One Shock, Not One Cure: Electroporation Reveals Disease-Specific Constraints in Hepatocyte Gene Editing Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Animal Care
2.2. Hepatocyte Electroporation and Transplantation
2.3. In Vitro LDL-C Uptake Assay
2.4. APAP Selection
2.5. Histology and Immunofluorescence Staining
2.6. Metabolic Analysis
2.7. Quantification of Atherosclerosis
2.8. Immunoblot
2.9. Real-Time Quantitative PCR (RT-PCR) Analysis
2.10. Statistical Analysis
3. Results
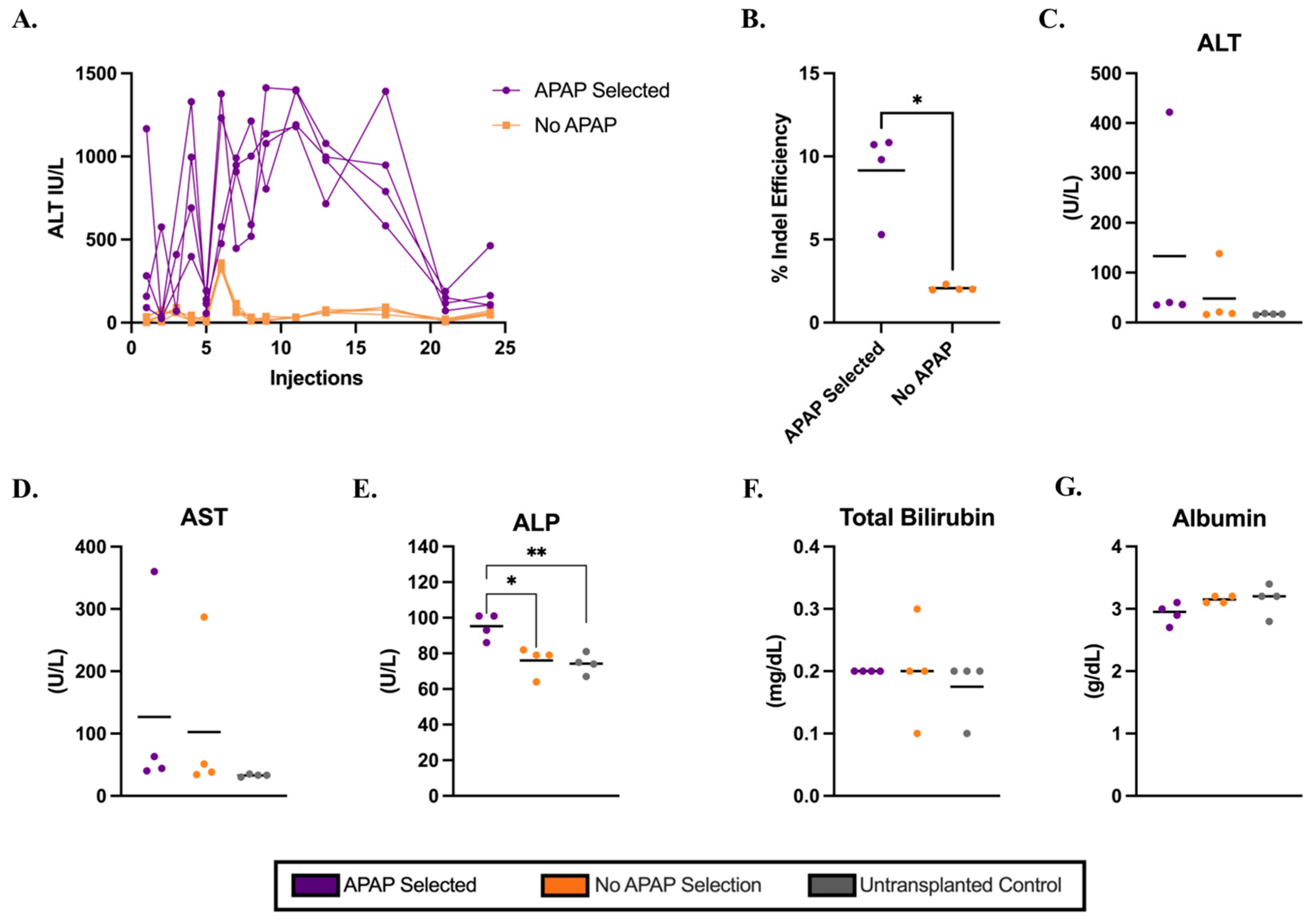
3.1. APAP-Mediated Selection of Electroporated Hepatocytes in C57BL/6J Mice
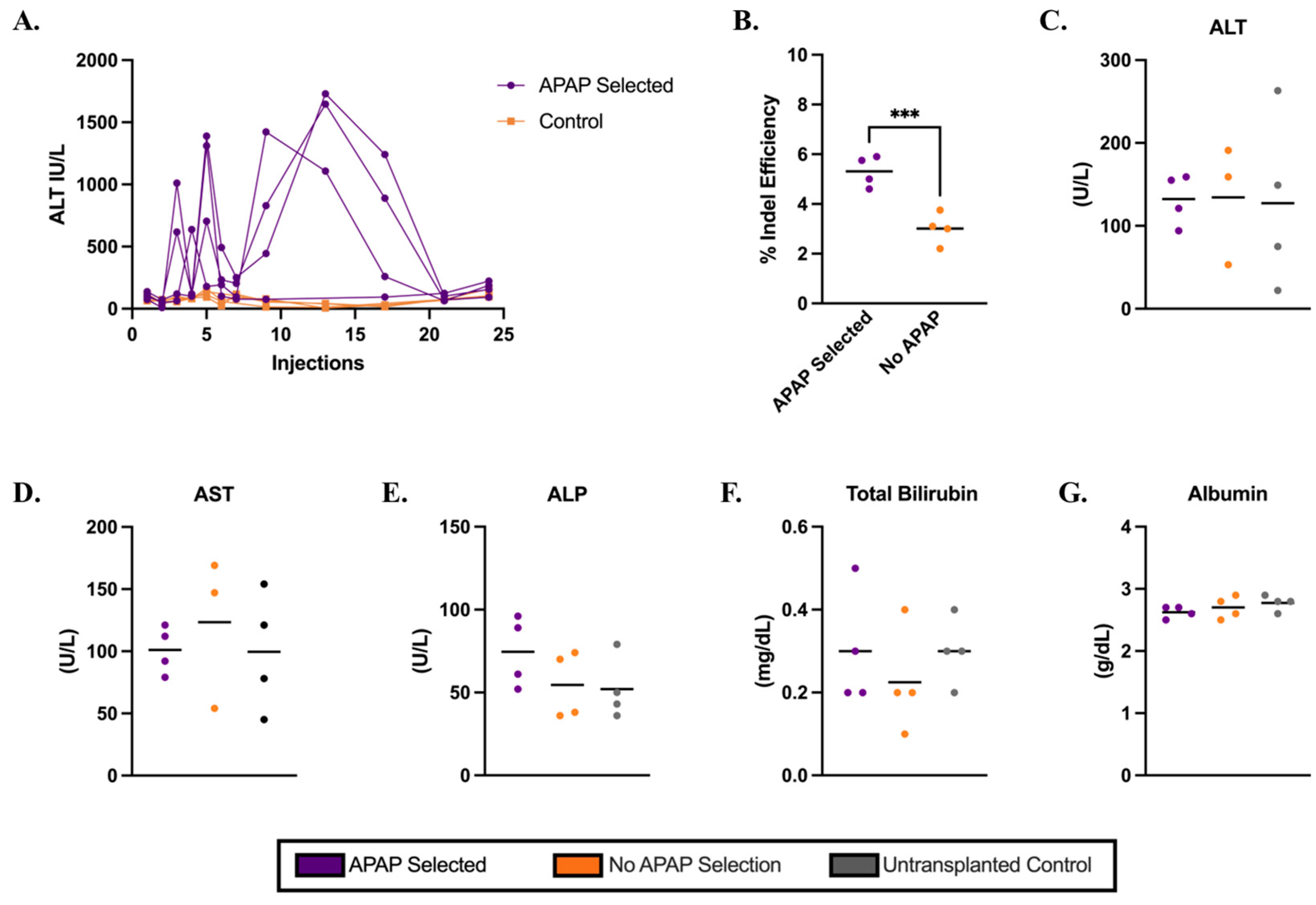
3.2. Cypor-Deficient Hepatocytes Selected in Ldlr−/− Mice Using APAP Treatment
3.3. Metabolic Biomarkers and Histology Analysis in Ldlr−/− Mice on a Regular Diet
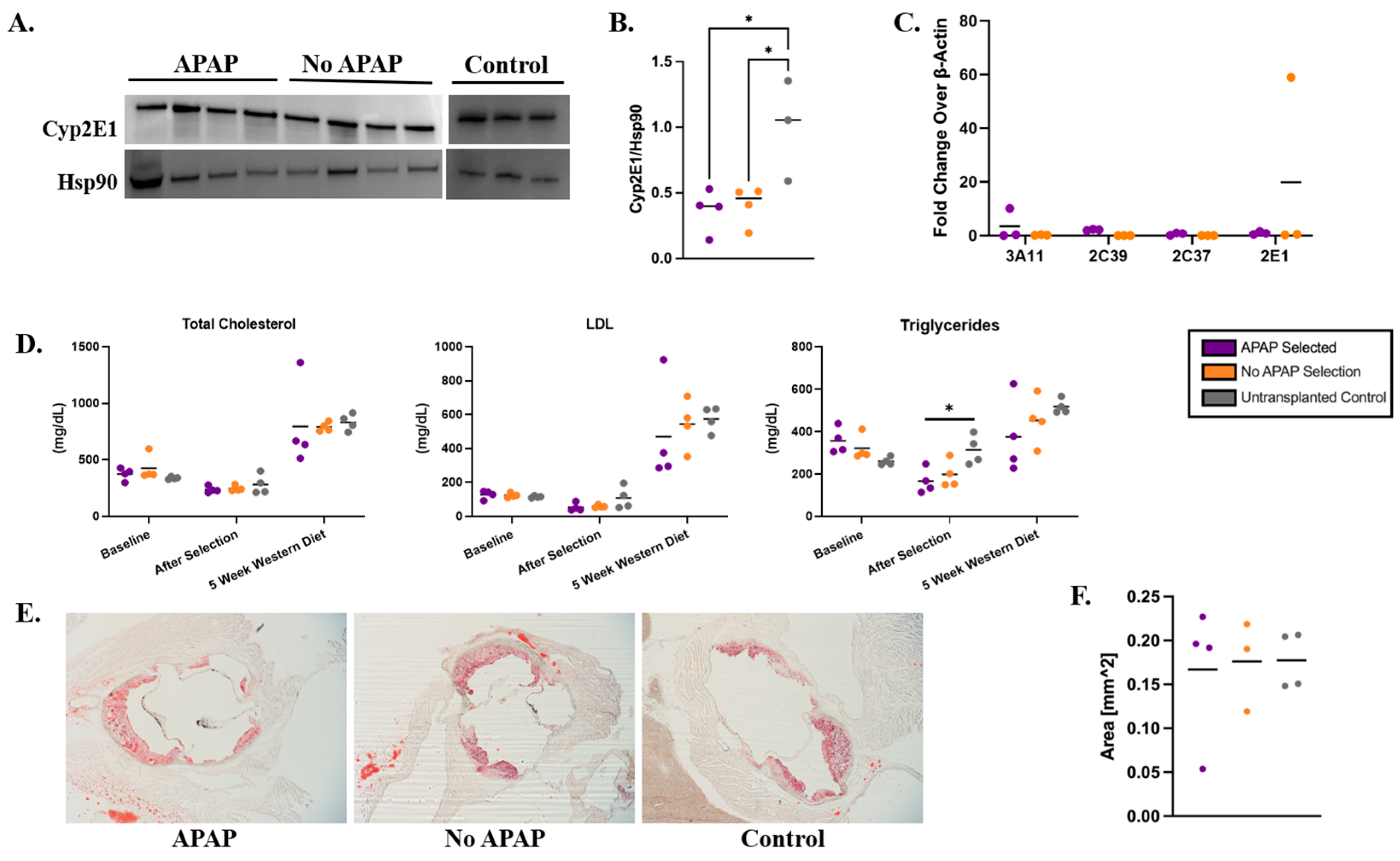
3.4. Persistence of Gene-Edited Hepatocytes in the Liver After Western Diet
3.5. Metabolic Markers and Atherosclerosis in Ldlr−/− Recipient Mice on a Western Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALB | Albumin |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| APAP | Acetaminophen |
| AST | Aspartate aminotransferase |
| Cypor | Cytochrome P450 oxidoreductase |
| Fah | Fumarylacetoacetate hydrolase |
| FH | Familial hypercholesterolemia |
| H&E | Hematoxylin and eosin |
| HoFH | Homozygous familial hypercholesterolemia |
| IF | Immunofluorescent |
| IHC | Immunohistochemistry |
| IP | Intraperitoneal |
| LDL-C | Low density lipoprotein—cholesterol |
| Ldlr | Low density lipoprotein receptor |
| NTBC | 2-nitro-4-trifluoromethylbenzoyl-1,3-cyclohexanedione |
| RNP | Ribonucleoprotein |
| RT-PCR | Real-time quantitative PCR |
| TBIL | Total bilirubin |
| TG | Triglycerides |
| WT | Wild-type |
References
- Cuchel, M.; Bruckert, E.; Ginsberg, H.N.; Raal, F.J.; Santos, R.D.; Hegele, R.A.; Kuivenhoven, J.A.; Nordestgaard, B.G.; Descamps, O.S.; Steinhagen-Thiessen, E.; et al. Homozygous familial hypercholesterolaemia: New insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 2014, 35, 2146–2157. [Google Scholar] [CrossRef]
- Chaudhry, A.; Trinder, M.; Vesely, K.; Cermakova, L.; Jackson, L.; Wang, J.; Hegele, R.A.; Brunham, L.R. Genetic Identification of Homozygous Familial Hypercholesterolemia by Long-Read Sequencing Among Patients With Clinically Diagnosed Heterozygous Familial Hypercholesterolemia. Circ. Genom. Precis. Med. 2023, 16, e003887. [Google Scholar] [CrossRef]
- Bajaj, A.; Cuchel, M. Advancements in the Treatment of Homozygous Familial Hypercholesterolemia. J. Atheroscler. Thromb. 2022, 29, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Hemphill, L.C. Familial hypercholesterolemia and estimation of US patients eligible for low-density lipoprotein apheresis after maximally tolerated lipid-lowering therapy. J. Clin. Lipidol. 2014, 8, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ 1991, 303, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, L.; Goldberg, A.; Hovingh, K.; Cohen, J.; Karalis, D.G. Recognition and Treatment of Homozygous Familial Hypercholesterolemia by Primary Care Physicians: A Survey from the National Lipid Association. J. Gen. Intern. Med. 2020, 35, 2225–2227. [Google Scholar] [CrossRef]
- Kayikcioglu, M.; Tokgozoglu, L. Current Treatment Options in Homozygous Familial Hypercholesterolemia. Pharmaceuticals 2022, 16, 64. [Google Scholar] [CrossRef]
- Santos, R.D.; Gidding, S.S.; Hegele, R.A.; Cuchel, M.A.; Barter, P.J.; Watts, G.F.; Baum, S.J.; Catapano, A.L.; Chapman, M.J.; Defesche, J.C.; et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: A consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016, 4, 850–861. [Google Scholar] [CrossRef]
- Raal, F.J.; Pilcher, G.J.; Panz, V.R.; van Deventer, H.E.; Brice, B.C.; Blom, D.J.; Marais, A.D. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation 2011, 124, 2202–2207. [Google Scholar] [CrossRef]
- Kroon, A.A.; van’t Hof, M.A.; Demacker, P.N.; Stalenhoef, A.F. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis 2000, 152, 519–526. [Google Scholar] [CrossRef]
- Serrano, M.T.; Sabroso, S.; Esteban, L.M.; Berenguer, M.; Fondevila, C.; Lorente, S.; Cortés, L.; Sanchez-Antolin, G.; Nuño, J.; De la Rosa, G.; et al. Mortality and Causes of Death After Liver Transplantation: Analysis of Sex Differences in a Large Nationwide Cohort. Transpl. Int. 2022, 35, 10263. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, A.; Nobili, V.; Calandra, S.; Francalanci, P.; Bernabei, S.; El Hachem, M.; Monti, L.; Gennari, F.; Torre, G.; de Ville de Goyet, J.; et al. Preemptive liver transplantation in a child with familial hypercholesterolemia. Pediatr. Transplant. 2011, 15, E25-29. [Google Scholar] [CrossRef] [PubMed]
- Arnon, R.; Kerkar, N.; Davis, M.K.; Anand, R.; Yin, W.; Gonzalez-Peralta, R.P.; for the SPLIT Research Group. Liver transplantation in children with metabolic diseases: The studies of pediatric liver transplantation experience. Pediatr. Transplant. 2010, 14, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hou, Y.; Zhao, W.; Yang, B. Recent progress in gene therapy for familial hypercholesterolemia treatment. iScience 2024, 27, 110641. [Google Scholar] [CrossRef]
- Grossman, M.; Rader, D.J.; Muller, D.W.M.; Kolansky, D.M.; Kozarsky, K.; Clark, B.J.; Stein, E.A.; Lupien, P.J.; Brewer, H.B.; Raper, S.E.; et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat. Med. 1995, 1, 1148–1154. [Google Scholar] [CrossRef]
- Williams, R.S. Human gene therapy—Of tortises and hares. Nat. Med. 1995, 1, 1137–1138. [Google Scholar] [CrossRef]
- Vonada, A.; Grompe, M. In vivo selection of hepatocytes. Hepatology 2024. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, X.; Wu, J.; Wang, C.; Zhang, K.; Zhang, L.; Hui, L. Hepatocyte transplantation: The progress and the challenges. Hepatol. Commun. 2023, 7, e0266. [Google Scholar] [CrossRef]
- Gerriets, V.; Anderson, J.; Patel, P.; Nappe, T.M. Acetaminophen. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mazaleuskaya, L.L.; Sangkuhl, K.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet. Genom. 2015, 25, 416–426. [Google Scholar] [CrossRef]
- Xia, C.; Panda, S.P.; Marohnic, C.C.; Martasek, P.; Masters, B.S.; Kim, J.J. Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc. Natl. Acad. Sci. USA 2011, 108, 13486–13491. [Google Scholar] [CrossRef]
- Agrawal, S.; Murray, B.P.; Khazaeni, B. Acetaminophen Toxicity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Barahman, M.; Asp, P.; Roy-Chowdhury, N.; Kinkhabwala, M.; Roy-Chowdhury, J.; Kabarriti, R.; Guha, C. Hepatocyte Transplantation: Quo Vadis? Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Vonada, A.; Wakefield, L.; Martinez, M.; Harding, C.O.; Grompe, M.; Tiyaboonchai, A. Complete correction of murine phenylketonuria by selection-enhanced hepatocyte transplantation. Hepatology 2024, 79, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Vonada, A.; Tiyaboonchai, A.; Nygaard, S.; Posey, J.; Peters, A.M.; Winn, S.R.; Cantore, A.; Naldini, L.; Harding, C.O.; Grompe, M. Therapeutic liver repopulation by transient acetaminophen selection of gene-modified hepatocytes. Sci. Transl. Med. 2021, 13, eabg3047. [Google Scholar] [CrossRef]
- Gibson, J.; Dhungana, A.; Pokhrel, M.; Arthur, B.; Suresh, P.; Adebayo, O.; Cottle, R.N. Validation of Clinical-Grade Electroporation Systems for CRISPR-Cas9-Mediated Gene Therapy in Primary Hepatocytes for the Correction of Inherited Metabolic Liver Disease. Cells 2025, 14, 711. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, T.; Ates, I.; Fernando, L.; Addlestone, E.; Lee, C.M.; Richards, V.P.; Cottle, R.N. Electroporation-Mediated Delivery of Cas9 Ribonucleoproteins Results in High Levels of Gene Editing in Primary Hepatocytes. Cris. J. 2022, 5, 397–409. [Google Scholar] [CrossRef]
- Ates, I.; Stuart, C.; Rathbone, T.; Barzi, M.; He, G.; Major, A.M.; Shankar, V.; Lyman, R.A.; Angner, S.S.; Mackay, T.F.C.; et al. Ex vivo gene editing and cell therapy for hereditary tyrosinemia type 1. Hepatol. Commun. 2024, 8, e0424. [Google Scholar] [CrossRef]
- Rathbone, T.; Ates, I.; Stuart, C.; Parker, T.; Cottle, R.N. Electroporation-mediated Delivery of Cas9 Ribonucleoproteins and mRNA into Freshly Isolated Primary Mouse Hepatocytes. J. Vis. Exp. 2022, e63828. [Google Scholar] [CrossRef]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Han, R. BEAT: A Python Program to Quantify Base Editing from Sanger Sequencing. Cris. J. 2019, 2, 223–229. [Google Scholar] [CrossRef]
- Grompe, M. Principles of therapeutic liver repopulation. J. Inherit. Metab. Dis. 2006, 29, 421–425. [Google Scholar] [CrossRef]
- Daugherty, A.; Tall, A.R.; Daemen, M.; Falk, E.; Fisher, E.A.; Garcia-Cardena, G.; Lusis, A.J.; Owens, A.P., 3rd; Rosenfeld, M.E.; Virmani, R.; et al. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e131–e157. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Dunbar, D.; Kaminsky, L.S. Characterization of mouse small intestinal cytochrome P450 expression. Drug Metab. Dispos. 2003, 31, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Oinonen, T.; Lindros, K.O. Zonation of hepatic cytochrome P-450 expression and regulation. Biochem. J. 1998, 329 Pt 1, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Loeb, W.F.; Quimbly, F.W. The Clinical Chemistry of Laboratory Animals; CRC Press: Philadelphia, PA, USA, 1999; Volume Second Edition. [Google Scholar]
- Getz, G.S.; Reardon, C.A. Do the Apoe−/− and Ldlr−/− Mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar] [CrossRef]
- Gu, J.; Weng, Y.; Zhang, Q.Y.; Cui, H.; Behr, M.; Wu, L.; Yang, W.; Zhang, L.; Ding, X. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: Impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J. Biol. Chem. 2003, 278, 25895–25901. [Google Scholar] [CrossRef]
- Weng, Y.; DiRusso, C.C.; Reilly, A.A.; Black, P.N.; Ding, X. Hepatic gene expression changes in mouse models with liver-specific deletion or global suppression of the NADPH-cytochrome P450 reductase gene. Mechanistic implications for the regulation of microsomal cytochrome P450 and the fatty liver phenotype. J. Biol. Chem. 2005, 280, 31686–31698. [Google Scholar] [CrossRef]
- Henderson, C.J.; Otto, D.M.; Carrie, D.; Magnuson, M.A.; McLaren, A.W.; Rosewell, I.; Wolf, C.R. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J. Biol. Chem. 2003, 278, 13480–13486. [Google Scholar] [CrossRef]
- Stromstedt, M.; Rozman, D.; Waterman, M.R. The ubiquitously expressed human CYP51 encodes lanosterol 14 alpha-demethylase, a cytochrome P450 whose expression is regulated by oxysterols. Arch. Biochem. Biophys. 1996, 329, 73–81. [Google Scholar] [CrossRef]
- Schwarz, M.; Russell, D.W.; Dietschy, J.M.; Turley, S.D. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 1998, 39, 1833–1843. [Google Scholar] [CrossRef]
- Zaher, H.; Buters, J.T.; Ward, J.M.; Bruno, M.K.; Lucas, A.M.; Stern, S.T.; Cohen, S.D.; Gonzalez, F.J. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol. Appl. Pharmacol. 1998, 152, 193–199. [Google Scholar] [CrossRef]
- Zong, H.; Armoni, M.; Harel, C.; Karnieli, E.; Pessin, J.E. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E532–E539. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, C.; Pokhrel, M.; Arthur, B.; Suresh, P.; Ates, I.; Gibson, J.; Dhungana, A.; Mehlem, R.; Boysia, A.; Padalkar, M.V.; et al. One Shock, Not One Cure: Electroporation Reveals Disease-Specific Constraints in Hepatocyte Gene Editing Therapy. Biology 2025, 14, 1091. https://doi.org/10.3390/biology14081091
Clark C, Pokhrel M, Arthur B, Suresh P, Ates I, Gibson J, Dhungana A, Mehlem R, Boysia A, Padalkar MV, et al. One Shock, Not One Cure: Electroporation Reveals Disease-Specific Constraints in Hepatocyte Gene Editing Therapy. Biology. 2025; 14(8):1091. https://doi.org/10.3390/biology14081091
Chicago/Turabian StyleClark, Callie, Menam Pokhrel, Benjamin Arthur, Pramita Suresh, Ilayda Ates, Justin Gibson, Abishek Dhungana, Ryan Mehlem, Andrew Boysia, Mugdha V. Padalkar, and et al. 2025. "One Shock, Not One Cure: Electroporation Reveals Disease-Specific Constraints in Hepatocyte Gene Editing Therapy" Biology 14, no. 8: 1091. https://doi.org/10.3390/biology14081091
APA StyleClark, C., Pokhrel, M., Arthur, B., Suresh, P., Ates, I., Gibson, J., Dhungana, A., Mehlem, R., Boysia, A., Padalkar, M. V., Pokhrel, A., Echesabal-Chen, J., Vonada, A., Stamatikos, A., Savinova, O. V., Grompe, M., & Cottle, R. N. (2025). One Shock, Not One Cure: Electroporation Reveals Disease-Specific Constraints in Hepatocyte Gene Editing Therapy. Biology, 14(8), 1091. https://doi.org/10.3390/biology14081091




