Eyes Wide Open: Assessing Early Visual Behavior in Zebrafish Larvae
Simple Summary
Abstract
1. Introduction
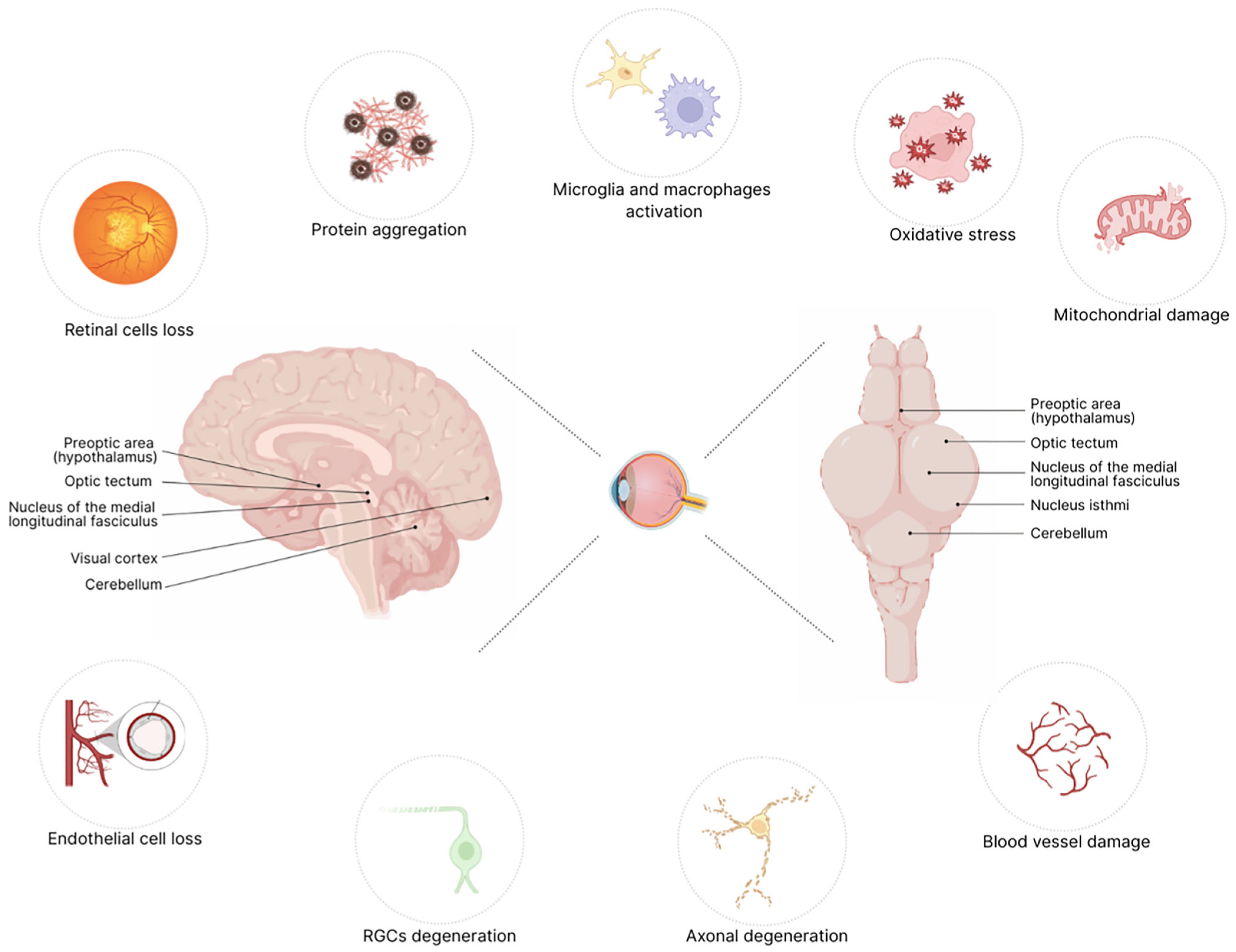
2. Mechanisms of Retinal Degeneration in Neurodegenerative Diseases
3. Zebrafish Retinal Development Overview
4. Behavioral Assays to Study Retinal Function in Zebrafish from 24 h Post-Fertilization to the Juvenile Stage
4.1. Visual Background Adaptation (VBA) Assay
4.2. Prey Tracking and Capture Assay
4.3. Escape Response (ER) Assay
4.4. Fundamental Visual Reflexes: The OKR and OMR
4.5. Visual Startle Response (VSR) and Visual Motor Response (VMR) Assays
4.6. The Light/Dark Preference Test in Zebrafish: Insights into Visual Processing and Neural Adaptation
4.7. Habituation to Repeated Stimuli: A Model for Sensory Adaptation and Neural Plasticity
4.8. The Phototactic Behavior Assay: Evaluating Visual Function and Light-Driven Responses
4.9. Color Perception Assay: Investigating Wavelength Sensitivity and Visual Processing
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection Between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.E.; Balendra, S.I.; Almonte, M.T.; Cordeiro, M.F. Retinal correlates of neurological disorders. Ther. Adv. Chronic Dis. 2019, 10, 2040622319882205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Paracha, S.S.; Agrawal, S.; Wu, Z.; Sung, C.-H. Recent Advances in Visual Dysfunction and Ocular Biomarkers in Neurological Disorders. Eye Brain 2025, 17, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage 2011, 54, S204–S217. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.P.; Pires, L.A.; Cruzeiro, M.M.; Almeida, A.L.M.; Martins, L.C.; Martins, P.N.; Shigaeff, N.; Vale, T.C. Optical coherence tomography in neurodegenerative disorders. Arq. Neuro-Psiquiatria 2022, 80, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Salobrar-García, E.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Rojas, P.; Vazirani, R.; Amarante, C.; Yubero, R.; Gil, P.; Pinazo-Durán, M.D.; et al. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PLoS ONE 2019, 14, e0220535. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Golomb, A. Vision in Alzheimer’s Disease. Gerontologist 1995, 35, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Iseri, P.K.; Altinaş, Ö.; Tokay, T.; Yüksel, N. Relationship Between Cognitive Impairment and Retinal Morphological and Visual Functional Abnormalities in Alzheimer Disease. J. Neuro-Ophthalmol. 2006, 26, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Johnson, N.; Ayala, A.; Thompson, A.C. Optical Coherence Tomography in Patients with Alzheimer’s Disease: What Can It Tell Us? Eye Brain 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, P.; Lenka, A.; Pal, P.K. The role of Optical Coherence Tomography in Parkinsonism: A critical review. J. Neurol. Sci. 2019, 403, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Visual signs and symptoms of Parkinson’s disease. Clin. Exp. Optom. 2007, 91, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Feldman, R.G.; Adelberg, D.; Kayne, H. Abnormalities in color vision and contrast sensitivity in Parkinson’s disease. Neurology 1992, 42, 887. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, P.; Battu, R.; Khanna, A.; Lenka, A.; Yadav, R.; Pal, P.K. Optical coherence tomography as a tool to evaluate retinal changes in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tao, J.; Li, J. Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Neurol. 2020, 28, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, K.; Li, F.; Xu, Y.; Shen, L.; Xu, H. Combination of optical coherence tomography (OCT) and OCT angiography increases diagnostic efficacy of Parkinson’s disease. Quant. Imaging Med. Surg. 2020, 10, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Manni, G.; Spadaro, M.; Colacino, G.; Restuccia, R.; Marchi, S.; Bucci, M.G.; Pierelli, F. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2520–2527. [Google Scholar]
- Garcia-Martin, E.; Pueyo, V.; Ara, J.; Almarcegui, C.; Martin, J.; Pablo, L.; Dolz, I.; Sancho, E.; Fernandez, F. Effect of optic neuritis on progressive axonal damage in multiple sclerosis patients. Mult. Scler. J. 2011, 17, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Kersten, H.M.; Danesh-Meyer, H.V.; Kilfoyle, D.H.; Roxburgh, R.H. Optical coherence tomography findings in Huntington’s disease: A potential biomarker of disease progression. J. Neurol. 2015, 262, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Beato, J.; Monteiro, A.; Costa, A.; Penas, S.; Guimarães, J.; Reis, F.F.; Garrett, C. Spectral-Domain Optical Coherence Tomography as a Potential Biomarker in Huntington’s Disease. Mov. Disord. 2016, 31, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Sevim, D.G.; Unlu, M.; Gultekin, M.; Karaca, C. Retinal single-layer analysis with optical coherence tomography shows inner retinal layer thinning in Huntington’s disease as a potential biomarker. Int. Ophthalmol. 2018, 39, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Hata, T.; Fukao, T.; Fukao, T.; Chen, W.; Kurita, T.; Natori, T.; Takiyama, Y. MFN2-related Charcot-Marie-Tooth Disease with Atypical Ocular Manifestations. Intern. Med. 2021, 60, 3969–3974. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ruan, G.; Yang, S.; Li, H.; Sun, Z.; Tian, B.; Yan, P.; Li, Y.; Yang, H.; Zhong, Y.; et al. Ocular manifestations of mitochondrial neurogastrointestinal encephalomyopathy: A case report and literature review. Am. J. Med. Genet. Part A 2023, 191, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Huang, S.-C.; Magnani, G.; Ambrosi, A.; Comi, G.; Leocani, L. Optical Coherence Tomography Reveals Retinal Neuroaxonal Thinning in Frontotemporal Dementia as in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 56, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.L.; Greaves, C.V.; Convery, R.S.; Bocchetta, M.; Warren, J.D.; Kaski, D.; Rohrer, J.D. Eye movements in frontotemporal dementia: Abnormalities of fixation, saccades and anti-saccades. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2021, 7, e12218. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Robbins, C.B.; Joseph, S.; Hemesat, A.; Kundu, A.; Ma, J.P.; Haystead, A.; Winslow, L.; Agrawal, R.; Johnson, K.G.; et al. Angioarchitectural alterations in the retina and choroid in frontotemporal dementia. PLoS ONE 2024, 19, e0312118. [Google Scholar] [CrossRef]
- Lee, M.J.; Abraham, A.G.; Swenor, B.K.; Sharrett, A.R.; Ramulu, P.Y. Application of Optical Coherence Tomography in the Detection and Classification of Cognitive Decline. J. Curr. Glaucoma Pract. 2018, 12, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dağ, E.; Örnek, N.; Örnek, K.; Erbahçeci-Timur, I.E. Optical Coherence Tomography and Visual Field Findings in Patients with Friedreich Ataxia. J. Neuro-Ophthalmol. 2014, 34, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, M.H.; Bartmann, A.P.; Clayton, L.M.S.; Nethisinghe, S.; Pfundt, R.; Chapple, J.P.; Reilly, M.M.; Manji, H.; Wood, N.J.; Bremner, F.; et al. Optical coherence tomography in autosomal recessive spastic ataxia of Charlevoix-Saguenay. Brain 2018, 141, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Joo, K.; Woo, S.J. Ophthalmic Manifestations and Genetics of the Polyglutamine Autosomal Dominant Spinocerebellar Ataxias: A Review. Front. Neurosci. 2020, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R. Visual evoked potentials in spinocerebellar degenerations. Clin. Neurol. Neurosurg. 1983, 85, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, H.; Ishiguro, H.; Shibano, K.; Hara, K.; Sugawara, M.; Enomoto, K.; Miyata, H. Primary degeneration of oculomotor, motor, and somatosensory systems and auditory and visual pathways in spinocerebellar ataxia type 7: A clinicopathological study in a Japanese autopsy case. Neuropathology 2022, 43, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Ihl, T.; Kadas, E.M.; Oberwahrenbrock, T.; Endres, M.; Klockgether, T.; Schroeter, J.; Brandt, A.U.; Paul, F.; Minnerop, M.; Doss, S.; et al. Investigation of Visual System Involvement in Spinocerebellar Ataxia Type 14. Cerebellum 2020, 19, 469–482. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, J.L.; Rezende Filho, F.M.; Sallum, J.M.; França Jr, M.C.; Pedroso, J.L.; Barsottini, O.G.P. Ophthalmological changes in hereditary spastic paraplegia and other genetic diseases with spastic paraplegia. J. Neurol. Sci. 2020, 409, 116620. [Google Scholar] [CrossRef] [PubMed]
- Orsini, A.; Ferrari, D.; Riva, A.; Santangelo, A.; Macrì, A.; Freri, E.; Canafoglia, L.; D’aNiello, A.; Di Gennaro, G.; Massimetti, G.; et al. Ocular phenotype and electroretinogram abnormalities in Lafora disease and correlation with disease stage. J. Neurol. 2022, 269, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Zivadinov, R.; Ramanathan, M.; Weinstock-Guttman, B. Optical coherence tomography and neurodegeneration: Are eyes the windows to the brain? Expert Rev. Neurother. 2016, 16, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Gupta, V.B.; Chitranshi, N.; Gangoda, S.; Wall, R.V.; Abbasi, M.; Golzan, M.; Dheer, Y.; Shah, T.; Avolio, A.; et al. One protein, multiple pathologies: Multifaceted involvement of amyloid β in neurodegenerative disorders of the brain and retina. Cell. Mol. Life Sci. 2016, 73, 4279–4297. [Google Scholar] [CrossRef] [PubMed]
- Firmani, G.; Salducci, M.; Testa, F.; Covelli, G.P.; Sagnelli, P.; Lambiase, A. Ocular Biomarkers in Alzheimer’s Disease: Insights into Early Detection Through Eye-Based Diagnostics—A Literature Review. La Clin. Ter. 2024, 175, 352–361. [Google Scholar] [CrossRef]
- Hong, Y.; Luo, Y. Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals 2021, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.C.L.; MacDonald, I.M.; Allison, W.T. Zebrafish Models of Photoreceptor Dysfunction and Degeneration. Biomolecules 2021, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Chang, C.-Y.; Kao, A.; Hsi, B.; Lee, S.-H.; Chen, Y.-H.; Wang, I.-J. Hypoxia-Induced Retinal Neovascularization in Zebrafish Embryos: A Potential Model of Retinopathy of Prematurity. PLoS ONE 2015, 10, e0126750. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira-Mello, L.; Baronio, D.; Panula, P. Zebrafish embryonically exposed to valproic acid present impaired retinal development and sleep behavior. Autism Res. 2023, 16, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Keller Sarmiento, I.J.; Bustos, B.I.; Blackburn, J.; Hac, N.E.; Ruzhnikov, M.; Monroe, M.; Levy, R.J.; Kinsley, L.; Li, M.; Silani, V.; et al. De novo FRMD5 Missense Variants in Patients with Childhood-Onset Ataxia, Prominent Nystagmus, and Seizures. Mov. Disord. 2024, 39, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Gestri, G.; Link, B.A.; Neuhauss, S.C. The visual system of zebrafish and its use to model human ocular Diseases. Dev. Neurobiol. 2011, 72, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—on the move towards ophthalmological research. Eye 2014, 28, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a Better Understanding of Human Eye Disease: Insights from the Zebrafish, Danio rerio. In Progress in Molecular Biology and Translational Science, 1st ed.; Chang, K., Min, K.-T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 100, pp. 287–330. [Google Scholar]
- Avanesov, A.; Malicki, J. Analysis of the Retina in the Zebrafish Model. In Methods in Cell Biology, 1st ed.; Harris, W.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 100, pp. 153–204. [Google Scholar]
- Sharma, S.; You, Y. Editorial: Retinal Changes in Neurological Diseases. Front. Neurosci. 2022, 15, 813044. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Asanad, S.; Chan, J.W.; Singer, M.B.; Zhang, J.; Sharifi, M.; Khansari, M.M.; Abdolahi, F.; Shi, Y.; Biffi, A.; et al. Past, present and future role of retinal imaging in neurodegenerative disease. Prog. Retin. Eye Res. 2021, 83, 100938. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Wong, R. Development of Cell Types and Synaptic Connections in the Retina by Josh Morgan and Rachel Wong. In Webvision: The Organization of the Retina and Visual System, 1st ed.; Kolb, H., Fernandez, E., Jones, B., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995; Available online: https://webvision.med.utah.edu/book/part-vi-development-of-cell-types-and-synaptic-connections-in-the-retina/development-of-cell-types-and-synaptic-connections-in-the-retina/ (accessed on 29 May 2025).
- Boff, J.M.; Shrestha, A.P.; Madireddy, S.; Viswaprakash, N.; Della Santina, L.; Vaithianathan, T. The Interplay Between Neurotransmitters and Calcium Dynamics in Retinal Synapses During Development, Health, and Disease. Int. J. Mol. Sci. 2024, 25, 2226. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, Y.; Hahn, J.; Sappington, A.; Stemmer, M.; Fernandes, A.M.; Helmbrecht, T.O.; Lele, S.; Butrus, S.; Laurell, E.; Arnold-Ammer, I.; et al. Molecular classification of zebrafish retinal ganglion cells links genes to cell types to behavior. Neuron 2020, 109, 645–662.e9. [Google Scholar] [CrossRef] [PubMed]
- Cogan, D.G.; Kuwabara, T. Comparison of retinal and cerebral vasculature in trypsin digest preparations. Br. J. Ophthalmol. 1984, 68, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, S.; Yang, L. Methylmercury-induced visual deficits involve loss of GABAergic cells in the zebrafish embryo retina. Sci. Total Environ. 2025, 959, 178329. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Ackerman, S.L. Oxidative Stress, Cell Cycle, and Neurodegeneration. J. Clin. Investig. 2003, 111, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Moda, F.; Ciullini, A.; Dellarole, I.L.; Lombardo, A.; Campanella, N.; Bufano, G.; Cazzaniga, F.A.; Giaccone, G. Secondary Protein Aggregates in Neurodegenerative Diseases: Almost the Rule Rather than the Exception. Front. Biosci. 2023, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khoury, Z.S.; Sohail, F.; Wang, J.; Mendoza, M.; Raake, M.; Silat, M.T.; Bathinapatta, M.R.; Sadeghzadegan, A.; Meghana, P.; Paul, J. Neuroinflammation: A Critical Factor in Neurodegenerative Disorders. Cureus 2024, 16, e62310. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.-W.; Zhang, D.-D.; Fan, Z.-G. Blood-retinal barrier as a converging pivot in understanding the initiation and development of retinal diseases. Chin. Med. J. 2020, 133, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.D.; Inglese, M.; Casaccia, P. Axonal Damage in Multiple Sclerosis. Mt. Sinai J. Med. J. Transl. Pers. Med. 2011, 78, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, Y.; Kitaoka, Y. Molecular mechanisms of retinal ganglion cell degeneration in glaucoma and future prospects for cell body and axonal protection. Front. Cell. Neurosci. 2013, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.R.; Scasny, A.J.; Kolodkin, A.L. Development of the vertebrate retinal direction-selective circuit. Dev. Biol. 2021, 477, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Seritrakul, P.; Gross, J.M. Genetic and epigenetic control of retinal development in zebrafish. Curr. Opin. Neurobiol. 2019, 59, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Easter, S.S., Jr.; Nicola, G.N. The Development of Vision in the Zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, V.C.; Neuhauss, S.C.F. Visual Behavior in Zebrafish. Zebrafish 2006, 3, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.L.; Geathers, J.S.; Weber, S.R.; Grillo, M.A.; Barber, A.J.; Sundstrom, J.M.; Grillo, S.L. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells 2021, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Fadool, J. Studying rod photoreceptor development in zebrafish. Physiol. Behav. 2005, 86, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, T.; Schröder, C.; Nevala, N.E.; Berens, P.; Baden, T. Fovea-like Photoreceptor Specializations Underlie Single UV Cone Driven Prey-Capture Behavior in Zebrafish. Neuron 2020, 107, 320–337.e6. [Google Scholar] [CrossRef] [PubMed]
- Baier, H.; Scott, E.K. The Visual Systems of Zebrafish. Annu. Rev. Neurosci. 2024, 47, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hinz, J.; Zhang, Y.; Thiele, T.R.; Arrenberg, A.B. Parallel Channels for Motion Feature Extraction in the Pretectum and Tectum of Larval Zebrafish. Cell Rep. 2020, 30, 442–453.e6. [Google Scholar] [CrossRef] [PubMed]
- Isa, T.; Marquez-Legorreta, E.; Grillner, S.; Scott, E.K. The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action. Curr. Biol. 2021, 31, R741–R762. [Google Scholar] [CrossRef] [PubMed]
- Baier, H.; Wullimann, M.F. Anatomy and function of retinorecipient arborization fields in zebrafish. J. Comp. Neurol. 2021, 529, 3454–3476. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Labrada, R.; Vázquez-Mojena, Y.; Velázquez-Pérez, L. Eye Movement Abnormalities in Neurodegenerative Diseases. In Eye Motility, 1st ed.; Mravicic, I., Ed.; IntechOpen: London, UK, 2019; pp. 65–84. [Google Scholar]
- Bollmann, J.H. The Zebrafish Visual System: From Circuits to Behavior. Annu. Rev. Vis. Sci. 2019, 5, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.G.S.; Lopes-Ferreira, M.; Lima, C. An Overview Towards Zebrafish Larvae as a Model for Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 5387. [Google Scholar] [CrossRef] [PubMed]
- Tuma, M.C.; Gelfand, V.I. Molecular Mechanisms of Pigment Transport in Melanophores. Pigment Cell Res. 1999, 12, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wagle, M.; Mathur, P.; Guo, S. Corticotropin-Releasing Factor Critical for Zebrafish Camouflage Behavior Is Regulated by Light and Sensitive to Ethanol. J. Neurosci. 2011, 31, 214–224. [Google Scholar] [CrossRef] [PubMed]
- A Prakash, B.; Toro, C.P. Modulating the Zebrafish Camouflage Response Pathway to Illustrate Neuroendocrine Control over a Robust and Quantifiable Behavior. J. Undergrad. Neurosci. Educ. 2019, 18, A57–A64. [Google Scholar]
- Mizusawa, K.; Kasagi, S.; Takahashi, A. Melanin-concentrating hormone is a major substance mediating light wavelength-dependent skin color change in larval zebrafish. Gen. Comp. Endocrinol. 2018, 269, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.Q.; McCarthy, W.R.; Zhang, H.; Adolph, A.R.; Li, L. Behavioral visual responses of wild-type and hypopigmented zebrafish. Vis. Res. 2002, 42, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Kawakami, K. Prey capture in zebrafish larvae serves as a model to study cognitive functions. Front. Neural Circuits 2013, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.I.; Goodhill, G.J. From perception to behavior: The neural circuits underlying prey hunting in larval zebrafish. Front. Neural Circuits 2023, 17, 1087993. [Google Scholar] [CrossRef] [PubMed]
- Borla, M.A.; Palecek, B.; Budick, S.; O’mAlley, D.M. Prey Capture by Larval Zebrafish: Evidence for Fine Axial Motor Control. Brain Behav. Evol. 2002, 60, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Jaesiri, O.-M.; Lazarte, I.P.; Li, Y.; Tian, G.; Zhao, P.; Zhao, Y.; Ho, V.D.; Semmelhack, J.L. Zebrafish larvae use stimulus intensity and contrast to estimate distance to prey. Curr. Biol. 2023, 33, 3179–3191.e4. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Lazarte, I.P.; Jaesiri, O.-M.; Zhao, P.; Semmelhack, J.L. Protocol for using UV stimuli to evoke prey capture strikes in head-fixed zebrafish larvae. STAR Protoc. 2023, 5, 102780. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, C.S.; Grossrubatscher, I.; Chávez, M.; Hoagland, A.; Huth, A.R.; Carroll, E.C.; Prendergast, A.; Qu, T.; Gallant, J.L.; Wyart, C.; et al. Experience, circuit dynamics, and forebrain recruitment in larval zebrafish prey capture. eLife 2020, 9, 56619. [Google Scholar] [CrossRef] [PubMed]
- Bianco, I.H.; Kampff, A.R.; Engert, F. Prey Capture Behavior Evoked by Simple Visual Stimuli in Larval Zebrafish. Front. Syst. Neurosci. 2011, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Bianco, I.H.; Engert, F. Visuomotor Transformations Underlying Hunting Behavior in Zebrafish. Curr. Biol. 2015, 25, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Förster, D.; Helmbrecht, T.O.; Mearns, D.S.; Jordan, L.; Mokayes, N.; Baier, H. Retinotectal circuitry of larval zebrafish is adapted to detection and pursuit of prey. eLife 2020, 9, 58596. [Google Scholar] [CrossRef] [PubMed]
- Smear, M.C.; Tao, H.W.; Staub, W.; Orger, M.B.; Gosse, N.J.; Liu, Y.; Takahashi, K.; Poo, M.-M.; Baier, H. Vesicular Glutamate Transport at a Central Synapse Limits the Acuity of Visual Perception in Zebrafish. Neuron 2007, 53, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dowling, J.E. A dominant form of inherited retinal degeneration caused by a non-photoreceptor cell-specific mutation. Proc. Natl. Acad. Sci. USA 1997, 94, 11645–11650. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Legorreta, E.; Piber, M.; Scott, E.K. Visual Escape in Larval Zebrafish: Stimuli, Circuits, and Behavior. In Behavioral and Neural Genetics of Zebrafish, 1st ed.; Gerlai, R.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–71. [Google Scholar]
- Li, L.; Dowling, J.E. Zebrafish visual sensitivity is regulated by a circadian clock. Vis. Neurosci. 1998, 15, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dowling, J.E. Disruption of the Olfactoretinal Centrifugal Pathway May Relate to the Visual System Defect in night blindness bMutant Zebrafish. J. Neurosci. 2000, 20, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Hale, D.; Reich, S.; Gold, D. Optokinetic Nystagmus: Five Reasons Why Every Neurologist Should Evaluate It (P3-9.006). Neurology 2023, 100, 1696. [Google Scholar] [CrossRef]
- Maurer, C.M.; Huang, Y.-Y.; Neuhauss, S.C.F. Application of zebrafish oculomotor behavior to model human disorders. Prog. Neurobiol. 2011, 22, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Neuhauss, S.C.F. The optokinetic response in zebrafish and its applications. Front. Biosci. 2008, 13, 1899. [Google Scholar] [CrossRef] [PubMed]
- Branchek, T.; Bremiller, R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J. Comp. Neurol. 1984, 224, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Kubo, F. Circuit Organization Underlying Optic Flow Processing in Zebrafish. Front. Neural Circuits 2021, 15, 709048. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Zebrafish mutants: Behavioral genetic studies of visual system defects. Dev. Dyn. 2001, 221, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rodwell, V.; Patil, M.; Kuht, H.J.; Neuhauss, S.C.F.; Norton, W.H.J.; Thomas, M.G. Zebrafish Optokinetic Reflex: Minimal Reporting Guidelines and Recommendations. Biology 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Kist, A.M.; Portugues, R. Optomotor Swimming in Larval Zebrafish Is Driven by Global Whole-Field Visual Motion and Local Light-Dark Transitions. Cell Rep. 2019, 29, 659–670.e3. [Google Scholar] [CrossRef] [PubMed]
- Naumann, E.A.; Fitzgerald, J.E.; Dunn, T.W.; Rihel, J.; Sompolinsky, H.; Engert, F. From Whole-Brain Data to Functional Circuit Models: The Zebrafish Optomotor Response. Cell 2016, 167, 947–960.e20. [Google Scholar] [CrossRef] [PubMed]
- Orger, M.B.; Baier, H. Channeling of red and green cone inputs to the zebrafish optomotor response. Vis. Neurosci. 2005, 22, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chen, J.; Rajagopalan, A.; Chen, D.F.; Cho, K.-S. Testing Visual Function by Assessment of the Optomotor Reflex in Glaucoma. In Glaucoma: Methods and Protocols, 2nd ed.; Jakobs, T., Liton, P.B., Eds.; Springer: New York, NY, USA, 2024; Volume 2858, pp. 219–227. [Google Scholar]
- Portugues, R.; Engert, F. The neural basis of visual behaviors in the larval zebrafish. Curr. Opin. Neurobiol. 2009, 19, 644–647. [Google Scholar] [CrossRef] [PubMed]
- LeFauve, M.K.; Rowe, C.J.; Crowley-Perry, M.; Wiegand, J.L.; Shapiro, A.G.; Connaughton, V.P. Using a variant of the optomotor response as a visual defect detection assay in zebrafish. J. Biol. Methods 2021, 8, e144. [Google Scholar] [CrossRef] [PubMed]
- Neuhauss, S.C.F.; Biehlmaier, O.; Seeliger, M.W.; Das, T.; Kohler, K.; Harris, W.A.; Baier, H. Genetic Disorders of Vision Revealed by a Behavioral Screen of 400 Essential Loci in Zebrafish. J. Neurosci. 1999, 19, 8603–8615. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Dunn, C.; Ramos, M.F. Zebrafish as an Animal Model for Ocular Toxicity Testing: A Review of Ocular Anatomy and Functional Assays. Toxicol. Pathol. 2020, 49, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Goodbourn, P.; Sztal, T.; Jusuf, P.R. Neural Endophenotype Assessment in Zebrafish Larvae Using Optomotor and ZebraBox Locomotion Assessment. In Neurobiology, 1st ed.; Dworkin, S., Ed.; Springer: New York, NY, USA, 2023; Volume 2746, pp. 213–224. [Google Scholar]
- Hill, B.N.; Britton, K.N.; Hunter, D.L.; Olin, J.K.; Lowery, M.; Hedge, J.M.; Knapp, B.R.; Jarema, K.A.; Rowson, Z.; Padilla, S. Inconsistencies in variable reporting and methods in larval zebrafish behavioral assays. Neurotoxicol. Teratol. 2023, 96, 107163. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, J. Effects of abnormal lighting on the development of zebrafish visual behavior. Behav. Brain Res. 2000, 116, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Emran, F.; Rihel, J.; Dowling, J.E. A Behavioral Assay to Measure Responsiveness of Zebrafish to Changes in Light Intensities. J. Vis. Exp. 2008, 20, e923. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, G.; Jelfs, B.; Carmer, R.; Venkatraman, P.; Ghadami, M.; Brown, S.A.; Pang, C.P.; Leung, Y.F.; Chan, R.H.M.; et al. Computational classification of different wild-type zebrafish strains based on their variation in light-induced locomotor response. Comput. Biol. Med. 2016, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ingebretson, J.J.; Masino, M.A. Quantification of locomotor activity in larval zebrafish: Considerations for the design of high-throughput behavioral studies. Front. Neural Circuits 2013, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Colwill, R.M.; Creton, R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav. Process. 2011, 86, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carmer, R.; Zhang, G.; Venkatraman, P.; Brown, S.A.; Pang, C.-P.; Zhang, M.; Ma, P.; Leung, Y.F. Statistical Analysis of Zebrafish Locomotor Response. PLoS ONE 2015, 10, e0139521. [Google Scholar] [CrossRef] [PubMed]
- Deeti, S.; O’FArrell, S.; Kennedy, B.N. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiang, L.; Liu, Y.; Venkatraman, P.; Chong, L.; Cho, J.; Bonilla, S.; Jin, Z.-B.; Pang, C.P.; Ko, K.M.; et al. Correction: A Naturally-Derived Compound Schisandrin B Enhanced Light Sensation in the pde6c Zebrafish Model of Retinal Degeneration. PLoS ONE 2016, 11, e0154552. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.; MacPhail, R.; Hunter, D.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Ma, Y.; Ma, J.; Yu, H.; Zhang, K.; Jin, L.; Yang, Q.; Sun, D.; Wu, D. Rapid Assessment of Ocular Toxicity from Environmental Contaminants Based on Visually Mediated Zebrafish Behavior Studies. Toxics 2023, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.; Kearn, J.; Parker, M.O. Protocol for investigating light/dark locomotion in larval stage zebrafish using a standardized behavioral assay. STAR Protoc. 2024, 5, 103346. [Google Scholar] [CrossRef] [PubMed]
- Beppi, C.; Beringer, G.; Straumann, D.; Bögli, S.Y. Light-stimulus intensity modulates startle reflex habituation in larval zebrafish. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.C.; Reichl, J.; Song, M.Y.; Dearinger, A.D.; Moridzadeh, N.; Lu, E.D.; Pearce, K.; Esdin, J.; Glanzman, D.L. Habituation of the C-Start Response in Larval Zebrafish Exhibits Several Distinct Phases and Sensitivity to NMDA Receptor Blockade. PLoS ONE 2011, 6, e29132. [Google Scholar] [CrossRef] [PubMed]
- Fotowat, H.; Engert, F. Neural circuits underlying habituation of visually evoked escape behaviors in larval zebrafish. eLife 2023, 12, e82916. [Google Scholar] [CrossRef] [PubMed]
- Agetsuma, M.; Aizawa, H.; Aoki, T.; Nakayama, R.; Takahoko, M.; Goto, M.; Sassa, T.; Amo, R.; Shiraki, T.; Kawakami, K.; et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010, 13, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Granato, M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. Exp. Biol. 2007, 210, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.B.; Li, J.M.; Orger, M.B.; Robson, D.N.; Schier, A.F.; Engert, F.; Portugues, R. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 2012, 485, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.C.; Pearce, K.C.; Choe, R.C.; Alzagatiti, J.B.; Yeung, A.K.; Bill, B.R.; Glanzman, D.L. Long-term habituation of the C-start escape response in zebrafish larvae. Neurobiol. Learn. Mem. 2016, 134, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. The Light–Dark Preference Test for Larval Zebrafish. In Zebrafish Protocols for Neurobehavioral Research, 1st ed.; Kalueff, A.V., Stewart, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 66, pp. 21–35. [Google Scholar]
- Guggiana-Nilo, D.A.; Engert, F. Properties of the Visible Light Phototaxis and UV Avoidance Behaviors in the Larval Zebrafish. Front. Behav. Neurosci. 2016, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Balay, S.D.; Widen, S.A.; Waskiewicz, A.J. Analysis of zebrafish cryptochrome 2 and 4 expression in UV cone photoreceptors. Gene Expr. Patterns 2020, 35, 119100. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-F.; Chen, Z.-F.; Lin, Z.-C.; Liao, X.-L.; Zou, T.; Qi, Z.; Cai, Z. Toxic effects of triclocarban on larval zebrafish: A focus on visual dysfunction. Aquat. Toxicol. 2021, 241, 106013. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.B.; Deb, D.; Bahl, A.; Engert, F. Algorithms underlying flexible phototaxis in larval zebrafish. J. Exp. Biol. 2021, 224, jeb238386. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.R.; Leathery, M.; Peck, M.; Barr, A.; Cunill, A.; Hageter, J.; Horstick, E.J. Light wavelength modulates search behavior performance in zebrafish. Sci. Rep. 2024, 14, 16533. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Nelson, R.; Connaughton, V.P. Color Processing in Zebrafish Retina. Front. Cell. Neurosci. 2018, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Avdesh, A.; Martin-Iverson, M.T.; Mondal, A.; Chen, M.; Askraba, S.; Morgan, N.; Lardelli, M.; Groth, D.M.; Verdile, G.; Martins, R.N. Evaluation of Color Preference in Zebrafish for Learning and Memory. J. Alzheimer’s Dis. 2012, 28, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Siregar, P.; Juniardi, S.; Audira, G.; Lai, Y.-H.; Huang, J.-C.; Chen, K.H.-C.; Chen, J.-R.; Hsiao, C.-D. Method Standardization for Conducting Innate Color Preference Studies in Different Zebrafish Strains. Biomedicines 2020, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Ryu, J.-H.; Choi, T.-I.; Bae, Y.-K.; Lee, S.; Kang, H.J.; Kim, C.-H. Innate Color Preference of Zebrafish and Its Use in Behavioral Analyses. Mol. Cells 2016, 39, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.J.Y.; Nevala, N.E.; Yoshimatsu, T.; Osorio, D.; Nilsson, D.-E.; Berens, P.; Baden, T. Zebrafish Differentially Process Color Across Visual Space to Match Natural Scenes. Curr. Biol. 2018, 28, 2018–2032.e5. [Google Scholar] [CrossRef] [PubMed]
- A Blose, B.; Lai, A.; Crosta, C.; Thompson, J.L.; Silverstein, S.M. Retinal Neurodegeneration as a Potential Biomarker of Accelerated Aging in Schizophrenia Spectrum Disorders. Schizophr. Bull. 2023, 49, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yan, Y.; Wu, X.; Geng, Z.; Wu, Y.; Xiao, G.; Wang, L.; Zhou, S.; Wei, L.; Wang, K.; et al. The correlation of retinal neurodegeneration and brain degeneration in patients with Alzheimer’s disease using optical coherence tomography angiography and MRI. Front. Aging Neurosci. 2023, 15, 1089188. [Google Scholar] [CrossRef] [PubMed]
- Chaitanuwong, P.; Singhanetr, P.; Chainakul, M.; Arjkongharn, N.; Ruamviboonsuk, P.; Grzybowski, A. Potential Ocular Biomarkers for Early Detection of Alzheimer’s Disease and Their Roles in Artificial Intelligence Studies. Neurol. Ther. 2023, 12, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Christinaki, E.; Kulenovic, H.; Hadoux, X.; Baldassini, N.; Van Eijgen, J.; De Groef, L.; Stalmans, I.; van Wijngaarden, P. Retinal imaging biomarkers of neurodegenerative diseases. Clin. Exp. Optom. 2021, 105, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z.; Pisani, S.; Nderitu, P.; Venkataraman, A.V.; Guu, T.-W.; Aarsland, D.; Jackson, T.; Ffytche, D. Using Retinal Diagnostics as a Biomarker for Neurodegenerative Diseases: Protocol for a Systematic Review. medRxiv 2024. 2024-05. [Google Scholar] [CrossRef]
- Souto, E.B.; Sanchez-Lopez, E.; Campos, J.R.; da Ana, R.; Espina, M.; Garcia, M.L.; Severino, P.; Batain, F.; Alves, T.F.R.; Crescencio, K.M.M.; et al. Retinal Drug Delivery: Rethinking Outcomes for the Efficient Replication of Retinal Behavior. Appl. Sci. 2020, 10, 4258. [Google Scholar] [CrossRef]
- Saleem, S.; Kannan, R.R. Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Ochenkowska, K.; Herold, A.; Samarut, É. Zebrafish Is a Powerful Tool for Precision Medicine Approaches to Neurological Disorders. Front. Mol. Neurosci. 2022, 15, 944693. [Google Scholar] [CrossRef] [PubMed]
- Link, B.A.; Collery, R.F. Zebrafish Models of Retinal Disease. Annu. Rev. Vis. Sci. 2015, 1, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Ameen-Ali, K.E.; Allen, C. The 3Rs in Zebrafish Research. In Zebrafish: A Practical Guide to Husbandry, Welfare and Research Methodology, 1st ed.; Allen, C., Mocho, J.-P., Eds.; CABI: Wallingford, UK, 2024; pp. 226–250. [Google Scholar]
- Geisler, R.; Köhler, A.; Dickmeis, T.; Strähle, U. Archiving of zebrafish lines can reduce animal experiments in biomedical research. Embo Rep. 2016, 18, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ganzen, L.; Venkatraman, P.; Pang, C.P.; Leung, Y.F.; Zhang, M. Utilizing Zebrafish Visual Behaviors in Drug Screening for Retinal Degeneration. Int. J. Mol. Sci. 2017, 18, 1185. [Google Scholar] [CrossRef] [PubMed]
- Ganzen, L.; Ko, M.J.; Zhang, M.; Xie, R.; Chen, Y.; Zhang, L.; James, R.; Mumm, J.; van Rijn, R.M.; Zhong, W.; et al. Drug screening with zebrafish visual behavior identifies carvedilol as a potential treatment for an autosomal dominant form of retinitis pigmentosa. Sci. Rep. 2021, 11, 11432. [Google Scholar] [CrossRef] [PubMed]
- Orger, M.B.; de Polavieja, G.G. Zebrafish Behavior: Opportunities and Challenges. Annu. Rev. Neurosci. 2017, 40, 125–147. [Google Scholar] [CrossRef] [PubMed]


| Disease | Retinal Alterations/Visual Dysfunction | References |
|---|---|---|
| Alzheimer’s disease (AD) | Retinal nerve fiber layer (RNFL) thinning, reduced blood perfusion, macular vessel density and inner retina thinning. | [4,5,6,7,8,9] |
| Parkinson’s Disease (PD) | RNFL thinning, optic neuritis, microcystic macular edema, cataracts, vision loss, photopic contrast sensitivity. | [10,11,12,13,14,15] |
| Multiple Sclerosis (MS) | RNFL thinning, optic neuritis, microcystic macular edema, decreased visual acuity, and nystagmus. | [16,17,18] |
| Huntington’s Disease (HD) | RNFL thinning, retinal ganglion cell loss, impaired visual processing. | [19,20,21] |
| Charcot-Marie-Tooth Disease (CMT) | Optic atrophy. | [22] |
| Mitochondrial Disorders | Ocular involvement, vision loss, ocular motility alterations. | [23] |
| Frontotemporal dementia | RNFL thinning, difficulty with eye movements, changes in visual perception. | [24,25,26] |
| Mild Cognitive impairment (MCI) | Reduced peripapillary RNFL and macular thickness, retinal microvascular density loss, correlated with cognitive impairment severity. | [5,27] |
| Hereditary Ataxias: Friedreich’s ataxia (FRDA) and spastic ataxia of Charlevoix-Saguenay (ARSACS) | Retinal ganglion cell loss and abnormal visual evoked potentials. | [5,28,29,30] |
| Spinocerebellar Ataxias (SCA) | Retinal ganglion cell loss, abnormal visual evoked potentials, progressive visual loss, macular degeneration, and optic atrophy. | [30,31,32,33] |
| Hereditary Spastic Paraparesis (HSP) | Pigmentary retinal degeneration, ophthalmoplegia, optic atrophy, cataracts, and nystagmus. | [34] |
| Lafora Disease | Rods and cones dysfunction, progressive visual impairment. | [35] |
| Test | Developmental Stage | Purpose | Method | Visual Function Assessed |
|---|---|---|---|---|
| Visual Background Adaptation (VBA) | from–3 dpf | Assesses neuroendocrine response to ambient light | In bright light, melanophores contract; in darkness, melanin disperses | Retinal ganglion cell function, light adaptation |
| Visual Motor Response (VMR) | 3–5 dpf | Assesses time to return to base activity after sudden visual stimuli | Larvae are exposed to multiple rounds of on-off light transitions, each lasting 30 min | Motion detection and visual motor coordination |
| Prey Tracking and Capture | from4 dpf | Tests visual tracking and object recognition | Larvae are introduced to moving prey (e.g., rotifers), and capture success is observed | Visual acuity, contrast sensitivity, motion tracking |
| Escape response (ER) | 5–7 dpf | Evaluates visual threat detection and sensorimotor integration | Zebrafish are exposed to a looming stimulus (e.g., black stripe in a rotating drum, virtual predator, or robotic model), and their escape behavior (latency, trajectory, response probability) is analyzed | Motion perception, threat avoidance, visual processing, and circadian modulation of visual sensitivity |
| Optokinetic Response (OKR) | from 4 to 5 dpf | Measures visual motion processing | Zebrafish are exposed to rotating striped patterns, and eye movements (nystagmus) are recorded | Retinal function, motion detection, visual acuity |
| Optomotor Response (OMR) | 5–7 dpf | Evaluates motion detection and visuomotor coordination | Zebrafish swim in response to moving patterns (e.g., drifting gratings) | Motion perception, contrast sensitivity, visual-motor integration |
| Visual Startle Response (VSR) | 5–6 dpf | Tests reaction to sudden visual stimuli | Sudden flashes or moving objects elicit a startle reflex | Motion detection, contrast sensitivity, visual-motor coordination |
| Light/Dark Preference Test | 4–5 dpf | Assesses innate preference for light or dark environments | Zebrafish are placed in a tank with light and dark zones, and their position is recorded | Light perception, photoreceptor function, scotopic/photopic vision |
| Phototactic Behavior | 6–7 dpf | Measures attraction or aversion to light stimuli | Zebrafish are given a choice between illuminated and dark areas | Light sensitivity, retinal function, visual-driven locomotion |
| Color Perception Assay | 8–9 dpf | Evaluates the ability to discriminate colors | Zebrafish are trained to associate colored stimuli with a reward, or tested for innate color preference | Cone photoreceptor function, color discrimination, photopic vision |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacich, M.; Marchese, M.; Damiani, D.; Santorelli, F.M.; Naef, V. Eyes Wide Open: Assessing Early Visual Behavior in Zebrafish Larvae. Biology 2025, 14, 934. https://doi.org/10.3390/biology14080934
Giacich M, Marchese M, Damiani D, Santorelli FM, Naef V. Eyes Wide Open: Assessing Early Visual Behavior in Zebrafish Larvae. Biology. 2025; 14(8):934. https://doi.org/10.3390/biology14080934
Chicago/Turabian StyleGiacich, Michela, Maria Marchese, Devid Damiani, Filippo Maria Santorelli, and Valentina Naef. 2025. "Eyes Wide Open: Assessing Early Visual Behavior in Zebrafish Larvae" Biology 14, no. 8: 934. https://doi.org/10.3390/biology14080934
APA StyleGiacich, M., Marchese, M., Damiani, D., Santorelli, F. M., & Naef, V. (2025). Eyes Wide Open: Assessing Early Visual Behavior in Zebrafish Larvae. Biology, 14(8), 934. https://doi.org/10.3390/biology14080934







