Characterization of Small Extracellular Vesicles Isolated from Aurelia aurita
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection
2.3. Isolation of EVs
2.4. Bicinchoninic Acid Assay (BCA)
2.5. Tunable Resistive Pulse Sensing (TRPS)
2.6. Electrophoretic Light Scattering (ELS)
2.7. Capillary Electrophoresis (CE)
2.8. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
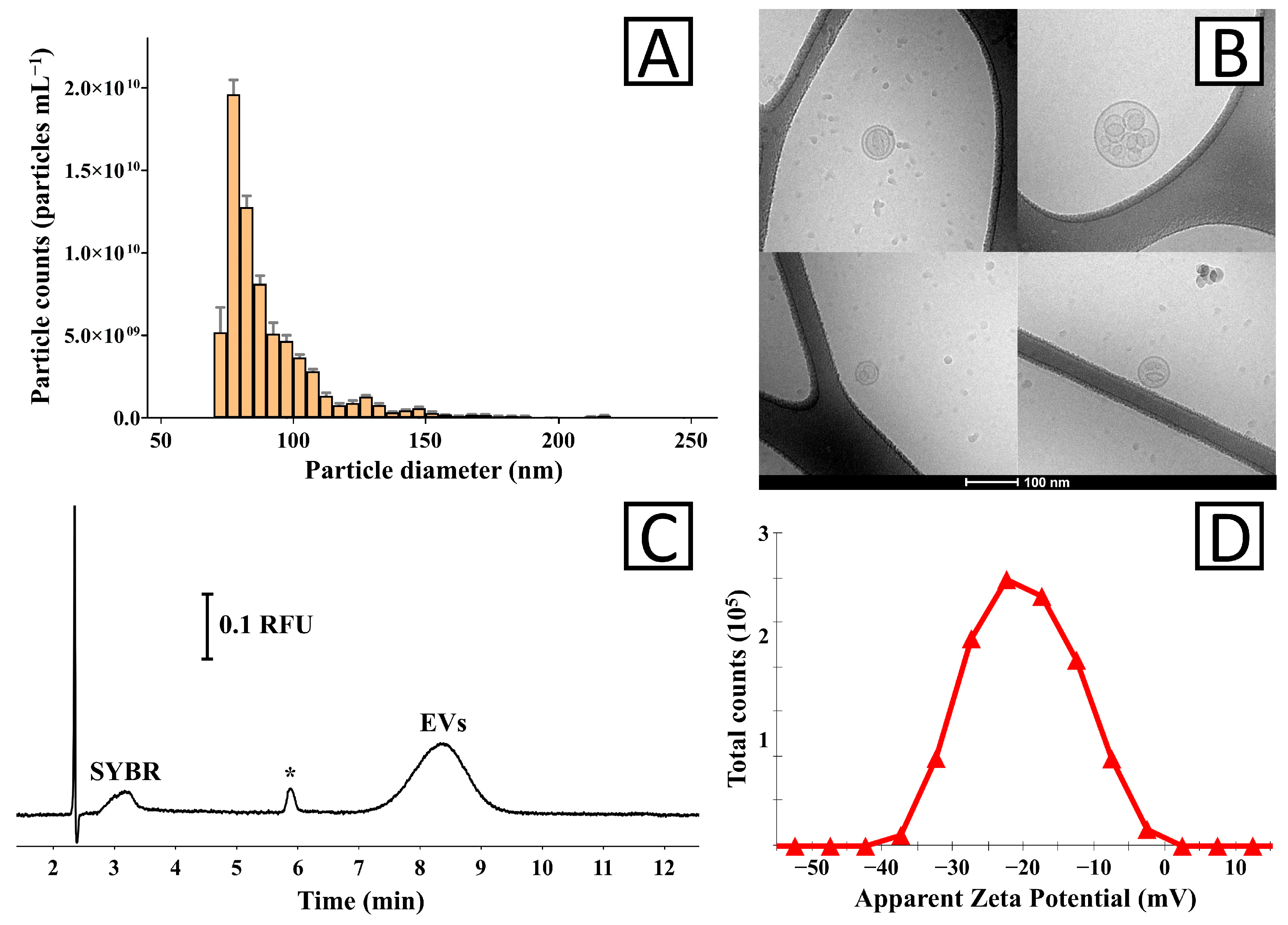
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | Bicinchoninic acid assay |
| BGE | Background electrolyte |
| BSA | Bovine serum albumin |
| CE | Capillary electrophoresis |
| Cryo-TEM | Cryogenic Transmission Electron Microscopy |
| ELS | Electrophoretic light scattering |
| EVs | Extracellular vesicles |
| PBS | Phosphate-buffered saline |
| SDS | Sodium dodecyl sulfate |
| SEC | Size-exclusion chromatography |
| TRPS | Tunable resistive pulse sensing |
| UF | Ultrafiltration |
References
- Holstein, T.W.; Watanabe, H.; Özbek, S. Signaling Pathways and Axis Formation in the Lower Metazoa. Current Topics in Developmental Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 97. [Google Scholar]
- Bosch, T.C.G. Emergence of Immune System Components in Cnidarians. In Encyclopedia of Immunobiology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Takahashi, T. Comparative Aspects of Structure and Function of Cnidarian Neuropeptides. Front. Endocrinol. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Kuranaga, E.; Nakajima, Y. Regeneration potential of jellyfish: Cellular mechanisms and molecular insights. Genes 2021, 12, 758. [Google Scholar] [CrossRef] [PubMed]
- Schmid, V.; Alder, H. Isolated, mononucleated, striated muscle can undergo pluripotent transdifferentiation and form a complex regenerate. Cell 1984, 38, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Piraino, S.; De Vito, D.; Schmich, J.; Bouillon, J.; Boero, F. Reverse development in Cnidaria. Can. J. Zool. 2004, 82, 1748–1754. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Cadar, E.; Pesterau, A.M.; Sirbu, R.; Negreanu-Pirjol, B.S.; Tomescu, C.L. Jellyfishes—Significant Marine Resources with Potential in the Wound-Healing Process: A Review. Mar. Drugs 2023, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.K. From Jellyfish to the Nobel Prize: The Discovery and Uses of Green Fluorescent Protein. Malays. J. Biochem. Mol. Biol. 2010, 18, 1–6. [Google Scholar]
- Amreen Nisa, S.A.; Vinu, D.; Krupakar, P.; Govindaraju, K.; Sharma, D.; Vivek, R. Jellyfish venom proteins and their pharmacological potentials: A review. Int. J. Biol. Macromol. 2021, 176, 424–436. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; León-Félix, C.M.; Amorim, C.A. Extracellular vesicles in nanomedicine and regenerative medicine: A review over the last decade. Bioact. Mater. 2024, 36, 126–156. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.H.; Choi, Y.; Kim, G.B.; Kim, S.; A Kim, S.; Kim, I. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Adv. Mater. 2020, 32, 2002440. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Deng, Q.; Zhu, C.; Zhang, B. Application of Extracellular Vesicles in Aquatic Animals: A Review of the Latest Decade. Rev. Fish. Sci. Aquac. 2022, 30, 447–466. [Google Scholar] [CrossRef]
- Fernández-Cervantes, I.; Rodríguez-Fuentes, N.; León-Deniz, L.V.; Quintana, L.E.A.; Cervantes-Uc, J.M.; Kao, W.A.H.; Cerón-Espinosa, J.D.; Cauich-Rodríguez, J.V.; Castaño-Meneses, V.M. Cell-free scaffold from jellyfish Cassiopea andromeda (Cnidaria; Scyphozoa) for skin tissue engineering. Mater. Sci. Eng. C 2020, 111, 110748. [Google Scholar] [CrossRef] [PubMed]
- Lesh-Laurie, G.E.; Corriel, R. Scyphistoma regeneration from isolated tentacles in aurelia aurita. J. Mar. Biol. Assoc. United Kingd. 1973, 53, 885–894. [Google Scholar] [CrossRef]
- Lesh-Laurie, G.E.; Hujer, A.; Suchy, P. Polyp regeneration from isolated tentacles of Aurelia scyphistomae: A role for gating mechanisms and cell division. Hydrobiologia 1991, 216, 91–97. [Google Scholar] [CrossRef]
- Moros, M.; Fergola, E.; Marchesano, V.; Mutarelli, M.; Tommasini, G.; Miedziak, B.; Palumbo, G.; Ambrosone, A.; Tino, A.; Tortiglione, C. The Aquatic Invertebrate Hydra vulgaris Releases Molecular Messages Through Extracellular Vesicles. Front. Cell Dev. Biol. 2021, 9, 788117. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Afshar, A.; Zare, A.; Salehpour, A.; Hashemi, A.; Zendehboudi, F.; Farrar, Z.; Mahdipour, M.; Khoradmehr, A.; Jahanfar, F.; et al. Proliferating and migrating effects of regenerating sea anemone Aulactinia stella cells-derived exosomes on human skin fibroblasts. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steć, A.; Chodkowska, M.; Kasprzyk-Pochopień, J.; Mielczarek, P.; Piekoszewski, W.; Lewczuk, B.; Płoska, A.; Kalinowski, L.; Wielgomas, B.; Dziomba, S. Isolation of Citrus lemon extracellular vesicles: Development and process control using capillary electrophoresis. Food Chem. 2023, 424, 136333. [Google Scholar] [CrossRef] [PubMed]
- Steć, A.; Klocek, K.; Czyrski, G.; Heinz, A.; Dziomba, S. Identity confirmation of extracellular vesicles by capillary electrophoresis using non-specific dyes. Microchem. J. 2024, 207, 112288. [Google Scholar] [CrossRef]
- Pascucci, L.; Scattini, G. Imaging extracelluar vesicles by transmission electron microscopy: Coping with technical hurdles and morphological interpretation. Biochim. Biophys. Acta—Gen. Subj. 2021, 1865, 129648. [Google Scholar] [CrossRef] [PubMed]
- Steć, A.; Heinz, A.; Dziomba, S. Characterization of extracellular vesicles by capillary zone electrophoresis: A novel concept for characterization of a next-generation drug delivery platform. J. Pharm. Anal. 2024, 14, 101004. [Google Scholar] [CrossRef] [PubMed]
- Hays, G.C.; Doyle, T.K.; Houghton, J.D.R. A Paradigm Shift in the Trophic Importance of Jellyfish? Trends Ecol. Evol. 2018, 33, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Brekhman, V.; Malik, A.; Haas, B.; Sher, N.; Lotan, T. Transcriptome profiling of the dynamic life cycle of the scypohozoan jellyfish Aurelia aurita. BMC Genom. 2015, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.H. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 2001, 451, 229–246. [Google Scholar] [CrossRef]
- Brulińska, D.; Olenycz, M.; Ziółkowska, M.; Mudrak-Cegiołka, S.; Wołowicz, M. Moon Jellyfish, Aurelia Aurita, in the Gulf of Gdansk: Threatening predator or not? Boreal Environ. Res. 2016, 21, 528–540. [Google Scholar]
- Janßen, H.; Augustin, C.B.; Hinrichsen, H.H.; Kube, S. Impact of secondary hard substrate on the distribution and abundance of Aurelia aurita in the western Baltic Sea. Mar. Pollut. Bull. 2013, 75, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Mihneva, V.; Raykov, V.; Dimitrov, D.P. European Sprat (Sprattus sprattus) and Moon Jellyfish (Aurelia aurita) in the Western Part of the Black Sea. Animals 2023, 13, 3691. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Fu, W.; Fan, M.; Li, K. Ecological effect of seasonally changing temperature on the life cycle of Aurelia aurita. Ecol. Modell. 2025, 501, 111014. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Wang, H. Effects of temperature on asexual reproduction and jellyfish booms of Aurelia aurita: Insights from mathematical modeling. Ecol. Modell. 2025, 499, 110915. [Google Scholar] [CrossRef]
- Balikci, E.; Baran, E.T.; Tahmasebifar, A.; Yilmaz, B. Characterization of Collagen from Jellyfish Aurelia aurita and Investigation of Biomaterials Potentials. Appl. Biochem. Biotechnol. 2024, 196, 6200–6221. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Sukhikh, S.; Zhikhreva, A.; Cheliubeeva, E.; Kapitunova, A.; Malkov, D.; Babich, O.; Kulikova, Y. Aurelia aurita jellyfish collagen: Recovery properties. Foods Raw Mater. 2025, 13, 296–305. [Google Scholar] [CrossRef]
- Steć, A.; Targońska, M.; Karkosińska, E.; Słowik, M.; Płoska, A.; Kalinowski, L.; Wielgomas, B.; Waleron, K.; Jasiecki, J.; Dziomba, S. Protein overproduction alters exosome secretion in Chinese hamster ovary cells. Anal. Bioanal. Chem. 2023, 415, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Ghirardello, A.; Bertazza, L.; Gasparotto, M.; Zanatta, E.; Iaccarino, L.; Valadi, H.; Doria, A.; Gatto, M. Size-Exclusion Chromatography Combined with Ultrafiltration Efficiently Isolates Extracellular Vesicles from Human Blood Samples in Health and Disease. Int. J. Mol. Sci. 2023, 24, 3663. [Google Scholar] [CrossRef] [PubMed]
- Tsering, T.; Nadeau, A.; Wu, T.; Dickinson, K.; Burnier, J.V. Extracellular vesicle-associated DNA: Ten years since its discovery in human blood. Cell Death Dis. 2024, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Yoshioka, Y.; Zayasu, Y.; Satoh, N.; Shinzato, C. Transcriptome Analyses of Immune System Behaviors in Primary Polyp of Coral Acropora digitifera Exposed to the Bacterial Pathogen Vibrio coralliilyticus under Thermal Loading. Mar. Biotechnol. 2020, 22, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Pyell, U. Characterization of nanoparticles by capillary electromigration separation techniques. Electrophoresis 2010, 31, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Kato, K.; Hanamura, N.; Kobayashi, M.; Ichiki, T. Evaluation of desialylation effect on zeta potential of extracellular vesicles secreted from human prostate cancer cells by on-chip microcapillary electrophoresis. Jpn. J. Appl. Phys. 2014, 53, 06JL01. [Google Scholar] [CrossRef]
- Khong, N.M.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, P.G.; Suh, J.H.; Pegg, R.B.; Chen, J.; Solval, K.M. The emergence of jellyfish collagen: A comprehensive review on research progress, industrial applications, and future opportunities. Trends Food Sci. Technol. 2023, 141, 104206. [Google Scholar] [CrossRef]
- Ding, J.F.; Li, Y.-Y.; Xu, J.-J.; Su, X.-R.; Gao, X.; Yue, F.-P. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocoll. 2011, 25, 1350–1353. [Google Scholar] [CrossRef]
- Sugahara, T.; Ueno, M.; Goto, Y.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S. Immunostimulation effect of jellyfish collagen. Biosci. Biotechnol. Biochem. 2006, 70, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzycka-Krahel, A.; Steć, A.; Czyrski, G.S.; Heinz, A.; Dziomba, S. Characterization of Small Extracellular Vesicles Isolated from Aurelia aurita. Biology 2025, 14, 922. https://doi.org/10.3390/biology14080922
Dobrzycka-Krahel A, Steć A, Czyrski GS, Heinz A, Dziomba S. Characterization of Small Extracellular Vesicles Isolated from Aurelia aurita. Biology. 2025; 14(8):922. https://doi.org/10.3390/biology14080922
Chicago/Turabian StyleDobrzycka-Krahel, Aldona, Aleksandra Steć, Grzegorz S. Czyrski, Andrea Heinz, and Szymon Dziomba. 2025. "Characterization of Small Extracellular Vesicles Isolated from Aurelia aurita" Biology 14, no. 8: 922. https://doi.org/10.3390/biology14080922
APA StyleDobrzycka-Krahel, A., Steć, A., Czyrski, G. S., Heinz, A., & Dziomba, S. (2025). Characterization of Small Extracellular Vesicles Isolated from Aurelia aurita. Biology, 14(8), 922. https://doi.org/10.3390/biology14080922





