Coordinated Regulation of Photosynthesis, Stomatal Traits, and Hormonal Dynamics in Camellia oleifera During Drought and Rehydration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
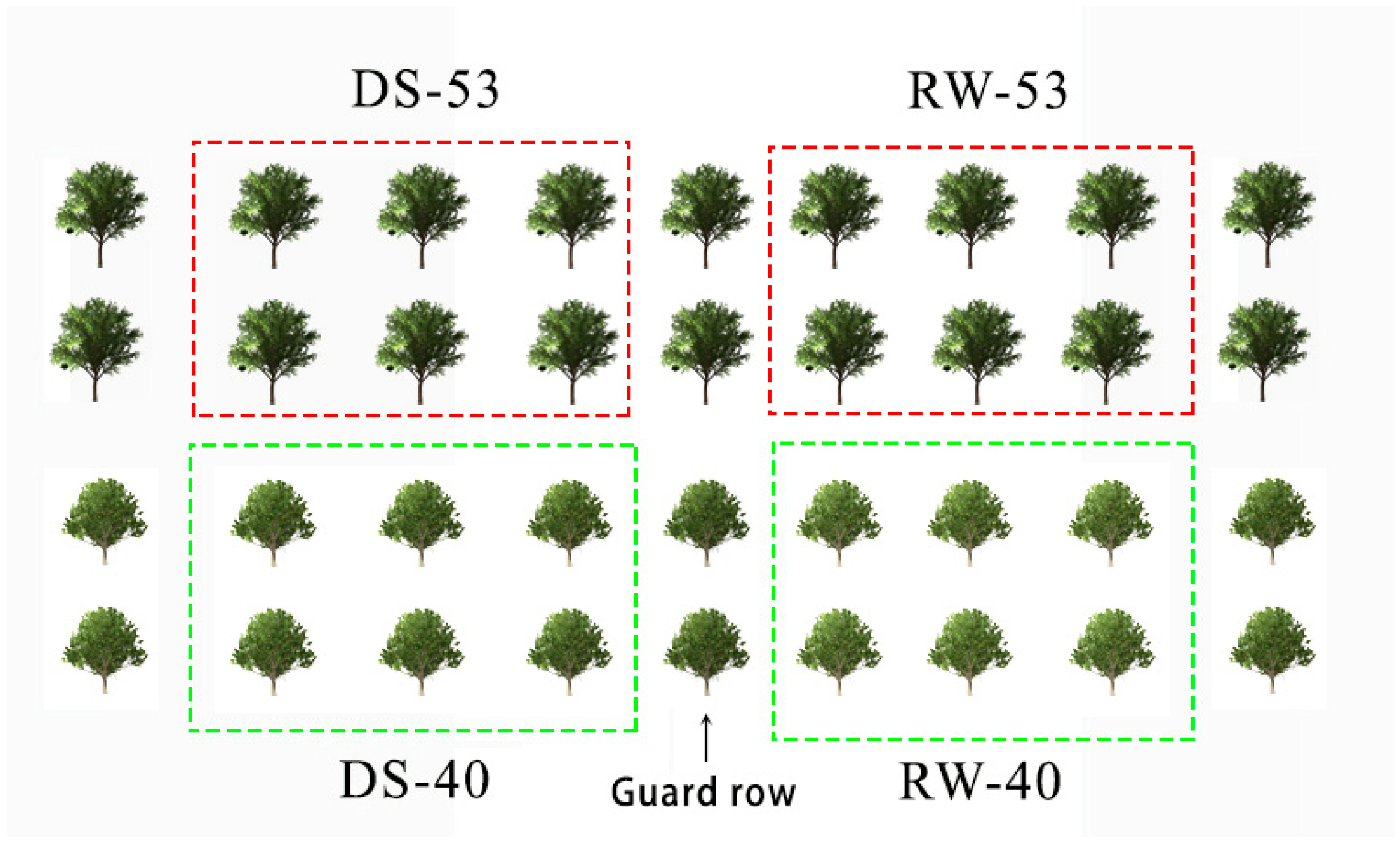
2.1. Study Area and Plant Material
2.2. Experimental Design
2.3. Determination of Leaf Relative Water Content
2.4. Measurement of Photosynthetic Parameters
2.5. Stomatal Characteristic Measurements
2.6. Endogenous Hormone Assays
2.7. Data Analysis
3. Results
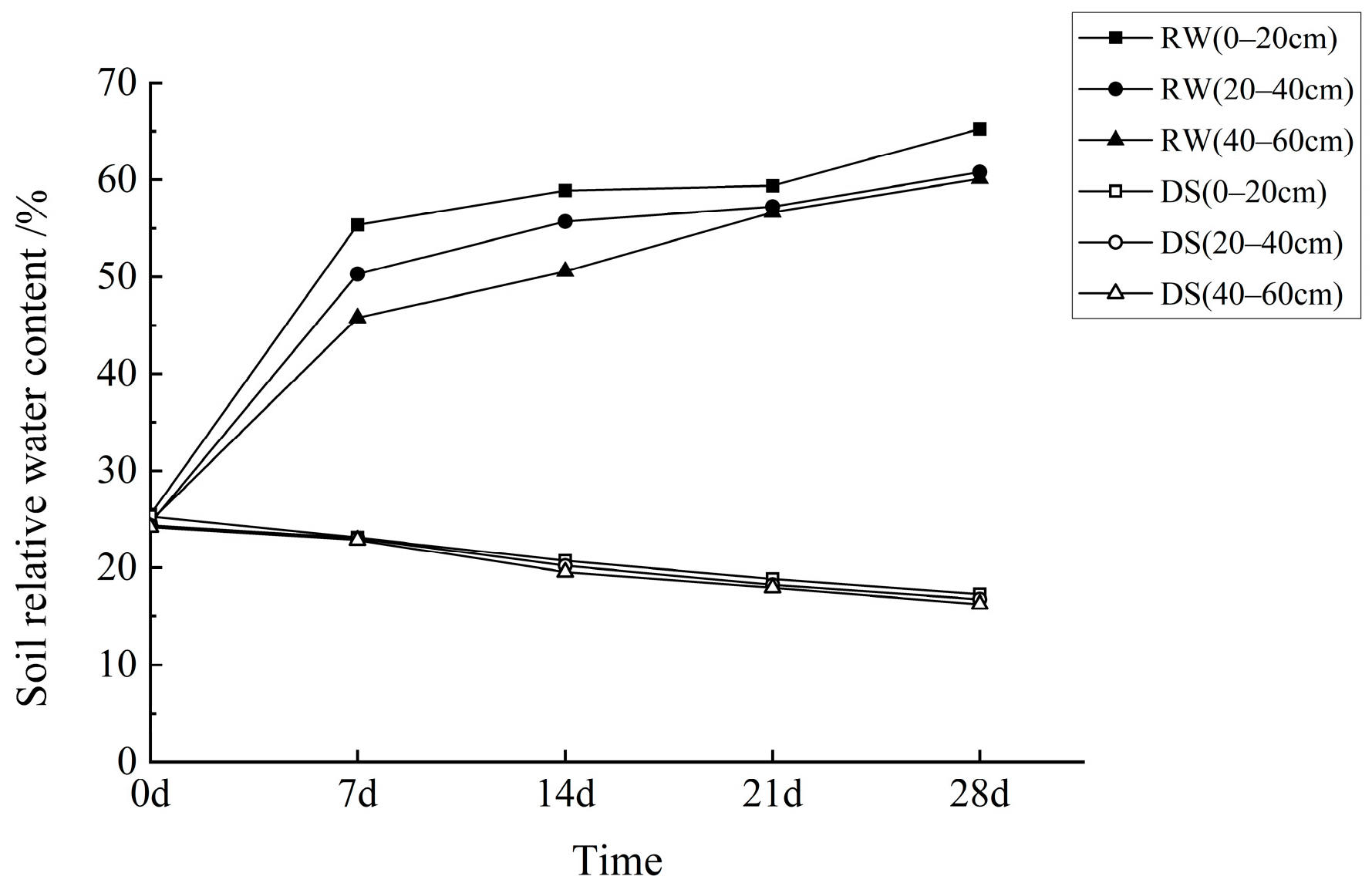
3.1. Effects of Drought and Rehydration on Leaf Relative Water Content in C. oleifera
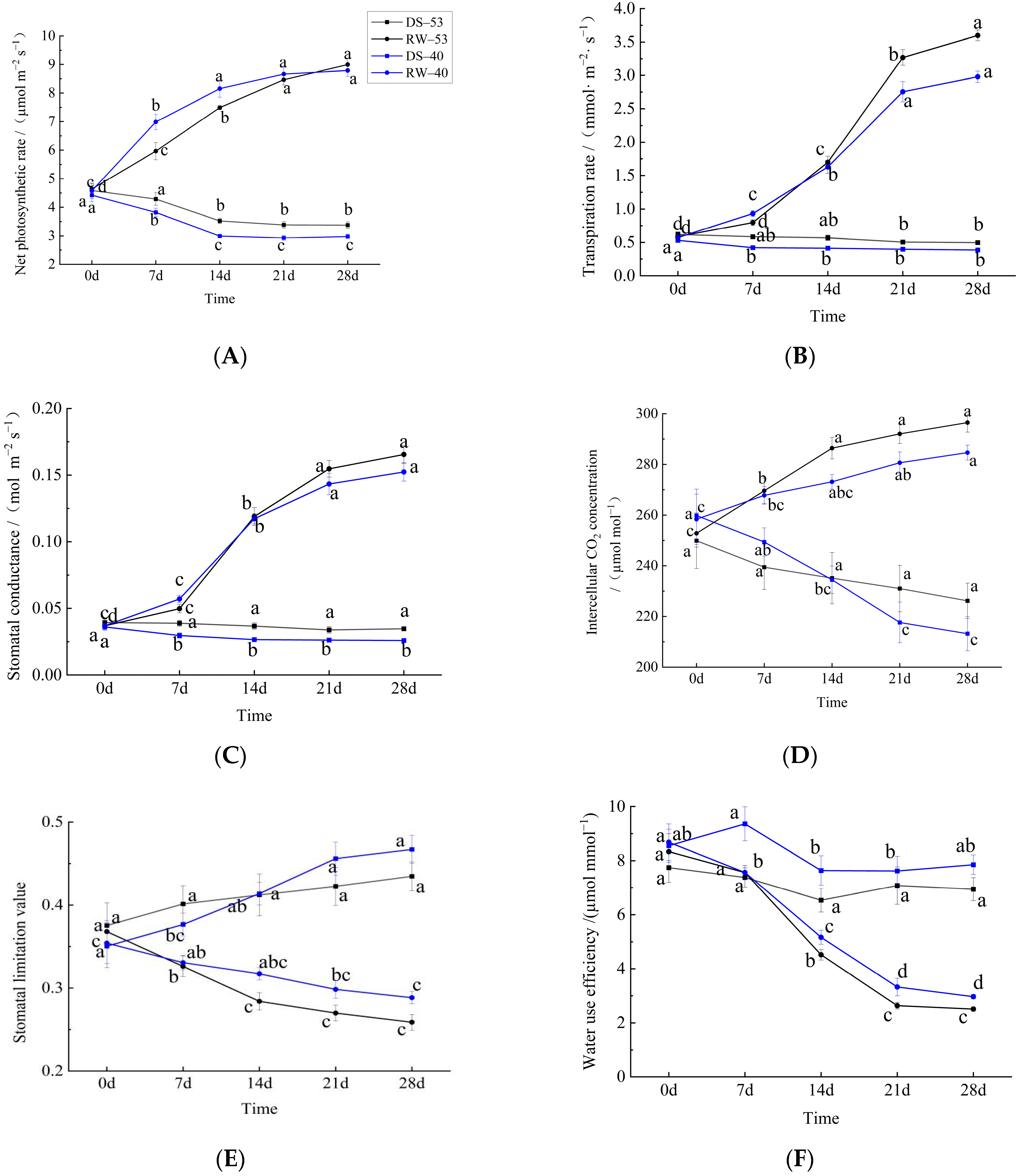
3.2. Effects of Drought and Rehydration on Photosynthetic Parameters of C. oleifera
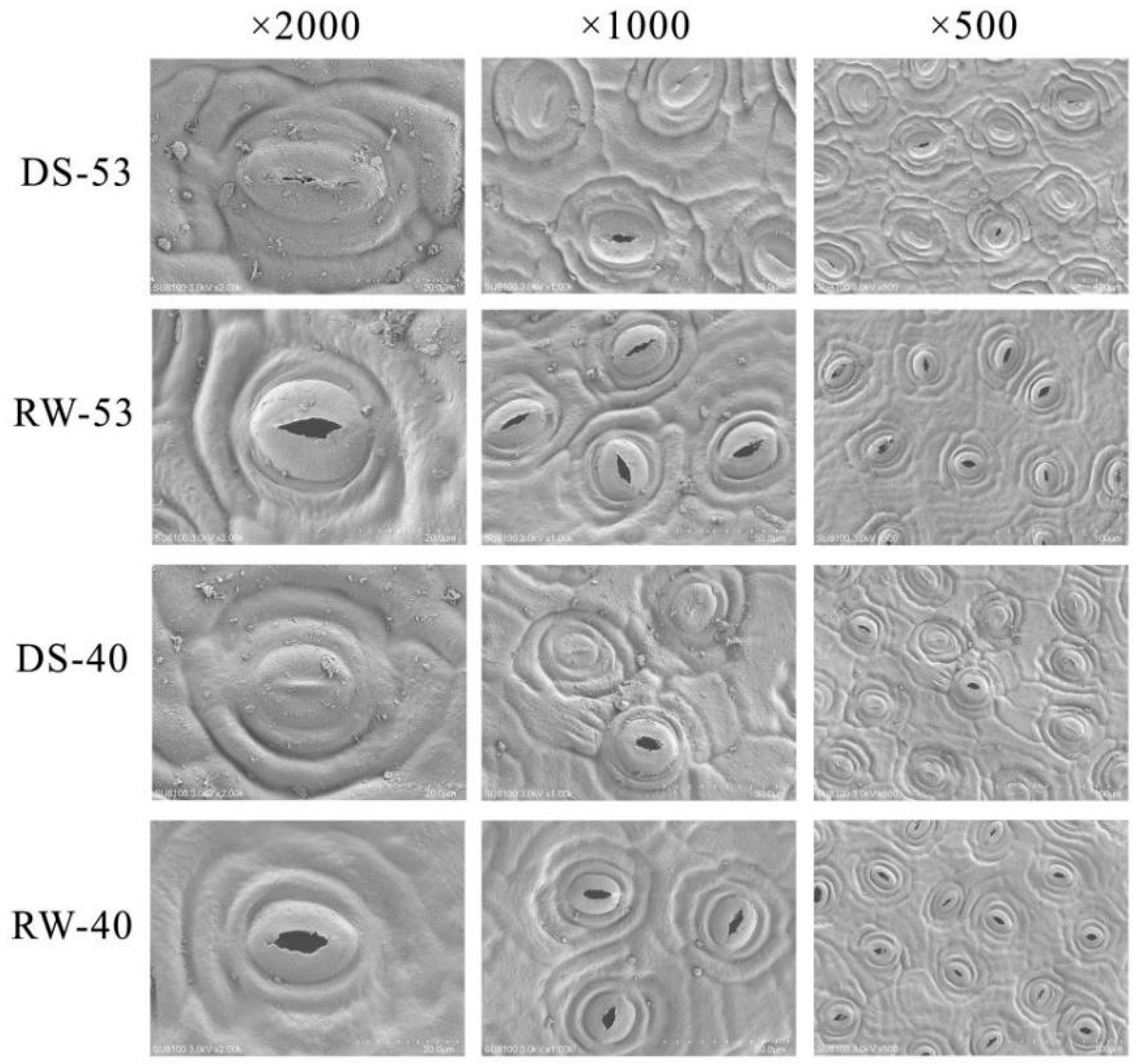
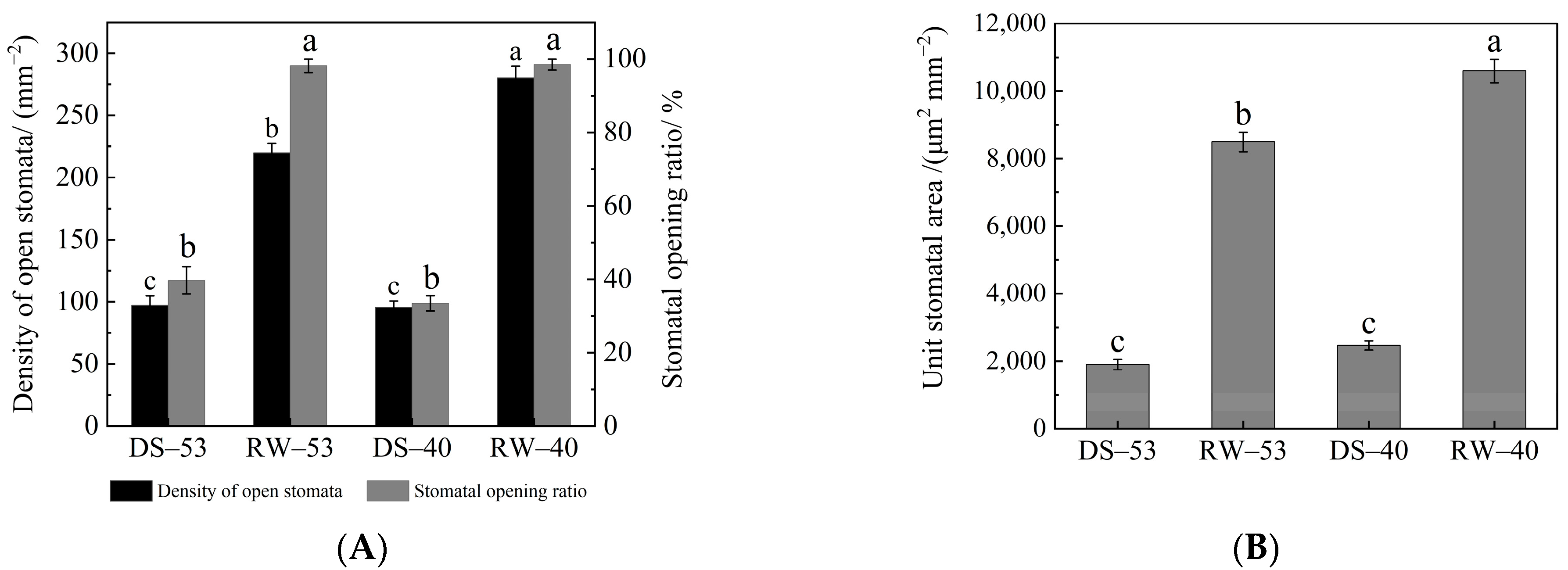
3.3. Effects of Drought and Rehydration on Stomatal Characteristics of C. oleifera
3.4. Effects of Drought and Rewatering on Endogenous Hormones in C. oleifera
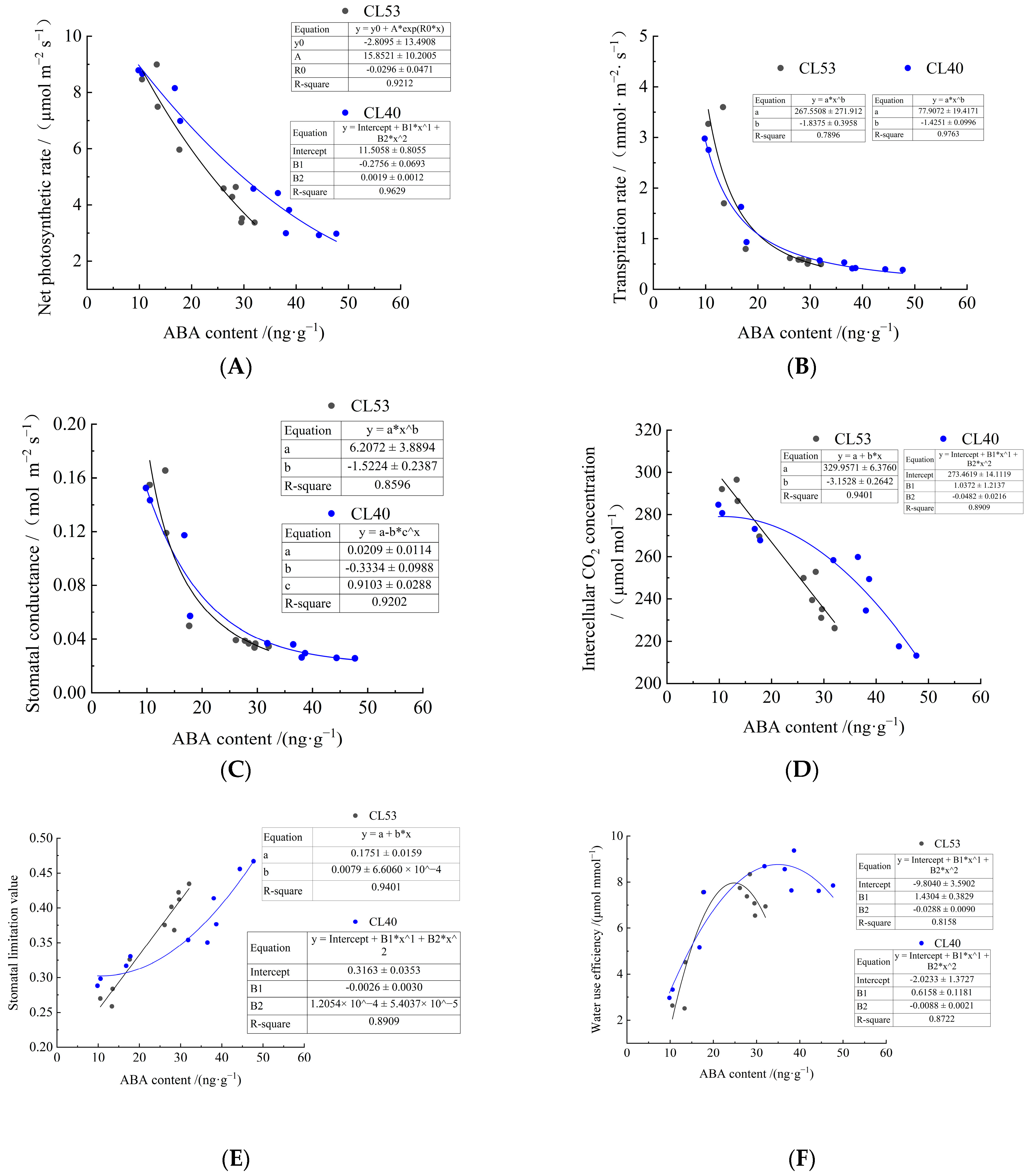
3.5. Correlation Between Photosynthetic Characteristics and ABA Content in C. oleifera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| ANOVA | analysis of variance |
| Ca | ambient CO2 concentration |
| Ci | intercellular CO2 concentration |
| CL53 | Camellia oleifera cultivar ‘CL53′ |
| CL40 | Camellia oleifera cultivar ‘CL40′ |
| DS | drought-stressed treatment |
| FAA | formalin–acetic acid–alcohol fixative |
| GA3 | gibberellin A3 |
| Gs | stomatal conductance |
| LRWC | leaf relative water content |
| Ls | stomatal limitation value |
| PB | phosphate buffer |
| Pn | net photosynthetic rate |
| POD | peroxidase |
| PSII | photosystem II |
| RWC | soil relative water content |
| SA | individual stomatal area |
| SOD | superoxide dismutase |
| SEM | scanning electron microscopy |
| SL | stomatal length |
| SW | stomatal width |
| Tr | transpiration rate |
| WUE | water-use efficiency |
References
- Zhou, Q.; Li, Y.; Wang, X.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Effects of different drought degrees on physiological characteristics and endogenous hormones of soybean. Plants 2022, 11, 2282. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- He, F.; Liu, P.; Wang, L.; Du, L.; Qing, J.; Du, Q.; Du, H. Effect of drought stress and rewatering on physiological characteristics of Eucommia ulmoides seedling. Plant Physiol. J. 2021, 57, 661–671. [Google Scholar]
- Jin, S.; Peng, Z. Changes in response of carbon and water physiological parameters of Robinia pseudoacacia seedlings to long-term drought and re-hydration. J. Beijing For. Univ. 2023, 45, 43–56. [Google Scholar]
- Yu, X.; Wang, J.; Ma, W.; Yi, F.; Zhang, P. Synergy of osmotic adjustment and antioxidant activity in Catalpa bungei: Alleviating persistent drought stress from SL to NSL. Front. Plant Sci. 2025, 16, 1536795. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. Int. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Driesen, E.; De Proft, M.; Saeys, W. Drought stress triggers alterations of adaxial and abaxial stomatal development in basil leaves increasing water-use efficiency. Hortic. Res. 2023, 10, uhad075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef]
- Gao, G.; Feng, Q.; Zhang, X.; Si, J.; Yu, T. An overview of stomatal and non-stomatal limitations to photosynthesis of plants. Arid. Zone Res. 2018, 35, 929–937. [Google Scholar]
- Hu, L.; Wang, Z.; Huang, B. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C perennial grass species. Physiol. Plant. 2010, 139, 93–106. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, Q.; Cao, L.; Guo, H.; Yan, C.; Yuan, T.; Yuan, Y.; Luo, S. Effects of drought stress on photosynthesis of adult Camellia oleifera forest. Nonwood For. Res. 2017, 35, 72–79. [Google Scholar]
- Diao, Z.; Chen, H.; Feng, J.; Liu, W.; Wang, Y. Effects of drought stress on physiological and biochemical characteristics of Camellia oleifera seedlings. J. Anhui Agric. Univ. 2014, 41, 642–646. [Google Scholar]
- Zhao, N.; Lyu, J.; Li, S.; Xu, X.; Li, B.; Zhao, J.; Lu, S. Relationship between photosynthetic characteristics and endogenous abscisic acid content of Robinia pseudoacacia and Platyclus orientalis under different drought treatments. Acta Ecol. Sin. 2024, 44, 2100–2114. [Google Scholar]
- Tan, X.; Yan, C.; Zhong, Q.; Wan, X.; Yuan, Y.; Guo, H.; Cao, L.; Wang, J.; Ge, X.; Wang, J.; et al. Breeding, Promotion and application of improved oil-tea cultivar in China. World For. Res. 2023, 36, 108–113. [Google Scholar]
- Guo, P.R.; Wu, L.L.; Wang, Y.; Liu, D.; Li, J.A. Effects of drought stress on the morphological structure and flower organ physiological characteristics of Camellia oleifera flower buds. Plants 2023, 12, 2585. [Google Scholar] [CrossRef]
- Ding, S.; Zhong, Q.; Yuan, T.; Cao, L.; Yan, C.; Yuan, Y. Effects of endogenous hormones on Camellia oleifera leaves and fruit growth under drought stress. For. Res. 2016, 29, 933–939. [Google Scholar]
- Wang, R.; Zhong, F.; Liao, W.; Li, T. Effects of soil moisture on fruit growth of Camellia oleifera. Sci. Silvae Sin. 2014, 50, 40–46. [Google Scholar]
- Cao, L.; Zhong, Q.; Luo, S.; Yuan, T.; Guo, H.; Yan, C.; Yuan, Y. Variation in leaf structure of Camellia oleifera under drought stress. For. Res. 2018, 31, 136–143. [Google Scholar]
- Ding, S.; Zhong, Q.; Yuan, T.; Cao, L.; Yan, C.; Yuan, Y.; Z, X.; Lin, J. Effects of drought stress on flower bud growth and yield in Camellia oleifera. J. Nanjing For. Univ. (Natl. Sci. Ed.) 2017, 41, 197–202. [Google Scholar]
- Luo, Z.; Wang, H.; Chen, M.; Yuan, J. Effects of drought stress on physiological and biochemical characteristics of four Camellia oleifera varieties seedlings. Nonwood For. Res. 2019, 37, 104–113. [Google Scholar]
- Dong, B.; Hong, W.; Huang, Y.; Xue, L.; Gong, H.; Wei, X.; Lan, L. Physiological responses of four Camellia oleifera clones of Guangxi province in seeding stage under drought stress. J. Cent. South Univ. For. Technol. 2018, 38, 1–8. [Google Scholar]
- Feng, S.; Cheng, H.; Li, Q.; Zhou, Q.; Jia, X.; Ding, C. Physiological responses of three Camellia oleifera in seedling stage under drought stress. Acta Bot. Boreali-Occident. Sin. 2013, 33, 1651–1657. [Google Scholar]
- Yang, D.; Chen, Y.; Wang, R.; He, Y.; Ma, X.; Shen, J.; He, Z.; Lai, H. Effects of exogenous abscisic acid on the physiological and biochemical responses of Camellia oleifera seedlings under drought stress. Plants 2024, 13, 225. [Google Scholar] [CrossRef]
- Cao, R.; Li, Z.; Ouyang, W.; Hu, D.; Zhou, Z.; Su, W.; Chen, X.; Liu, J. Identification of NAC gene in Camellia oleifera and analysis of its response to drought stress. Acta Agric. Univ. Jiangxiensis 2021, 43, 1357–1370. [Google Scholar]
- Zhang, Z.; Xu, Y.; Liu, C.; Chen, L.; Zhang, Y.; He, Z.; Wang, R.; Xun, C.; Ma, Y.; Yuan, X.; et al. Cataloging the genetic response: Unveiling drought-responsive gene expression in oil tea camellia (Camellia oleifera Abel.) through transcriptomics. Life 2024, 14, 989. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Zhang, Z.; Wang, R.; Chen, Y. Integration of mRNA and miRNA analysis reveals the differentially regulatory network in two different Camellia oleifera cultivars under drought stress. Front. Plant Sci. 2022, 13, 1001357. [Google Scholar] [CrossRef]
- Zhang, Q. Studies on the Mechanisms of the Responses of Stomatal Conductance and Photosynthesis to Different Light Environments in Oryza. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Liu, N.; Zhao, H.; Hou, L.; Zhang, C.; Bo, W.; Pang, X.; Li, Y. HPLC-MS/MS-based and transcriptome analysis reveal the effects of ABA and MeJA on jujube (Ziziphus jujuba Mill.) cracking. Food Chem. 2023, 421, 136155. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Daniel, V.C.; Eloy, N.L.; María, J.I.R.; Begoña, B.; Juan, M.R. Enhancing drought tolerance in lettuce: The efficacy of the seaweed-derived biostimulant cytolan® stress applied at different growth stages. Horticulturae 2025, 11, 157. [Google Scholar] [CrossRef]
- Lu, K.; Gu, Y.; Du, Y.; Yao, Y.; Tan, X.; Wu, L.; Zhou, J.; Yuan, J. NAC transcription factors are key regulators of brassinolide-enhanced drought tolerance in Camellia oil tree. BMC Plant biol. 2025, 25, 625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, P.R.; Sheng, S.; Li, J.; Yan, J. Identification of codreb genes for drought and cold tolerance in Camellia oleifera. Int. J. Plant Biol. 2023, 14, 228–241. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, J.; Li, S.; Li, B.; Lv, J.; Gao, X.; Xu, X.; Lu, S. The response of endogenous ABA and soluble sugars of Platycladus orientalis to drought and post-drought rehydration. Biology 2024, 13, 194. [Google Scholar] [CrossRef]
- Yang, F.; Lv, G. Responses of Calligonum leucocladum to prolonged drought stress through antioxidant system activation, soluble sugar accumulation, and maintaining photosynthetic homeostasis. Int. J. Mol. Sci. 2025, 26, 4403. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, G.; Hao, W. Physiological responses and transcriptome analysis of Ammopiptanthus nanus to drought stress. Acta Ecol. Sin. 2025, 45, 854–865. [Google Scholar]
- Chen, X.; Wu, X.; Liu, S.; Hu, X.; Liu, C. Effects of AMF on photosynthetic characteristics and gene expressions of Tea plants under drought stress. Acta Hortic. Sin. 2024, 51, 2358–2370. [Google Scholar]
- Liu, B.; Liang, J.; Tang, G.; Wang, X.; Liu, F.; Zhao, D. Drought stress affects on growth, water use efficiency, gas exchange andchlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019, 250, 230–235. [Google Scholar] [CrossRef]
- Meng, Z.; Song, F.; Liu, Z.; Zhang, F. Effects of drought and re-watering at seedling stage on photosynthesis and chlorophyll fluorescence characteristics in rapeseed. Chin. J. Oil Crop Sci. 2012, 34, 40–47. [Google Scholar]
- Li, S. Study on the Mechanisms Underlying the Effects of Drought on Leaf Photosynthesis and Carbon Dioxide Diffusion Efficiency. Ph.D. Dissertation, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Chen, M.; Li, Y.; Wang, L.; Zhang, L.; Li, D.; Ju, J.; Yu, H. Photosynthetic responses to drought and subsequent re-watering in seedlings from two different provenances of Quercus variabilis Bl. Chin. J. Ecol. 2019, 38, 2950–2958. [Google Scholar]
- You, R.; Deng, X.; Hu, Y.; Ouyang, S.; Chen, L.; Xiang, W. Progress on physiological and ecological responses of trees to drought stress and re-watering. Sci. Silvae Sin. 2023, 59, 124–136. [Google Scholar]
- Chen, M.; Chen, L.; Li, S.; Xie, H.; Song, Y. Effects of drought stress on physiological characteristics and stomatal morphology of different cassava seedlings. J. Sichuan Agric. Univ. 2024, 42, 1049–1056+1084. [Google Scholar]
- Jin, S.; Peng, Z.; Zhang, S. The impact of varying degress of drought stress and re-hydration treatment on the physiological indicators of Robinia pseudoacacia seedlings. J. Northeast. For. Univ. 2024, 52, 27–39. [Google Scholar]
- He, X.; Zhou, W.; Qiu, F.; Gong, C.; Xu, L.; Xiao, X.; Wang, Y. Responses of different Camellia oleifera varieties to drought stress and the comprehensive evaluation of their drought resistance. J. Cent. South Univ. For. Technol. 2023, 43, 1–14. [Google Scholar]
- Zhong, Z.; Zhang, L.; Gao, S.; Peng, T. Leaf cytological characteristics and resistance comparison of four citrus rootstocks under drought stress. Acta Hortic. Sin. 2021, 48, 1579–1588. [Google Scholar]
- Zhou, Y.; Liu, H.; Zhang, S.; Liu, F.; Liu, N. Adaptation strategies of 29 species to sropical soral islands based on leaf anatomical traits. J. Trop. Subtrop. Bot. 2023, 31, 747–756. [Google Scholar]
- Cheng, L. Morphological Difference in Leaves of Various Apple (Malus Domestic) Cultivars and Its Correlation with Water-Use Efficiency Under Drought Stress. Master’s Thesis, Northwest A&F University, Xi’an, China, 2011. [Google Scholar]
- Zhu, B.; Xu, Q.; Ma, S.; Liu, B.; Duan, M.; Wang, L. Effect of potassium deficiency on endogenous hormones, photosynthesis and characteristics of chlorophyll fluorescence in Brassica napus under drought stress. Chin. J. Oil Crop Sci. 2022, 44, 570–580. [Google Scholar]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L.N. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, T.; Li, X.; Song, C.P.; Zhu, J.K.; Chen, L.; Zhao, Y. Phosphorylation of SWEET sucrose transporters regulates plant root:shoot ratio under drought. Nat. Plants 2022, 8, 68–77. [Google Scholar] [CrossRef]
- Hu, J.; Cui, S.; Yan, X.; Xu, H.; Huang, X. Effects of exogenous gibberellic acid on physiological characteristics of Hedera nepalensis var. sinensis under drought stress. J. Yunnan Agric. Univ. (Nat. Sci.) 2024, 39, 110–118. [Google Scholar]
- Liao, Z.; Zhang, Y.; Yu, Q.; Fang, W.; Chen, M.; Li, T.; Liu, Y.; Liu, Z.; Chen, L.; Yu, S.; et al. Coordination of growth and drought responses by GA-ABA signaling in rice. New Phytol. 2023, 240, 1149–1161. [Google Scholar] [CrossRef] [PubMed]

| Variety | Forest Age (a) | Average Base Diameter (cm) | Average Tree Height (cm) | Average Crown Width (cm) |
|---|---|---|---|---|
| CL53 | 7 | 6.2 ± 0.40 | 135.4 ± 11.3 | 162 |
| CL40 | 7 | 5.7 ± 0.35 | 172.8 ± 13.5 | 134 |
| Treatment | Leaf Relative Water Content (%) | ||||
|---|---|---|---|---|---|
| 0 d | 7 d | 14 d | 21 d | 28 d | |
| DS-53 | 76.61 ± 0.64 ab | 75.95 ± 1.02 ab | 77.44 ± 1.11 a | 74.17 ± 1.80 b | 71.43 ± 2.26 b |
| RW-53 | 75.37 ± 1.28 b | 81.69 ± 1.34 a | 81.46 ± 0.75 a | 81.36 ± 1.17 a | 82.48 ± 0.85 a |
| DS-40 | 75.54 ± 0.50 a | 75.86 ± 0.61 a | 74.08 ± 1.24 ab | 72.27 ± 2.11 b | 72.54 ± 1.29 b |
| RW-40 | 75.21 ± 0.66 d | 78.34 ± 1.38 c | 80.19 ± 1.39 bc | 81.24 ± 1.87 b | 83.39 ± 1.00 a |
| Variety | Stomatal Density (mm−2) | Stomatal Apparatus Length (μm) | Stomatal Aperture Width (μm) | Stomatal Apparatus Area (μm2) |
|---|---|---|---|---|
| CL53 | 230.37 ± 11.94 b | 24.25 ± 1.39 a | 20.19 ± 1.49 a | 383.97 ± 32.47 a |
| CL40 | 285.37 ± 12.23 a | 21.87 ± 1.06 b | 18.60 ± 1.88 b | 319.91 ± 40.43 b |
| Treatment | ABA Content/(ng·g−1) | ||||
|---|---|---|---|---|---|
| 0 d | 7 d | 14 d | 21 d | 28 d | |
| DS-53 | 26.12 ± 0.73 d | 27.77 ± 0.52 c | 29.65 ± 1.21 b | 29.50 ± 0.49 b | 32.06 ± 1.01 a |
| RW-53 | 28.45 ± 0.89 a | 17.66 ± 0.46 b | 13.51 ± 0.90 c | 10.51 ± 0.51 d | 13.33 ± 0.55 c |
| DS-40 | 36.51 ± 0.84 d | 38.65 ± 0.30 c | 38.04 ± 1.09 c | 44.36 ± 1.02 b | 47.70 ± 0.69 a |
| RW-40 | 31.83 ± 0.36 a | 17.82 ± 0.72 b | 16.79 ± 0.73 c | 10.57 ± 0.58 d | 9.83 ± 0.17 d |
| Treatment | GA3 Content/(ng·g−1) | ||||
|---|---|---|---|---|---|
| 0 d | 7 d | 14 d | 21 d | 28 d | |
| DS-53 | 2.96 ± 0.12 a | 2.79 ± 0.15 a | 2.68 ± 0.13 ab | 2.66 ± 0.13 ab | 2.42 ± 0.28 b |
| RW-53 | 2.58 ± 0.11 c | 3.39 ± 0.16 b | 3.98 ± 0.08 a | 3.80 ± 0.19 a | 3.89 ± 0.36 a |
| DS-40 | 6.77 ± 0.31 a | 6.58 ± 0.30 a | 6.49 ± 0.24 a | 6.50 ± 0.29 a | 5.91 ± 0.34 b |
| RW-40 | 6.42 ± 0.25 b | 6.45 ± 0.24 b | 7.15 ± 0.27 a | 7.43 ± 0.18 a | 7.51 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Yan, C.; He, T.; Zhong, Q.; Yuan, Y.; Cao, L. Coordinated Regulation of Photosynthesis, Stomatal Traits, and Hormonal Dynamics in Camellia oleifera During Drought and Rehydration. Biology 2025, 14, 965. https://doi.org/10.3390/biology14080965
Cao L, Yan C, He T, Zhong Q, Yuan Y, Cao L. Coordinated Regulation of Photosynthesis, Stomatal Traits, and Hormonal Dynamics in Camellia oleifera During Drought and Rehydration. Biology. 2025; 14(8):965. https://doi.org/10.3390/biology14080965
Chicago/Turabian StyleCao, Linqing, Chao Yan, Tieding He, Qiuping Zhong, Yaqi Yuan, and Lixian Cao. 2025. "Coordinated Regulation of Photosynthesis, Stomatal Traits, and Hormonal Dynamics in Camellia oleifera During Drought and Rehydration" Biology 14, no. 8: 965. https://doi.org/10.3390/biology14080965
APA StyleCao, L., Yan, C., He, T., Zhong, Q., Yuan, Y., & Cao, L. (2025). Coordinated Regulation of Photosynthesis, Stomatal Traits, and Hormonal Dynamics in Camellia oleifera During Drought and Rehydration. Biology, 14(8), 965. https://doi.org/10.3390/biology14080965






