Zebrafish as a Model Organism for Post-Traumatic Stress Disorder: Insights into Stress Mechanisms and Behavioral Assays
Simple Summary
Abstract
1. Introduction
2. (Patho)physiology of Stress Response in Zebrafish
3. Environmental and Developmental Factors Affecting Stress Response
3.1. Abiotic Stress-Induced Factors: Light Cycle and Circadian Rhythm
3.2. Abiotic Stress-Induced Factors: Thermal Stressing
3.3. Age-Related Differences in Stress Responses
3.4. Sex Differences in Stress Response in Zebrafish
3.5. Stress Coping Styles
3.6. Environmental Enrichment and Physical Exercise
3.7. Feeding Regimens
3.8. Social Interactions
3.9. Learning Capabilities
4. Standard Tests Used to Measure Stress Response in Zebrafish Studies
4.1. Behavioral Assessment
4.2. Biochemical and Molecular-Genetic Assays
4.2.1. Hypothalamic–Pituitary–Intervertebral Axis Assessment
4.2.2. Neurotransmitters
4.3. Advances in Optogenetics and Real Time Neuroimaging
5. Zebrafish Models for PTSD Research
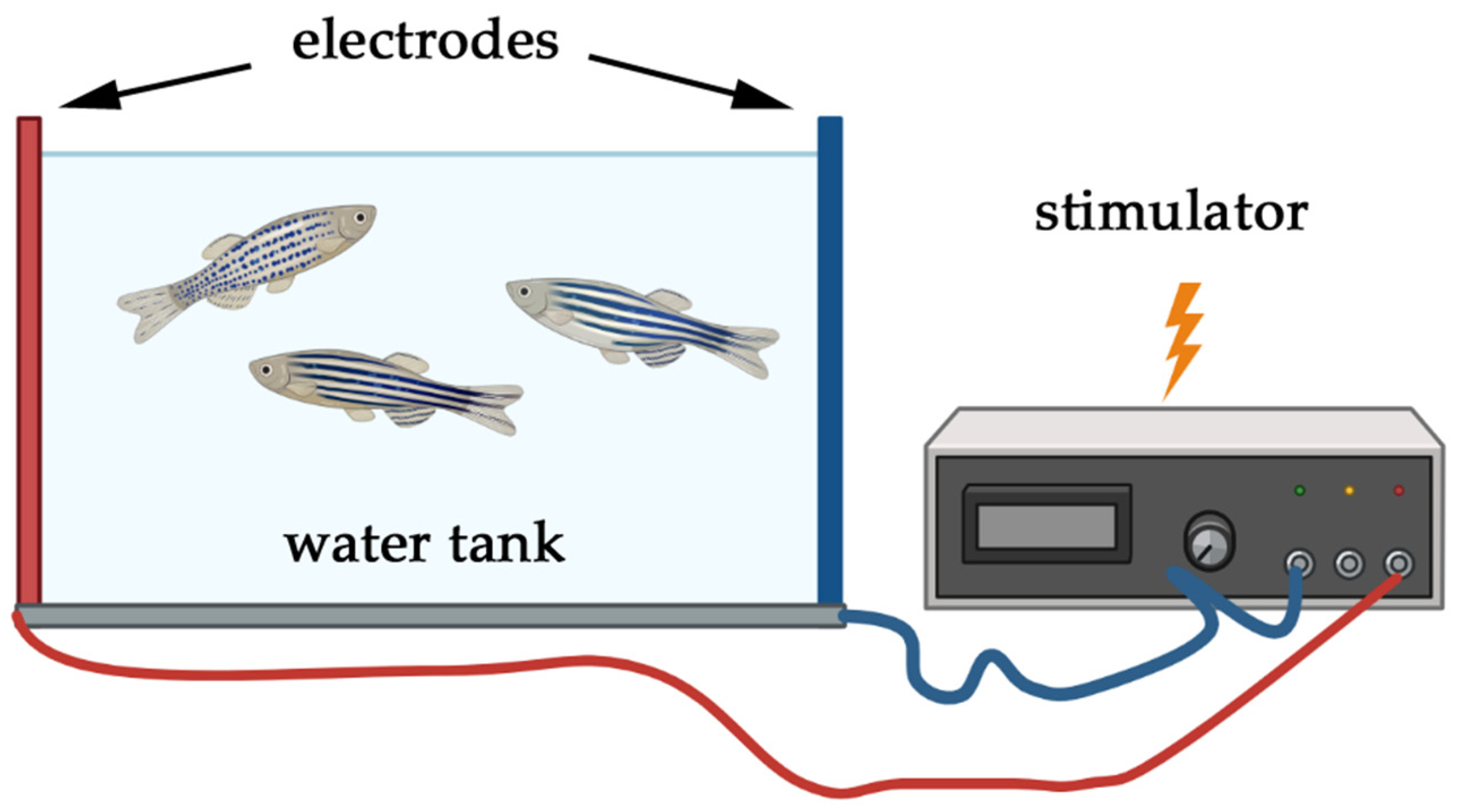
5.1. Electric Shock Models
5.1.1. Application of Electric Shock
5.1.2. Relevance of Electric Shock Models to PTSD
5.2. Immobilization Stress Models
5.2.1. Application of Immobilization Stress
5.2.2. Immobilization Stress and PTSD
5.3. Confinement Stress Models
5.3.1. Application of Confinement Stress
5.3.2. Confinement Stress and PTSD
5.4. Exposure to Acute and Prolonged Stressors
5.4.1. Application of Stressors
5.4.2. Acute Stress and PTSD
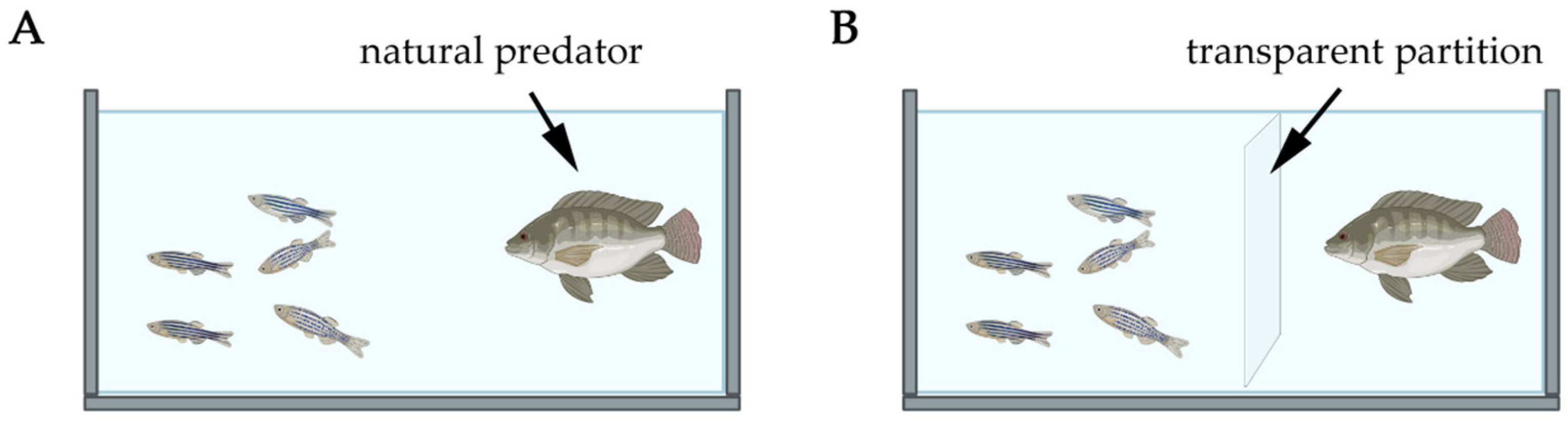
6. Predator-Based Models in Zebrafish PTSD Research
6.1. Behavioral and Hormonal Responses Induced by Predator Exposure
6.2. Influence of Predator Type on Zebrafish Responses
6.3. Artificial Predator Models: Looming Dot Stimulus (LDS)
6.4. Conspecific Alarm Substance (CAS) as a Predator-Based PTSD Model
6.5. Relevance and Applications to PTSD Research
7. Complex Stress Models for PTSD in Zebrafish
7.1. Chronic Unpredictable Stress (CUS/UCS)
7.2. Time-Dependent Sensitization (TDS) as a Distinct Method or Phenomenon
8. Early Life Interventions
8.1. Environmental and Mechanical Stressors
8.2. Pharmacological Stressors and Epigenetic Programming
8.3. Considerations in Modeling and Paradigm Classification
9. Distinct Features of Zebrafish Compared to Rodents
9.1. Color
9.2. Shoal Cohesion
9.3. Neural Injury Response in Zebrafish and Its Implications for PTSD Modeling
10. Limitations of Zebrafish Models in PTSD Research
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| ACTH | Adrenocorticotropic Hormone |
| BDNF | Brain-Derived Neurotrophic Factor |
| CAS | Conspecific Alarm Substance |
| CRH | Corticotropin-Releasing Hormone |
| CUS/UCS | Chronic Unpredictable Test |
| DA | Dopamine |
| dpf | days post-fertilization |
| EE | Environmental Enrichment |
| GABA | Gamma-Aminobutyric Acid |
| GC | Glucocorticoid |
| HPA | Hypothalamic–Pituitary–Adrenal (axis) |
| HPI | Hypothalamic–Pituitary–Interrenal (axis) |
| LD | Light–Dark (cycle) |
| LDS | Looming Dot Stimulus |
| LDT | Light–Dark Tank Test |
| NA | Noradrenaline |
| NPO | Neurosecretory Preoptic (area) |
| NTT | Novel Tank Test |
| PTSD | Post-Traumatic Stress Disorder |
| PVN | Paraventricular Nucleus |
| TDS | Time-Dependent Sensitization |
References
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, M.S.; Demin, K.A.; Giacomini, A.C.V.V.; Amstislavskaya, T.G.; Strekalova, T.; Maslov, G.O.; Kositsin, Y.; Petersen, E.V.; Kalueff, A.V. Understanding how stress responses and stress-related behaviors have evolved in zebrafish and mammals. Neurobiol. Stress. 2021, 15, 100405. [Google Scholar] [CrossRef] [PubMed]
- Theodoridi, A.; Dinarello, A.; Badenetti, L.; Pavlidis, M.; Dalla Valle, L.; Tsalafouta, A. Knockout of the hsd11b2 Gene Extends the Cortisol Stress Response in Both Zebrafish Larvae and Adults. Int. J. Mol. Sci. 2021, 22, 12525. [Google Scholar] [CrossRef]
- Al-Zoubi, R.M.; Abu-Hijleh, H.; Zarour, A.; Zakaria, Z.Z.; Yassin, A.; Al-Ansari, A.A.; Al-Asmakh, M.; Bawadi, H. Zebrafish Model in Illuminating the Complexities of Post-Traumatic Stress Disorders: A Unique Research Tool. Int. J. Mol. Sci. 2024, 25, 4895. [Google Scholar] [CrossRef]
- Caramillo, E.M.; Khan, K.M.; Collier, A.D.; Echevarria, D.J. Modeling PTSD in the zebrafish: Are we there yet? Behav. Brain Res. 2015, 276, 151–160. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Wang, D.; Hu, G.; Liu, Z.; Yan, D.; Serikuly, N.; Alpyshov, E.T.; Demin, K.A.; Strekalova, T.; et al. Delayed behavioral and genomic responses to acute combined stress in zebrafish, potentially relevant to PTSD and other stress-related disorders: Focus on neuroglia, neuroinflammation, apoptosis and epigenetic modulation. Behav. Brain Res. 2020, 389, 112644. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Klee, E.W.; Schneider, H.; Clark, K.J.; Cousin, M.A.; Ebbert, J.O.; Hooten, W.M.; Karpyak, V.M.; Warner, D.O.; Ekker, S.C. Zebrafish: A model for the study of addiction genetics. Hum. Genet. 2012, 131, 977–1008. [Google Scholar] [CrossRef]
- Niederriter, A.R.; Davis, E.E.; Golzio, C.; Oh, E.C.; Tsai, I.C.; Katsanis, N. In vivo modeling of the morbid human genome using Danio rerio. J. Vis. Exp. 2013, 78, e50338. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Fang, Y.; Ma, J.; Wang, J.; Qu, L.; Yang, Q.; Wu, W.; Jin, L.; Sun, D. Advances in Zebrafish as a Comprehensive Model of Mental Disorders. Depress. Anxiety 2023, 2023, 6663141. [Google Scholar] [CrossRef]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. Part C Embryo Today 2013, 99, 14–23. [Google Scholar] [CrossRef]
- Hasani, H.; Sun, J.; Zhu, S.I.; Rong, Q.; Willomitzer, F.; Amor, R.; McConnell, G.; Cossairt, O.; Goodhill, G.J. Whole-brain imaging of freely-moving zebrafish. Front. Neurosci. 2023, 17, 1127574. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.M.; Ang, R.J.W.; Wee, C.L. Larval Zebrafish as a Model for Mechanistic Discovery in Mental Health. Front. Mol. Neurosci. 2022, 15, 900213. [Google Scholar] [CrossRef]
- Tea, J.; Alderman, S.L.; Gilmour, K.M. Social stress increases plasma cortisol and reduces forebrain cell proliferation in subordinate male zebrafish (Danio rerio). J. Exp. Biol. 2019, 222 Pt 2, jeb194894. [Google Scholar] [CrossRef]
- Golla, A.; Østby, H.; Kermen, F. Chronic unpredictable stress induces anxiety-like behaviors in young zebrafish. Sci. Rep. 2020, 10, 10339. [Google Scholar] [CrossRef]
- Clark, K.J.; Boczek, N.J.; Ekker, S.C. Stressing zebrafish for behavioral genetics. Rev. Neurosci. 2011, 22, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Hou, T.; Sun, T.; Zhu, L.; Zhang, S.; Tang, K.; Wang, Z. Starvation stress affects the maternal development and larval fitness in zebrafish (Danio rerio). Sci. Total Environ. 2019, 695, 133897. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.G.; Silva, R.X.; Silva Sde, N.; Rodrigues Ldo, S.; Oliveira, K.R.; Batista Ede, J.; Maximino, C.; Herculano, A.M. Time-dependent sensitization of stress responses in zebrafish: A putative model for post-traumatic stress disorder. Behav. Process. 2016, 128, 70–82. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Gusev, E.; Hu, D.; Komelkova, M. Experimental PTSD Models in Zebrafish: A Systematic Review of Behavioral, Neurochemical, and Molecular Outcomes. Biology 2025, 14, 456. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Castillo-Ramírez, L.A.; Herget, U.; Ryu, S.; De Marco, R.J. Early-life challenge enhances cortisol regulation in zebrafish larvae. Biol. Open 2024, 13, bio061684. [Google Scholar] [CrossRef]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 1–27. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, F.J.; López-Olmeda, J.F.; Vera, L.M.; Migaud, H.; López-Patiño, M.A.; Míguez, J.M. Environmental Cycles, Melatonin, and Circadian Control of Stress Response in Fish. Front. Endocrinol. 2019, 10, 279. [Google Scholar] [CrossRef]
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Vijayan, M.M. Maternal stress and fish reproduction: The role of cortisol revisited. Fish Fish. 2018, 19, 1016–1030. [Google Scholar] [CrossRef]
- Carbajal, A.; Tallo-Parra, O.; Monclús, L.; Vinyoles, D.; Solé, M.; Lacorte, S.; Lopez-Bejar, M. Variation in scale cortisol concentrations of a wild freshwater fish: Habitat quality or seasonal influences? Gen. Comp. Endocrinol. 2019, 275, 44–50. [Google Scholar] [CrossRef]
- Ghazal, A.; Paul, R.; Tarkan, A.S.; Britton, J.R. Influence of season, capture method, sample age and extraction protocols on the scale cortisol concentrations of three species of freshwater fish. Gen. Comp. Endocrinol. 2025, 362, 114671. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.J.; Kim, S.Y.; Kim, H.B.; Baek, H.J. Changes in Plasma Sex Steroid and Cortisol Levels during Annual Reproductive Cycle of Ribbed Gunnel, Dictyosoma burgeri. Dev. Reprod. 2012, 16, 279–287. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Coordinated Action of Corticotropin-Releasing Hormone and Cortisol Shapes the Acute Stress-Induced Behavioural Response in Zebrafish. Neuroendocrinology 2022, 112, 74–87. [Google Scholar] [CrossRef]
- Castillo-Ramírez, L.A.; Ryu, S.; De Marco, R.J. Cortisol dynamics and GR-dependent feedback regulation in zebrafish larvae exposed to repeated stress. Biol. Open 2024, 13, bio061683. [Google Scholar] [CrossRef]
- Eto, K.; Mazilu-Brown, J.K.; Henderson-MacLennan, N.; Dipple, K.M.; McCabe, E.R. Development of catecholamine and cortisol stress responses in zebrafish. Mol. Genet. Metab. Rep. 2014, 1, 373–377. [Google Scholar] [CrossRef]
- Pavlidis, M.; Theodoridi, A.; Tsalafouta, A. Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 121–131. [Google Scholar] [CrossRef]
- Fanouraki, E.; Mylonas, C.C.; Papandroulakis, N.; Pavlidis, M. Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen. Comp. Endocrinol. 2011, 173, 313–322. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Vieira, R.S.F.; Venâncio, C.; Félix, L. Cortisol Quantification for Assessing Stress-Induced Changes in Zebrafish Larvae. Methods Mol. Biol. 2024, 2753, 483–493. [Google Scholar] [CrossRef]
- Young, E.A.; Breslau, N. Cortisol and catecholamines in posttraumatic stress disorder: An epidemiologic community study. Arch. Gen. Psychiatry 2004, 61, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Cay, M.; Ucar, C.; Senol, D.; Cevirgen, F.; Ozbag, D.; Altay, Z.; Yildiz, S. Effect of increase in cortisol level due to stress in healthy young individuals on dynamic and static balance scores. North. Clin. Istanb. 2018, 5, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Fuzzen, M.L.; Van Der Kraak, G.; Bernier, N.J. Stirring up new ideas about the regulation of the hypothalamic-pituitary-interrenal axis in zebrafish (Danio rerio). Zebrafish 2010, 7, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, J.; Eachus, H.; Lityagina, O.; Ryu, S. Optogenetic induction of chronic glucocorticoid exposure in early-life leads to blunted stress-response in larval zebrafish. Eur. J. Neurosci. 2024, 59, 3134–3146. [Google Scholar] [CrossRef]
- Wilson, K.S.; Matrone, G.; Livingstone, D.E.; Al-Dujaili, E.A.; Mullins, J.J.; Tucker, C.S.; Hadoke, P.W.; Kenyon, C.J.; Denvir, M.A. Physiological roles of glucocorticoids during early embryonic development of the zebrafish (Danio rerio). J. Physiol. 2013, 591, 6209–6220. [Google Scholar] [CrossRef]
- Wilson, K.S.; Baily, J.; Tucker, C.S.; Matrone, G.; Vass, S.; Moran, C.; Chapman, K.E.; Mullins, J.J.; Kenyon, C.; Hadoke, P.W.; et al. Early-life perturbations in glucocorticoid activity impacts on the structure, function and molecular composition of the adult zebrafish (Danio rerio) heart. Mol. Cell. Endocrinol. 2015, 414, 120–131. [Google Scholar] [CrossRef]
- Dinarello, A.; Licciardello, G.; Fontana, C.M.; Tiso, N.; Argenton, F.; Dalla Valle, L. Glucocorticoid receptor activities in the zebrafish model: A review. J. Endocrinol. 2020, 247, R63–R82. [Google Scholar] [CrossRef]
- Henríquez Martínez, A.; Ávila, L.C.; Pulido, M.A.; Ardila, Y.A.; Akle, V.; Bloch, N.I. Age-Dependent Effects of Chronic Stress on Zebrafish Behavior and Regeneration. Front. Physiol. 2022, 13, 856778. [Google Scholar] [CrossRef]
- Yeramilli, V.; Rizek, C.S.; Graham, J.; Taylor, C.; Cheddadi, R.; Patterson, S.; Watts, S.; Martin, C. Parental preconception stress in zebrafish induces long-lasting anxiety in offspring. Physiol. Behav. 2024, 277, 114477. [Google Scholar] [CrossRef] [PubMed]
- Collymore, C.; Tolwani, R.J.; Rasmussen, S. The Behavioral Effects of Single Housing and Environmental Enrichment on Adult Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 280–285. [Google Scholar] [PubMed]
- Stevens, C.H.; Reed, B.T.; Hawkins, P. Enrichment for Laboratory Zebrafish-A Review of the Evidence and the Challenges. Animals 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Roques, J.A.C.; Aliti, G.M.; Ademar, K.; Sundh, H.; Sundell, K.; Ericson, M.; Kettunen, P. Low Holding Densities Increase Stress Response and Aggression in Zebrafish. Biology 2022, 11, 725. [Google Scholar] [CrossRef]
- Shishis, S.; Tsang, B.; Gerlai, R. The effect of fish density and tank size on the behavior of adult zebrafish: A systematic analysis. Front. Behav. Neurosci. 2022, 16, 934809. [Google Scholar] [CrossRef]
- Sen Sarma, O.; Frymus, N.; Axling, F.; Thörnqvist, P.O.; Roman, E.; Winberg, S. Optimizing zebrafish rearing-Effects of fish density and environmental enrichment. Front. Behav. Neurosci. 2023, 17, 1204021. [Google Scholar] [CrossRef]
- Schlegel, A. Zebrafish Models for Dyslipidemia and Atherosclerosis Research. Front. Endocrinol. 2016, 7, 159. [Google Scholar] [CrossRef]
- Meguro, S.; Hosoi, S.; Hasumura, T. High-fat diet impairs cognitive function of zebrafish. Sci. Rep. 2019, 9, 17063. [Google Scholar] [CrossRef]
- Wu, M.M.; Zhang, H.; Yang, Y.; Wang, Y.; Luk, P.K.; Xia, I.F.; Wong, K.H.; Kwok, K.W. Food emulsifiers aggravate inflammation and oxidative stress induced by food contaminants in zebrafish. Food Chem. Toxicol. 2024, 191, 114850. [Google Scholar] [CrossRef]
- Giacomini, A.C.; de Abreu, M.S.; Koakoski, G.; Idalêncio, R.; Kalichak, F.; Oliveira, T.A.; da Rosa, J.G.; Gusso, D.; Piato, A.L.; Barcellos, L.J. My stress, our stress: Blunted cortisol response to stress in isolated housed zebrafish. Physiol. Behav. 2015, 139, 182–187. [Google Scholar] [CrossRef]
- Shams, S.; Khan, A.; Gerlai, R. Early social deprivation does not affect cortisol response to acute and chronic stress in zebrafish. Stress. 2021, 24, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, V.; Frigato, E.; López-Olmeda, J.F.; Sánchez-Vázquez, F.J.; Bertolucci, C. The Light Wavelength Affects the Ontogeny of Clock Gene Expression and Activity Rhythms in Zebrafish Larvae. PLoS ONE 2015, 10, e0132235. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vives, B.; Aliaga-Guerrero, M.; Cañavate, J.P.; García-Mateos, G.; Martín-Robles, A.J.; Herrera-Pérez, P.; Muñoz-Cueto, J.A.; Sánchez-Vázquez, F.J. Metamorphosis induces a light-dependent switch in Senegalese sole (Solea senegalensis) from diurnal to nocturnal behavior. J. Biol. Rhythm. 2012, 27, 135–144. [Google Scholar] [CrossRef]
- Liu, S.T.; Chang, C.Y.; Lee, K.Y.; Tong, S.K.; Huang, H.L.; Chen, H.; Horng, J.L.; Chou, M.Y. Alternation of social behaviors for zebrafish (Danio rerio) in response to acute cold stress. Fish Physiol. Biochem. 2024, 50, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.M.; Hurd, M.W.; Batchelor, M.M. Circadian rhythmicity in the locomotor activity of larval zebrafish. NeuroReport 1998, 9, 3445–3449. [Google Scholar] [CrossRef]
- Wong, R.Y.; French, J.; Russ, J.B. Differences in stress reactivity between zebrafish with alternative stress coping styles. R. Soc. Open Sci. 2019, 6, 181797. [Google Scholar] [CrossRef]
- Baker, M.R.; Wong, R.Y. Contextual fear learning and memory differ between stress coping styles in zebrafish. Sci. Rep. 2019, 9, 9935. [Google Scholar] [CrossRef]
- Hurd, M.W.; Cahill, G.M. Entraining signals initiate behavioral circadian rhythmicity in larval zebrafish. J. Biol. Rhythm. 2002, 17, 307–314. [Google Scholar] [CrossRef]
- Kopp, R.; Legler, J.; Legradi, J. Alterations in locomotor activity of feeding zebrafish larvae as a consequence of exposure to different environmental factors. Environ. Sci. Pollut. Res. Int. 2018, 25, 4085–4093. [Google Scholar] [CrossRef]
- Mracek, P.; Pagano, C.; Fröhlich, N.; Idda, M.L.; Cuesta, I.H.; Lopez-Olmeda, J.F.; Sánchez-Vázquez, F.J.; Vallone, D.; Foulkes, N.S. ERK Signaling Regulates Light-Induced Gene Expression via D-Box Enhancers in a Differential, Wavelength-Dependent Manner. PLoS ONE 2013, 8, e67858. [Google Scholar] [CrossRef]
- Lunkes, L.C.; Paiva, I.M.; Egger, R.C.; Braga, W.F.; Alvarez-Leite, J.I.; da Cunha Barreto-Vianna, A.R.; Murgas, L.D.S. Melatonin administration attenuates acute stress by inducing sleep state in zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 246, 109044. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.B.; Medeiros, B.Z.; da Silva Lemos, I.; da Silva, G.L.; Alano, C.G.; Dondossola, E.R.; Torres, C.A.; Effting, P.S.; Rico, E.P.; Streck, E.L. Melatonin improves behavioral parameters and oxidative stress in zebrafish submitted to a leucine-induced MSUD protocol. Metab. Brain Dis. 2023, 38, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Taranov, A.S.; Ilyin, N.P.; Lakstygal, A.M.; Volgin, A.D.; de Abreu, M.S.; Strekalova, T.; Kalueff, A.V. Understanding neurobehavioral effects of acute and chronic stress in zebrafish. Stress 2021, 24, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.V.; Izvekov, E.I.; Pavlova, V.V.; Pankova, N.A.; Osipova, E.A. Circadian rhythms in zebrafish (Danio rerio) behaviour and the sources of their variability. Biol. Rev. Camb. Philos. Soc. 2021, 96, 785–797. [Google Scholar] [CrossRef]
- Tan, Y.; DeBruyne, J.; Cahill, G.M.; Wells, D.E. Identification of a mutation in the Clock1 gene affecting zebrafish circadian rhythms. J. Neurogenet. 2008, 22, 149–166. [Google Scholar] [CrossRef]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Guerra-Librero, A.; Rodríguez-Santana, C.; Escames, G.; Acuña-Castroviejo, D. The Zebrafish, an Outstanding Model for Biomedical Research in the Field of Melatonin and Human Diseases. Int. J. Mol. Sci. 2022, 23, 7438. [Google Scholar] [CrossRef]
- Feugere, L.; Bates, A.; Emagbetere, T.; Chapman, E.; Malcolm, L.E.; Bulmer, K.; Hardege, J.; Beltran-Alvarez, P.; Wollenberg Valero, K.C. Heat induces multiomic and phenotypic stress propagation in zebrafish embryos. PNAS Nexus 2023, 2, pgad137. [Google Scholar] [CrossRef]
- Rajeswari, J.J.; Gilbert, G.N.Y.; Khalid, E.; Vijayan, M.M. Brain monoamine changes modulate the corticotropin-releasing hormone receptor 1-mediated behavioural response to acute thermal stress in zebrafish larvae. Mol. Cell. Endocrinol. 2025, 600, 112494. [Google Scholar] [CrossRef] [PubMed]
- Feugere, L.; Scott, V.F.; Rodriguez-Barucg, Q.; Beltran-Alvarez, P.; Wollenberg Valero, K.C. Thermal stress induces a positive phenotypic and molecular feedback loop in zebrafish embryos. J. Therm. Biol. 2021, 102, 103114. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.C.; Wu, Y.H.; Ho, T.N.; Huang, Y.Y.; Hsu, T. Heat stress modulates nucleotide excision repair capacity in zebrafish (Danio rerio) early and mid-early embryos via distinct mechanisms. Chemosphere 2020, 238, 124653. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.V.; Sihite, A.C.; Hsu, T. Susceptibility of DNA damage recognition activities linked to nucleotide excision and mismatch repair in zebrafish (Danio rerio) early and mid-early embryos to 2.5 to 4.5 °C heat stress. Fish Physiol. Biochem. 2023, 49, 515–527. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Lei, Y.; Liang, Q.; Wang, Y.; Zheng, Y.; Gan, X.; Bai, C.; Chen, J. Cold stress during the perinatal period leads to developmental and neurobehavioral toxicity in zebrafish larvae. Neurotoxicol. Teratol. 2023, 96, 107164. [Google Scholar] [CrossRef]
- Hung, I.C.; Hsiao, Y.C.; Sun, H.S.; Chen, T.M.; Lee, S.J. MicroRNAs regulate gene plasticity during cold shock in zebrafish larvae. BMC Genom. 2016, 17, 922. [Google Scholar] [CrossRef]
- Ren, J.; Long, Y.; Liu, R.; Song, G.; Li, Q.; Cui, Z. Characterization of Biological Pathways Regulating Acute Cold Resistance of Zebrafish. Int. J. Mol. Sci. 2021, 22, 3028. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Z.; Zhong, S.; Cui, Z. Cloning of down-regulated genes under cold stress and identification of important genes related to cold tolerance in zebrafish (Danio rerio). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 298, 111739. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Song, G.; Zhou, T.; Long, Y.; Li, Q.; Zhong, S.; Cui, Z. Deficiency in the membrane protein Tmbim3a/Grinaa initiates cold-induced ER stress and cell death by activating an intrinsic apoptotic pathway in zebrafish. J. Biol. Chem. 2019, 294, 11445–11457. [Google Scholar] [CrossRef]
- Liu, R.; Long, Y.; Liu, R.; Song, G.; Li, Q.; Yan, H.; Cui, Z. Understanding the Function and Mechanism of Zebrafish Tmem39b in Regulating Cold Resistance. Int. J. Mol. Sci. 2022, 23, 11442. [Google Scholar] [CrossRef]
- Long, Y.; Yan, J.; Song, G.; Li, X.; Li, X.; Li, Q.; Cui, Z. Transcriptional events co-regulated by hypoxia and cold stresses in Zebrafish larvae. BMC Genom. 2015, 16, 385. [Google Scholar] [CrossRef]
- Wu, S.M.; Liu, J.H.; Shu, L.H.; Chen, C.H. Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 202–213. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.M.; Wang, W.B.; Wang, G.; Li, Y. Transcriptome analysis reveals the early resistance of zebrafish larvae to oxidative stress. Fish Physiol. Biochem. 2022, 48, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, S.; Liu, Y.; Chu, P.; Han, B.; Ning, X.; Wang, T.; Yin, S. Acute cold stress leads to zebrafish ovarian dysfunction by regulating miRNA and mRNA. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101139. [Google Scholar] [CrossRef]
- Ge, G.; Long, Y.; Song, G.; Li, Q.; Cui, Z.; Yan, H. Transcriptomic Profiling Revealed Signaling Pathways Associated with the Spawning of Female Zebrafish under Cold Stress. Int. J. Mol. Sci. 2022, 23, 7494. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Li, Z.Q.; Bu, M.D.; Li, J.Z.; Chen, L.B. Metabolomic-based analysis reveals bile acid-mediated ovarian failure induced by low temperature in zebrafish. Zool. Res. 2024, 45, 791–804. [Google Scholar] [CrossRef]
- Kishi, S.; Slack, B.E.; Uchiyama, J.; Zhdanova, I.V. Zebrafish as a genetic model in biological and behavioral gerontology: Where development meets aging in vertebrates—A mini-review. Gerontology 2009, 55, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.O.; Gordillo-Martinez, F.; Ramos, A.; Lau, I.H. Effects of Noise Exposure and Ageing on Anxiety and Social Behaviour in Zebrafish. Biology 2023, 12, 1165. [Google Scholar] [CrossRef]
- Evans, J.R.; Torres-Pérez, J.V.; Miletto Petrazzini, M.E.; Riley, R.; Brennan, C.H. Stress reactivity elicits a tissue-specific reduction in telomere length in aging zebrafish (Danio rerio). Sci. Rep. 2021, 11, 339. [Google Scholar] [CrossRef]
- Hudock, J.; Kenney, J.W. Aging in zebrafish is associated with reduced locomotor activity and strain dependent changes in bottom dwelling and thigmotaxis. PLoS ONE 2024, 19, e0300227. [Google Scholar] [CrossRef]
- Coimbra, B.M.; Carvalho, C.M.; Moretti, P.N.; Mello, M.F.; Belangero, S.I. Stress-related telomere length in children: A systematic review. J. Psychiatr. Res. 2017, 92, 47–54. [Google Scholar] [CrossRef]
- Ruhl, T.; Jonas, A.; Seidel, N.I.; Prinz, N.; Albayram, O.; Bilkei-Gorzo, A.; von der Emde, G. Oxidation and Cognitive Impairment in the Aging Zebrafish. Gerontology 2015, 62, 47–57. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.-Y.; Shen, Y.-J.; Chen, Q.-L.; Liu, Z.-H. Endogenous 17β-estradiol regulates sexually dimorphic anxiety responses in zebrafish via the HPI axis and 5-HT/DA pathways. Front. Mar. Sci. 2025, 12, 1541351. [Google Scholar] [CrossRef]
- Cantabella, E.; Camilleri, V.; Cavalie, I.; Dubourg, N.; Gagnaire, B.; Charlier, T.D.; Adam-Guillermin, C.; Cousin, X.; Armant, O. Revealing the Increased Stress Response Behavior through Transcriptomic Analysis of Adult Zebrafish Brain after Chronic Low to Moderate Dose Rates of Ionizing Radiation. Cancers 2022, 14, 3793. [Google Scholar] [CrossRef]
- Vera-Chang, M.N.; St-Jacques, A.D.; Lu, C.; Moon, T.W.; Trudeau, V.L. Fluoxetine Exposure During Sexual Development Disrupts the Stress Axis and Results in Sex- and Time- Dependent Effects on the Exploratory Behavior in Adult Zebrafish Danio rerio. Front. Neurosci. 2019, 13, 1015. [Google Scholar] [CrossRef]
- Giacomini, A.C.; Abreu, M.S.; Zanandrea, R.; Saibt, N.; Friedrich, M.T.; Koakoski, G.; Gusso, D.; Piato, A.L.; Barcellos, L.J. Environmental and Pharmacological Manipulations Blunt the Stress Response of Zebrafish in a Similar Manner. Sci. Rep. 2016, 6, 28986. [Google Scholar] [CrossRef]
- Rambo, C.L.; Mocelin, R.; Marcon, M.; Villanova, D.; Koakoski, G.; de Abreu, M.S.; Oliveira, T.A.; Barcellos, L.J.G.; Piato, A.L.; Bonan, C.D. Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol. Behav. 2017, 171, 50–54. [Google Scholar] [CrossRef]
- Hubená, P.; Benrejdal, L.; Brodin, D.; Axling, J.; Sen Sarma, O.; Bergman, P.; Winberg, S. Effects of stress coping styles and social defeat on zebrafish behaviour and brain transcriptomics. bioRxiv 2025. [Google Scholar] [CrossRef]
- Leite, G.O.; Rodrigues Santos, S.A.A.; de Castro Ribeiro, A.D.; Bezerra, F.M.D.H.; Campos, A.R. Impact of sex and environmental conditions on the responses to pain in zebrafish. Braz. J. Pain. 2021, 4, 9–14. [Google Scholar] [CrossRef]
- Yuan, M.; Chen, Y.; Huang, Y.; Lu, W. Behavioral and Metabolic Phenotype Indicate Personality in Zebrafish (Danio rerio). Front. Physiol. 2018, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Puty, B.; Matos Oliveira, K.R.; Herculano, A.M. Behavioral and neurochemical changes in the zebrafish leopard strain. Genes. Brain Behav. 2013, 12, 576–582. [Google Scholar] [CrossRef]
- Gorissen, M.; Manuel, R.; Pelgrim, T.N.; Mes, W.; de Wolf, M.J.; Zethof, J.; Flik, G.; van den Bos, R. Differences in inhibitory avoidance, cortisol and brain gene expression in TL and AB zebrafish. Genes Brain Behav. 2015, 14, 428–438. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Loosli, F.; Conti, F.; Foulkes, N.S.; Bertolucci, C. Comparison of anxiety-like and social behaviour in medaka and zebrafish. Sci. Rep. 2022, 12, 10926. [Google Scholar] [CrossRef] [PubMed]
- Marcon, M.; Mocelin, R.; Benvenutti, R.; Costa, T.; Herrmann, A.P.; de Oliveira, D.L.; Koakoski, G.; Barcellos, L.J.G.; Piato, A. Environmental enrichment modulates the response to chronic stress in zebrafish. J. Exp. Biol. 2018, 221 Pt 4, jeb176735. [Google Scholar] [CrossRef]
- Lee, C.J.; Paull, G.C.; Tyler, C.R. Effects of environmental enrichment on survivorship, growth, sex ratio and behaviour in laboratory maintained zebrafish Danio rerio. J. Fish Biol. 2019, 94, 86–95. [Google Scholar] [CrossRef]
- Buenhombre, J.; Daza-Cardona, E.A.; Sousa, P.; Gouveia, A., Jr. Different influences of anxiety models, environmental enrichment, standard conditions and intraspecies variation (sex, personality and strain) on stress and quality of life in adult and juvenile zebrafish: A systematic review. Neurosci. Biobehav. Rev. 2021, 131, 765–791. [Google Scholar] [CrossRef] [PubMed]
- Pusic, K.M.; Pusic, A.D.; Kraig, R.P. Environmental Enrichment Stimulates Immune Cell Secretion of Exosomes that Promote CNS Myelination and May Regulate Inflammation. Cell. Mol. Neurobiol. 2016, 36, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.G.; Shaykin, J.D.; Stairs, D.J.; Bardo, M.T. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability: An updated review. Pharmacol. Biochem. Behav. 2022, 221, 173471. [Google Scholar] [CrossRef]
- DePasquale, C.; Leri, J. The influence of exercise on anxiety-like behavior in zebrafish (Danio rerio). Behav. Process. 2018, 157, 638–644. [Google Scholar] [CrossRef]
- da Rosa, J.G.; Barcellos, H.H.; Idalencio, R.; Marqueze, A.; Fagundes, M.; Rossini, M.; Variani, C.; Balbinoti, F.; Tietböhl, T.M.; Rosemberg, D.B.; et al. Just Keep Swimming: Neuroendocrine, Metabolic, and Behavioral Changes After a Forced Swimming Test in Zebrafish. Zebrafish 2017, 14, 51–59. [Google Scholar] [CrossRef]
- Heinkele, F.J.; Lou, B.; Erben, V.; Bennewitz, K.; Poschet, G.; Sticht, C.; Kroll, J. Metabolic and Transcriptional Adaptations Improve Physical Performance of Zebrafish. Antioxidants 2021, 10, 1581. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.S.A.; Carneiro, W.F.; Monteiro, K.S.; Souza, S.P.; Vianna, A.R.D.C.B.; Murgas, L.D.S. Metabolic effects of physical exercise on zebrafish (Danio rerio) fed a high-fat diet. J. Comp. Physiol. B 2024, 194, 793–804. [Google Scholar] [CrossRef]
- Piato, Â.L.; Capiotti, K.M.; Tamborski, A.R.; Oses, J.P.; Barcellos, L.J.; Bogo, M.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 561–567. [Google Scholar] [CrossRef]
- Dametto, F.S.; Fior, D.; Idalencio, R.; Rosa, J.G.S.; Fagundes, M.; Marqueze, A.; Barreto, R.E.; Piato, A.; Barcellos, L.J.G. Feeding regimen modulates zebrafish behavior. PeerJ 2018, 6, e5343. [Google Scholar] [CrossRef]
- del Pozo, A.; Sánchez-Férez, J.A.; Sánchez-Vázquez, F.J. Circadian rhythms of self-feeding and locomotor activity in zebrafish (Danio rerio). Chronobiol. Int. 2011, 28, 39–47. [Google Scholar] [CrossRef]
- Averill, L.A.; Purohit, P.; Averill, C.L.; Boesl, M.A.; Krystal, J.H.; Abdallah, C.G. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci. Lett. 2017, 649, 147–155. [Google Scholar] [CrossRef]
- Nicosia, N.; Giovenzana, M.; Misztak, P.; Mingardi, J.; Musazzi, L. Glutamate-Mediated Excitotoxicity in the Pathogenesis and Treatment of Neurodevelopmental and Adult Mental Disorders. Int. J. Mol. Sci. 2024, 25, 6521. [Google Scholar] [CrossRef] [PubMed]
- Clift, D.; Richendrfer, H.; Thorn, R.J.; Colwill, R.M.; Creton, R. High-throughput analysis of behavior in zebrafish larvae: Effects of feeding. Zebrafish 2014, 11, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.; Fontana, B.D.; Amstislavskaya, T.G.; Gorbunova, M.A.; Altenhofen, S.; Barthelson, K.; Bastos, L.M.; Borba, J.V.; Bonan, C.D.; Brennan, C.H.; et al. Housing and Husbandry Factors Affecting Zebrafish (Danio rerio) Novel Tank Test Responses: A Global Multi-Laboratory Study. Res. Sq. 2024, rs.3, rs-4849877. [Google Scholar] [CrossRef]
- Licitra, R.; Fronte, B.; Verri, T.; Marchese, M.; Sangiacomo, C.; Santorelli, F.M. Zebrafish feed intake: A systematic review for standardizing feeding management in laboratory conditions. Biology 2024, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Beckmann, H.; Azap, R.; Ryu, S. Acute Stress Modulates Social Approach and Social Maintenance in Adult Zebrafish. eNeuro 2023, 10, ENEURO.0491-22.2023. [Google Scholar] [CrossRef]
- Geng, Y.; Peterson, R.T. The zebrafish subcortical social brain as a model for studying social behavior disorders. Dis. Model. Mech. 2019, 12, dmm039446. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, M.; Sundvik, M.; Chen, Y.C.; Panula, P. Adaptive changes in zebrafish brain in dominant-subordinate behavioral context. Behav. Brain Res. 2011, 225, 529–537. [Google Scholar] [CrossRef]
- Bozi, B.; Rodrigues, J.; Lima-Maximino, M.; de Siqueira-Silva, D.H.; Soares, M.C.; Maximino, C. Social Stress Increases Anxiety-Like Behavior Equally in Male and Female Zebrafish. Front. Behav. Neurosci. 2021, 15, 785656. [Google Scholar] [CrossRef] [PubMed]
- Filby, A.L.; Paull, G.C.; Bartlett, E.J.; Van Look, K.J.; Tyler, C.R. Physiological and health consequences of social status in zebrafish (Danio rerio). Physiol. Behav. 2010, 101, 576–587. [Google Scholar] [CrossRef]
- Maruska, K.P. Social Transitions Cause Rapid Behavioral and Neuroendocrine Changes. Integr. Comp. Biol. 2015, 55, 294–306. [Google Scholar] [CrossRef]
- Ressler, K.J.; Berretta, S.; Bolshakov, V.Y.; Rosso, I.M.; Meloni, E.G.; Rauch, S.L.; Carlezon, W.A., Jr. Post-traumatic stress disorder: Clinical and translational neuroscience from cells to circuits. Nat. Rev. Neurol. 2022, 18, 273–288. [Google Scholar] [CrossRef]
- Sison, M.; Gerlai, R. Associative learning in zebrafish (Danio rerio) in the plus maze. Behav. Brain Res. 2010, 207, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Reemst, K.; Shahin, H.; Shahar, O.D. Learning and memory formation in zebrafish: Protein dynamics and molecular tools. Front. Cell Dev. Biol. 2023, 11, 1120984. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Myggland, A.; Duperreault, E.; May, Z.; Gallup, J.; Powell, R.A.; Schalomon, M.; Digweed, S.M. Episodic-like memory in zebrafish. Anim. Cogn. 2016, 19, 1071–1079. [Google Scholar] [CrossRef]
- Arey, R.N.; Murphy, C.T. Conserved regulators of cognitive aging: From worms to humans. Behav. Brain Res. 2017, 322 Pt B, 299–310. [Google Scholar] [CrossRef]
- Wang, J.; Cao, H. Zebrafish and Medaka: Important Animal Models for Human Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 10766. [Google Scholar] [CrossRef]
- Shenoy, A.; Banerjee, M.; Upadhya, A.; Bagwe-Parab, S.; Kaur, G. The Brilliance of the Zebrafish Model: Perception on Behavior and Alzheimer’s Disease. Front. Behav. Neurosci. 2022, 16, 861155. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, E.; Iacob, D.; Rarinca, V.; Plavan, G.; Ureche, D.; Jijie, R.; Nicoara, M. Zebrafish as a Potential Model for Neurodegenerative Diseases: A Focus on Toxic Metals Implications. Int. J. Mol. Sci. 2023, 24, 3428. [Google Scholar] [CrossRef] [PubMed]
- Lodetti, G.; Baldin, S.L.; de Farias, A.C.S.; de Pieri Pickler, K.; Teixeira, A.G.; Dondossola, E.R.; Bernardo, H.T.; Maximino, C.; Rico, E.P. Repeated exposure to ethanol alters memory acquisition and neurotransmission parameters in zebrafish brain. Pharmacol. Biochem. Behav. 2025, 246, 173915. [Google Scholar] [CrossRef]
- Zhdanov, A.V.; Khatsko, S.L.; Zabegalov, K.N.; Bytov, M.V.; Demin, K.A.; Galstyan, D.S.; de Abreu, M.S.; Amstislavskaya, T.G.; Kalueff, A.V. Modeling Stress-Related Disorders in Zebrafish Using Prolonged Predator Exposure and Prolonged Unpredictable Stress. J. Neurosci. Res. 2025, 103, e70048. [Google Scholar] [CrossRef]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, L.J.G.; Ritter, F.; Kreutz, L.C.; Quevedo, R.M.; da Silva, L.B.; Bedin, A.C.; Finco, J.; Cericato, L. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 2007, 272, 774–778. [Google Scholar] [CrossRef]
- Wong, M.I.; Lau, I.H.; Gordillo-Martinez, F.; Vasconcelos, R.O. The effect of time regime in noise exposure on the auditory system and behavioural stress in the zebrafish. Sci. Rep. 2022, 12, 15353. [Google Scholar] [CrossRef]
- Soh, Z.; Matsuno, M.; Yoshida, M.; Tsuji, T. Real-Time Cameraless Measurement System Based on Bioelectrical Ventilatory Signals to Evaluate Fear and Anxiety. Zebrafish 2018, 15, 133–144. [Google Scholar] [CrossRef]
- Yoshida, M. Recording the ventilation activity of free-swimming zebrafish and its application to novel tank tests. Physiol. Behav. 2022, 244, 113665. [Google Scholar] [CrossRef]
- Soh, Z.; Matsuno, M.; Yoshida, M.; Furui, A.; Tsuji, T. Measurement of emotional states of zebrafish through integrated analysis of motion and respiration using bioelectric signals. Sci. Rep. 2021, 11, 187. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.; Kalueff, A.V. A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef]
- Ajuwon, V.; Cruz, B.F.; Carriço, P.; Champalimaud Research Scientific Hardware Platform; Kacelnik, A.; Monteiro, T. GoFish: A low-cost, open-source platform for closed-loop behavioural experiments on fish. Behav. Res. Methods 2024, 56, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.V.; Del Rosario Hernández, T.; Creton, R. Behavioral effects of visual stimuli in adult zebrafish using a novel eight-tank imaging system. Front. Behav. Neurosci. 2024, 18, 1320126. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Collins, C.; Kyzar, E.J.; Pham, M.; Roth, A.; Gaikwad, S.; Cachat, J.; Stewart, A.M.; Landsman, S.; Grieco, F.; et al. Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 2012, 210, 266–271. [Google Scholar] [CrossRef] [PubMed]
- DePasquale, C.; Franklin, K.; Jia, Z.; Jhaveri, K.; Buderman, F.E. The effects of exploratory behavior on physical activity in a common animal model of human disease, zebrafish (Danio rerio). Front. Behav. Neurosci. 2022, 16, 1020837. [Google Scholar] [CrossRef]
- Boorse, G.C.; Denver, R.J. Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen. Comp. Endocrinol. 2006, 146, 9–18. [Google Scholar] [CrossRef]
- Herget, U.; Wolf, A.; Wullimann, M.F.; Ryu, S. Molecular neuroanatomy and chemoarchitecture of the neurosecretory preoptic-hypothalamic area in zebrafish larvae. J. Comp. Neurol. 2014, 522, 1542–1564, Erratum in J. Comp. Neurol. 2014, 522, 3139. [Google Scholar] [CrossRef] [PubMed]
- Grone, B.P.; Maruska, K.P. Divergent evolution of two corticotropin-releasing hormone (CRH) genes in teleost fishes. Front. Neurosci. 2015, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.; Vijayan, M. The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endocrinol. 2009, 161, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Alderman, S.L.; Bernier, N.J. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen. Comp. Endocrinol. 2009, 164, 61–69. [Google Scholar] [CrossRef]
- Vom Berg-Maurer, C.M.; Trivedi, C.A.; Bollmann, J.H.; De Marco, R.J.; Ryu, S. The Severity of Acute Stress Is Represented by Increased Synchronous Activity and Recruitment of Hypothalamic CRH Neurons. J. Neurosci. 2016, 36, 3350–3362. [Google Scholar] [CrossRef]
- Alderman, S.L.; Bernier, N.J. Localization of corticotropin-releasing factor, urotensin I, and CRF-binding protein gene expression in the brain of the zebrafish, Danio rerio. J. Comp. Neurol. 2007, 502, 783–793. [Google Scholar] [CrossRef]
- Chakravarty, S.; Reddy, B.R.; Sudhakar, S.R.; Saxena, S.; Das, T.; Meghah, V.; Brahmendra Swamy, C.V.; Kumar, A.; Idris, M.M. Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: Altered brain proteome profile implicates mitochondrial dysfunction. PLoS ONE 2013, 8, e63302. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.R.; Zhang, Y.; Rao, Y.B.; Chen, X.; Lou, H.F.; Zhang, Y.; Xie, H.Y.; Fang, P.; Hu, L.W. The changes in, and relationship between, plasma nitric oxide and corticotropin-releasing hormone in patients with major depressive disorder. Clin. Exp. Pharmacol. Physiol. 2018, 45, 10–15. [Google Scholar] [CrossRef]
- Stevens, A.; White, A. ACTH: Cellular peptide hormone synthesis and secretory pathways. Results Probl. Cell Differ. 2010, 50, 63–84. [Google Scholar] [CrossRef]
- Navarro, S.; Crespo, D.; Schulz, R.W.; Ge, W.; Rotllant, J.; Cerdá-Reverter, J.M.; Rocha, A. Role of the Melanocortin System in Gonadal Steroidogenesis of Zebrafish. Animals 2022, 12, 2737. [Google Scholar] [CrossRef]
- Lee, C.J.; Paull, G.C.; Tyler, C.R. Improving zebrafish laboratory welfare and scientific research through understanding their natural history. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1038–1056. [Google Scholar] [CrossRef]
- Lee, H.B.; Shams, S.; Dang Thi, V.H.; Boyum, G.E.; Modhurima, R.; Hall, E.M.; Green, I.K.; Cervantes, E.M.; Miguez, F.E.; Clark, K.J. Key HPI axis receptors facilitate light adaptive behavior in larval zebrafish. Sci. Rep. 2024, 14, 7759. [Google Scholar] [CrossRef]
- Rajeswari, J.J.; Faught, E.; Santos, H.; Vijayan, M.M. Mineralocorticoid receptor activates postnatal adiposity in zebrafish lacking proopiomelanocortin. J. Cell. Physiol. 2024, 239, e31428. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Iwanowicz, L.R.; Blazer, V.S.; McCormick, S.D.; Vanveld, P.A.; Ottinger, C.A. Aroclor 1248 exposure leads to immunomodulation, decreased disease resistance and endocrine disruption in the brown bullhead, Ameiurus nebulosus. Aquat. Toxicol. 2009, 93, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Houdelet, C.; Bessa, E.; Geffroy, B.; Sadoul, B. Water temperature explains part of the variation in basal plasma cortisol level within and between fish species. J. Fish Biol. 2023, 103, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.B.; Schoonheim, P.J.; Ziv, L.; Voelker, L.; Baier, H.; Gahtan, E. A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Front. Behav. Neurosci. 2012, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Gesto, M.; Hernández, J.; López-Patiño, M.A.; Soengas, J.L.; Míguez, J.M. Is gill cortisol concentration a good acute stress indicator in fish? A study in rainbow trout and zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 188, 65–69. [Google Scholar] [CrossRef]
- O’Daniel, M.P.; Petrunich-Rutherford, M.L. Effects of chronic prazosin, an alpha-1 adrenergic antagonist, on anxiety-like behavior and cortisol levels in a chronic unpredictable stress model in zebrafish (Danio rerio). PeerJ 2020, 8, e8472. [Google Scholar] [CrossRef]
- Sic, A.; Bogicevic, M.; Brezic, N.; Nemr, C.; Knezevic, N.N. Chronic Stress and Headaches: The Role of the HPA Axis and Autonomic Nervous System. Biomedicines 2025, 13, 463. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Wong, A. The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 361–379. [Google Scholar] [CrossRef]
- Su, C.; Huang, T.; Zhang, M.; Zhang, Y.; Zeng, Y.; Chen, X. Glucocorticoid receptor signaling in the brain and its involvement in cognitive function. Neural Regen. Res. 2025, 20, 2520–2537. [Google Scholar] [CrossRef]
- Ramos-Ramírez, P.; Tliba, O. Glucocorticoid Receptor β (GRβ): Beyond Its Dominant-Negative Function. Int. J. Mol. Sci. 2021, 22, 3649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caratti, G.; Matthews, L.; Poolman, T.; Kershaw, S.; Baxter, M.; Ray, D. Glucocorticoid receptor function in health and disease. Clin. Endocrinol. 2015, 83, 441–448. [Google Scholar] [CrossRef]
- Deuter, C.E.; Kaczmarczyk, M.; Hellmann-Regen, J.; Kuehl, L.K.; Wingenfeld, K.; Otte, C. The influence of pharmacological mineralocorticoid and glucocorticoid receptor blockade on the cortisol response to psychological stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 129, 110905. [Google Scholar] [CrossRef]
- Ziv, L.; Muto, A.; Schoonheim, P.J.; Meijsing, S.H.; Strasser, D.; Ingraham, H.A.; Schaaf, M.J.; Yamamoto, K.R.; Baier, H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 2013, 18, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Natsaridis, E.; Perdikaris, P.; Fokos, S.; Dermon, C.R. Neuronal and Astroglial Localization of Glucocorticoid Receptor GRα in Adult Zebrafish Brain (Danio rerio). Brain Sci. 2023, 13, 861. [Google Scholar] [CrossRef] [PubMed]
- Xin, N.; Wang, D.T.; Zhang, L.; Zhou, Y.; Cheng, Y. Early developmental stage glucocorticoid exposure causes DNA methylation and behavioral defects in adult zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109301. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, P.O.; Goodyer, I.M. Childhood adversity and allostatic overload of the hypothalamic-pituitary-adrenal axis: A vulnerability model for depressive disorders. Dev. Psychopathol. 2011, 23, 1017–1037. [Google Scholar] [CrossRef]
- Herget, U.; Ryu, S.; De Marco, R.J. Altered glucocorticoid reactivity and behavioral phenotype in rx3-/- larval zebrafish. Front. Endocrinol. 2023, 14, 1187327. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef]
- Varga, Z.K.; Pejtsik, D.; Biró, L.; Zsigmond, Á.; Varga, M.; Tóth, B.; Salamon, V.; Annus, T.; Mikics, É.; Aliczki, M. Conserved Serotonergic Background of Experience-Dependent Behavioral Responsiveness in Zebrafish (Danio rerio). J. Neurosci. 2020, 40, 4551–4564. [Google Scholar] [CrossRef]
- Wasel, O.; Freeman, J.L. Chemical and Genetic Zebrafish Models to Define Mechanisms of and Treatments for Dopaminergic Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5981. [Google Scholar] [CrossRef]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Cinosi, E.; Davies, S.; Domschke, K.; Fineberg, N.; et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef]
- Tay, T.L.; Ronneberger, O.; Ryu, S.; Nitschke, R.; Driever, W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat. Commun. 2011, 2, 171. [Google Scholar] [CrossRef]
- Schweitzer, J.; Lohr, H.; Filippi, A.; Driever, W. Dopaminergic and noradrenergic circuit development in zebrafish. Dev. Neurobiol. 2012, 72, 256–268. [Google Scholar] [CrossRef] [PubMed]
- van Staden, C.; Weinshenker, D.; Finger-Baier, K.; Botha, T.L.; Brand, L.; Wolmarans, W. Posttraumatic anxiety-like behaviour in zebrafish is dose-dependently attenuated by the alpha-2A receptor agonist, guanfacine. Behav. Pharmacol. 2025, 36, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.C.; Raskind, M.A. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp. Neurol. 2016, 284 Pt B, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Wang, L.P.; Tsien, J.Z. Dopamine Rebound-Excitation Theory: Putting Brakes on PTSD. Front. Psychiatry 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.J.; Akhurst, J.; Bruno, R.; Laing, P.A.F.; Matthews, A.; Felmingham, K.L. Dopamine, endocannabinoids and their interaction in fear extinction and negative affect in PTSD. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110118. [Google Scholar] [CrossRef]
- Stewart, A.M.; Ullmann, J.F.; Norton, W.H.; Parker, M.O.; Brennan, C.H.; Gerlai, R.; Kalueff, A.V. Molecular psychiatry of zebrafish. Mol. Psychiatry 2015, 20, 2–17. [Google Scholar] [CrossRef]
- Tu, X.; Li, Y.W.; Chen, Q.L.; Shen, Y.J.; Liu, Z.H. Tributyltin enhanced anxiety of adult male zebrafish through elevating cortisol level and disruption in serotonin, dopamine and gamma-aminobutyric acid neurotransmitter pathways. Ecotoxicol. Environ. Saf. 2020, 203, 111014. [Google Scholar] [CrossRef]
- Näslund, J.; Landin, J.; Hieronymus, F.; Banote, R.K.; Kettunen, P. Anxiolytic-like effects of acute serotonin-releasing agents in zebrafish models of anxiety: Experimental study and systematic review. Acta Neuropsychiatr. 2024, 37, e35. [Google Scholar] [CrossRef]
- DU, Y.; Li, Z.; Zhao, Y.; Han, J.; Hu, W.; Liu, Z. Role of 5-hydroxytryptamine type 3 receptors in the regulation of anxiety reactions. J. Zhejiang Univ. Sci. B 2024, 25, 23–37. [Google Scholar] [CrossRef]
- Southwick, S.M.; Paige, S.; Morgan, C.A., 3rd; Bremner, J.D.; Krystal, J.H.; Charney, D.S. Neurotransmitter alterations in PTSD: Catecholamines and serotonin. Semin. Clin. Neuropsychiatry 1999, 4, 242–248. [Google Scholar]
- Kovacic, Z.; Henigsberg, N.; Pivac, N.; Nedic, G.; Borovecki, A. Platelet serotonin concentration and suicidal behavior in combat related posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Altermatt, M.; Zhang, R.W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701.e8. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.; Kanady, J.C.; Neylan, T.C. Sleep disturbance in PTSD and other anxiety-related disorders: An updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology 2020, 45, 55–73, Erratum in Neuropsychopharmacology 2020, 45, 240–241. https://doi.org/10.1038/s41386-019-0529-y. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, C.X.; Gu, Y.; Zhao, Y.; Ren, W.; Wang, Y.; Chen, J.; Guan, N.N.; Song, J. Serotonergic modulation of vigilance states in zebrafish and mice. Nat. Commun. 2024, 15, 2596. [Google Scholar] [CrossRef]
- Domingues, I.; Gravato, C. Oxidative Stress Assessment in Zebrafish Larvae. Methods Mol. Biol. 2018, 1797, 477–486. [Google Scholar] [CrossRef]
- Guo, D.; Luo, L.; Kong, Y.; Kuang, Z.; Wen, S.; Zhao, M.; Zhang, W.; Fan, J. Enantioselective neurotoxicity and oxidative stress effects of paclobutrazol in zebrafish (Danio rerio). Pestic. Biochem. Physiol. 2022, 185, 105136. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, B.; Zhou, S.; Zhao, M.; Li, R.; Zhou, Y.; Shi, X.; Han, J.; Zhang, W.; Zhou, B. Mitochondrial Dysfunction Was Involved in Decabromodiphenyl Ethane-Induced Glucolipid Metabolism Disorders and Neurotoxicity in Zebrafish Larvae. Environ. Sci. Technol. 2023, 57, 11043–11055. [Google Scholar] [CrossRef]
- De Marco, R.J.; Groneberg, A.H.; Yeh, C.M.; Castillo Ramírez, L.A.; Ryu, S. Optogenetic elevation of endogenous glucocorticoid level in larval zebrafish. Front. Neural Circuits 2013, 7, 82. [Google Scholar] [CrossRef]
- De Marco, R.J.; Thiemann, T.; Groneberg, A.H.; Herget, U.; Ryu, S. Optogenetically enhanced pituitary corticotroph cell activity post-stress onset causes rapid organizing effects on behaviour. Nat. Commun. 2016, 7, 12620. [Google Scholar] [CrossRef]
- Eachus, H.; Choi, M.K.; Tochwin, A.; Kaspareit, J.; Ho, M.; Ryu, S. Elevated glucocorticoid alters the developmental dynamics of hypothalamic neurogenesis in zebrafish. Commun. Biol. 2024, 7, 416. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Long, Y.; Xie, J.; Ren, J.; Zhou, T.; Song, G.; Li, Q.; Cui, Z. Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress. Int. J. Mol. Sci. 2021, 22, 5551. [Google Scholar] [CrossRef] [PubMed]
- Duque, M.; Chen, A.B.; Narayan, S.; Olson, D.E.; Fishman, M.C.; Engert, F.; Ahrens, M.B. Astroglial mediation of fast-acting antidepressant effect in zebrafish. bioRxiv 2022. [Google Scholar] [CrossRef]
- Piatkevich, K.D.; Jung, E.E.; Straub, C.; Linghu, C.; Park, D.; Suk, H.J.; Hochbaum, D.R.; Goodwin, D.; Pnevmatikakis, E.; Pak, N.; et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 2018, 14, 352–360, Erratum in Nat. Chem. Biol. 2018, 14, 901. https://doi.org/10.1038/s41589-018-0023-6. [Google Scholar] [CrossRef]
- Doszyn, O.; Dulski, T.; Zmorzynska, J. Diving into the zebrafish brain: Exploring neuroscience frontiers with genetic tools, imaging techniques, and behavioral insights. Front. Mol. Neurosci. 2024, 17, 1358844. [Google Scholar] [CrossRef]
- Deslauriers, J.; Toth, M.; Der-Avakian, A.; Risbrough, V.B. Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol. Psychiatry 2018, 83, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Yang, E.; Nguyen, M.; Kalueff, A.V. Developing zebrafish models relevant to PTSD and other trauma- and stressor-related disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 55, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Kigar, S.L.; Cuarenta, A.; Zuniga, C.L.; Chang, L.; Auger, A.P.; Bakshi, V.P. Brain, behavior, and physiological changes associated with predator stress-An animal model for trauma exposure in adult and neonatal rats. Front. Mol. Neurosci. 2024, 17, 1322273. [Google Scholar] [CrossRef]
- Baisley, S.K.; Cloninger, C.L.; Bakshi, V.P. Fos expression following regimens of predator stress versus footshock that differentially affect prepulse inhibition in rats. Physiol. Behav. 2011, 104, 796–803. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.; Achterberg, E.J.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef]
- Bailey, J.M.; Oliveri, A.N.; Levin, E.D. Pharmacological analyses of learning and memory in zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2015, 139 Pt B, 103–111. [Google Scholar] [CrossRef]
- Tan, J.K.; Nazar, F.H.; Makpol, S.; Teoh, S.L. Zebrafish: A Pharmacological Model for Learning and Memory Research. Molecules 2022, 27, 7374. [Google Scholar] [CrossRef]
- Yashina, K.; Tejero-Cantero, Á.; Herz, A.; Baier, H. Zebrafish Exploit Visual Cues and Geometric Relationships to Form a Spatial Memory. iScience 2019, 19, 119–134. [Google Scholar] [CrossRef]
- Blank, M.; Guerim, L.D.; Cordeiro, R.F.; Vianna, M.R. A one-trial inhibitory avoidance task to zebrafish: Rapid acquisition of an NMDA-dependent long-term memory. Neurobiol. Learn. Mem. 2009, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Scott-Scheiern, T.; Kempker, L.; Simons, K. Active avoidance conditioning in zebrafish (Danio rerio). Neurobiol. Learn. Mem. 2007, 87, 72–77. [Google Scholar] [CrossRef]
- Kenney, J.W.; Scott, I.C.; Josselyn, S.A.; Frankland, P.W. Contextual fear conditioning in zebrafish. Learn. Mem. 2017, 24, 516–523. [Google Scholar] [CrossRef]
- Khalili, A.; van Wijngaarden, E.; Zoidl, G.R.; Rezai, P. Zebrafish larva’s response and habituation to electric signal: Effects of voltage, current and pulsation studied in a microfluidic device. Sens. Actuators A Phys. 2021, 332, 113070. [Google Scholar] [CrossRef]
- Steenbergen, P.J. Response of zebrafish larvae to mild electrical stimuli: A 96-well setup for behavioural screening. J. Neurosci. Methods 2018, 301, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Teulier, L.; Guillard, L.; Leon, C.; Romestaing, C.; Voituron, Y. Consequences of electroshock-induced narcosis in fish muscle: From mitochondria to swim performance. J. Fish Biol. 2018, 92, 1805–1818. [Google Scholar] [CrossRef]
- Burkhardt, D.S.; Leyden, C.; Thomas, C.; Brysch, C.; Dehmelt, F.A.; Arrenberg, A.B. Behavioral and neurophysiological effects of electrical stunning on zebrafish larvae. Lab Anim 2025, 54, 50–58. [Google Scholar] [CrossRef]
- Haney, W.A.; Moussaoui, B.; Strother, J.A. Prolonged exposure to stressors suppresses exploratory behavior in zebrafish larvae. J. Exp. Biol. 2020, 223 Pt 22, jeb224964. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.W.; Canzian, J.; Resmim, C.M.; Fontana, B.D.; Rosemberg, D.B. Contextual fear conditioning in zebrafish: Influence of different shock frequencies, context, and pharmacological modulation on behavior. Neurobiol. Learn. Mem. 2024, 214, 107963. [Google Scholar] [CrossRef]
- Wang, C.; Kang, X.; Zhou, L.; Chai, Z.; Wu, Q.; Huang, R.; Xu, H.; Hu, M.; Sun, X.; Sun, S.; et al. Synaptotagmin-11 is a critical mediator of parkin-linked neurotoxicity and Parkinson’s disease-like pathology. Nat. Commun. 2018, 9, 81. [Google Scholar] [CrossRef]
- Kuroda, T.; Mizutani, Y.; Cançado, C.R.X.; Podlesnik, C.A. Predator videos and electric shock function as punishers for zebrafish (Danio rerio). J. Exp. Anal. Behav. 2019, 111, 116–129. [Google Scholar] [CrossRef]
- Piato, A.L.; Rosemberg, D.B.; Capiotti, K.M.; Siebel, A.M.; Herrmann, A.P.; Ghisleni, G.; Vianna, M.R.; Bogo, M.R.; Lara, D.R.; Bonan, C.D. Acute restraint stress in zebrafish: Behavioral parameters and purinergic signaling. Neurochem. Res. 2011, 36, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Dal Santo, G.; Conterato, G.M.; Barcellos, L.J.; Rosemberg, D.B.; Piato, A.L. Acute restraint stress induces an imbalance in the oxidative status of the zebrafish brain. Neurosci. Lett. 2014, 558, 103–108. [Google Scholar] [CrossRef]
- Assad, N.; Luz, W.L.; Santos-Silva, M.; Carvalho, T.; Moraes, S.; Picanço-Diniz, D.L.W.; Bahia, C.P.; Oliveira Batista, E.J.; da Conceição Passos, A.; Oliveira, K.R.H.M.; et al. Acute Restraint Stress Evokes Anxiety-Like Behavior Mediated by Telencephalic Inactivation and GabAergic Dysfunction in Zebrafish Brains. Sci. Rep. 2020, 10, 5551. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.Y.; Marçal, R.M.; Champagne, D.L.; van der Kooy, F.; Verpoorte, R.; Choi, Y.H. Effect of acute stresses on zebra fish (Danio rerio) metabolome measured by NMR-based metabolomics. Planta Med. 2014, 80, 1227–1233. [Google Scholar] [CrossRef]
- Li, H.Q.; Jiang, W.; Ling, L.; Pratelli, M.; Chen, C.; Gupta, V.; Godavarthi, S.K.; Spitzer, N.C. Generalized fear after acute stress is caused by change in neuronal cotransmitter identity. Science 2024, 383, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, Z. Neurotransmitter Switching: A Novel Mechanism for Fear Generalization. Neurosci. Bull. 2024, 40, 2015–2018. [Google Scholar] [CrossRef]
- Lucas Luz, W.; Santos-Silva, M.; Cardoso, P.B.; Assad, N.; Moraes, E.R.D.S.; Grisólia, A.B.A.; Braga, D.V.; Leão, L.K.R.; de Moraes, S.A.S.; Passos, A.D.C.; et al. Putative Activation of the CB1 Cannabinoid Receptors Prevents Anxiety-Like Behavior, Oxidative Stress, and GABA Decrease in the Brain of Zebrafish Submitted to Acute Restraint Stress. Front. Behav. Neurosci. 2021, 14, 598812. [Google Scholar] [CrossRef]
- Martins, M.L.; Pinheiro, E.F.; Saito, G.A.; Lima, C.A.C.; Leão, L.K.R.; Batista, E.J.O.; Passos, A.D.C.F.; Gouveia, A., Jr.; Oliveira, K.R.H.M.; Herculano, A.M. Distinct acute stressors produce different intensity of anxiety-like behavior and differential glutamate release in zebrafish brain. Front. Behav. Neurosci. 2024, 18, 1464992. [Google Scholar] [CrossRef]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; Goodspeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 450–457. [Google Scholar] [CrossRef]
- Wilson Alphonse, C.R.; Rajaretinam, R.K. Habituation and Behavioural Response of Confinement-Induced Anxiety Conditions in a Zebrafish Model. Appl. Biosci. 2022, 1, 315–323. [Google Scholar] [CrossRef]
- Müller, T.E.; Dos Santos, M.M.; Ferreira, S.A.; Claro, M.T.; de Macedo, G.T.; Fontana, B.D.; Barbosa, N.V. Negative impacts of social isolation on behavior and neuronal functions are recovered after short-term social reintroduction in zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 134, 111038. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.R.; Mendo, T.; Broell, F.; Webster, M.M. No experimental evidence of stress-induced hyperthermia in zebrafish (Danio rerio). J. Exp. Biol. 2019, 222 Pt 2, jeb192971. [Google Scholar] [CrossRef]
- Rey, S.; Huntingford, F.A.; Boltaña, S.; Vargas, R.; Knowles, T.G.; Mackenzie, S. Fish can show emotional fever: Stress-induced hyperthermia in zebrafish. Proc. Biol. Sci. 2015, 282, 20152266. [Google Scholar] [CrossRef]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Maximino, C.; da Silva, A.W.; Gouveia, A., Jr.; Herculano, A.M. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 624–631. [Google Scholar] [CrossRef]
- Maximino, C.; da Silva, A.W.; Araújo, J.; Lima, M.G.; Miranda, V.; Puty, B.; Benzecry, R.; Picanço-Diniz, D.L.; Gouveia, A., Jr.; Oliveira, K.R.; et al. Fingerprinting of psychoactive drugs in zebrafish anxiety-like behaviors. PLoS ONE 2014, 9, e103943. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Ma, Q.; Sun, S.X.; Zhang, H.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Reduced oxidative stress increases acute cold stress tolerance in zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 235, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lara, R.A.; Vasconcelos, R.O. Impact of noise on development, physiological stress and behavioural patterns in larval zebrafish. Sci. Rep. 2021, 11, 6615. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.P.; Zhang, Y.P.; Peng, Z.; Wang, J.; Collier, A.D.; Echevarria, D.J.; Savelieva, K.V.; Lawrence, R.F.; Rex, C.S.; et al. Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 384–394. [Google Scholar] [CrossRef]
- Kotova, M.M.; Amikishiev, S.V.; Apukhtin, K.V.; Galstyan, D.S.; de Abreu, M.S.; Stewart, A.M.; Yang, L.; Kalueff, A.V. Prolonged 5-week and 12-week chronic stress differentially modulates CNS expression of pro- and anti-neuroinflammatory biomarkers, brain monoamines and affective behavior in adult zebrafish. J. Comp. Physiol. B 2025. [Google Scholar] [CrossRef]
- Angiulli, E.; Pagliara, V.; Cioni, C.; Frabetti, F.; Pizzetti, F.; Alleva, E.; Toni, M. Increase in environmental temperature affects exploratory behaviour, anxiety and social preference in Danio rerio. Sci. Rep. 2020, 10, 5385. [Google Scholar] [CrossRef]
- Lee, H.B.; Schwab, T.L.; Sigafoos, A.N.; Gauerke, J.L.; Krug, R.G., 2nd; Serres, M.R.; Jacobs, D.C.; Cotter, R.P.; Das, B.; Petersen, M.O.; et al. Novel zebrafish behavioral assay to identify modifiers of the rapid, nongenomic stress response. Genes Brain Behav. 2019, 18, e12549. [Google Scholar] [CrossRef] [PubMed]
- Eachus, H.; Ryu, S.; Placzek, M.; Wood, J. Zebrafish as a model to investigate the CRH axis and interactions with DISC1. Curr. Opin. Endocr. Metab. Res. 2022, 26, 100383. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Aponte, A.; Petrunich-Rutherford, M.L. Acute net stress of young adult zebrafish (Danio rerio) is not sufficient to increase anxiety-like behavior and whole-body cortisol. PeerJ 2019, 7, e7469. [Google Scholar] [CrossRef]
- Chin, J.S.R.; Phan, T.N.; Albert, L.T.; Keene, A.C.; Duboué, E.R. Long lasting anxiety following early life stress is dependent on glucocorticoid signaling in zebrafish. Sci. Rep. 2022, 12, 12826. [Google Scholar] [CrossRef]
- Demin, K.A.; Lakstygal, A.M.; Krotova, N.A.; Masharsky, A.; Tagawa, N.; Chernysh, M.V.; Ilyin, N.P.; Taranov, A.S.; Galstyan, D.S.; Derzhavina, K.A.; et al. Understanding complex dynamics of behavioral, neurochemical and transcriptomic changes induced by prolonged chronic unpredictable stress in zebrafish. Sci. Rep. 2020, 10, 19981. [Google Scholar] [CrossRef]
- Demin, K.A.; Kolesnikova, T.O.; Galstyan, D.S.; Krotova, N.A.; Ilyin, N.P.; Derzhavina, K.A.; Levchenko, N.A.; Strekalova, T.; de Abreu, M.S.; Petersen, E.V.; et al. Modulation of behavioral and neurochemical responses of adult zebrafish by fluoxetine, eicosapentaenoic acid and lipopolysaccharide in the prolonged chronic unpredictable stress model. Sci. Rep. 2021, 11, 14289. [Google Scholar] [CrossRef]
- Liu, B.; Dong, K.; Chen, X.; Dong, H.; Zhao, Y.; Wang, X.; Sun, Z.; Xie, F.; Qian, L. Inhibition of Glycolysis Alleviates Chronic Unpredictable Mild Stress Induced Neuroinflammation and Depression-like Behavior. Brain Sci. 2024, 14, 1098. [Google Scholar] [CrossRef]
- Archard, G.A.; Earley, R.L.; Hanninen, A.F.; Braithwaite, V.A. Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct. Ecol. 2012, 26, 637–645. [Google Scholar] [CrossRef]
- Bass, S.L.; Gerlai, R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008, 186, 107–117. [Google Scholar] [CrossRef]
- Yilmaz, M.; Meister, M. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 2013, 23, 2011–2015. [Google Scholar] [CrossRef]
- Dunn, T.W.; Gebhardt, C.; Naumann, E.A.; Riegler, C.; Ahrens, M.B.; Engert, F.; Del Bene, F. Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron 2016, 89, 613–628. [Google Scholar] [CrossRef]
- Cléry, J.C.; Schaeffer, D.J.; Hori, Y.; Gilbert, K.M.; Hayrynen, L.K.; Gati, J.S.; Menon, R.S.; Everling, S. Looming and receding visual networks in awake marmosets investigated with fMRI. Neuroimage 2020, 215, 116815. [Google Scholar] [CrossRef]
- Liu, X.; Feng, X.; Huang, H.; Huang, K.; Xu, Y.; Ye, S.; Tseng, Y.T.; Wei, P.; Wang, L.; Wang, F. Male and female mice display consistent lifelong ability to address potential life-threatening cues using different post-threat coping strategies. BMC Biol. 2022, 20, 281. [Google Scholar] [CrossRef]
- Heinemans, M.; Moita, M.A. Looming stimuli reliably drive innate defensive responses in male rats, but not learned defensive responses. Sci. Rep. 2024, 14, 21578. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Liu, Z.; Chen, Z.; Shi, Y.; Wang, Q.; Liu, S.; Li, D.; Cao, P. BRAIN CIRCUITS. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 2015, 348, 1472–1477. [Google Scholar] [CrossRef]
- Wei, P.; Liu, N.; Zhang, Z.; Liu, X.; Tang, Y.; He, X.; Wu, B.; Zhou, Z.; Liu, Y.; Li, J.; et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 2015, 6, 6756. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Meinerz, D.L.; Fontana, B.D.; Mezzomo, N.J.; Stefanello, F.V.; de S Prestes, A.; Batista, C.B.; Rubin, M.A.; Barbosa, N.V.; Rocha, J.B.T.; et al. Extending the analysis of zebrafish behavioral endophenotypes for modeling psychiatric disorders: Fear conditioning to conspecific alarm response. Behav. Process. 2018, 149, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.G.; Silva Sde, N.; Silva, R.X.; Oliveira, K.R.; Batista Ede, J.; Maximino, C.; Herculano, A.M. Putative involvement of the nitrergic system on the consolidation, but not initiation, of behavioral sensitization after conspecific alarm substance in zebrafish. Pharmacol. Biochem. Behav. 2015, 139 Pt B, 127–133. [Google Scholar] [CrossRef]
- Canzian, J.; Fontana, B.D.; Quadros, V.A.; Rosemberg, D.B. Conspecific alarm substance differently alters group behavior of zebrafish populations: Putative involvement of cholinergic and purinergic signaling in anxiety- and fear-like responses. Behav. Brain Res. 2017, 320, 255–263. [Google Scholar] [CrossRef]
- Ding, J.X.; Rudak, P.T.; Inoue, W.; Haeryfar, S.M.M. Physical restraint mouse models to assess immune responses under stress with or without habituation. STAR Protoc. 2021, 2, 100838. [Google Scholar] [CrossRef]
- Chun, E.K.; Donovan, M.; Liu, Y.; Wang, Z. Behavioral, neurochemical, and neuroimmune changes associated with social buffering and stress contagion. Neurobiol. Stress 2022, 16, 100427. [Google Scholar] [CrossRef] [PubMed]
- Sarapultsev, A.; Komelkova, M.; Lookin, O.; Khatsko, S.; Gusev, E.; Trofimov, A.; Tokay, T.; Hu, D. Rat Models in Post-Traumatic Stress Disorder Research: Strengths, Limitations, and Implications for Translational Studies. Pathophysiology 2024, 31, 709–760. [Google Scholar] [CrossRef]
- Eraslan, E.; Castelhano-Carlos, M.J.; Amorim, L.; Soares-Cunha, C.; Rodrigues, A.J.; Sousa, N. Physiological and behavioral contagion/buffering effects of chronic unpredictable stress in a socially enriched environment: A preliminary study. Neurobiol. Stress 2024, 30, 100635. [Google Scholar] [CrossRef]
- Manuel, R.; Gorissen, M.; Zethof, J.; Ebbesson, L.O.; van de Vis, H.; Flik, G.; van den Bos, R. Unpredictable chronic stress decreases inhibitory avoidance learning in Tuebingen long-fin zebrafish: Stronger effects in the resting phase than in the active phase. J. Exp. Biol. 2014, 217 Pt 21, 3919–3928. [Google Scholar] [CrossRef]
- Quadros, V.A.; Costa, F.V.; Canzian, J.; Nogueira, C.W.; Rosemberg, D.B. Modulatory role of conspecific alarm substance on aggression and brain monoamine oxidase activity in two zebrafish populations. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 322–330. [Google Scholar] [CrossRef]
- de Sousa, E.B.; Heymbeeck, J.A.A.; Feitosa, L.M.; Xavier, A.G.O.; Dos Santos Campos, K.; do Socorro Dos Santos Rodrigues, L.; de Freitas, L.M.; do Carmo Silva, R.X.; Ikeda, S.R.; de Nazaré Dos Santos Silva, S.; et al. Activation of NOS-cGMP pathways promotes stress-induced sensitization of behavioral responses in zebrafish. Pharmacol. Biochem. Behav. 2024, 243, 173816. [Google Scholar] [CrossRef]
- Roberts, A.C.; Alzagatiti, J.B.; Ly, D.T.; Chornak, J.M.; Ma, Y.; Razee, A.; Zavradyan, G.; Khan, U.; Lewis, J.; Natarajan, A.; et al. Induction of Short-Term Sensitization by an Aversive Chemical Stimulus in Zebrafish Larvae. eNeuro 2020, 7, ENEURO.0336-19.2020, Erratum in eNeuro 2022, 9, ENEURO.0514-21.2021. https://doi.org/10.1523/ENEURO.0514-21.2021. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Sellés-Egea, A.; Riesco, M.F.; De Garnica, M.G.; Martínez-Fernández, B.; Herráez, M.P.; Robles, V. Early stress exposure on zebrafish development: Effects on survival, malformations and molecular alterations. Fish Physiol. Biochem. 2024, 50, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Radtke, K.M.; Ruf, M.; Gunter, H.M.; Dohrmann, K.; Schauer, M.; Meyer, A.; Elbert, T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 2011, 1, e21. [Google Scholar] [CrossRef]
- van den Bos, R.; Althuizen, J.; Tschigg, K.; Bomert, M.; Zethof, J.; Filk, G.; Gorissen, M. Early life exposure to cortisol in zebrafish (Danio rerio): Similarities and differences in behaviour and physiology between larvae of the AB and TL strains. Behav. Pharmacol. 2019, 30, 260–271. [Google Scholar] [CrossRef]
- Miller, N.Y.; Gerlai, R. Shoaling in zebrafish: What we don’t know. Rev. Neurosci. 2011, 22, 17–25. [Google Scholar] [CrossRef]
- Miller, N.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef]
- Saraswathy, V.M.; Zhou, L.; Mokalled, M.H. Single-cell analysis of innate spinal cord regeneration identifies intersecting modes of neuronal repair. Nat. Commun. 2024, 15, 6808. [Google Scholar] [CrossRef] [PubMed]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Herculano, A.M.; Maximino, C. Serotonergic modulation of zebrafish behavior: Towards a paradox. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 55, 50–66. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Margiotta-Casaluci, L.; Owen, S.F.; Cumming, R.I.; de Polo, A.; Winter, M.J.; Panter, G.H.; Rand-Weaver, M.; Sumpter, J.P. Quantitative cross-species extrapolation between humans and fish: The case of the anti-depressant fluoxetine. PLoS ONE 2014, 9, e110467. [Google Scholar] [CrossRef]
- Cellini, B.R.; Edachola, S.V.; Faw, T.D.; Cigliola, V. Blueprints for healing: Central nervous system regeneration in zebrafish and neonatal mice. BMC Biol. 2025, 23, 115. [Google Scholar] [CrossRef]
- Mitra, S.; Lee, W.; Hayashi, K.; Boyd, J.; Milloy, M.J.; Dong, H.; Wood, E.; Kerr, T. A gender comparative analysis of post-traumatic stress disorder among a community-based cohort of people who use drugs in Vancouver, Canada. Addict. Behav. 2021, 115, 106793. [Google Scholar] [CrossRef]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 2018, 299 Pt A, 157–171. [Google Scholar] [CrossRef]
- Costa, F.V.; Kolesnikova, T.O.; Galstyan, D.S.; Ilyin, N.P.; de Abreu, M.S.; Petersen, E.V.; Demin, K.A.; Yenkoyan, K.B.; Kalueff, A.V. Current State of Modeling Human Psychiatric Disorders Using Zebrafish. Int. J. Mol. Sci. 2023, 24, 3187. [Google Scholar] [CrossRef]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef]
- Keller, P.J. In vivo imaging of zebrafish embryogenesis. Methods 2013, 62, 268–278. [Google Scholar] [CrossRef]
- Kettunen, P. Calcium Imaging in the Zebrafish. Adv. Exp. Med. Biol. 2020, 1131, 901–942. [Google Scholar] [CrossRef]
- Astell, K.R.; Sieger, D. Zebrafish In Vivo Models of Cancer and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037077. [Google Scholar] [CrossRef]
- Castranova, D.; Samasa, B.; Venero Galanternik, M.; Gore, A.V.; Goldstein, A.E.; Park, J.S.; Weinstein, B.M. Long-term imaging of living adult zebrafish. Development 2022, 149, dev199667. [Google Scholar] [CrossRef]
- Deng, P.; Liu, S.; Zhao, Y.; Zhang, X.; Kong, Y.; Liu, L.; Xiao, Y.; Yang, S.; Hu, J.; Su, J.; et al. Long-working-distance high-collection-efficiency three-photon microscopy for in vivo long-term imaging of zebrafish and organoids. iScience 2024, 27, 110554. [Google Scholar] [CrossRef]
- Luchiari, A.C.; Maximino, C. Fish personality: Meta-theoretical issues, personality dimensions, and applications to neuroscience and psychopathology. Personal. Neurosci. 2023, 6, e9. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Torigoe, M.; Tanimoto, Y.; Okamoto, H. Adult zebrafish can learn Morris water maze-like tasks in a two-dimensional virtual reality system. Cell Rep. Methods 2024, 4, 100863. [Google Scholar] [CrossRef]



| Factor | Type of Influence | Outcome | Specific Example | Complexity |
|---|---|---|---|---|
| Light Cycles |  (Environmental Cycle) |  (Circadian rhythm establishment) | Larvae raised in darkness fail to develop normal rhythms [55,56] |  |
| Temperature |  (Environmental Cycle) |  (Stress sensitivity) | Cold stress increases shoaling behavior [57] |  |
| Circadian Rhythms |  (Biological Rhythms) |  (Behavioral and stress regulation) | Adults maintain strong circadian rhythms under constant conditions [58] |  |
| Age-Related Differences |  (Biological Aging) |  (Stress response changes) | Older zebrafish show reduced locomotor activity and different cortisol responses [2] |  |
| Stress Coping Styles |  (Behavioral Trait) |  (Stress response, learning, and memory) | Reactive zebrafish exhibit higher cortisol levels, more rapid learning, and longer retention of fear memories, compared to proactive individuals [59,60] |  |
 ; Non-Physical Stressor:
; Non-Physical Stressor:  ; Cognitive or Behavioral Regulation:
; Cognitive or Behavioral Regulation:  ; Fear:
; Fear:  ; Anxiety:
; Anxiety:  ; Simple Influence:
; Simple Influence:  ; Complex Influence:
; Complex Influence:  .
.| Aspect/Endpoint | Male Zebrafish | Female Zebrafish | References |
|---|---|---|---|
| Basal Anxiety-Like Behavior | Lower basal anxiety; greater exploration of upper tank; less freezing; lower plasma/brain estradiol and cortisol | Higher basal anxiety; more bottom dwelling, more freezing and immobility; higher estradiol and cortisol | [93,94] |
| Acute Stress-Induced Cortisol | Higher stress-induced cortisol surge | Lower stress-induced cortisol; enriched or group housing blunts any sex differences | [95,96] |
| Chronic/Unpredictable Stress (UCS) | UCS increases aggression and cortisol | UCS does not change aggression or cortisol; females more resilient/adapted | [97] |
| Social and Environmental Modulation | Group housing, enrichment, fluoxetine/diazepam lower cortisol and anxiety—but blunted in enriched/isolated tanks; males show greater modulation | Similar blunted stress response with enrichment/pharmacological agents | [95,96] |
| HPI Axis Gene Expression | Lower crha, crhr2, actha; higher crhbp (lower HPI reactivity) | Higher crha, crhr2, actha; lower crhbp (higher HPI reactivity) | [93] |
| Neurotransmitter Profiles (5HT, DA) | Higher brain serotonin and dopamine | Lower serotonin and dopamine | [93] |
| Aggression (Baseline and UCS Effect) | Lower baseline aggression; UCS increases aggression | Higher baseline aggression; UCS does not further increase aggression | [97] |
| Transcriptomic and Plasticity Response | Bold lines: strong transcriptomic reprogramming after social defeat; moderate behavioral flexibility | Shy lines: pronounced behavioral flexibility, minimal transcriptomic shifts | [98] |
| Pain/Nociception | No significant sex difference | Similarly to males; modulatory effects are environmentally mediated | [99] |
| Model | Type of Stressor | Outcome | Stressor | Ethological Validity | Etiological Validity | Model Complexity | Stress Paradigm (Duration, Predictability, Strength) |
|---|---|---|---|---|---|---|---|
| Predator Exposure Models |  |  |  | ●●● | ●●● |  | Chronic, Predictable or Unpredictable, Strong |
| Conspecific Alarm Substance (CAS) |  |  |  | ●● | ●● |  | Acute (Intermittent), Unpredictable, Mild |
| Chronic Unpredictable Stress (CUS/UCS) |  |  |  | ●● | ●●● |  | Chronic, Unpredictable, Mild or Strong |
| Looming Dot Stimulus (LDS) |  |  |  | ● | ● |  | Acute (Intermittent), Predictable or Unpredictable, Mild |
| Social Isolation Models |  |  |  | ●● | ●● |  | Chronic, Predictable, Mild |
| Pharmacological Stress Models |  |  |  | ● | ● |  | Acute (Intermittent) or Chronic, Predictable, Mild or Strong |
| Early Life Interventions |  |  |  | ● | ●●● |  | Acute (Intermittent) or Chronic, Predictable or Unpredictable, Mild or Strong |
 ; Non-Physical Stressor:
; Non-Physical Stressor:  ; Cognitive or Behavioral Regulation:
; Cognitive or Behavioral Regulation:  ; Fear:
; Fear:  ; Anxiety:
; Anxiety:  ; Depression:
; Depression:  ; Single Stressor:
; Single Stressor:  ; Multiple Stressors:
; Multiple Stressors:  ; ●●●—High Ethological or Etiological Validity; ●●—Medium Ethological or Etiological Validity; ●—Low Ethological or Etiological Validity; Simple Model:
; ●●●—High Ethological or Etiological Validity; ●●—Medium Ethological or Etiological Validity; ●—Low Ethological or Etiological Validity; Simple Model:  ; Complex Model:
; Complex Model:  .
.| Advantages | Description |
| Precise, Controllable Stressor | Electric shock intensity, duration, and frequency can be finely calibrated, enabling highly reproducible experiments and systematic manipulation of acute stress parameters. |
| Robust Behavioral Analysis | Enables detailed study of fear conditioning, contextual learning, avoidance behavior, and punishment sensitivity—core components of PTSD-related cognitive mechanisms. Suitable for high-throughput larval assays (e.g., 96-well setups). |
| Physiological and Molecular Insights | Activates the HPI axis and induces measurable changes in cortisol and stress-relevant gene expression (e.g., CRF, GR), providing insight into stress hormone dynamics and neuroendocrine activation. |
| Potential for Ethical Refinement | Under optimized conditions (e.g., brief pulses at ≤3 V), electric shocks can avoid long-term physiological damage, allowing for ethically refined protocols. |
| Mechanistic Specificity | Ideal for isolating single-event trauma effects, fear memory encoding, and extinction dynamics, especially in genetic or pharmacological manipulation studies. |
| Limitations | Description |
| Ethical and Welfare Concerns | Electrical stimulation can induce distress and is ethically sensitive. Requires justification, strict protocol optimization, and institutional oversight to ensure animal welfare. |
| Limited Syndromic Validity | Models acute trauma well but fails to reproduce chronicity, unpredictability, and emotional dysregulation of full PTSD. Less suited for modeling, generalized anxiety, or long-term neuroplasticity. |
| Technical Complexity | Applying shocks underwater demands specialized apparatus, voltage calibration, and shielding to prevent variability—barriers to standardization across labs. |
| Species-Specific Stress Physiology | Differences in neural conductivity, circuitry, and fear expression may limit direct translation to mammalian PTSD physiology, especially in complex affective domains. |
| Protocol Inconsistency Across Studies | Variation in shock waveform, duration, frequency, and delivery methods complicates replication and comparative synthesis; emphasizes the need for harmonized, transparent reporting standards. |
| Advantages | Description |
| Ethically Acceptable | Immobilization is generally less invasive than predator or electric shock exposure, resulting in fewer welfare concerns. |
| Relevance to PTSD | Simulates helplessness and uncontrollability—two core psychological dimensions in trauma—thereby enhancing face validity for PTSD-like states. |
| Physiological and Behavioral Insights | Elicits measurable changes in cortisol, oxidative stress markers, and GABAergic signaling, offering a clear window into acute stress neurobiology. |
| Simplicity of Setup | Does not require specialized equipment or complex protocols, enabling easier standardization and replication across laboratories. |
| Limitations | Description |
| Potentially Limited Stress Intensity | Stress severity may be lower than in predator or electric shock models, reducing symptom generalizability to severe PTSD phenotypes. |
| Habituation Effects | Repeated immobilization can lead to rapid desensitization, limiting its use in chronic or long-term stress modeling. |
| Restricted Scope of Behavioral Outcomes | Primarily models acute anxiety and passivity; does not capture chronic PTSD dimensions like hypervigilance or emotional numbing. |
| Advantages | Description |
| Simplicity | Easy to implement using basic labware; no specialized equipment needed. |
| Behavioral Observations | Allows study of social reintegration, altered dominance, and withdrawal, particularly upon return to group housing. |
| Ethical Considerations | Considered a low-burden stressor, which is advantageous in early screening or chronic low-dose stress protocols. |
| Limitations | Description |
| Stress Intensity | May not reach the threshold needed to induce robust or lasting PTSD-like symptoms. |
| Advantages | Description |
|---|---|
| Versatility | A wide range of physical, sensory, chemical, and social stressors can be applied to isolate specific aspects of the stress response. |
| Stressor Intensity | The intensity of stressor can be varied if necessary, e.g., to study strength-dependent effects. |
| Rapid Effects | Allows for immediate assessment of stress hormones, behavior, and neural activation following trauma onset. |
| Accumulated Effects | Allows for long-lasting outcomes of prolonged (chronic) stress, often applied in form of various stressors exchanging each other. |
| Ethical Ease | Typically low-intensity and non-invasive, making these paradigms suitable for early-stage studies and high-throughput screening. |
| Limitations | Description |
| Transient Responses | Effects may be short-lived and resolve themselves quickly, limiting relevance for chronic PTSD modeling. |
| Variability | Different stressors and exposure designs may yield inconsistent results across labs and strains. |
| Limited Scope | Models are not well-suited for studying delayed PTSD symptoms, emotional dysregulation, or fear generalization. |
| Protocol Duration | Anxiety-Like Behavior | Cortisol Dysregulation | Neuroinflammation | Translational Validity |
|---|---|---|---|---|
| 7 days | Moderate | Transient elevation | Mild IL-1β increase | Limited |
| 14–15 days | Severe/Persistent | Sustained hypercortisolemia | TNF-α, IL-6 upregulation | High |
| 21+ days | Variable | Hypocortisolemia | Adaptive tolerance | Low |
| Limitation | Type | Impact | Comparison to Mammals | Recommended Solutions |
|---|---|---|---|---|
| Simpler Nervous System | Physiological | Limits replication of complex PTSD symptoms (e.g., intrusive memories) | Lacks prefrontal cortex; divergent telencephalon organization [4] | Combine with mammalian models for higher-order cognitive validation |
| HPI vs. HPA Axis Differences | Endocrinological and Genetic | Altered cortisol kinetics and feedback regulation | Faster cortisol peaks (15–30 min vs. 30–60 min in humans) [30] | Use multi-species biomarker panels (CRH, GR, BDNF) |
| Methodological Variability | Experimental Control | Compromises reproducibility (e.g., CUS/UCS protocols differ across labs) | Less standardized than rodent models [2] | Adopt ARRIVE 2.0 guidelines; share protocol repositories |
| Sex-Specific Knowledge Gaps | Physiological | Overlooks sex differences in prevalence/mechanisms | Human PTSD shows 2:1 to 3:1 female predominance [292] | Stratify analyses by sex; study estrogen/androgen interactions |
| Environmental Sensitivity | Environmental | Confounds stress responses (pH, temperature, light) | More dependent on aquatic conditions | Standardize housing |
| Behavioral Simplification | Behavioral | Anxiety-like behaviors ≠ PTSD psychopathology | No equivalents to nightmares/guilt | Integrate AI-driven ethograms tracking fear generalization |
| Regenerative Capacity | Neural | May underestimate chronic neural sequelae | Zebrafish CNS regenerates; mammals (including humans) form glial scars [291] | Combine with rodent injury models |
| Parameter | Recommended Standard | Rationale/Data Support |
|---|---|---|
| Tank Size and Shape | NTT: 28 × 15 × 7 cm; OFT: 20 × 20 × 10 cm; LDT: 25 × 15 × 10 cm | No systematic data; proposed to ensure consistent arena geometry. |
| Lighting Conditions | 14 h light (200 lux white LED)/10 h dark, 28 °C water | No systematic data; aligns circadian cues across studies. |
| Stocking Density | 3–5 fish / L | No systematic data; balances social context with minimized crowding. |
| Water Quality | pH 7.0–7.5; conductivity 300–500 µS/cm; dissolved O2 > 6 mg/L | No systematic data; maintains baseline physiological homeostasis. |
| Stress Exposure Duration | 10–15 days; 2 stressors/day; 10–15 min each, random times | Supported by our systematic review as optimal for multi-dimensional PTSD-like phenotypes. |
| PostStress Testing Windows | 24 h, 48 h, 7 days (acute, subacute, persistent) | Common timepoints in CUS/UCS studies; ensures cross-study comparability. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarapultsev, A.; Komelkova, M.; Lookin, O.; Khatsko, S.; Zhdanov, A.; Fedorov, S.; Gusev, E.; Trofimov, A.; Tokay, T.; Hu, D. Zebrafish as a Model Organism for Post-Traumatic Stress Disorder: Insights into Stress Mechanisms and Behavioral Assays. Biology 2025, 14, 939. https://doi.org/10.3390/biology14080939
Sarapultsev A, Komelkova M, Lookin O, Khatsko S, Zhdanov A, Fedorov S, Gusev E, Trofimov A, Tokay T, Hu D. Zebrafish as a Model Organism for Post-Traumatic Stress Disorder: Insights into Stress Mechanisms and Behavioral Assays. Biology. 2025; 14(8):939. https://doi.org/10.3390/biology14080939
Chicago/Turabian StyleSarapultsev, Alexey, Maria Komelkova, Oleg Lookin, Sergey Khatsko, Alexander Zhdanov, Stanislav Fedorov, Evgenii Gusev, Alexander Trofimov, Tursonjan Tokay, and Desheng Hu. 2025. "Zebrafish as a Model Organism for Post-Traumatic Stress Disorder: Insights into Stress Mechanisms and Behavioral Assays" Biology 14, no. 8: 939. https://doi.org/10.3390/biology14080939
APA StyleSarapultsev, A., Komelkova, M., Lookin, O., Khatsko, S., Zhdanov, A., Fedorov, S., Gusev, E., Trofimov, A., Tokay, T., & Hu, D. (2025). Zebrafish as a Model Organism for Post-Traumatic Stress Disorder: Insights into Stress Mechanisms and Behavioral Assays. Biology, 14(8), 939. https://doi.org/10.3390/biology14080939







