Transcriptome Screening and Identification of Chemosensory Genes in the Goji Berry Psyllid, Bactericera gobica (Hemiptera: Psyllidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Tissue Collection
2.2. RNA Etraction, cDNA Library Construction, Sequencing, Assembly, and Annotation
2.3. Identification of Chemosensory Gene Families and Phylogenetic Analysis
2.4. Transcript Abundance Analysis and qPCR Validation
3. Results
3.1. Transcriptome Sequencing and Assembly
3.2. Mining of Chemosensory Genes
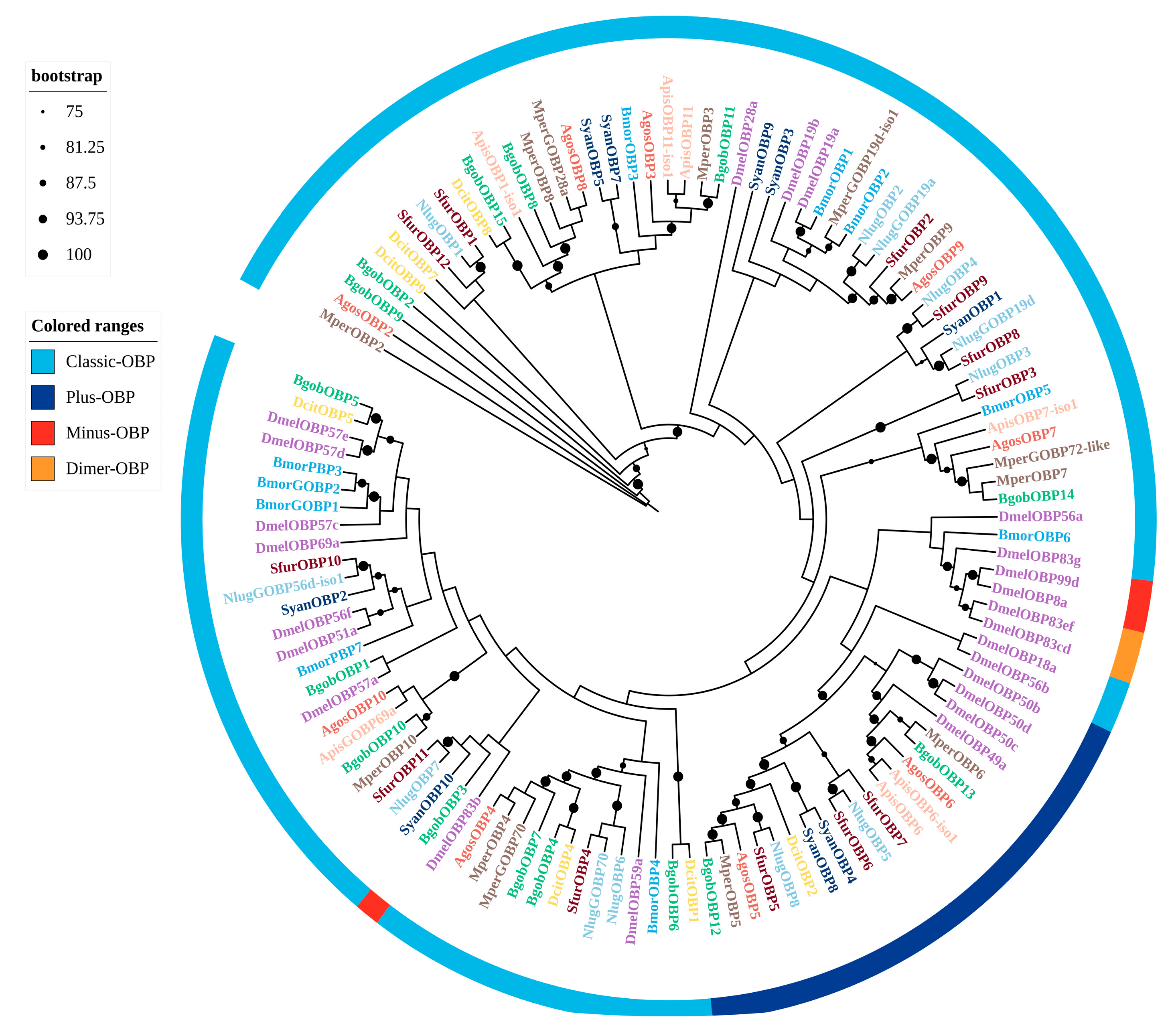
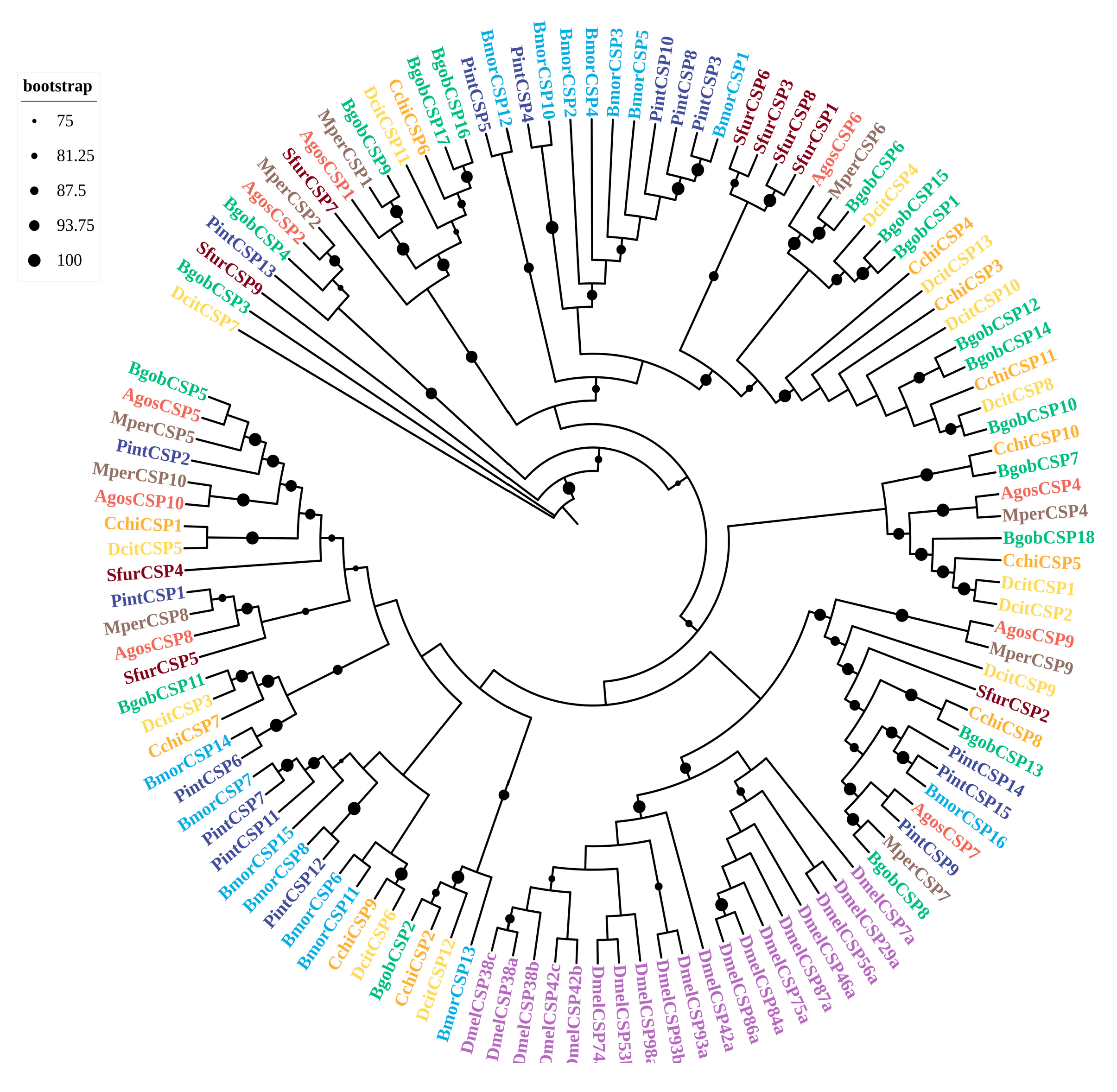
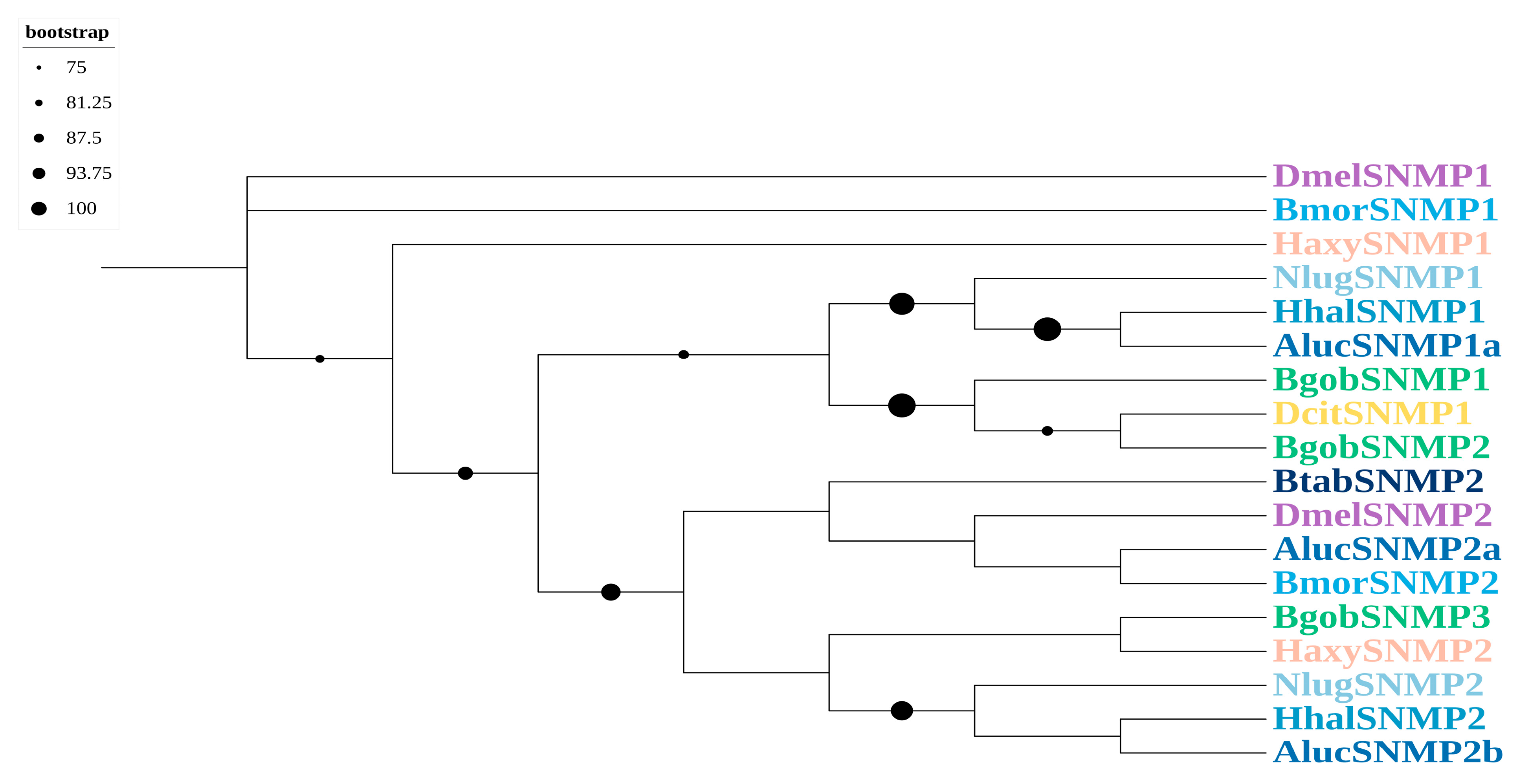
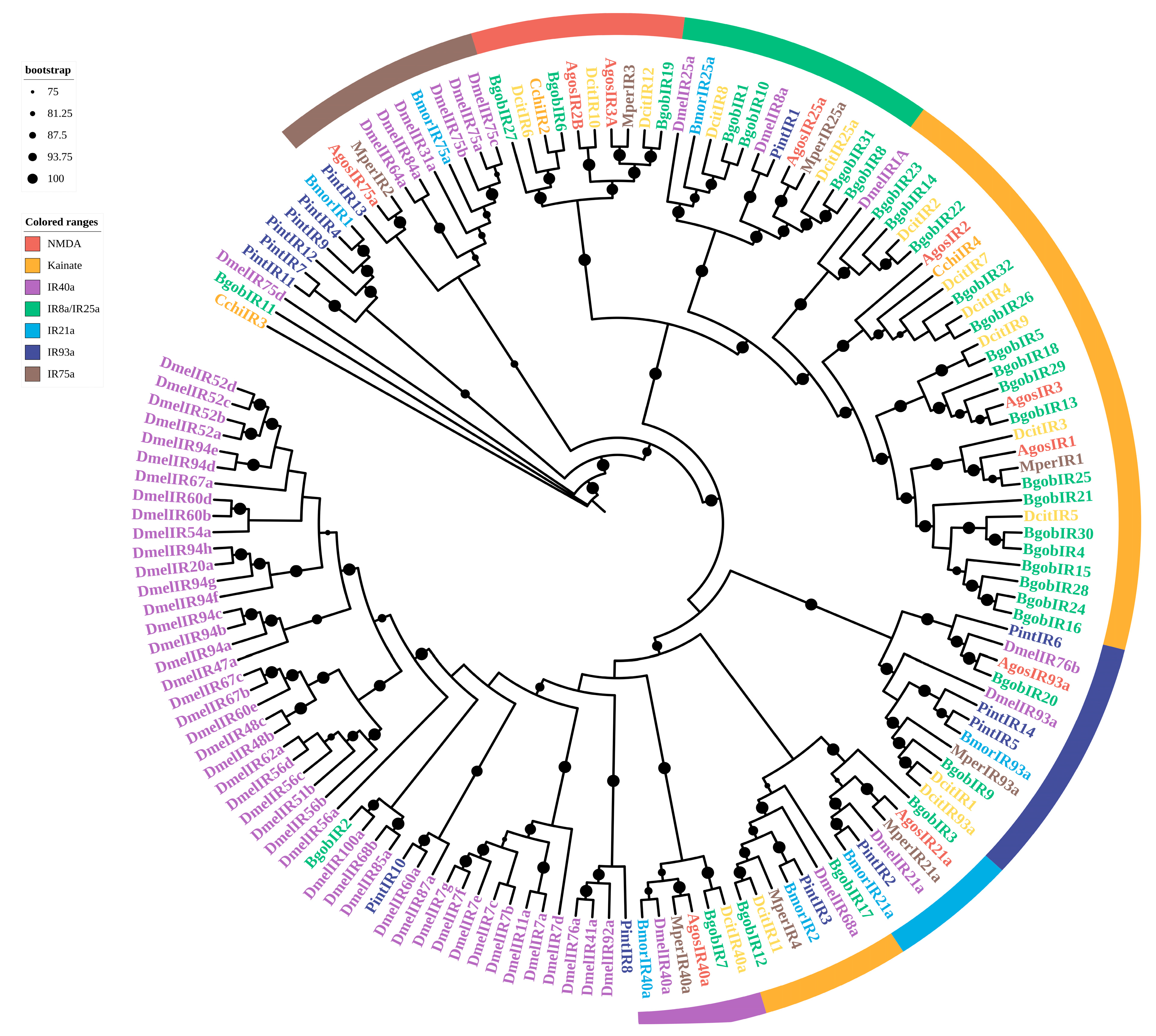
3.3. Identification and Phylogenetic Analysis of Chemosensory Genes in B. gobica
3.3.1. OBPs
3.3.2. CSPs
3.3.3. SNMPs
3.3.4. ORs
3.3.5. GRs
3.3.6. IRs
3.4. Expression Abundance of Chemosensory Genes in B. gobica
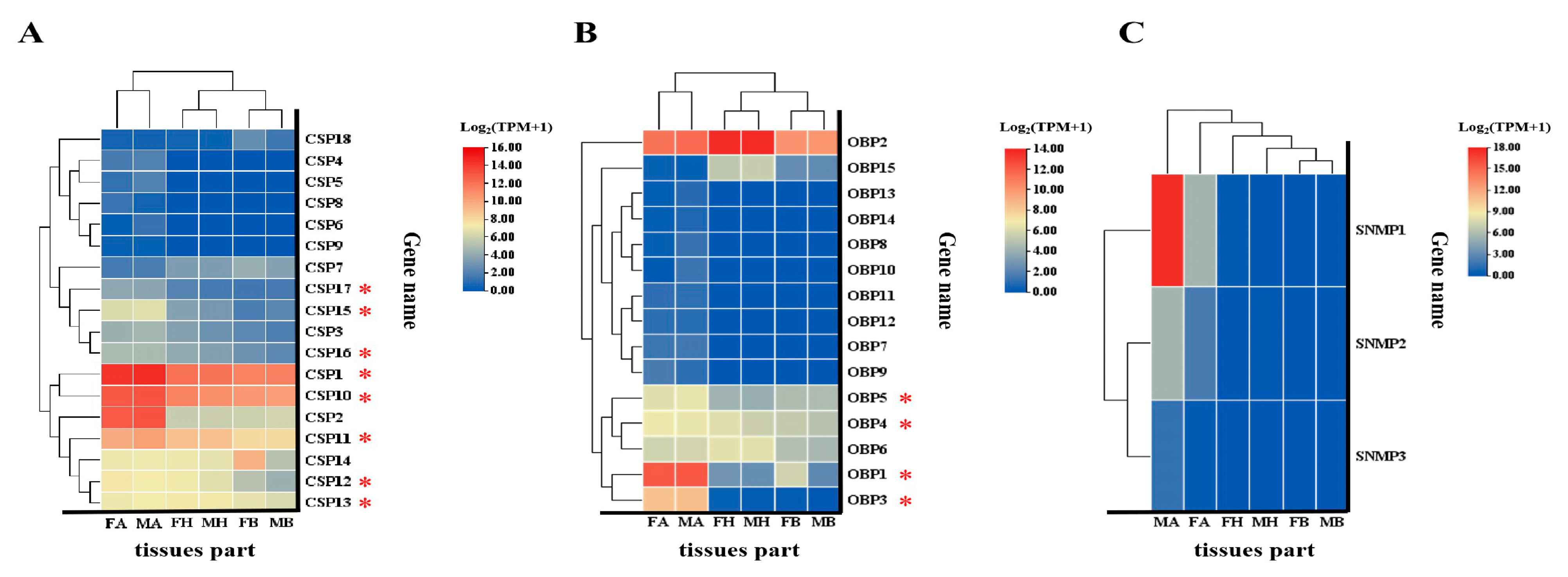
3.4.1. Expression Profiles of BgobOBPs, BgobCSPs, and BgobSNMPs
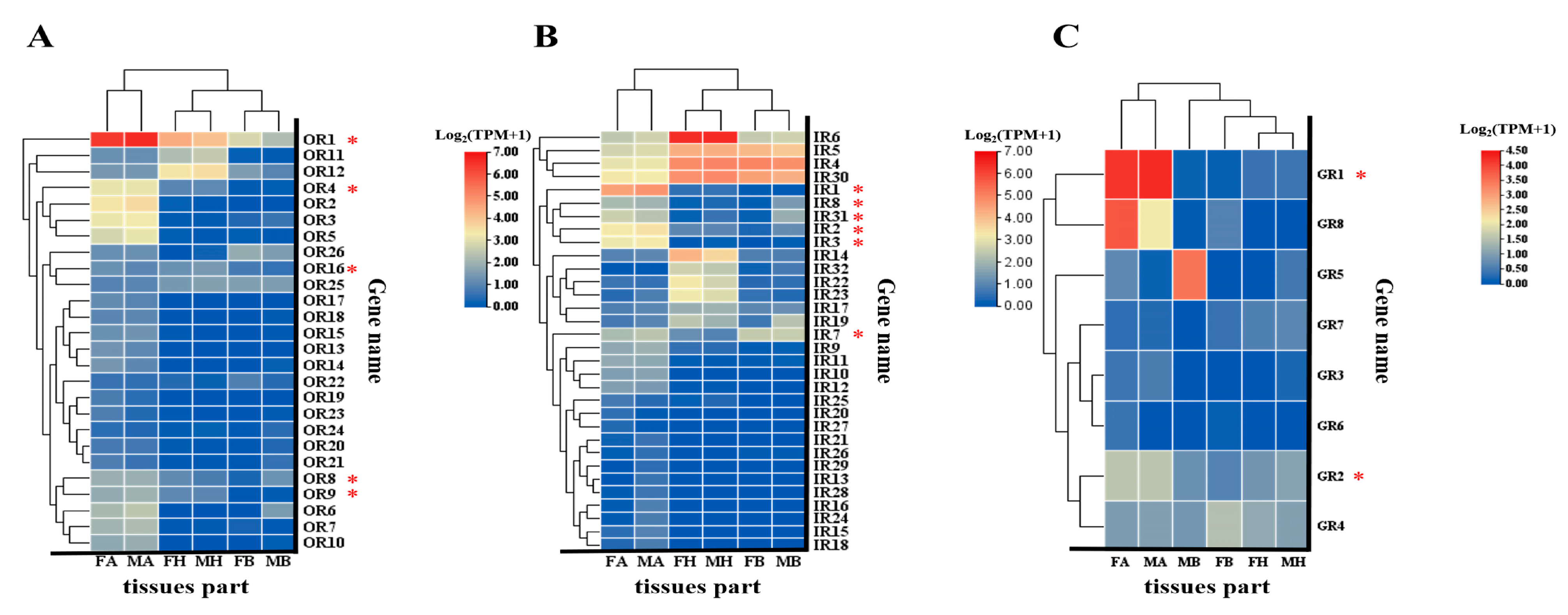
3.4.2. Expression Profiles of BgobORs, BgobGRs, and BgobIRs
3.4.3. Real-Time PCR Validation of the Expression of Chemosensory Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conchou, L.; Anderson, P.; Birgersson, G. Host plant species differentiation in a polyphagous moth: Olfaction is enough. J. Chem. Ecol. 2017, 43, 794–805. [Google Scholar] [CrossRef]
- Forêt, S.; Kevin, W.W.; Ryszard, M. Chemosensory proteins in the honey bee: Insights from the annotated genome, comparative analyses and expressional profiling. Insect Biochem. Mol. Biol. 2007, 37, 19–28. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Hassanali, A.; Njagi, P.G.N.; Bashir, M.O. Chemical ecology of locusts and related acridids. Annu. Rev. Entomol. 2005, 50, 223–245. [Google Scholar] [CrossRef]
- Kaissling, K.-E. Olfactory perireceptor and receptor events in moths: A kinetic model revised. J. Comp. Physiol. A 2009, 195, 895–922. [Google Scholar] [CrossRef] [PubMed]
- Van Der Goes Van Naters, W.; Carlson, J.R. Insects as chemosensors of humans and crops. Nature 2006, 444, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000. [Google Scholar] [CrossRef] [PubMed]
- Guidobaldi, F.; May-Concha, I.J.; Guerenstein, P.G. Morphology and physiology of the olfactory system of blood-feeding insects. J. Physiol. 2014, 108, 96–111. [Google Scholar] [CrossRef]
- Billeter, J.-C.; Levine, J.D. The role of cVA and the odorant binding protein Lush in social and sexual behavior in Drosophila melanogaster. Front. Ecol. Evol. 2015, 3, 75. [Google Scholar] [CrossRef]
- Große-Wilde, E.; Gohl, T.; Bouché, E.; Breer, H.; Krieger, J. Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur. J. Neurosci. 2007, 25, 2364–2373. [Google Scholar] [CrossRef]
- Ishida, Y.; Leal, W.S. Chiral discrimination of the Japanese beetle sex pheromone and a behavioral antagonist by a pheromone-degrading enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 9076–9080. [Google Scholar] [CrossRef]
- McKenzie, S.K.; Fetter-Pruneda, I.; Ruta, V.; Kronauer, D.J.C. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl. Acad. Sci. USA 2016, 113, 14091–14096. [Google Scholar] [CrossRef]
- Ronderos, D.S.; Lin, C.-C.; Potter, C.J.; Smith, D.P. Farnesol-detecting olfactory neurons in Drosophila. J. Neurosci. 2014, 34, 3959–3968. [Google Scholar] [CrossRef]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.L.; Reinberg, D.; Zwiebel, L.J.; Breit, B.; et al. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 2015, 12, 1261–1271. [Google Scholar] [CrossRef]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory perception: Receptors, cells, and circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.A.; Ignell, R.; Carlson, J.R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 2005, 25, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Schoonhoven, L.M.; Van Loon, J.J.A. An inventory of taste in caterpillars: Each species its own key. Acta Zool. Acad. Sci. Hung. 2002, 48 (Suppl. S1), 215–263. [Google Scholar]
- Kaissling, K. Chemo-electrical transduction in insect olfactory receptors. Annu. Rev. Neurosci. 1986, 9, 121–145. [Google Scholar] [CrossRef]
- Krieger, J.; Gänβle, H.; Raming, K.; Breer, H. Odorant binding proteins of Heliothis virescens. Insect Biochem. Mol. Biol. 1993, 23, 449–456. [Google Scholar] [CrossRef]
- Rützler, M.; Zwiebel, L. Molecular biology of insect olfaction: Recent progress and conceptual models. J. Comp. Physiol. A 2005, 191, 777–790. [Google Scholar] [CrossRef]
- Wicher, D.; Miazzi, F. Functional properties of insect olfactory receptors: Ionotropic receptors and odorant receptors. Cell Tissue Res. 2021, 383, 7–19. [Google Scholar] [CrossRef]
- Kaupp, U.B. Olfactory signalling in vertebrates and insects: Differences and commonalities. Nat. Rev. Neurosci. 2010, 11, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q. History of Goji Berries in China. In Phytochemicals in Goji Berries; CRC Press: Boca Raton, FL, USA, 2020; pp. 435–446. [Google Scholar]
- Li, J.; Liu, S.; Guo, K.; Zhang, F.; Qiao, H.; Chen, J.; Yang, M.; Zhu, X.; Xu, R.; Xu, C.; et al. Plant-mediated competition facilitates a phoretic association between a gall mite and a psyllid vector. Exp. Appl. Acarol. 2018, 76, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Smith, O.M.; Chen, W.; Liu, P.; Yuan, Q.; Kang, C.; Wang, T.; Sun, J.; Yan, B.; Liu, X.; et al. Morphological characterization and sexual dimorphism of the antennal sensilla in Bactericera gobica Loginova (Hemiptera: Psyllidae)—A scanning and transmission electron microscopic study. PeerJ 2022, 10, e12888. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Luo, Z.; Cai, X.; Bian, L.; Xiu, C.; Fu, N.; Liu, N.; Zhang, Z.; Li, Z. Transcriptome profiling of Euproctis pseudoconspersa reveals candidate olfactory genes for type III sex pheromone detection. Int. J. Mol. Sci. 2025, 26, 1405. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, J.; Yang, M.; Li, Y.; Guo, K.; Qiao, H.; Xu, R.; Liu, S.; Xu, C. Selection and validation of reference genes for gene expression in Bactericera gobica Loginova under different insecticide stresses. Int. J. Mol. Sci. 2024, 25, 2434. [Google Scholar] [CrossRef]
- Zhong, Y.; Cheng, C.; Jiang, B.; Jiang, N.; Zhang, Y.; Hu, M.; Zhong, G. Digital gene expression analysis of ponkan mandarin (Citrus reticulata blanco) in response to Asia citrus psyllid-vectored huanglongbing infection. Int. J. Mol. Sci. 2016, 17, 1063. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Bin, S.; Chen, L.; Han, Q.; Lin, J. Antennal and abdominal transcriptomes reveal chemosensory genes in the Asian citrus psyllid, Diaphorina citri. PLoS ONE 2016, 11, e0159372. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, H.B.; Fan, J.Y.; Liu, T.Y.; Meng, L.W.; Liu, Y.; Yang, M.; Zhu, X.; Xu, R.; Xu, C.; et al. An odorant-binding protein of Asian citrus psyllid, Diaphorina citri, participates in the response of host plant volatiles. Pest Manag. Sci. 2021, 77, 3068–3079. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.H.; Wang, Z.; Xiao, X.; Yin, X.H.; Hu, S.Y.; Miao, H.-N.; Zhang, Y.-J.; Liang, P.; Gu, S.-H. Omics analysis of odorant-binding proteins and cuticle-enriched SfruOBP18 confers multi-insecticide tolerance in Spodoptera frugiperda. J. Agric. Food Chem. 2024, 72, 22532–22544. [Google Scholar] [CrossRef]
- Xiao, X.; Yin, X.H.; Hu, S.Y.; Miao, H.N.; Wang, Z.; Li, H.; Zhang, Y.-J.; Liang, P.; Gu, S.H. Overexpression of two odorant binding proteins confers chlorpyrifos resistance in the green peach aphid Myzus persicae. J. Agric. Food Chem. 2024, 72, 20101–20113. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, H.B.; Liu, Y.; Fan, J.Y.; Ma, Y.J.; Yuan, C.Y.; Lou, B.-H.; Wang, J.J. Odorant binding protein 2 reduces imidacloprid susceptibility of Diaphorina citri. Pestic. Biochem. Physiol. 2020, 168, 104642. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, W.W.; Fang, A.; Wang, Z.B.; Yuan, X.F.; Sun, Y.; Zou, Z.-H.; Chen, N.; Jing, T.-X.; Liu, Y.-X.; et al. Chemosensory protein 8 confers thiamethoxam resistance in Diaphorina citri. Pestic. Biochem. Physiol. 2025, 208, 106264. [Google Scholar] [CrossRef] [PubMed]
- Mugford, S.T.; Gravino, M.; Kreuter, N.; Maqbool, A.; Joyce, J.; Canham, J.; Claire, D.; Christine, W.; Thomas, M.; Saskia, H. The Aphid Effector Mp10/CSP4 is Conserved Among Hemipteran Sap-Feeders and Targets Cell Surface Receptor Trafficking Processes in Plants. In Proceedings of the Molecular Plant-Microbe Interactions (MPMI), Providence, RI, USA, 16–20 July 2023; Zenodo: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Rao, W.; Ma, T.; Cao, J.; Zhang, Y.; Chen, S.; Lin, S.; Liu, X.; He, G.; Wan, L. Recognition of a salivary effector by the TNL protein RCSP promotes effector-triggered immunity and systemic resistance in Nicotiana benthamiana. J. Integr. Plant Biol. 2025, 67, 150–168. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Bargeton, B.; Abuin, L.; Bukar, N.; Reina, J.H.; Bartoi, T.; Graf, M.; Ong, H.; Ulbrich, M.H.; Masson, J.-F.; et al. A CD36 ectodomain mediates insect pheromone detection via a putative tunnelling mechanism. Nat. Commun. 2016, 7, 11866. [Google Scholar] [CrossRef]
- Nichols, Z.; Vogt, R.G. The SNMP/CD36 gene family in Diptera, Hymenoptera and Coleoptera: Drosophila melanogaster, D. pseudoobscura, Anopheles gambiae, Aedes aegypti, Apis mellifera, and Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 398–415. [Google Scholar] [CrossRef]
- Li, R.; Jiang, G.F.; Shu, X.H.; Wang, Y.Q.; Li, M.-J. Identification and expression profile analysis of chemosensory genes from the antennal transcriptome of bamboo locust (Ceracris kiangsu). Front. Physiol. 2020, 11, 889. [Google Scholar] [CrossRef]
- Gu, S.H.; Sun, L.; Yang, R.N.; Wu, K.M.; Guo, Y.Y.; Li, X.C.; Zhou, J.-J.; Zhang, Y.-J.; Newcomb, R.D. Molecular characterization and differential expression of olfactory genes in the antennae of the black cutworm moth Agrotis ipsilon. PLoS ONE 2014, 9, e103420. [Google Scholar] [CrossRef]
- Vogt, R.G.; Miller, N.E.; Litvack, R.; Fandino, R.A.; Sparks, J.; Staples, J.; Friedman, R.; Dickens, J.C. The insect SNMP gene family. Insect Biochem. Mol. Biol. 2009, 39, 448–456. [Google Scholar] [CrossRef]
- Liu, S.; Chang, H.; Liu, W.; Cui, W.; Liu, Y.; Wang, Y.; Ren, B.; Wang, G. Essential role for SNMP1 in detection of sex pheromones in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2020, 127, 103485. [Google Scholar] [CrossRef]
- Liu, J.; Xie, J.; Khashaveh, A.; Zhou, J.; Zhang, Y.; Dong, H.; Cong, B.; Gu, S. Identification and tissue expression profiles of odorant receptor genes in the green peach aphid Myzus persicae. Insects 2022, 13, 398. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Vidal, G.; Bouysset, C.; Gévar, J.; Mbouzid, H.; Nara, C.; Delaroche, J.; Golebiowski, J.; Montagné, N.; Fiorucci, S.; Jacquin-Joly, E. Reverse chemical ecology in a moth: Machine learning on odorant receptors identifies new behaviorally active agonists. Cell. Mol. Life Sci. 2021, 78, 6593–6603. [Google Scholar] [CrossRef] [PubMed]
- Vosshall, L.B. Olfactory/gustatory processing. In Cold Spring Harbor Monograph Archive; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2007; Volume 49, pp. 79–100. [Google Scholar]
- Clyne, P.J.; Warr, C.G.; Carlson, J.R. Candidate taste receptors in Drosophila. Science 2000, 287, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Z.; Xiao, Q.; Gao, Z.L.; Gu, J.; Huang, L.H. Identification and function analysis of two new gustatory receptors related to silkworm monophagy. Insect Sci. 2025, 1–14. [Google Scholar] [CrossRef]
- Chen, R.; Yan, J.; Wickham, J.D.; Gao, Y. Genomic identification and evolutionary analysis of chemosensory receptor gene families in two Phthorimaea pest species: Insights into chemical ecology and host adaptation. BMC Genom. 2024, 25, 493. [Google Scholar] [CrossRef]
- Silbering, A.F.; Rytz, R.; Grosjean, Y.; Abuin, L.; Ramdya, P.; Jefferis, G.S.; Benton, R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011, 31, 13357–13375. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef]
- Brown, E.B.; Shah, K.D.; Palermo, J.; Dey, M.; Dahanukar, A.; Keene, A.C. Ir56d-dependent fatty acid responses in Drosophila uncover taste discrimination between different classes of fatty acids. eLife 2021, 10, e67878. [Google Scholar] [CrossRef]
- Budelli, G.; Ni, L.; Berciu, C.; van Giesen, L.; Knecht, Z.A.; Chang, E.C.; Kaminski, B.; Silbering, A.F.; Samuel, A.; Klein, M.; et al. Ionotropic receptors specify the morphogenesis of phasic sensors controlling rapid thermal preference in Drosophila. Neuron 2019, 101, 738–747.e3. [Google Scholar] [CrossRef]
- Enjin, A.; Zaharieva, E.E.; Frank, D.D.; Mansourian, S.; Suh, G.S.; Gallio, M.; Stensmyr, M.C. Humidity sensing in Drosophila. Curr. Biol. 2016, 26, 1352–1358. [Google Scholar] [CrossRef]
- Bray, S.; Amrein, H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 2003, 39, 1019–1029. [Google Scholar] [CrossRef]
- Moon, S.J.; Lee, Y.; Jiao, Y.; Montell, C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 2009, 19, 1623–1627. [Google Scholar] [CrossRef]
- Miyamoto, T.; Amrein, H. Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 2008, 11, 874–876. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ge, Y.; Zhang, Z.; Liang, J.; Kang, C.; Zhang, C.; Chen, K.; Wan, X.; Zhang, L.; Shi, W.; et al. Transcriptome Screening and Identification of Chemosensory Genes in the Goji Berry Psyllid, Bactericera gobica (Hemiptera: Psyllidae). Biology 2025, 14, 1105. https://doi.org/10.3390/biology14081105
Liu Z, Ge Y, Zhang Z, Liang J, Kang C, Zhang C, Chen K, Wan X, Zhang L, Shi W, et al. Transcriptome Screening and Identification of Chemosensory Genes in the Goji Berry Psyllid, Bactericera gobica (Hemiptera: Psyllidae). Biology. 2025; 14(8):1105. https://doi.org/10.3390/biology14081105
Chicago/Turabian StyleLiu, Zhanghui, Yang Ge, Zekun Zhang, Jiayi Liang, Chuanzhi Kang, Chengcai Zhang, Kang Chen, Xiufu Wan, Liu Zhang, Wangpeng Shi, and et al. 2025. "Transcriptome Screening and Identification of Chemosensory Genes in the Goji Berry Psyllid, Bactericera gobica (Hemiptera: Psyllidae)" Biology 14, no. 8: 1105. https://doi.org/10.3390/biology14081105
APA StyleLiu, Z., Ge, Y., Zhang, Z., Liang, J., Kang, C., Zhang, C., Chen, K., Wan, X., Zhang, L., Shi, W., & Chen, H. (2025). Transcriptome Screening and Identification of Chemosensory Genes in the Goji Berry Psyllid, Bactericera gobica (Hemiptera: Psyllidae). Biology, 14(8), 1105. https://doi.org/10.3390/biology14081105





