Hypoxia Disrupted Serotonin Levels in the Prefrontal Cortex and Striatum, Leading to Depression-like Behavior
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Hypoxia Treatment
2.3. Behavior Test
2.4. Open Field Test
2.5. Forced Swimming Test
2.6. Anesthesia and Sacrifice Method
2.7. Molecular Investigation
2.8. Statistical Analysis
3. Results
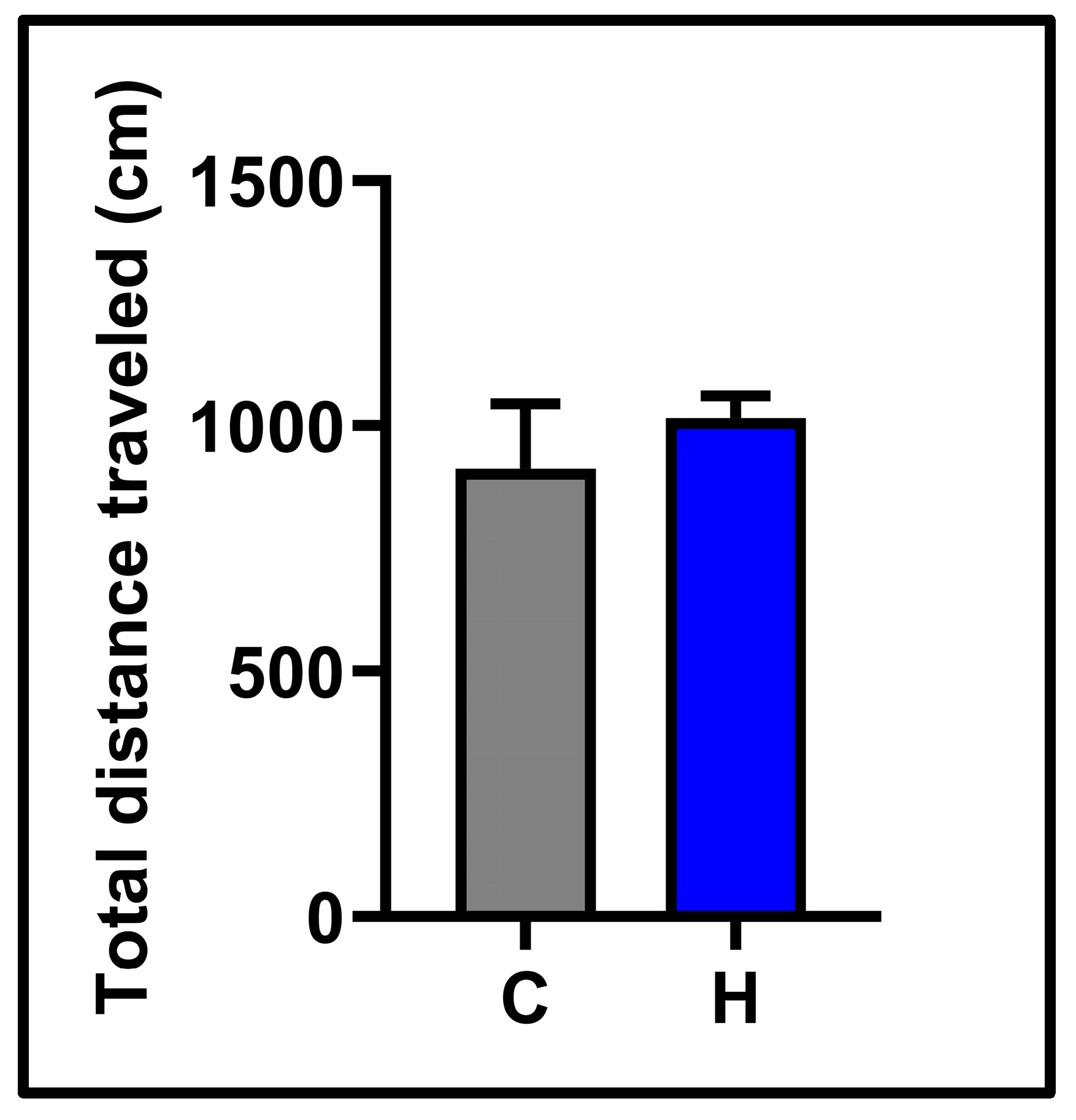
3.1. Behavioral Results
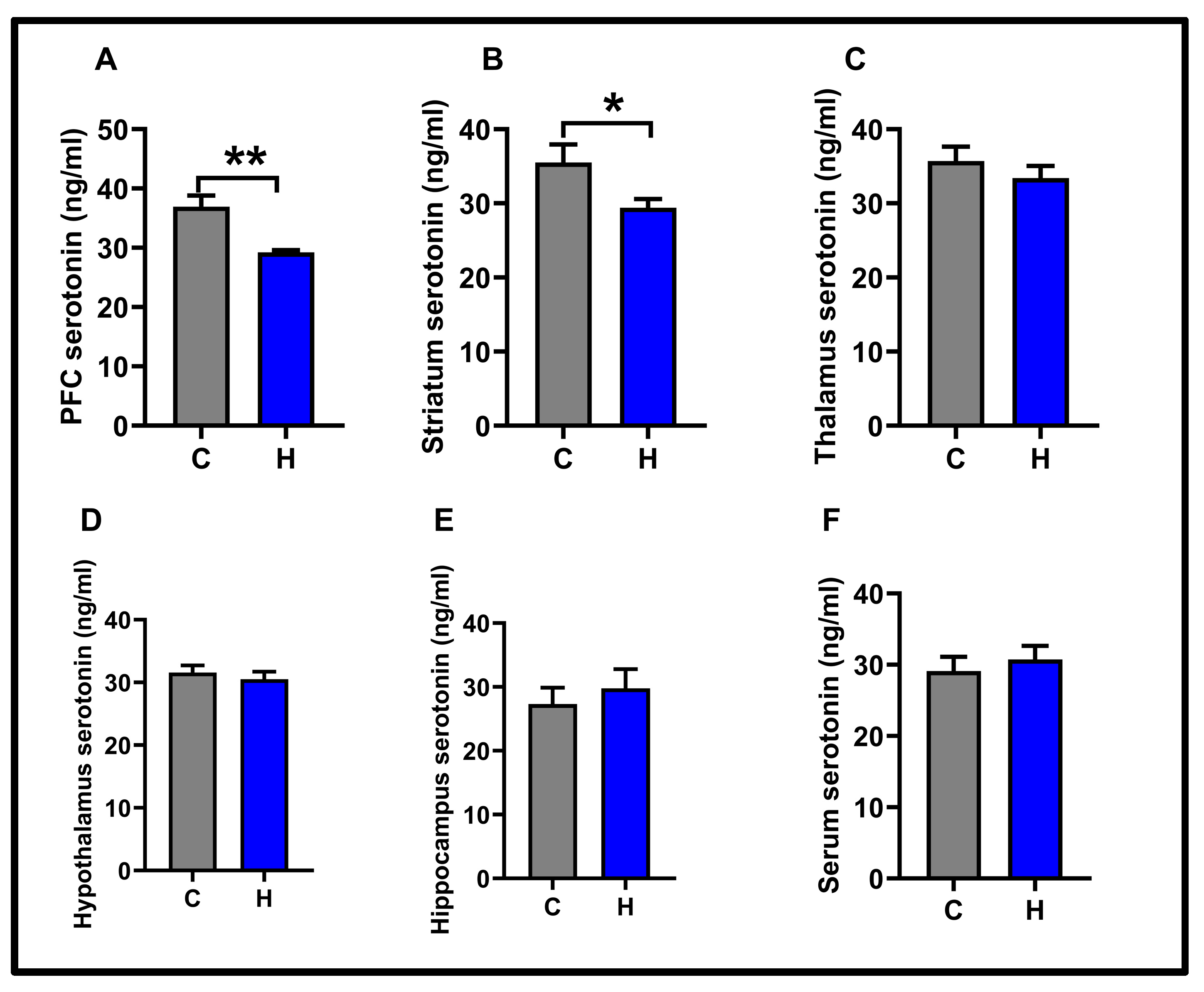
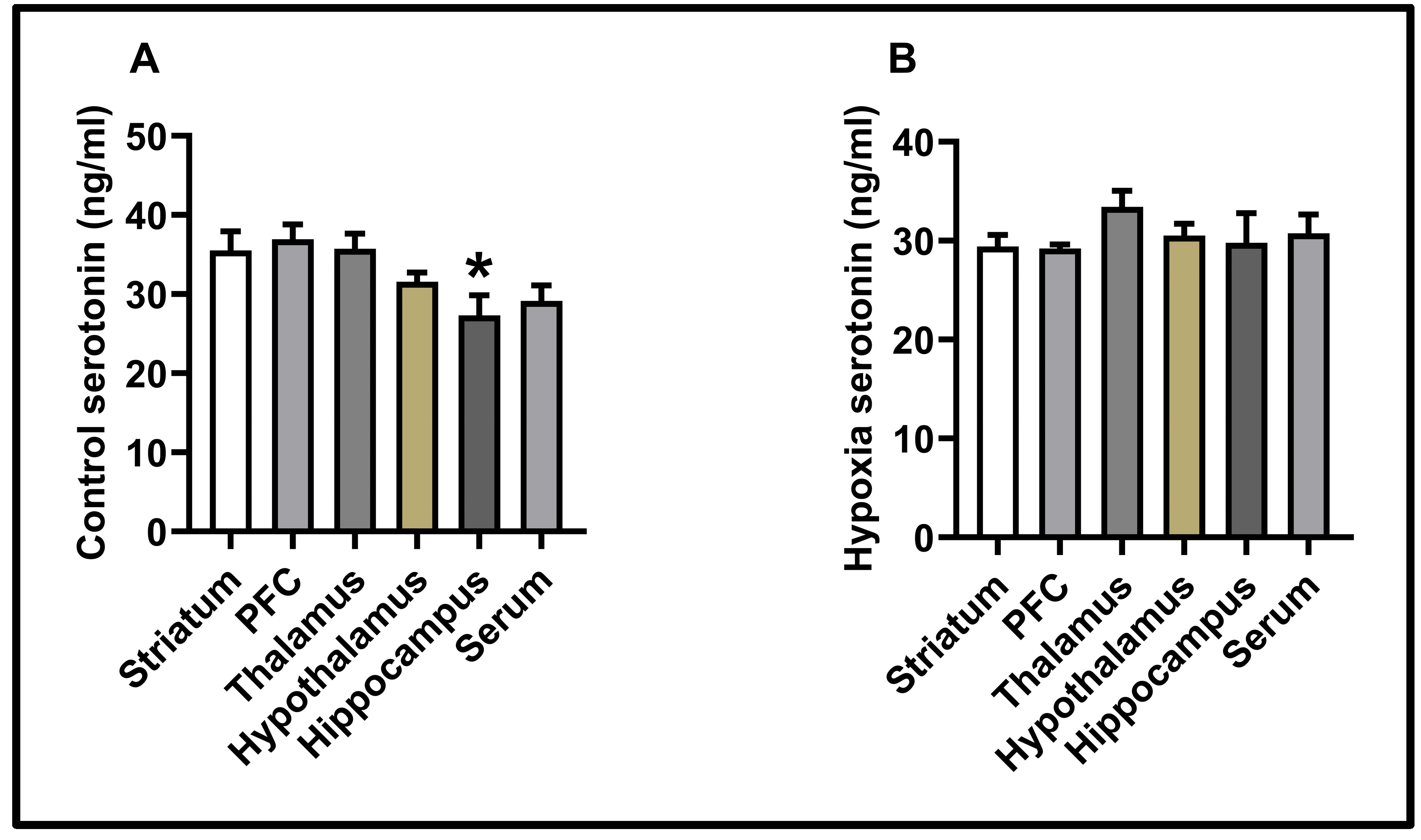
3.2. Molecular Results
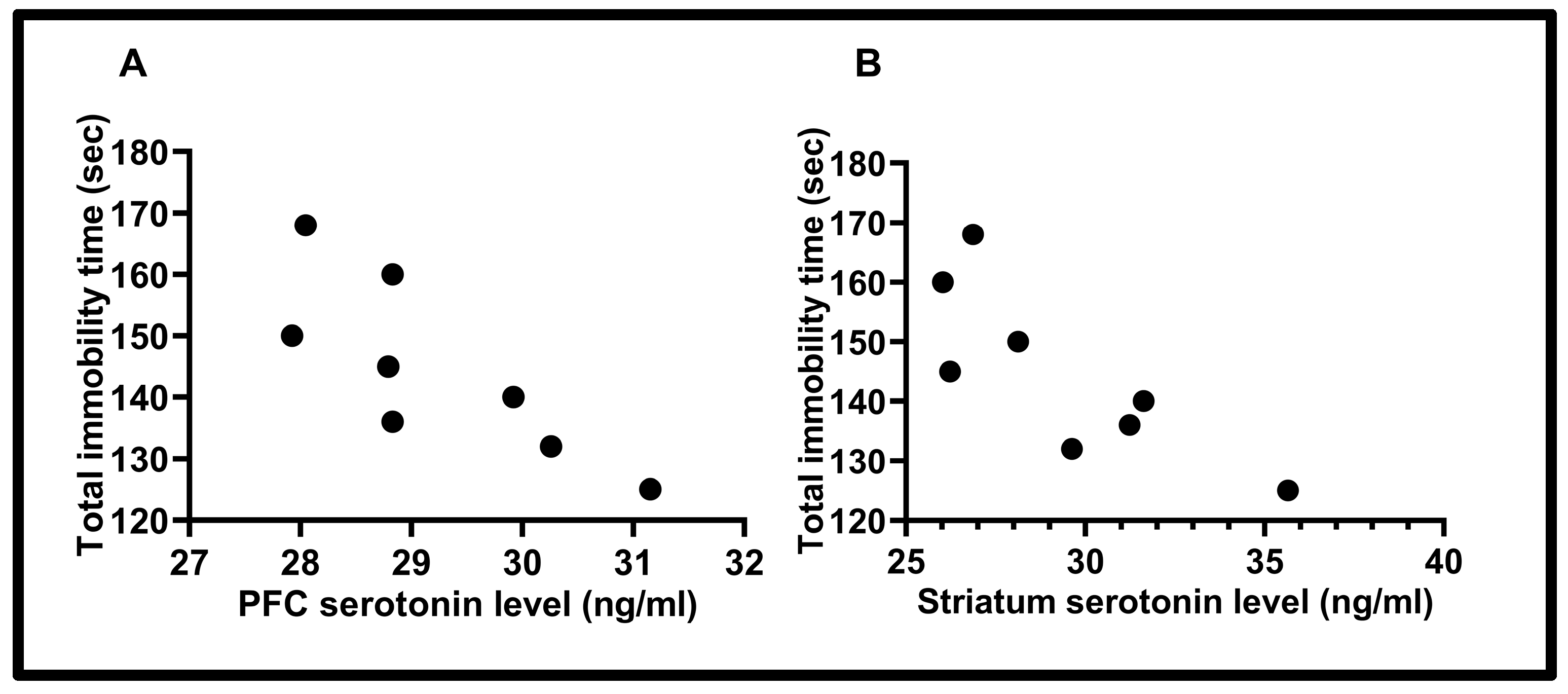
3.3. Correlation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| FST | Forced swimming test |
| IDO | Indoleamine 2,3-dioxygenase |
| PFC | Prefrontal cortex |
| LD | Linear dichroism |
References
- Irwin, M.R.; Miller, A.H. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007, 21, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Depression, W. Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; p. 24. [Google Scholar]
- Liu, J.; Liu, Y.; Ma, W.; Tong, Y.; Zheng, J. Temporal and spatial trend analysis of all-cause depression burden based on Global Burden of Disease (GBD) 2019 study. Sci. Rep. 2024, 14, 12346. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Hu, Z.; Xu, J.; Wang, C. The molecular pathophysiology of depression and the new therapeutics. MedComm 2022, 3, e156. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Jia, Y.; Chen, Z.; Li, S.; Qi, B.; Ma, A. Foods for Sleep Improvement: A Review of the Potential and Mechanisms Involved. Foods 2025, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhou, M.; Xiao, N.; Tan, Z.; Liang, X. Unveiling the Mystery of the Stimulatory Effects of Arecoline: Its Relevance to the Regulation of Neurotransmitters and the Microecosystem in Multi-Ecological Intestinal Sites. Int. J. Mol. Sci. 2025, 26, 3150. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Tian, L.; Wang, X.; Meng, T.; Yu, S.; Li, Y. Decoding serotonin: The molecular symphony behind depression. Front. Cell. Neurosci. 2025, 19, 1572462. [Google Scholar] [CrossRef] [PubMed]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, M.; Crucian, B.; Salam, A.P.; Rybka, A.; Kaufmann, I.; Moreels, M.; Quintens, R.; Schelling, G.; Thiel, M.; Baatout, S. Early adaption to the antarctic environment at dome C: Consequences on stress-sensitive innate immune functions. High Alt. Med. Biol. 2014, 15, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Rybnikova, E.A. Brain hypoxia and ischemia: New insights into neurodegeneration and neuroprotection. Front. Neurosci. 2019, 13, 770. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.R.; Dutta, G.; Ghosh, T. Naproxen, a Nonsteroidal Anti-Inflammatory Drug, Can Affect Daily Hypobaric Hypoxia-Induced Alterations of Monoamine Levels in Different Areas of the Brain in Male Rats. High Alt. Med. Biol. 2016, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Semenov, D.; Samoilov, M.; Lazarewicz, J. Preconditioning reduces hypoxia-evoked alterations in glutamatergic Ca2+ signaling in rat cortex. Acta Neurobiol. Exp. 2008, 68, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, J.A. Invited review: Physiological and pathophysiological responses to intermittent hypoxia. J. Appl. Physiol. 2001, 90, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.; Ben Mabrouk, F.; Mitchell, G. Unexpected benefits of intermittent hypoxia: Enhanced respiratory and nonrespiratory motor function. Physiology 2014, 29, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kanekar, S.; Bogdanova, O.V.; Olson, P.R.; Sung, Y.-H.; D’Anci, K.E.; Renshaw, P.F. Hypobaric hypoxia induces depression-like behavior in female Sprague-Dawley rats, but not in males. High Alt. Med. Biol. 2015, 16, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.X.; Wang, B.; Li, W.T. Intermittent hypoxia improves cognition and reduces anxiety-related behavior in APP/PS1 mice. Brain Behav. 2020, 10, e01513. [Google Scholar] [CrossRef] [PubMed]
- Institute of Laboratory Animal Resources (US); Committee on Care, and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2010.
- Akat, F.; Ficicilar, H.; Durak, A.; Tuncay, E.; Dursun, A.; Topal Çelikkan, F.; Sabuncuoğlu, B.; Turan, B.; Baştuğ, M. Intermittent hypoxia induces beneficial cardiovascular remodeling in left ventricular function of type 1 diabetic rat. Anatol. J. Cardiol. 2018, 19, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.S. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 1934, 18, 385. [Google Scholar] [CrossRef]
- Caliskan, H.; Akat, F.; Tatar, Y.; Zaloglu, N.; Dursun, A.D.; Bastug, M.; Ficicilar, H. Effects of exercise training on anxiety in diabetic rats. Behav. Brain Res. 2019, 376, 112084. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Detke, M.J.; Lucki, I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: The effects of water depth. Behav. Brain Res. 1995, 73, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Mikhailenko, V.A.; Butkevich, I.P.; Bagaeva, T.R.; Makukhina, G.V.; Otellin, V.A. Short-and long-term influences of hypoxia during early postnatal period of development on behavioral and hormonal responses in rats. Neurosci. Lett. 2009, 464, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-L.; Jiang, X.-F.; Cheng, Y.-C.; Liu, Y.-F.; Yang, K.; Zhu, S.-L.; Kong, X.-B.; Tu, Y.; Bian, K.-F.; Liu, Z.-L. Exendin-4 inhibits high-altitude cerebral edema by protecting against neurobiological dysfunction. Neural Regen. Res. 2018, 13, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Lu, B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology 2008, 33, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Bambico, F.R.; Nguyen, N.-T.; Gobbi, G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur. Neuropsychopharmacol. 2009, 19, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Fitzpatrick, P.F. Mechanisms of tryptophan and tyrosine hydroxylase. IUBMB Life 2013, 65, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Thomas, P. Restoration of tryptophan hydroxylase functions and serotonin content in the Atlantic croaker hypothalamus by antioxidant treatment during hypoxic stress. Front. Neurosci. 2014, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Criado, G.; Šimelyte, E.; Inglis, J.J.; Essex, D.; Williams, R.O. Indoleamine 2, 3 dioxygenase–mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009, 60, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Y.; Zhang, L.; Song, W.; Shi, L. Hypoxia enhances indoleamine 2, 3-dioxygenase production in dendritic cells. Oncotarget 2018, 9, 11572. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.C.; Maes, M. The role of indoleamine 2, 3-dioxygenase (IDO) in the pathophysiology of interferon-α-induced depression. J. Psychiatry Neurosci. 2004, 29, 11–17. [Google Scholar] [PubMed]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, H.; Önal, D.; Nalçacı, E. Darbepoetin alpha has an anxiolytic and anti-neuroinflammatory effect in male rats. BMC Immunol. 2024, 25, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, X.; Wang, R.; Sakagami, M. Functional connectivity between prefrontal cortex and striatum estimated by phase locking value. Cogn. Neurodyn. 2016, 10, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Marchand, W.R. Cortico-basal ganglia circuitry: A review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct. Funct. 2010, 215, 73–96. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.; Bertoux, M.; Hornberger, M. Beyond and below the cortex: The contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. J. Neurol. Neurosurg. Psychiatry 2014, 85, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, J.N.; van der Werff, S.J.; van Tol, M.; Veltman, D.J.; Aleman, A.; Zitman, F.G.; Rombouts, S.; van der Wee, N. Investigating distinct and common abnormalities of resting-state functional connectivity in depression, anxiety, and their comorbid states. Eur. Neuropsychopharmacol. 2015, 25, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A.; Cai, N.; Bliven, T.; Juhasz, M.; Conner, J.M.; Acheson, A.L.; Lindsay, R.M.; Wiegand, S.J. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997, 389, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, H.; Karabulut, G. Effects of the dexamethasone-induced insulin resistance model on self-care behaviors and brain-derived growth factor in rats. J. Endocrinol. 2025, 265, e250088. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; O’Donnell, C.P. Rodent models of sleep apnea. Respir. Physiol. Neurobiol. 2013, 188, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wu, N.; He, L.; Shao, J.; Ma, H. Establishment of an experimental rat model of high altitude cerebral edema by hypobaric hypoxia combined with temperature fluctuation. Brain Res. Bull. 2020, 165, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, K.; Falla, M.; Brugger, H.; Gatterer, H.; Strapazzon, G.; Tomazin, I.; Zafren, K.; Sperner-Unterweger, B.; Fusar-Poli, P. Isolated high altitude psychosis, delirium at high altitude, and high altitude cerebral edema: Are these diagnoses valid? Front. Psychiatry 2023, 14, 1221047. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Glozier, N.; Ratnavadivel, R.; Grunstein, R.R. Obstructive sleep apnea and depression. Sleep Med. Rev. 2009, 13, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kanekar, S.; Sheth, C.; Ombach, H.; Brown, J.; Hoffman, M.; Ettaro, R.; Renshaw, P. Sex-based changes in rat brain serotonin and behavior in a model of altitude-related vulnerability to treatment-resistant depression. Psychopharmacology 2021, 238, 2867–2881. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.; Jaromirska, J.; Gabryelska, A.; Sochal, M. Obstructive Sleep Apnea and Serotoninergic Signalling Pathway: Pathomechanism and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 9427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-H.; Yan, H.-C.; Zhang, J.; Qu, H.-D.; Qiu, X.-S.; Chen, L.; Li, S.-J.; Cao, X.; Bean, J.C.; Chen, L.-H. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J. Neurosci. 2010, 30, 12653–12663. [Google Scholar] [CrossRef] [PubMed]
- Duszczyk, M.; Gamdzyk, M.; Ziembowicz, A.; Boguszewski, P.; Łazarewicz, J.; Salińska, E. Antidepressant-like and anxiolytic-like effects of mild hypobaric hypoxia in mice: Possible involvement of neuropeptide Y. Acta Neurobiol. Exp. 2015, 75, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Jia, X.; Bade, R.; Liu, X.; Jiang, S.; Xie, Y.; Xie, W.; Gao, M.; Shao, G. Hypoxic preconditioning modulates BDNF signaling to alleviate depression-like behaviors in mice and its whole transcriptome sequencing analysis. Sci. Rep. 2025, 15, 15363. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, T.V.; Manukhina, E.B.; Smith, M.L.; Downey, H.F.; Mallet, R.T. Intermittent hypoxia: Cause of or therapy for systemic hypertension? Exp. Biol. Med. 2008, 233, 627–650. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, S.B.; Panjwani, U.; Yadav, D.K.; Amitabh, K.; Singh, S.; Selvamurthy, W. Effect of a carbohydrate supplement on feeding behaviour and exercise in rats exposed to hypobaric hypoxia. Appetite 2002, 39, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, O.V.; Abdullah, O.; Kanekar, S.; Bogdanov, V.B.; Prescot, A.P.; Renshaw, P.F. Neurochemical alterations in frontal cortex of the rat after one week of hypobaric hypoxia. Behav. Brain Res. 2014, 263, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sheth, C.; Ombach, H.; Olson, P.; Renshaw, P.F.; Kanekar, S. Increased anxiety and anhedonia in female rats following exposure to altitude. High Alt. Med. Biol. 2015, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çalışkan, H.; Cihan, K.H.; Koçak, S.; Karabulut, G.; Nalçacı, E. Hypoxia Disrupted Serotonin Levels in the Prefrontal Cortex and Striatum, Leading to Depression-like Behavior. Biology 2025, 14, 931. https://doi.org/10.3390/biology14080931
Çalışkan H, Cihan KH, Koçak S, Karabulut G, Nalçacı E. Hypoxia Disrupted Serotonin Levels in the Prefrontal Cortex and Striatum, Leading to Depression-like Behavior. Biology. 2025; 14(8):931. https://doi.org/10.3390/biology14080931
Chicago/Turabian StyleÇalışkan, Hasan, Koray Hamza Cihan, Seda Koçak, Gözde Karabulut, and Erhan Nalçacı. 2025. "Hypoxia Disrupted Serotonin Levels in the Prefrontal Cortex and Striatum, Leading to Depression-like Behavior" Biology 14, no. 8: 931. https://doi.org/10.3390/biology14080931
APA StyleÇalışkan, H., Cihan, K. H., Koçak, S., Karabulut, G., & Nalçacı, E. (2025). Hypoxia Disrupted Serotonin Levels in the Prefrontal Cortex and Striatum, Leading to Depression-like Behavior. Biology, 14(8), 931. https://doi.org/10.3390/biology14080931





