Sexual Dimorphism in the Skeletal Morphology of Asian Elephants (Elephas maximus): A Preliminary Morphometric Study of Skull, Scapula, and Pelvis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Anatomical Measurement
2.3. Study Design and Statistical Analysis
2.3.1. Study 1: Descriptive Analysis and Correlation of Morphometric Data for Skull, Scapula, and Pelvis
2.3.2. Study 2: Sex Prediction Using Morphometric Data
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haynes, G. Mammoths, Mastodonts, and Elephants: Biology, Behavior and the Fossil Record; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Bader, C.; Delapré, A.; Houssaye, A. Shape variation in the limb long bones of modern elephants reveals adaptations to body mass and habitat. J. Anat. 2023, 242, 806–830. [Google Scholar] [CrossRef] [PubMed]
- Yates, B.C.; Espinoza, E.O.; Baker, B.W. Forensic species identification of elephant (Elephantidae) and giraffe (Giraffidae) tail hair using light microscopy. Forensic Sci. Med. Pathol. 2010, 6, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Toneva, D.H.; Nikolova, S.Y.; Tasheva-Terzieva, E.D.; Zlatareva, D.K.; Lazarov, N.E. Sexual dimorphism in shape and size of the neurocranium. Int. J. Legal. Med. 2022, 136, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Smuts, M.M.; Bezuidenhout, A.J. Osteology of the pelvic limb of the African elephant (Loxodonta africana). Onderstepoort J. Vet. Res. 1994, 61, 51–66. [Google Scholar] [PubMed]
- Smuts, M.M.; Bezuidenhout, A.J. Osteology of the thoracic limb of the African elephant (Loxodonta africana). Onderstepoort J. Vet. Res. 1993, 60, 1–14. [Google Scholar] [PubMed]
- Ruscillo, D.A. A Morphometric Exploration of Sexual Dimorphism in Mammalian Skeletons for Applicability in Archaeology. Ph.D. Dissertation, Institute of Archaeology, University of London, London, UK, 2000. [Google Scholar]
- Pérez-Barbería, F.J.; Gordon, I.J.; Pagel, M. The Origins of Sexual Dimorphism in Body Size in Ungulates. Evolution 2002, 56, 1276–1285. [Google Scholar] [PubMed]
- Punzalan, D.; Hosken, D.J. Sexual dimorphism: Why the sexes are (and are not) different. Curr. Biol. 2010, 20, R972–R973. [Google Scholar] [CrossRef] [PubMed]
- Nganvongpanit, K.; Pitakarnnop, T.; Buddhachat, K.; Phatsara, M. Gender-Related Differences in Pelvic Morphometrics of the Retriever Dog Breed. Anat. Histol. Embryol. 2017, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pitakarnnop, T.; Buddhachat, K.; Euppayo, T.; Kriangwanich, W.; Nganvongpanit, K. Feline (Felis catus) Skull and Pelvic Morphology and Morphometry: Gender-Related Difference? Anat. Histol. Embryol. 2017, 46, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Nganvongpanit, K.; Buddhachat, K.; Kaewmong, P.; Cherdsukjai, P.; Kittiwatanawong, K. What the skull and scapular morphology of the dugong (Dugong dugon) can tell us: Sex, habitat and body length? Sci. Rep. 2017, 7, 1964. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Santos, J.C.; Perrault, B.J.; Morando, M.; Vásquez Almazán, C.R.; Sites, J.W., Jr. Sexual dimorphism, phenotypic integration, and the evolution of head structure in casque-headed lizards. Ecol. Evol. 2017, 7, 8989–8998. [Google Scholar] [CrossRef] [PubMed]
- Nganvongpanit, K.; Cherdsukjai, P.; Boonsri, B.; Buddhachat, K.; Kaewmong, P.; Kittiwattanawong, K. Pelvic bone morphometric analysis in the dugong (Dugong dugon). Sci. Rep. 2020, 10, 19350. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, K.; Sukumar, R. The role of tusks, musth and body size in male–male competition among Asian elephants, Elephas maximus. Anim. Behav. 2013, 86, 1207–1214. [Google Scholar] [CrossRef]
- Kurki, H.K. Pelvic dimorphism in relation to body size and body size dimorphism in humans. J. Hum. Evol. 2011, 61, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Siegal-Willott, J.; Isaza, R.; Johnson, R.; Blaik, M. Distal limb radiography, ossification, and growth plate closure in the juvenile Asian elephant (Elephas maximus). J. Zoo Wildl. Med. 2008, 39, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-López, E.; Casinos, A. Evolution of scapula size and shape in Carnivora: Locomotor habits and differential shape scaling. bioRxiv 2022, 2022.2008.2026.505396. [Google Scholar] [CrossRef]
- Göhlich, U.B. On a pelvis of the straight-tusked elephant Elephas antiquus (Proboscidea, Mammalia) from Binsfeld near Speyer (Rhineland-Palatinate, Germany). Paläontologische Zeitschrift 2000, 74, 205–214. [Google Scholar] [CrossRef]
- Todd, N.E. Qualitative comparison of the cranio-dental osteology of the extant elephants, Elephas Maximus (Asian Elephant) and Loxodonta Africana (African Elephant). Anat. Rec. 2010, 293, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Khanduri, S.; Malik, S.; Khan, N.; Patel, Y.D.; Khan, A.; Chawla, H.; Singh, V.; Gupta, A.; Shaikh, J.; Siddiqui, S. Establishment of cephalic index using cranial parameters by computed tomography in a sampled North Indian population. Cureus 2021, 13, e15421. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; McGreevy, P.; Valenzuela, M. Human induced rotation and reorganization of the brain of domestic dogs. PLoS ONE 2010, 5, e11946. [Google Scholar] [CrossRef] [PubMed]
- Iotchev, I.B.; Bognár, Z.; Tóth, K.; Reicher, V.; Kis, A.; Kubinyi, E. Sleep-physiological correlates of brachycephaly in dogs. Brain Struct. Funct. 2023, 228, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Oksa-Minaļto, J.; Maggs, D.J.; Akimova, J.; Ilgaža, A.; Sebbag, L. Ocular surface physiology and aqueous tear secretion in cats of diverse cephalic conformations. Vet. Ophthalmol. 2023, 26, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Schatz, K.Z.; Engelke, E.; Pfarrer, C. Comparative morphometric study of the mimic facial muscles of brachycephalic and dolichocephalic dogs. Anat. Histol. Embryol. 2021, 50, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Allanson, J.E.; Cunniff, C.; Hoyme, H.E.; McGaughran, J.; Muenke, M.; Neri, G. Elements of morphology: Standard terminology for the head and face. Am. J. Med. Genet. A 2009, 149A, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Kharuhadetch, P.; Wattanawaragorn, S.; Tiamtongon, C.; Wathanyutakon, T.; Navic, P.; Mahakkanukrauh, P. Sex determination using scapular and clavicular parameters in modern Thai population. Int. J. Morphol. 2022, 40, 768–773. [Google Scholar] [CrossRef]
- Krishan, K.; Kanchan, T.; DiMaggio, J.A. A study of limb asymmetry and its effect on estimation of stature in forensic case work. Forensic Sci. Int. 2010, 200, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Trevathan, W. Primate pelvic anatomy and implications for birth. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Bard, J. Fins into Limbs. J. Anat. 2008, 212, 331–332. [Google Scholar] [CrossRef]
- Ren, L.; Butler, M.; Miller, C.; Paxton, H.; Schwerda, D.; Fischer, M.S.; Hutchinson, J.R. The movements of limb segments and joints during locomotion in African and Asian elephants. J. Exp. Biol. 2008, 211, 2735–2751. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Schwendener, N.; Kanz, F.; Lösch, S.; Milella, M. What we see is what we touch? Sex estimation on the pelvis in virtual anthropology. Int. J. Legal Med. 2023, 137, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, R.; Rahardjo, P.; Ruriana, I.; Guglielmi, G. Anthropometric study using three-dimensional pelvic CT scan in sex determination among adult Indonesian population. Forensic Sci. Med. Pathol. 2023, 19, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Baca, K.; Bridge, B.; Snow, M. Three-dimensional geometric morphometric sex determination of the whole and modeled fragmentary human pubic bone. PLoS ONE 2022, 17, e0265754. [Google Scholar] [CrossRef] [PubMed]
- Joon, V.; Rohatgi, D. Sex determination by Pelvis: A mini literature review. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 1008–1017. [Google Scholar] [CrossRef]
- Sangchay, N.; Dzetkulicova, V.; Zuppello, M.; Chetsawang, J. Consideration of accuracy and observational error analysis in pelvic sex assessment: A study in a Thai cadaveric human population. Siriraj Med. J. 2022, 74, 330–339. [Google Scholar] [CrossRef]
- Fahrni, S.; Lorenzo, C.; Alejandro, D.; Tanya, U.; Fabrice, D.; Olivier, D.; Grabherr, S. CT-scan vs. 3D surface scanning of a skull: First considerations regarding reproducibility issues. Forensic Sci. Res. 2017, 2, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Demircioğlu, İ. Morphometric analysis and three-dimensional computed tomography reconstruction of the long bones of femoral and crural regions in Van cats. Folia Morphol. 2021, 80, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Phombut, C.; Rooppakhun, S.; Sindhupakorn, B. Morphometric Analysis and Three-Dimensional Computed Tomography Reconstruction of Thai Distal Femur. Appl. Sci. 2021, 11, 1052. [Google Scholar] [CrossRef]
- Waltenberger, L.; Rebay-Salisbury, K.; Mitteroecker, P. Three-dimensional surface scanning methods in osteology: A topographical and geometric morphometric comparison. Am. J. Phys. Anthropol. 2021, 174, 846–858. [Google Scholar] [CrossRef] [PubMed]
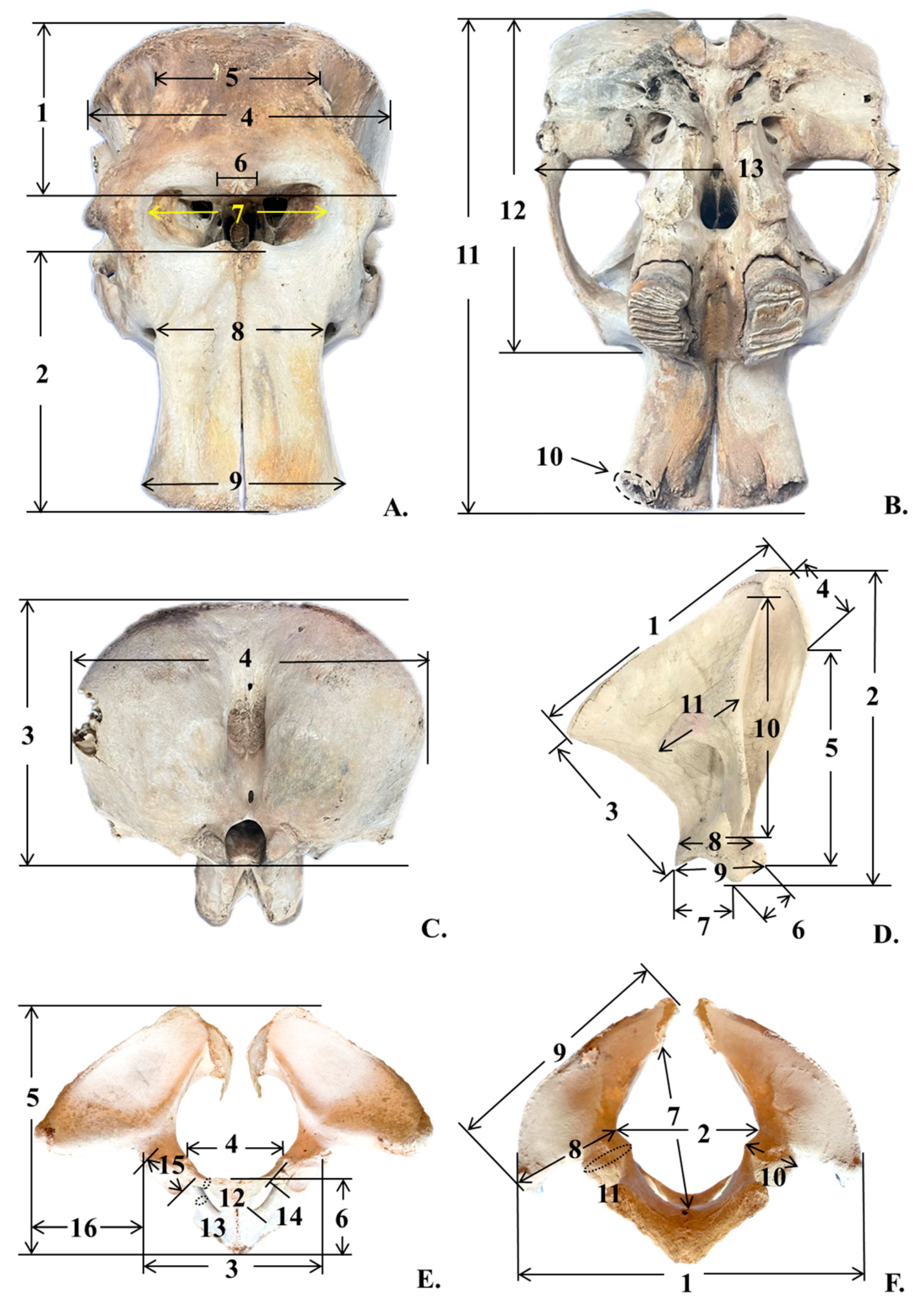
| Number | Parameter | Abbreviation | Key |
|---|---|---|---|
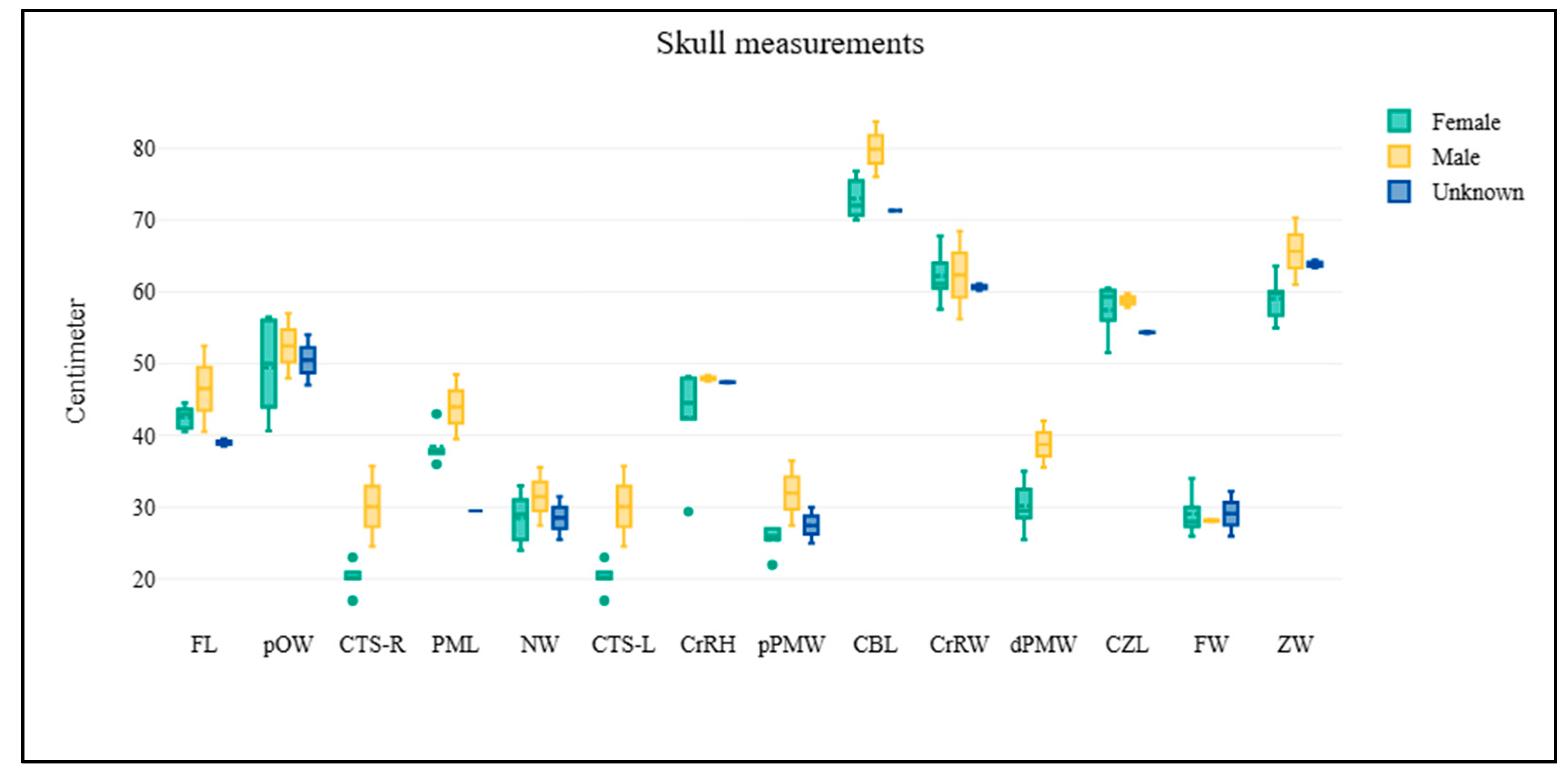
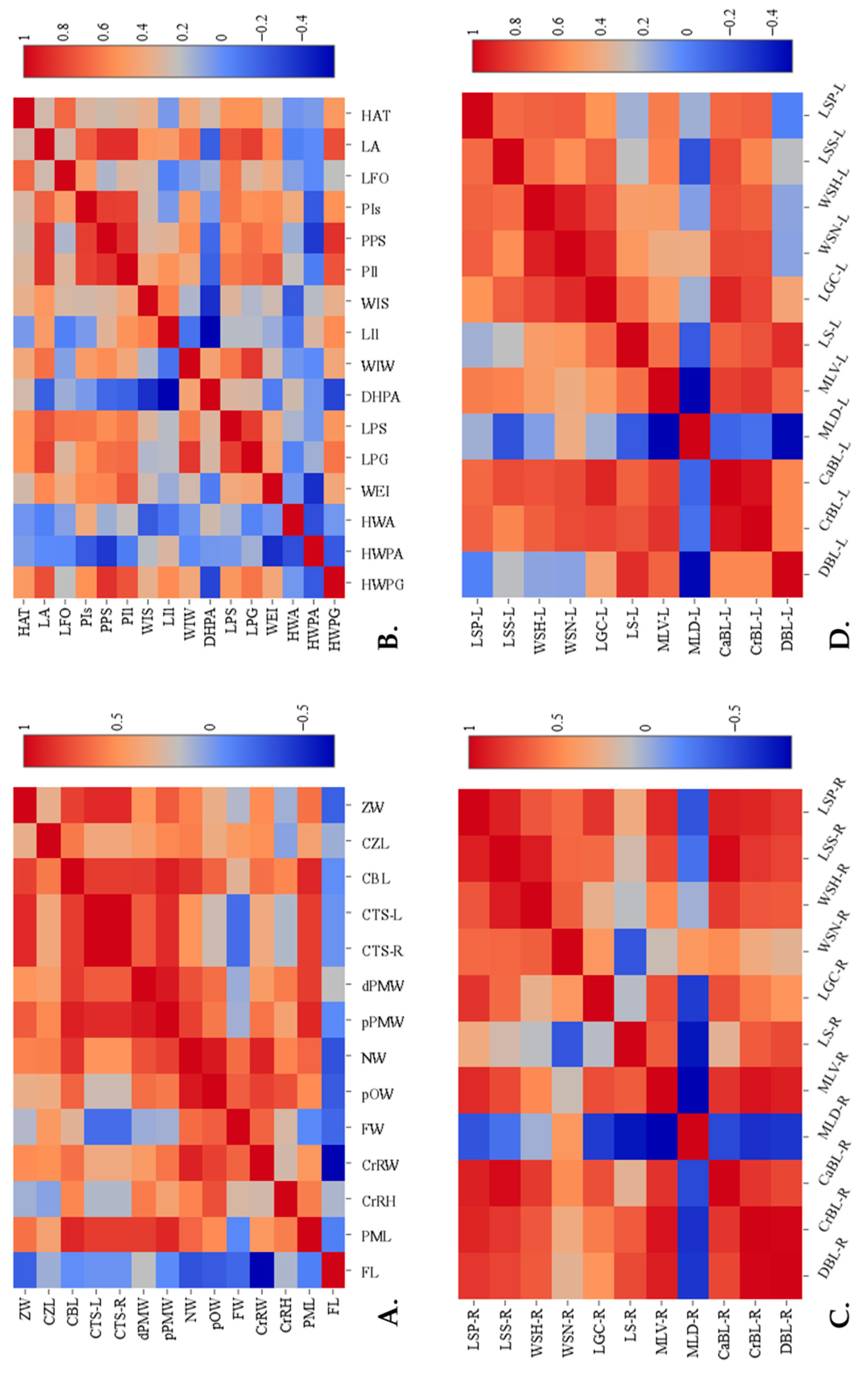
1 2 3 4 5 6 7 8 9 10-R 10-L 11 12 13 | Skull Frontal length Premaxillary length Cranial height Cranial width Frontal width Post-orbital width Nare width Proximal premaxillary width Distal premaxillary width Circumference inside tusk socket (Right) Circumference inside tusk socket (Left) Condylo-basal length Condylo-zygomatic length Zygomatic width | FL PML CrRH CrRW FW pOW NW pPMW dPMW CTS-R CTS-L CBL CZL ZW | Figure 1A Figure 1A Figure 1C Figure 1A,C Figure 1A Figure 1A Figure 1A Figure 1A Figure 1A Figure 1B Figure 1B Figure 1B Figure 1B Figure 1B |
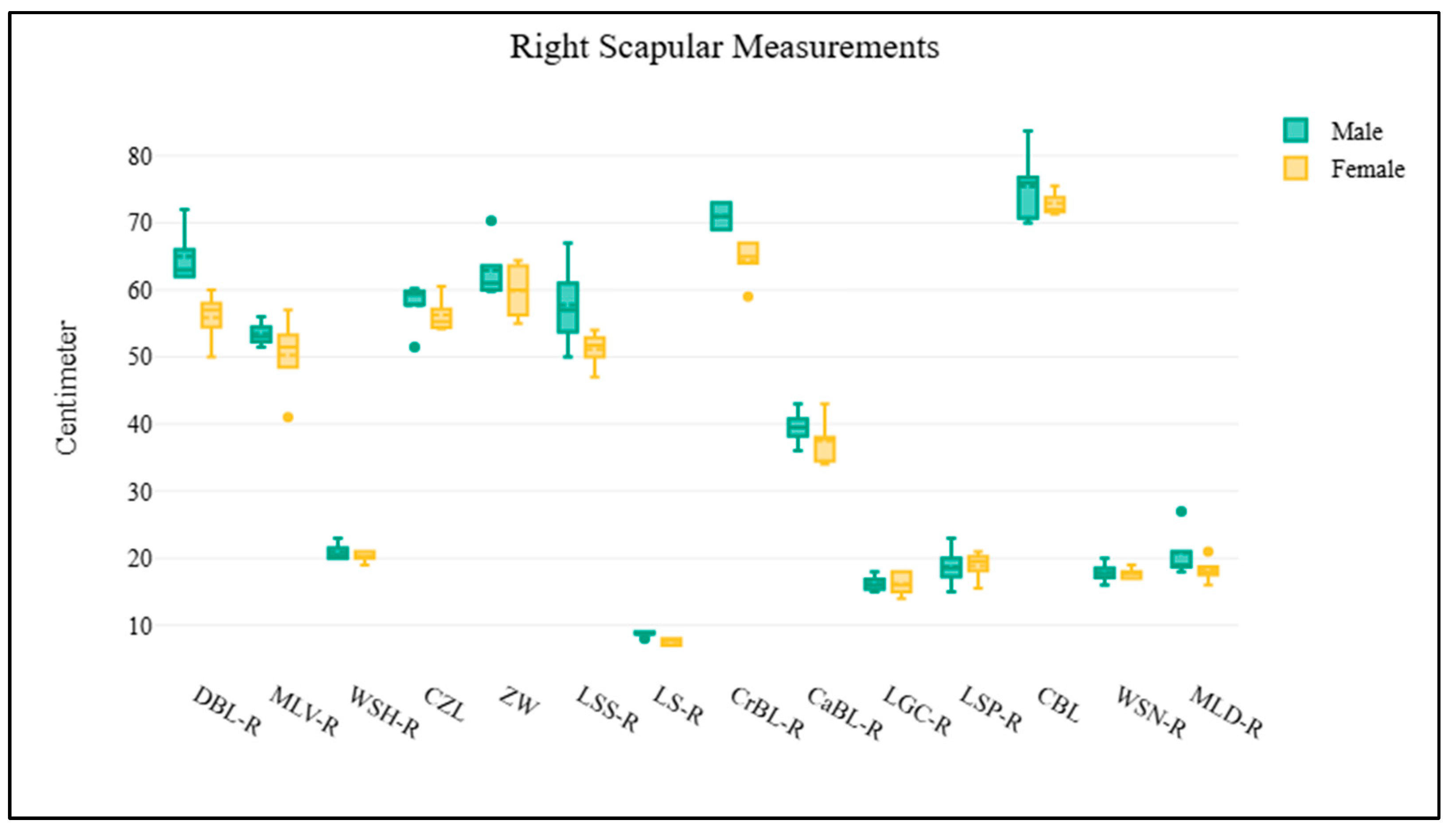
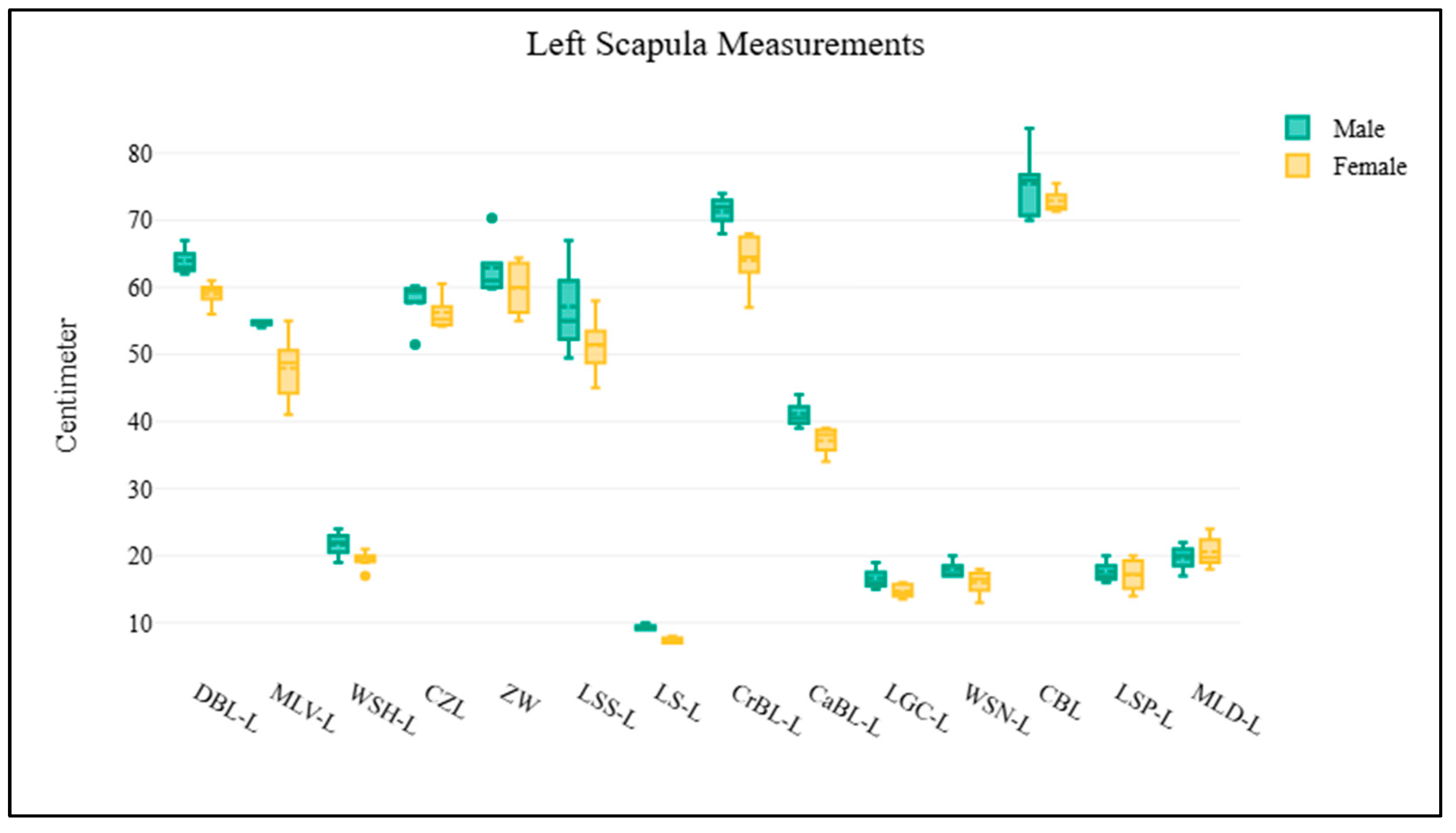
1 2 3 4 5 6 7 8 9 10 11 | Scapula Dorsal border length (right or left) Cranial border length (right or left) Caudal border length (right or left) Maximum length of dorsal part (right or left) Maximum length of ventral part (right or left) Length of supraglenoid (right or left) Length of glenoid cavity (right or left) Width of scapula neck (right or left) Width of scapula head (right or left) Length of scapula spine (right or left) Length of suprahematus process (right or left) | DBL-R or DBL-L CrBL-R or CrBL-L CaBL-R or CaBL-L MLD-R or MLD-L MLV-R or MLV-L LS-R or LS-L LGC-R or LGC-L WSN-R or WSN-L WSH-R or WSH-L LSS-R or LSS-L LSP-R or LSP-L | Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D |
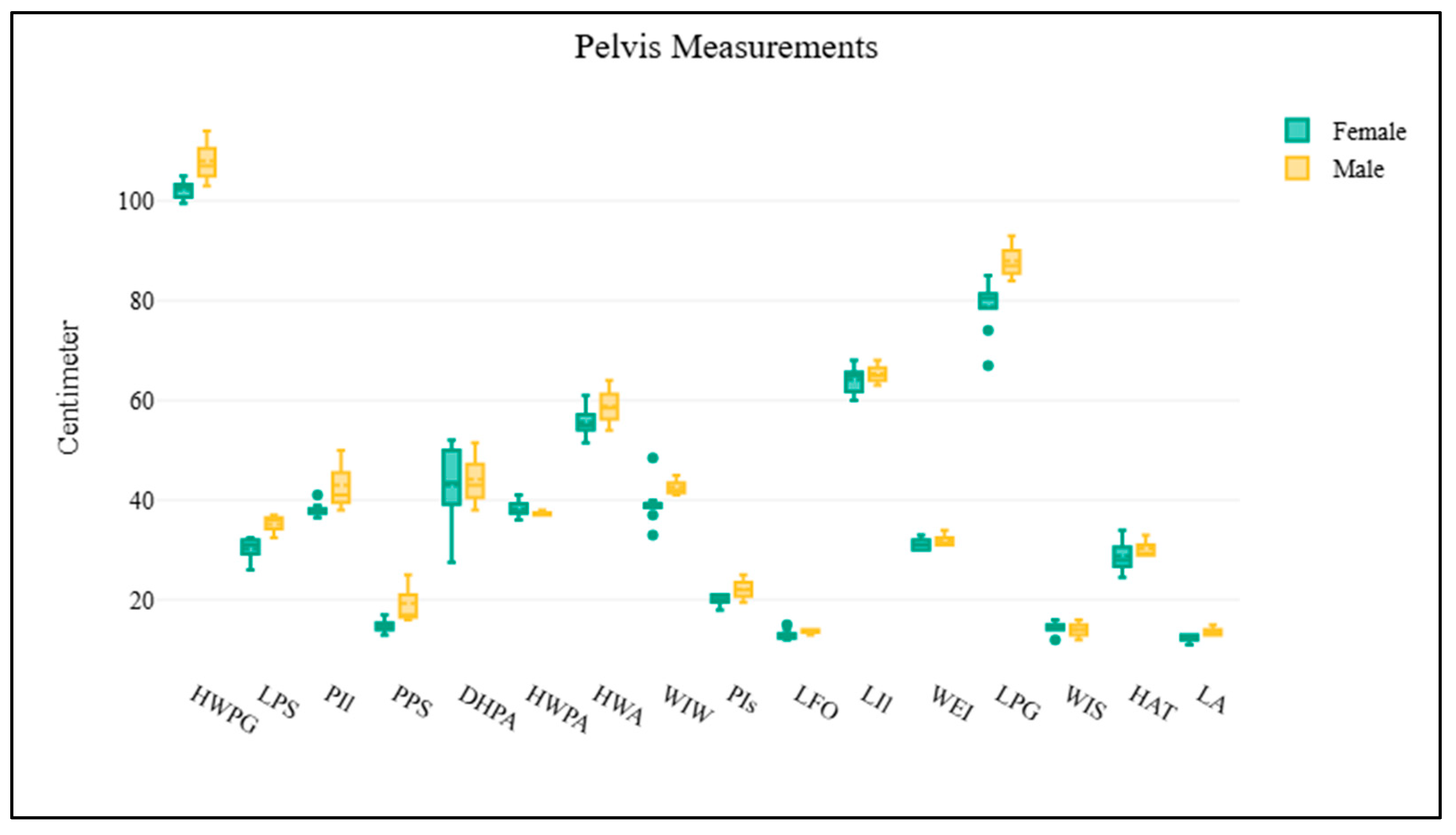
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 | Pelvis Horizontal width of pelvic girdle Horizontal width of pelvic aperture Horizontal width between the outer margins of the acetabula Width between eminentiae iliopubicae Length of pelvic girdle Length of the symphysis Diagonal height of pelvic aperture Width of ilium wing Length of ilium Minimum width of ilium shaft Minimum perimeter of ilium shaft Minimum perimeter of the pubis shaft Minimum perimeter of the ischium shaft Maximum inner length of foramen obturatum Maximum length of acetabulum Horizontal distance between outer margin of acetabulum and tuber coxae | HWPG HWPA HWA WEI LPG LPS DHPA WIW LIl WIS PIl PPS PIs LFO LA HAT | Figure 1F Figure 1F Figure 1F Figure 1D Figure 1F Figure 1D Figure 1D Figure 1D Figure 1F Figure 1F Figure 1F Figure 1D Figure 1D Figure 1D Figure 1D Figure 1D |
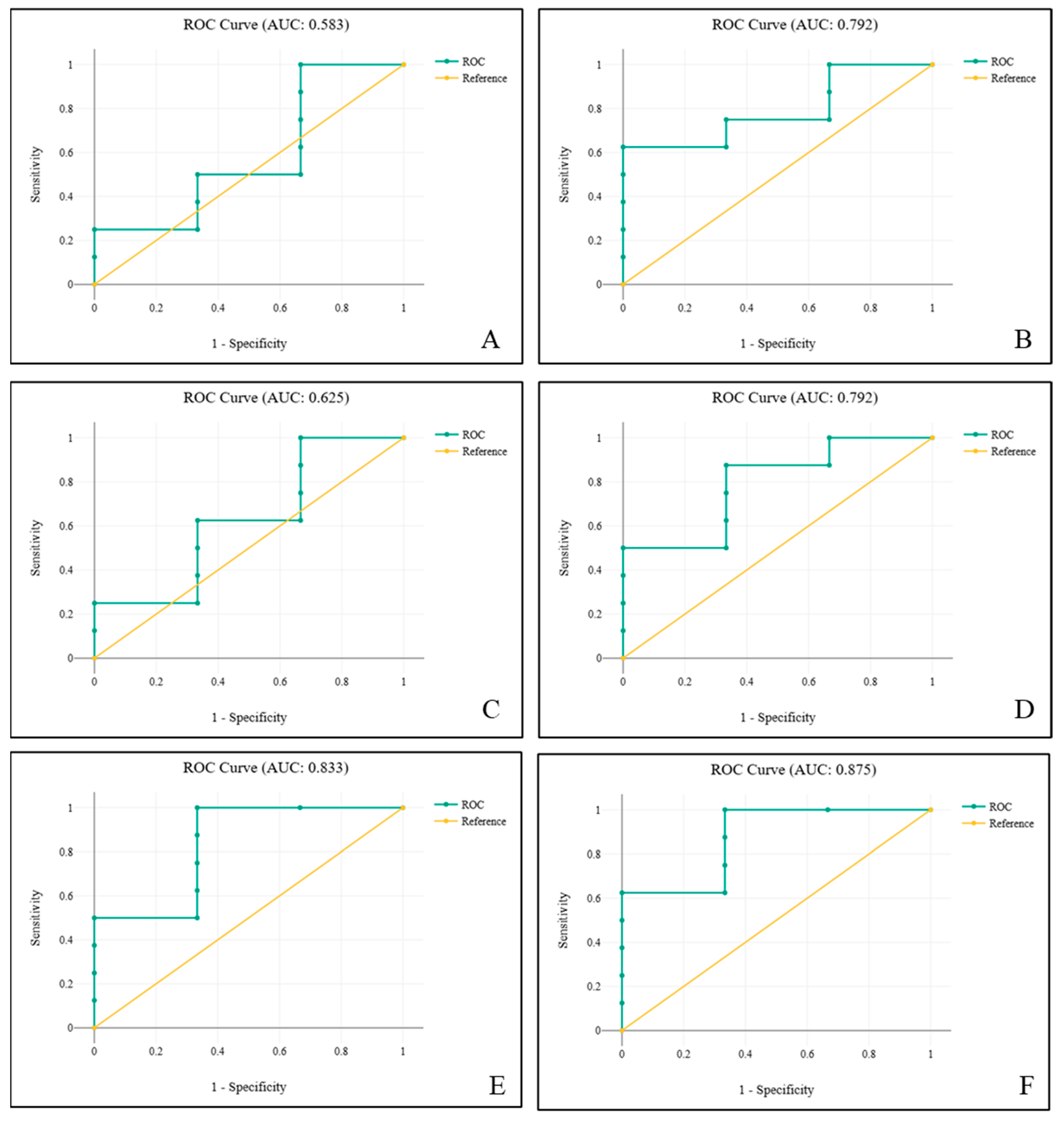
| Coefficient B | Standard Error | z | p | Odds Ratio | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| Constant | −12.54 | 13.31 | 0.94 | 0.346 | 0 | 0–769,660.75 |
| LPG/LPS | 5.27 | 5.21 | 1.01 | 0.312 | 194.65 | 0.01–5,346,092.68 |
| Constant | −7.6 | 6.64 | 1.14 | 0.253 | 0 | 0–225.81 |
| LPG/PPS | 1.7 | 1.32 | 1.28 | 0.199 | 5.48 | 0.41–73.54 |
| Constant | −3.33 | 5.29 | 0.63 | 0.53 | 0.04 | 0–1149.56 |
| LPS/PPS | 2.19 | 2.71 | 0.81 | 0.419 | 8.95 | 0.04–1815.33 |
| Female | Male | Correct | ||
|---|---|---|---|---|
| Observed | Female | 8 | 0 | 100% |
| Male | 2 | 1 | 33.33% | |
| Total | 81.82% |
| Coefficient B | Standard Error | z | p | Odds Ratio | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| Constant | −21.06 | 16.04 | 1.31 | 0.189 | 0 | 0–32,162.71 |
| LPG/LPS | 5.39 | 5.64 | 0.95 | 0.34 | 218.46 | 0–13,936,770.15 |
| LPG/PPS | 1.64 | 1.31 | 1.26 | 0.209 | 5.18 | 0.4–67.25 |
| Constant | −7.48 | 6.61 | 1.13 | 0.258 | 0 | 0–239.81 |
| LPG/PPS | 4.58 | 3.13 | 1.46 | 0.144 | 97.12 | 0.21–44,861.43 |
| LPS/PPS | −7.37 | 6.89 | 1.07 | 0.285 | 0 | 0–466.26 |
| Female | Male | Correct | ||
|---|---|---|---|---|
| Observed | Female | 7 | 1 | 87.5% |
| Male | 1 | 2 | 66.67% | |
| Total | 81.82% |
| Coefficient B | Standard Error | z | p | Odds Ratio | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| Constant | −309.65 | 229.07 | 1.35 | 0.176 | 0 | 0–3.251 × 1060 |
| LPG/LPS | 126.47 | 91.87 | 1.38 | 0.169 | 8.389 × 1054 | 0–1.331 × 10133 |
| LPG/PPS | −66.69 | 47.34 | 1.41 | 0.159 | 0 | 0–214,221,969,531.24 |
| LPS/PS | 162.42 | 117.5 | 1.38 | 0.167 | 3.457 × 1070 | 0–3.584 × 10170 |
| Female | Male | Correct | ||
|---|---|---|---|---|
| Observed | Female | 8 | 0 | 100% |
| Male | 1 | 2 | 66.67% | |
| Total | 90.91% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongtueng, P.; Piboon, P.; Klinhom, S.; Aunsan, I.; Tongser, N.; Angkawanish, T.; Nganvongpanit, K.; Boonsri, B. Sexual Dimorphism in the Skeletal Morphology of Asian Elephants (Elephas maximus): A Preliminary Morphometric Study of Skull, Scapula, and Pelvis. Biology 2025, 14, 933. https://doi.org/10.3390/biology14080933
Kongtueng P, Piboon P, Klinhom S, Aunsan I, Tongser N, Angkawanish T, Nganvongpanit K, Boonsri B. Sexual Dimorphism in the Skeletal Morphology of Asian Elephants (Elephas maximus): A Preliminary Morphometric Study of Skull, Scapula, and Pelvis. Biology. 2025; 14(8):933. https://doi.org/10.3390/biology14080933
Chicago/Turabian StyleKongtueng, Piyamat, Promporn Piboon, Sarisa Klinhom, Intorn Aunsan, Nontanan Tongser, Taweepoke Angkawanish, Korakot Nganvongpanit, and Burin Boonsri. 2025. "Sexual Dimorphism in the Skeletal Morphology of Asian Elephants (Elephas maximus): A Preliminary Morphometric Study of Skull, Scapula, and Pelvis" Biology 14, no. 8: 933. https://doi.org/10.3390/biology14080933
APA StyleKongtueng, P., Piboon, P., Klinhom, S., Aunsan, I., Tongser, N., Angkawanish, T., Nganvongpanit, K., & Boonsri, B. (2025). Sexual Dimorphism in the Skeletal Morphology of Asian Elephants (Elephas maximus): A Preliminary Morphometric Study of Skull, Scapula, and Pelvis. Biology, 14(8), 933. https://doi.org/10.3390/biology14080933









