrān 2014, 12, 65–72. [Google Scholar]
- Zhou, G.; An, Q.; Liu, Z.; Wan, Y.; Bao, W. Systematic analysis of NRAMP family genes in Areca catechu and its response to Zn/Fe deficiency stress. Int. J. Mol. Sci. 2023, 24, 7383. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, G.; Ludewig, U.; Erenoglu, B.E.; Mori, S.; Kitahara, T.; von Wirén, N. ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J. Biol. Chem. 2004, 279, 9091–9096. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H.; Ekiz, H.; Kalayci, M.; Yilmaz, A.; Braun, H.-J. Phytosiderophore release in bread and durum wheat genotypes differing in zinc efficiency. Plant Soil 1996, 180, 183–189. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Bashir, K.; Ishimaru, Y.; Nishizawa, N.K. Iron uptake and loading into rice grains. Rice 2010, 3, 122–130. [Google Scholar] [CrossRef]
- Swamy, B.P.M.; Rahman, M.A.; Inabangan-Asilo, M.A.; Amparado, A.; Manito, C.; Chadha-Mohanty, P.; Reinke, R.; Slamet-Loedin, I.H. Advances in breeding for high grain Zinc in Rice. Rice 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Keller, B.; Gruissem, W.; Bhullar, N.K. Rice nicotianamine synthase 2 expression improves dietary iron and zinc levels in wheat. TAG Theor. Appl. Genetics. Theor. Und Angew. Genet. 2017, 130, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Douchkov, D.; Gryczka, C.; Stephan, U.W.; Hell, R.; BÄumlein, H. Ectopic expression of nicotianamine synthase genes results in improved iron accumulation and increased nickel tolerance in transgenic tobacco. Plant Cell Environ. 2005, 28, 365–374. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Moran Lauter, A.N.; Peiffer, G.A.; Yin, T.; Whitham, S.A.; Cook, D.; Shoemaker, R.C.; Graham, M.A. Identification of candidate genes involved in early iron deficiency chlorosis signaling in soybean (Glycine max) roots and leaves. BMC Genom. 2014, 15, 702. [Google Scholar] [CrossRef]
- Wang, S.; Lu, B.; Wu, T.; Zhang, X.; Xu, X.; Han, Z.; Wang, Y. Transcriptomic analysis demonstrates the early responses of local ethylene and redox signaling to low iron stress in malus xiaojinensis. Tree Genet. Genomes 2014, 10, 573–584. [Google Scholar] [CrossRef]
- Roschzttardtz, H.; Séguéla-Arnaud, M.; Briat, J.F.; Vert, G.; Curie, C. The FRD3 citrate effluxer promotes iron nutrition between symplastically disconnected tissues throughout Arabidopsis development. Plant Cell 2011, 23, 2725–2737. [Google Scholar] [CrossRef]
- Chen, C.L.; Cui, Y.; Cui, M.; Zhou, W.J.; Wu, H.L.; Ling, H.Q. A FIT-binding protein is involved in modulating iron and zinc homeostasis in Arabidopsis. Plant Cell Env. 2018, 41, 1698–1714. [Google Scholar] [CrossRef]
- Chu, H.X.; Mu, W.Y.; Dang, H.Y.; Wang, T.; Sun, R.Q.; Hou, S.B.; Huang, T.M.; Huang, Q.N.; Shi, M.; Wang, Z.H. Evaluation on concentration and nutrition of micro-elements in wheat grains in major wheat production regions of China. Acta Agron. Sin. 2022, 48, 2853–2865. [Google Scholar]
- Huang, H.; Lu, R.; Zhan, J.; He, J.; Wang, Y.; Li, T. Role of root exudates in cadmium accumulation of a low-cadmium-accumulating tobacco line (Nicotiana tabacum L.). Toxics 2023, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT METHOD. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



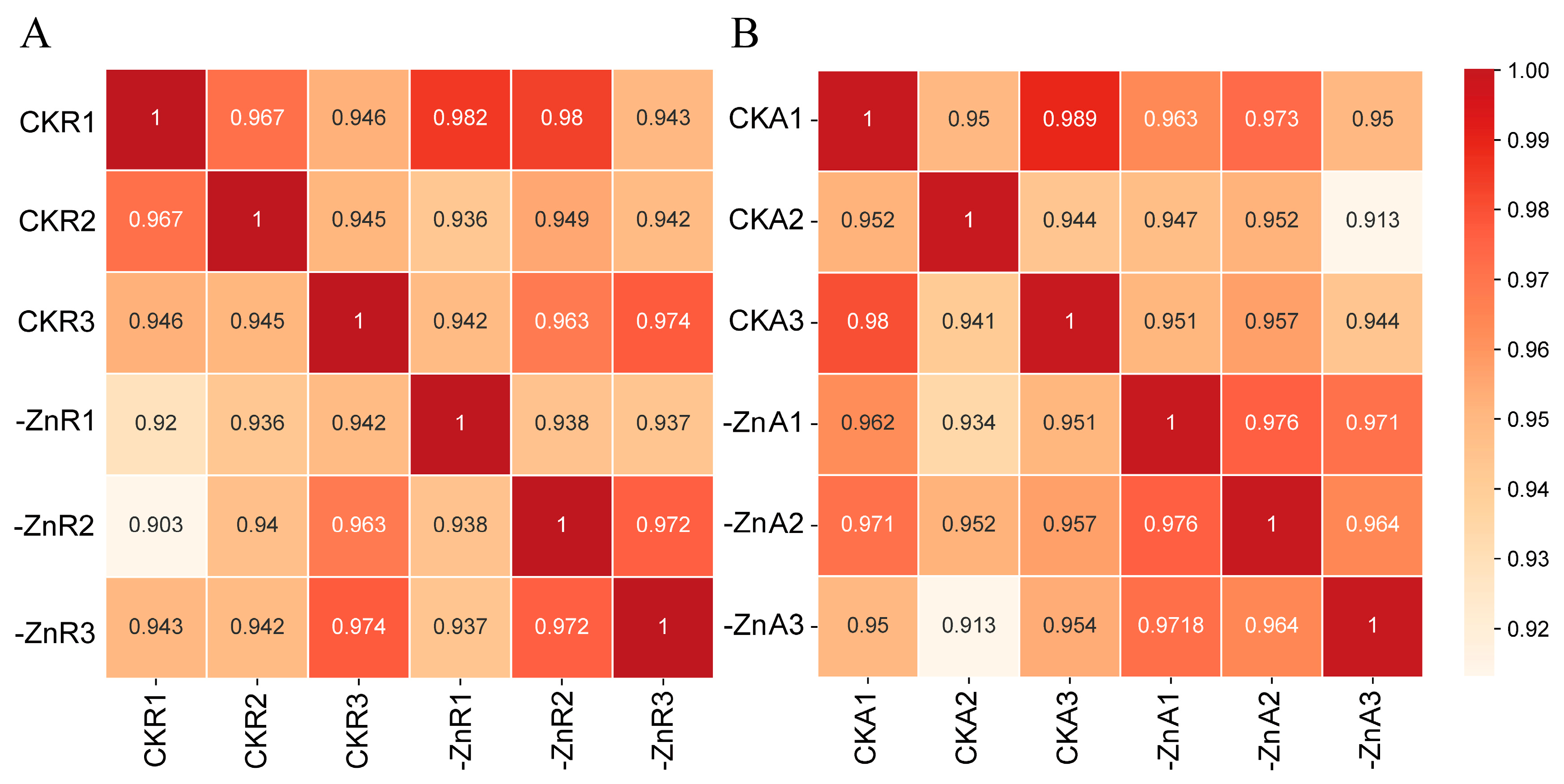
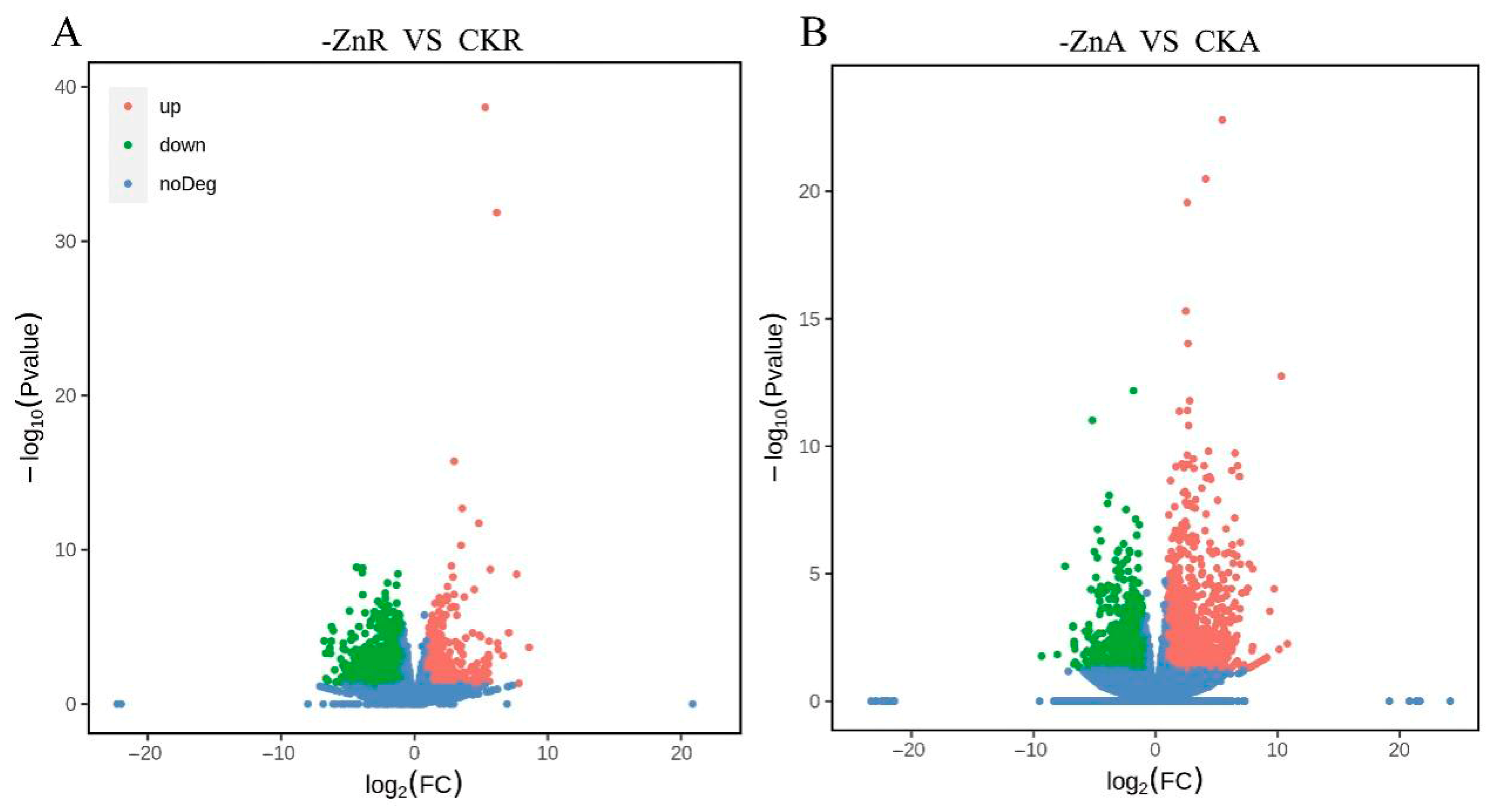

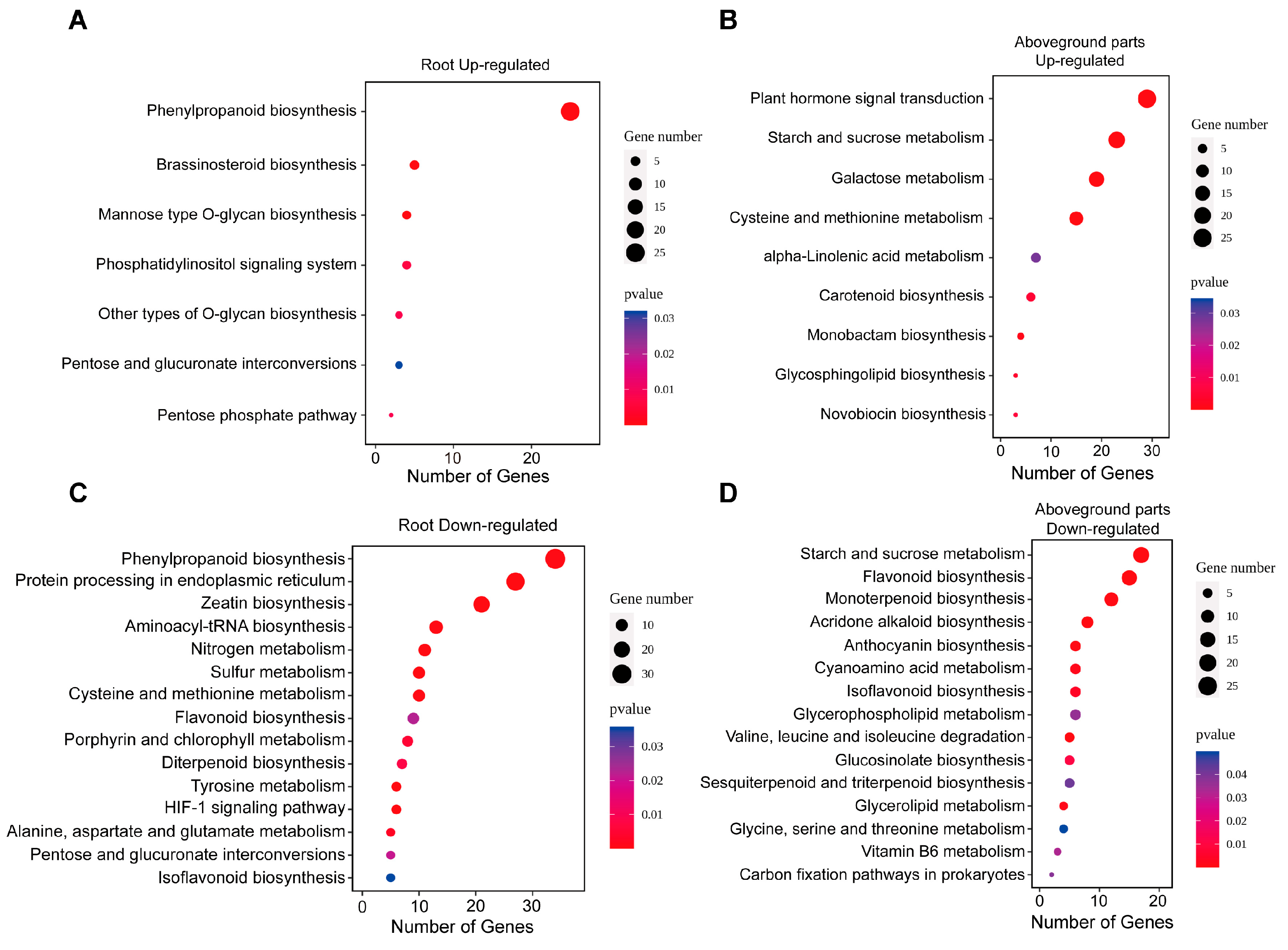


| Treatment | Total Root Length (cm) | Total Root Surface Area (cm2) | Total Root Volume (cm3) | Average Root Diameter (mm) | Number of Root Tips |
|---|---|---|---|---|---|
| CK | 418.32 b | 1.89 a | 72.32 a | 0.56 a | 724 a |
| -Zn | 489.03 a | 2.08 a | 81.19 a | 0.63 a | 827 a |
| Sample | Raw Reads | Clean Reads | Mapped Reads | Mapped (%) | Unique Mapped Reads (%) | Clean Q30 (%) | Exon (%) |
|---|---|---|---|---|---|---|---|
| CKR1 | 76,657,956 | 75,174,400 | 64,536,355 | 85.85 | 79.40 | 95.40 | 82.78 |
| CKR2 | 73,554,322 | 72,111,182 | 65,898,408 | 91.38 | 76.76 | 95.58 | 83.30 |
| CKR3 | 81,630,228 | 80,045,472 | 68,349,124 | 85.39 | 79.11 | 95.52 | 83.33 |
| -ZnR1 | 72,715,286 | 71,350,538 | 65,855,677 | 92.30 | 72.40 | 95.58 | 80.86 |
| -ZnR2 | 79,714,610 | 78,245,238 | 70,729,786 | 90.40 | 83.33 | 95.34 | 82.26 |
| -ZnR3 | 79,028,732 | 77,640,002 | 68,806,972 | 88.62 | 82.04 | 95.59 | 81.92 |
| CKA1 | 88,092,076 | 86,186,994 | 79,824,523 | 92.62 | 85.39 | 95.22 | 82.73 |
| CKA2 | 84,953,578 | 83,296,162 | 78,667,403 | 94.44 | 86.32 | 95.47 | 82.75 |
| CKA3 | 81,094,022 | 79,433,574 | 75,224,060 | 97.70 | 87.86 | 95.38 | 82.29 |
| -ZnA1 | 76,738,728 | 75,184,032 | 71,478,194 | 95.07 | 88.19 | 95.41 | 83.48 |
| -ZnA2 | 83,440,878 | 81,729,870 | 76,978,018 | 94.19 | 86.68 | 95.29 | 83.20 |
| -ZnA3 | 76,232,316 | 74,721,754 | 70,389,855 | 94.20 | 86.84 | 95.54 | 80.82 |
| Gene ID (-ZnR_vs._CKR_up) | Log2 (FC) | p Value | Gene Description |
| TraesCS7D02G413000 | 6.17 | 1.39 × 10−32 | vacuolar iron transporter 1 (VIT1-7D) |
| TraesCS5B02G202100 | 1.15 | 5.88 × 10−5 | vacuolar iron transporter 2 (VIT2-5B) |
| TraesCS5A02G552400 | 2.38 | 1.70 × 10−6 | nicotianamine synthase 2 (NAS2-5A) |
| TraesCS2D02G094200 | 2.97 | 1.86 × 10−16 | nicotianamine synthase 9 (NAS9-2D) |
| TraesCS2B02G111100 | 2.97 | 8.01 × 10−8 | nicotianamine synthase 9 (NAS9-2B) |
| TraesCS2A02G095700 | 1.75 | 3.06 × 10−5 | nicotianamine synthase 9 (NAS9-2A) |
| TraesCS2B02G023500 | 2.59 | 3.41 × 10−5 | deoxymugineic acid synthase (DMAS-2B) |
| TraesCS1D02G294000 | 2.89 | 5.75 × 10−9 | ZRT- and IRT-like proteins 9 (ZIP9-1D) |
| TraesCS4A02G025400 | 1.86 | 1.29 × 10−7 | ZRT- and IRT-like proteins 9 (ZIP9-4A) |
| TraesCS4D02G277100 | 1.81 | 7.62 × 10−5 | ZRT- and IRT-like proteins 9 (ZIP9-4D) |
| TraesCS7B02G321200 | 1.80 | 5.46 × 10−5 | ZRT- and IRT-like proteins 10 (ZIP10-7B) |
| TraesCS7A02G420600 | 2.41 | 1.03 × 10−7 | ZRT- and IRT-like proteins 10 (ZIP10-7A) |
| TraesCS7D02G412800 | 1.43 | 3.3 × 10−6 | cadmium/zinc-transporting ATPase (HMA2-7D) |
| TraesCS5B02G261800 | 2.40 | 8.80 × 10−5 | tonoplast dicarboxylate transporter (tDT-5B) |
| TraesCS5B02G261500 | 2.01 | 3.42 × 10−7 | tonoplast dicarboxylate transporter (tDT-5B) |
| TraesCS3A02G183800 | 1.75 | 4.42 × 10−5 | tonoplast dicarboxylate transporter (tDT-3A) |
| TraesCS3D02G188000 | 2.42 | 1.98 × 10−7 | tonoplast dicarboxylate transporter (tDT-3B) |
| Gene ID (-ZnA_vs._CKA_up) | Log2 (FC) | p Value | Gene Description |
| TraesCS2A02G143400 | 6.52 | 1.87 × 10−10 | ZRT- and IRT-like proteins 8 (ZIP8-2A) |
| TraesCS2D02G146800 | 4.84 | 5.03 × 10−5 | ZRT and IRT-like proteins 8 (ZIP8-2D) |
| TraesCS1A02G297400 | 2.91 | 6.55 × 10−6 | ZRT- and IRT-like proteins 9 (ZIP9-1A) |
| TraesCS1B02G306400 | 3.85 | 8.74 × 10−6 | ZRT- and IRT-like proteins 9 (ZIP9-1B) |
| TraesCS7D02G413300 | 5.11 | 1.33 × 10−8 | ZRT- and IRT-like proteins 10 (ZnT10-7D) |
| TraesCS5A02G230300 | 4.71 | 1.68 × 10−6 | cadmium/zinc-transporting ATPase (HMA1-5A) |
| TraesCS7D02G412800 | 2.67 | 2.12 × 10−6 | cadmium/zinc-transporting ATPase (HMA2-7D) |
| TraesCS7B02G448000 | 4.95 | 6.64 × 10−5 | basic leucine zipper 19 (bZIP19-7B) |
| TraesCS3D02G188000 | 10.32 | 1.78 × 10−13 | tonoplast dicarboxylate transporter (Tdt-3D) |
| TraesCS3A02G183800 | 6.97 | 4.18 × 10−6 | tonoplast dicarboxylate transporter (tDT-3A) |
| TraesCS5A02G263100 | 5.99 | 1.45 × 10−6 | tonoplast dicarboxylate transporter (tDT-5A) |
| TraesCS5D02G270600 | 2.89 | 4.52 × 10−5 | tonoplast dicarboxylate transporter (tDT-5D) |
| TraesCS3A02G183800 | 6.97 | 4.18 × 10−6 | tonoplast dicarboxylate transporter (tDT-3A) |
| TraesCS1B02G300600 | 3.02 | 4.78 × 10−5 | nicotianamine aminotransferase A (NAAT-1B) |
| Organic Matter (g·kg−1) | Total Nitrogen (g·kg−1) | Available Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) | Available Zinc (mg·kg−1) | pH |
|---|---|---|---|---|---|
| 21.36 | 1.16 | 22.10 | 150.56 | 0.85 | 6.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Zhang, S.; Wang, W.; Hu, N.; Shi, W. Transcriptome Analysis Reveals Candidate Pathways and Genes Involved in Wheat (Triticum aestivum L.) Response to Zinc Deficiency. Biology 2025, 14, 985. https://doi.org/10.3390/biology14080985
Zhu S, Zhang S, Wang W, Hu N, Shi W. Transcriptome Analysis Reveals Candidate Pathways and Genes Involved in Wheat (Triticum aestivum L.) Response to Zinc Deficiency. Biology. 2025; 14(8):985. https://doi.org/10.3390/biology14080985
Chicago/Turabian StyleZhu, Shoujing, Shiqi Zhang, Wen Wang, Nengbing Hu, and Wenjuan Shi. 2025. "Transcriptome Analysis Reveals Candidate Pathways and Genes Involved in Wheat (Triticum aestivum L.) Response to Zinc Deficiency" Biology 14, no. 8: 985. https://doi.org/10.3390/biology14080985
APA StyleZhu, S., Zhang, S., Wang, W., Hu, N., & Shi, W. (2025). Transcriptome Analysis Reveals Candidate Pathways and Genes Involved in Wheat (Triticum aestivum L.) Response to Zinc Deficiency. Biology, 14(8), 985. https://doi.org/10.3390/biology14080985






