Linarin and Hyperoside Inhibit lptD/msbA to Disrupt Membranes of Multidrug-Resistant Acinetobacter baumannii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
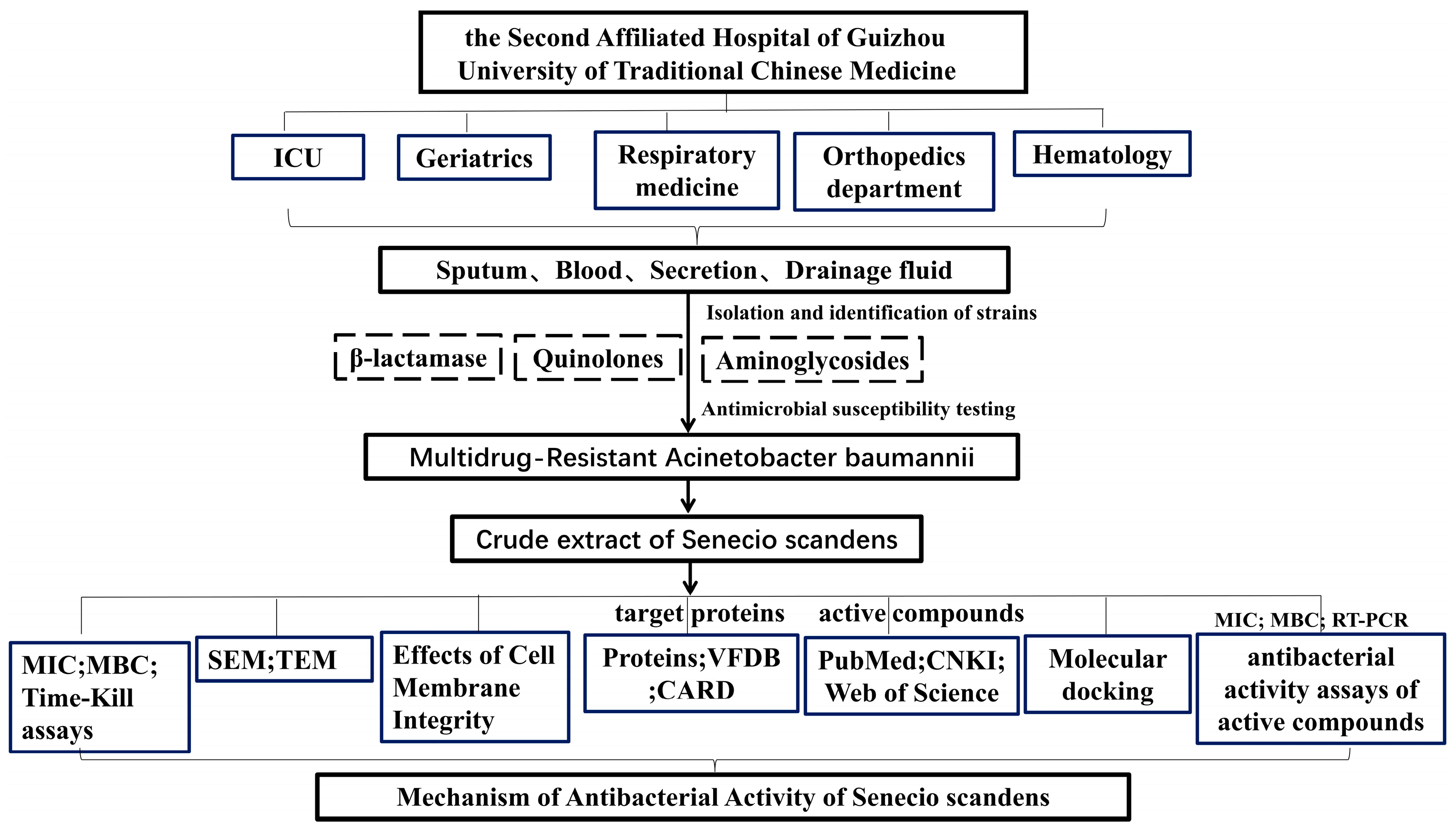
2.1. Drug and Bacterial Isolation
2.2. Determination of the MIC and MBC
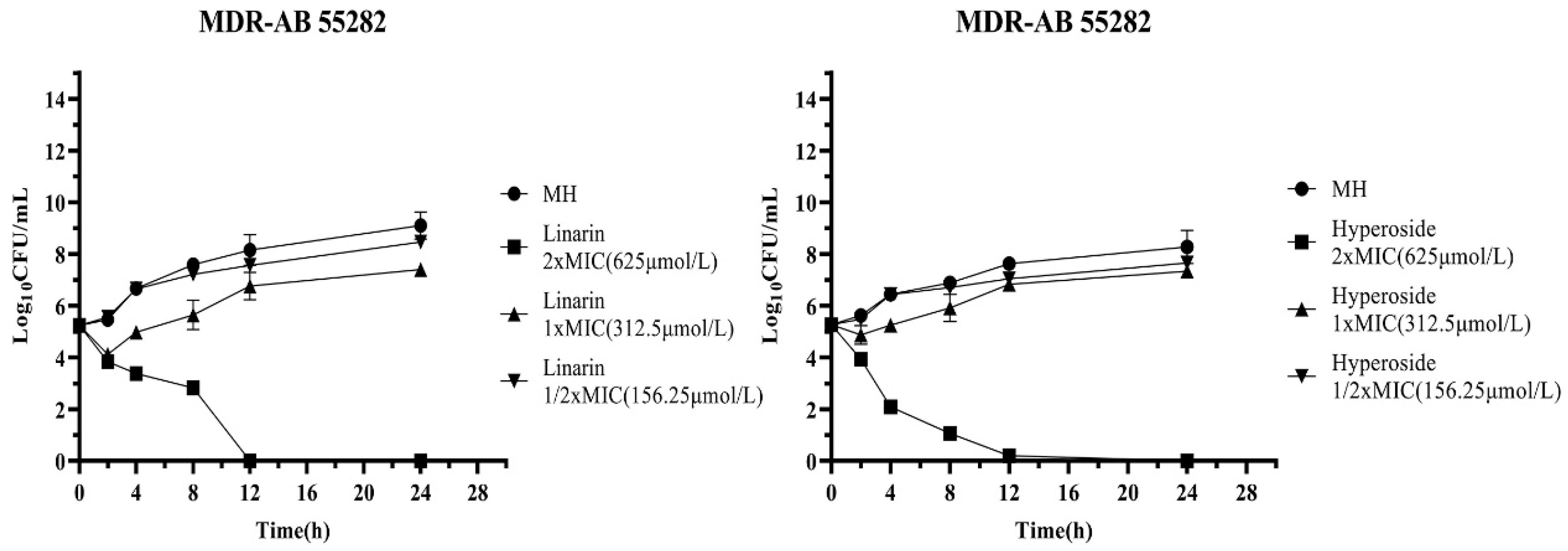
2.3. Time-Kill Curve Assay
2.4. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.5. Effects of Cell Membrane Integrity
2.6. Collection of Active Compounds in Senecio Scandens and Acquisition of Target Proteins
2.7. Molecular Docking
2.8. The mRNA Expression Levels of lptD and msbA in MDR AB 55282 Under the Action of Bioactive Components
2.9. Statistical Analysis
3. Results
3.1. Results of the Sensitivity Assay of Clinically Isolated MDR AB in Commonly Used Clinical Antibacterial Agents
3.2. Antimicrobial Activity of Colistin and Senecio scandens Against MDR AB
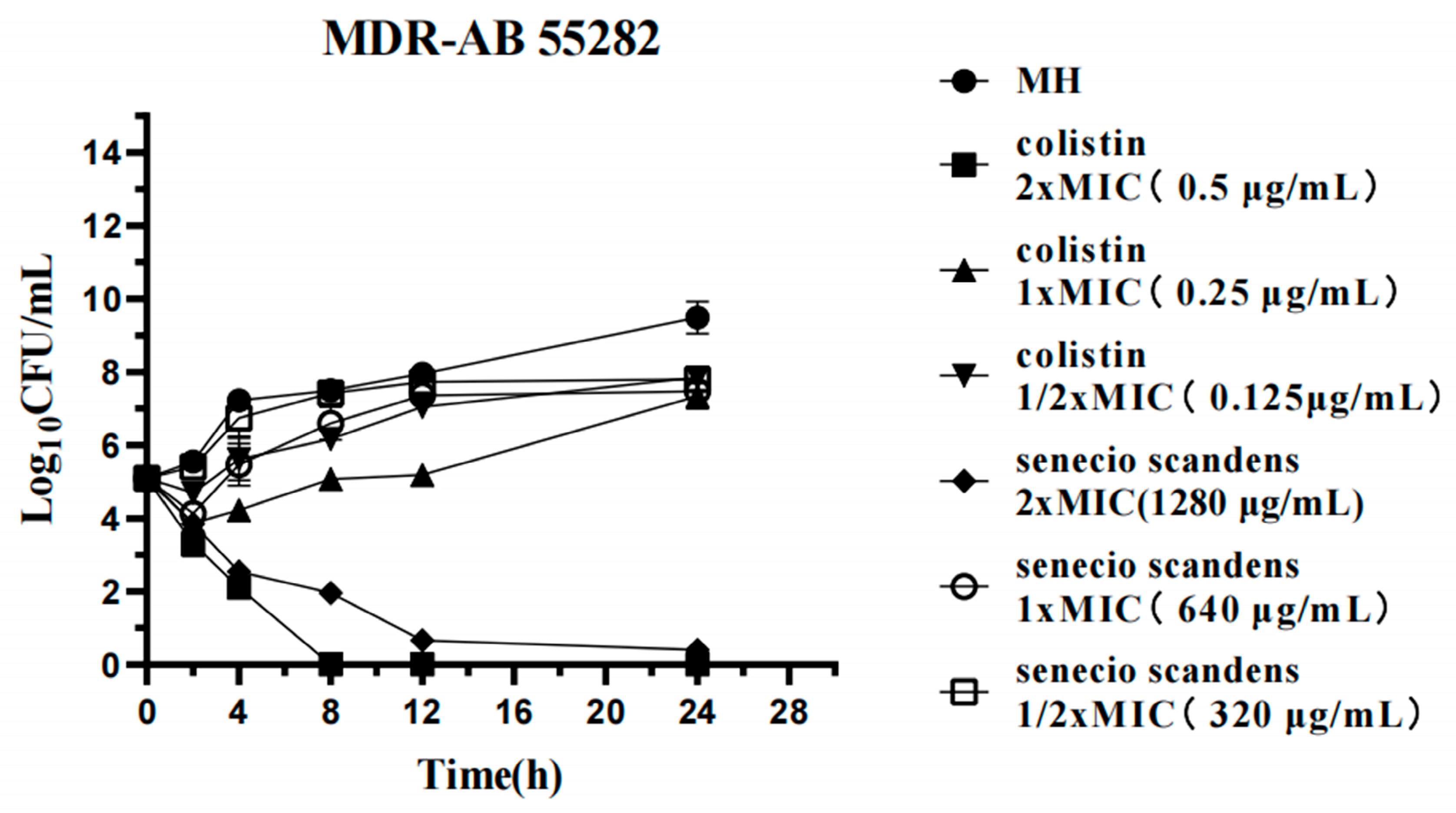
3.3. Time-Kill Assays
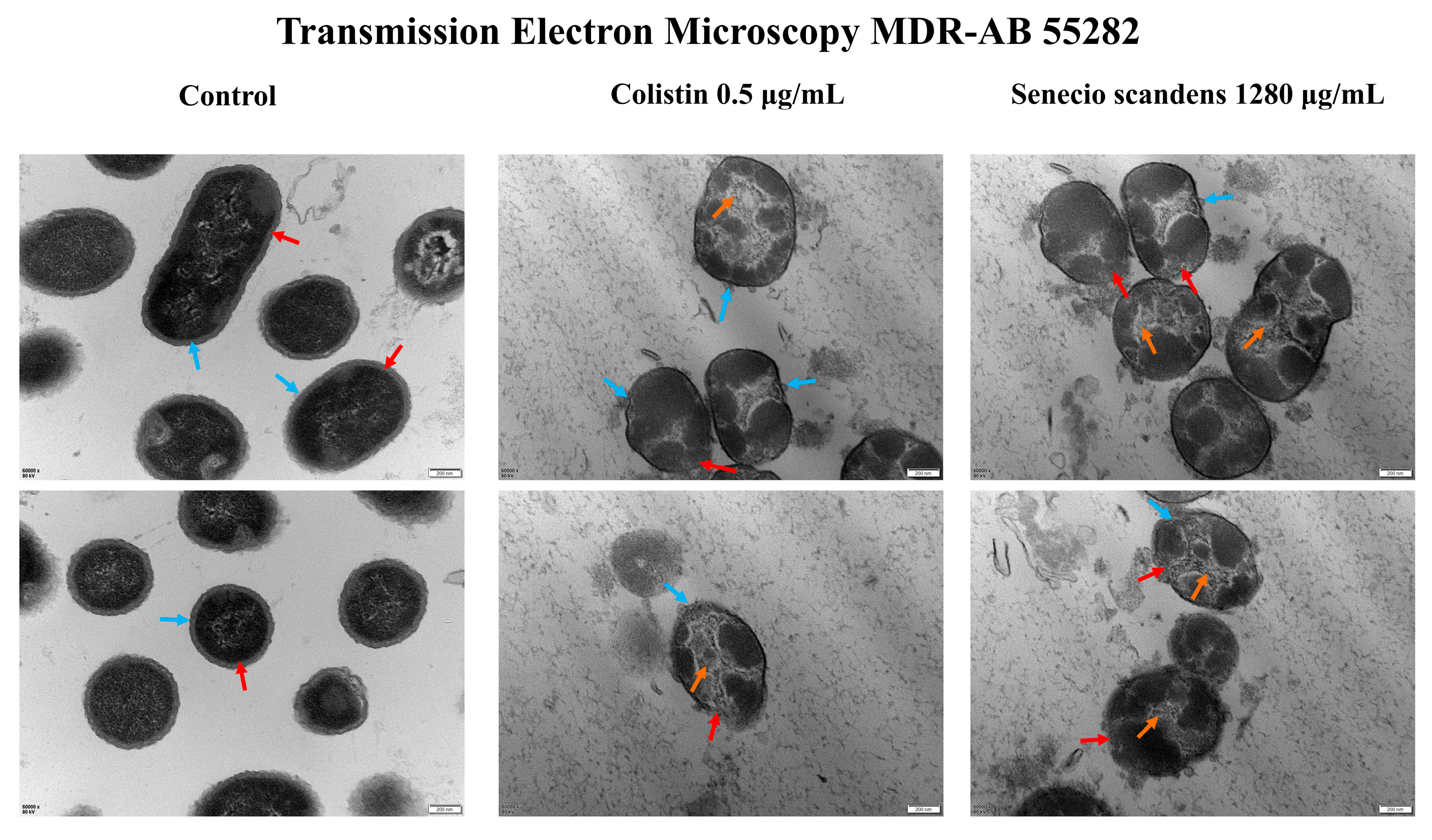
3.4. Ultrastructural Changes of MDR AB 55282 Induced by Senecio scandens
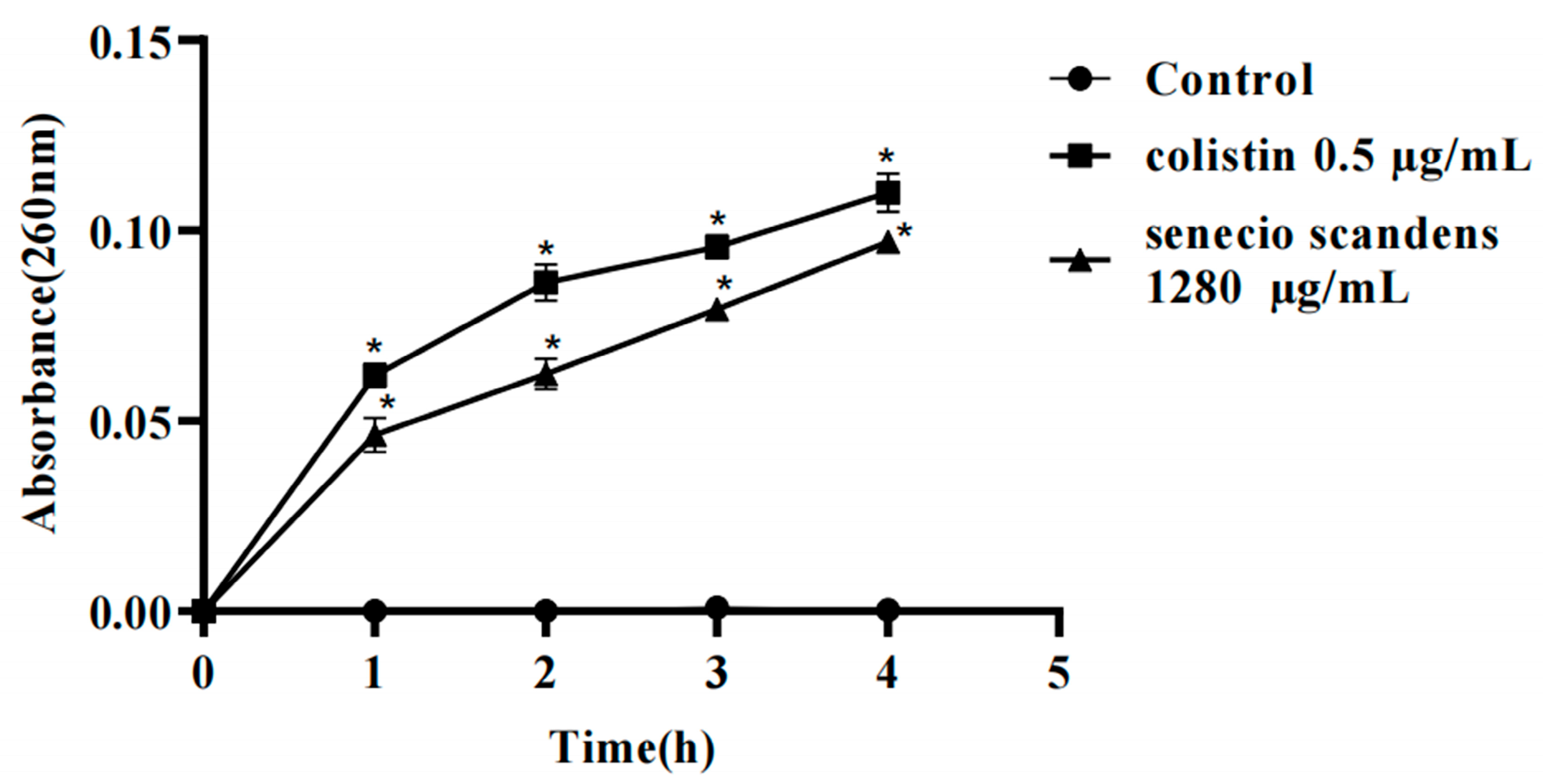
3.5. Effects of Colistin and Senecio scandens on Cell Membrane Integrity
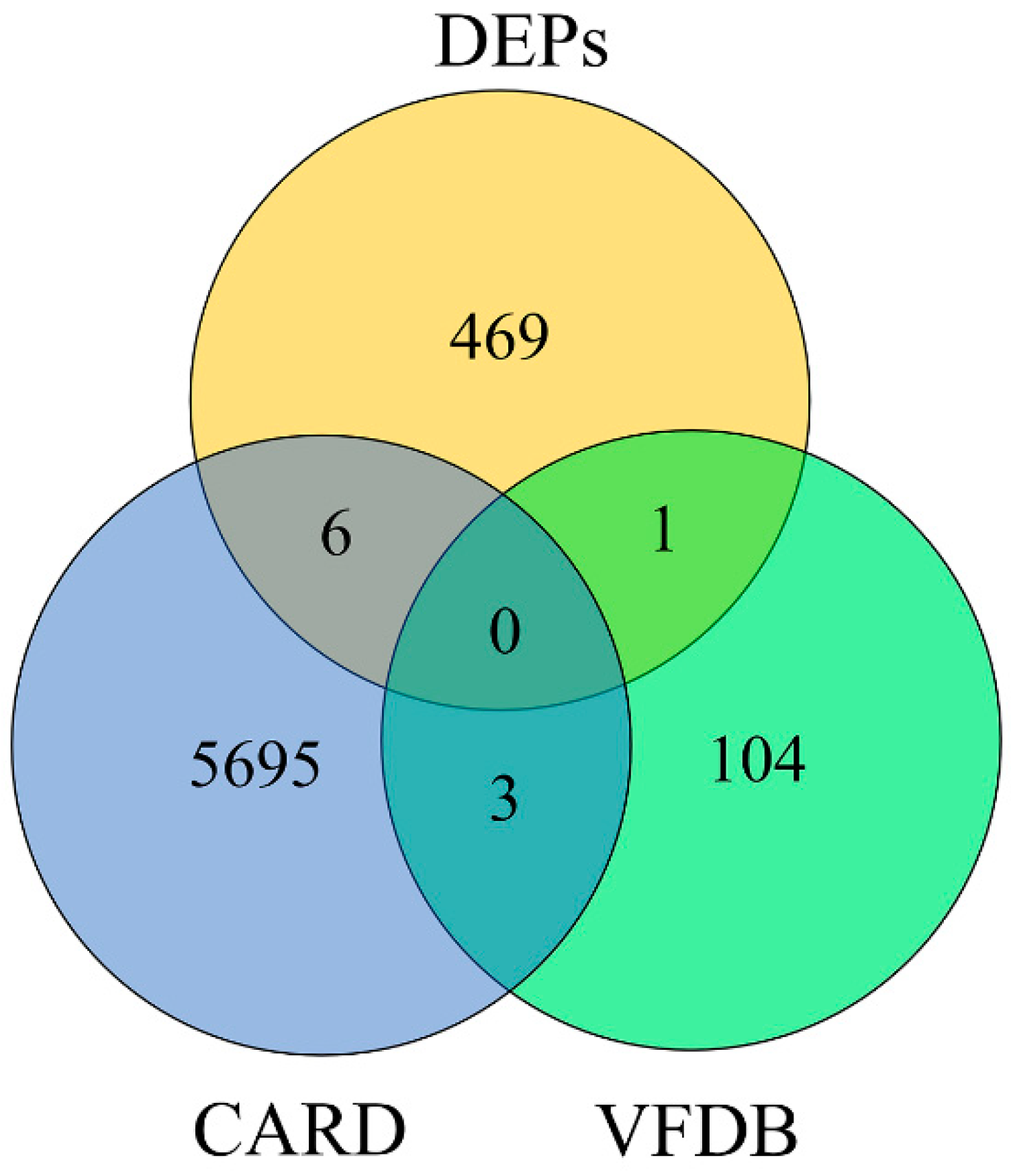
3.6. Collection of the Active Compounds in Senecio scandens
3.7. Obtaining the Target Protein
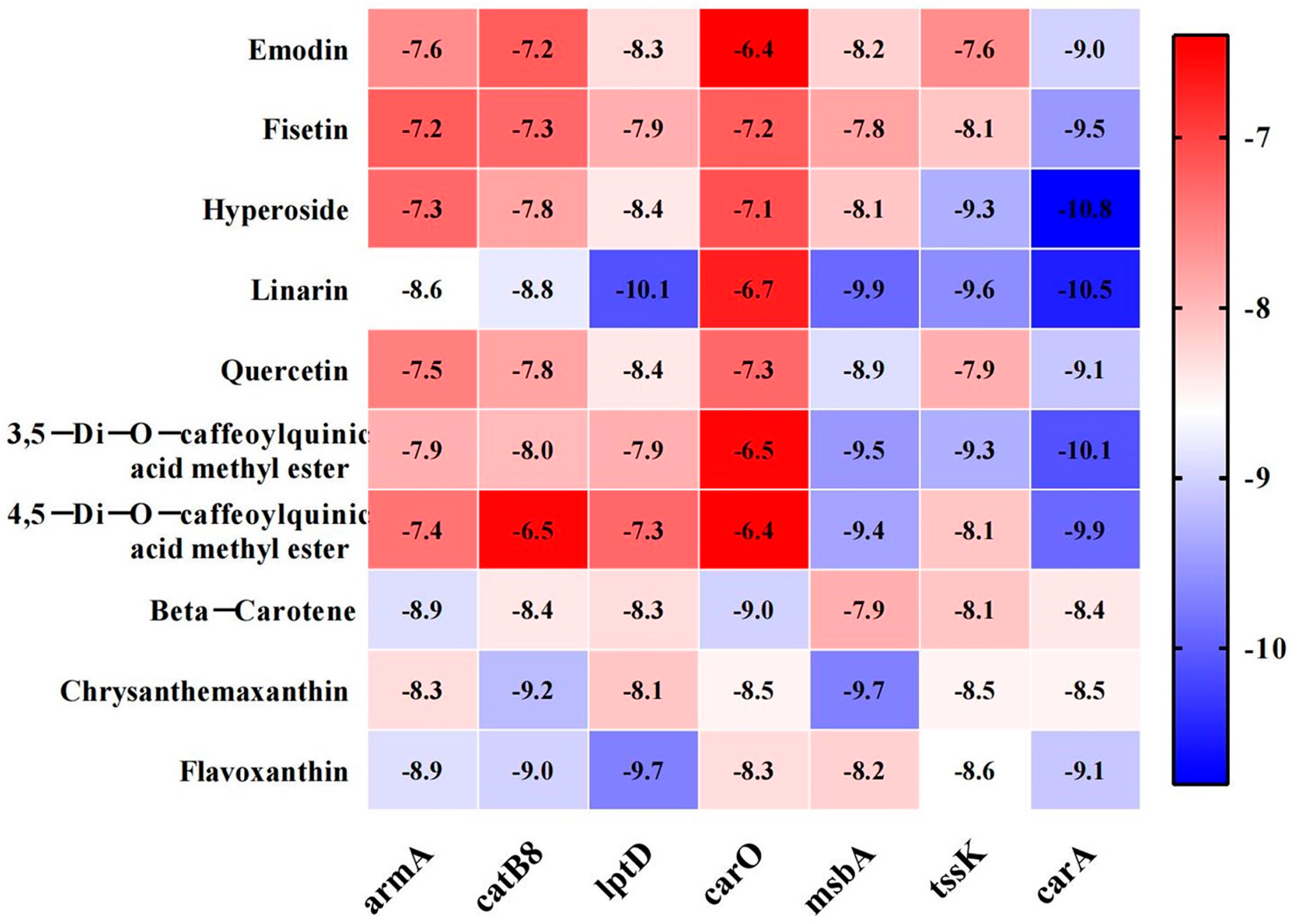
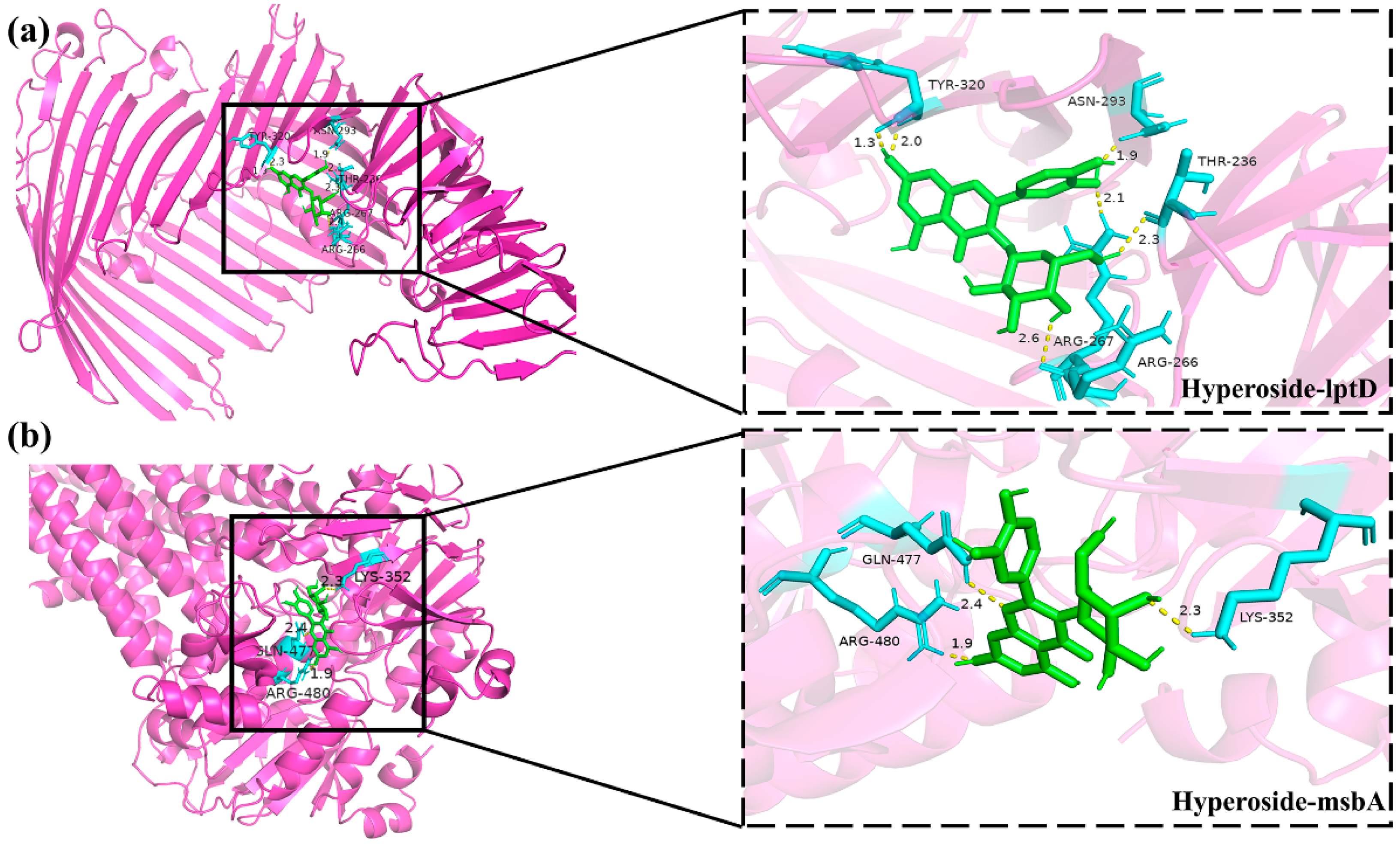
3.8. Molecular Docking Between Active Components and Key Target Points
3.9. Results of Antibacterial Activity Assays of Active Compounds Against MDR AB 55282
3.10. Results of the mRNA Expression Levels of lptD and msbA in MDR AB 55282 Affected by Active Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDR AB | multidrug-resistant Acinetobacter baumannii |
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
References
- Alsan, M.; Klompas, M. Acinetobacter baumannii: An Emerging and Important Pathogen. J. Clin. Outcomes Manag. 2010, 17, 363–369. [Google Scholar]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Dexter, C.; Murray, G.L.; Paulsen, I.T.; Peleg, A.Y. Community-acquired Acinetobacter baumannii: Clinical characteristics, epidemiology and pathogenesis. Expert. Rev. Anti Infect. Ther. 2015, 13, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef]
- Iwasawa, Y.; Hosokawa, N.; Harada, M.; Hayano, S.; Shimizu, A.; Suzuki, D.; Nakashima, K.; Yaegashi, M. Severe Community-acquired Pneumonia Caused by Acinetobacter baumannii Successfully Treated with the Initial Administration of Meropenem Based on the Sputum Gram Staining Findings. Intern. Med. 2019, 58, 301–305. [Google Scholar] [CrossRef]
- Muzahid, N.H.; Hussain, M.H.; Huet, M.A.L.; Dwiyanto, J.; Su, T.T.; Reidpath, D.; Mustapha, F.; Ayub, Q.; Tan, H.S.; Rahman, S. Molecular characterization and comparative genomic analysis of Acinetobacter baumannii isolated from the community and the hospital: An epidemiological study in Segamat, Malaysia. Microb. Genom. 2023, 9, mgen000977. [Google Scholar] [CrossRef]
- Xu, A.; Zhu, H.; Gao, B.; Weng, H.; Ding, Z.; Li, M.; Weng, X.; He, G. Diagnosis of severe community-acquired pneumonia caused by Acinetobacter baumannii through next-generation sequencing: A case report. BMC Infect. Dis. 2020, 20, 45. [Google Scholar] [CrossRef]
- WHO. WHO Bacterial Priority Pathogens List, 2024 Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Gordon, N.C.; Wareham, D.W. Multidrug-resistant Acinetobacter baumannii: Mechanisms of virulence and resistance. Int. J. Antimicrob. Agents 2010, 35, 219–226. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.I.; Kim, Y.R.; Hong, K.W.; Wie, S.H.; Park, Y.J.; Jeong, H.; Kang, M.W. Carbapenem-resistant Acinetobacter baumannii: Diversity of resistant mechanisms and risk factors for infection. Epidemiol. Infect. 2012, 140, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Cain, A.K.; Hamidian, M. Portrait of a killer: Uncovering resistance mechanisms and global spread of Acinetobacter baumannii. PLoS Pathog. 2023, 19, e1011520. [Google Scholar] [CrossRef]
- Hussain, I.; Janjua, M.R.S.A.; Haq, A.U.; Hassan, S.U.; Albaqami, F.M.K.; Alsuwat, M.A.; Alrashdi, B.M.; Alzwain, S.; Naqvi, S.A.R. Evaluation of Anti-Infection and Anti-Diabetic Activities in Methanolic and n-Hexane Plant Extracts of Indigenously Cultivated Chenopodium album. Agronomy 2024, 14, 1340. [Google Scholar] [CrossRef]
- Rafique, A.; Amjad, F.; Janjua, M.; Naqvi, S.A.R.; Hassan, S.U.; Abdullah, H.; Nazir, M.S.; Ali, Z.; Alshihri, A.A.; Momenah, M.A.; et al. Chia seed-mediated fabrication of ZnO/Ag/Ag2O nanocomposites: Structural, antioxidant, anticancer, and wound healing studies. Front. Chem. 2024, 12, 1405385. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yuan, Z.T.; Liang, X.L.; Xiong, Y.K.; Yang, M.; Ma, G.Q. Research advances in drug resistance mechanisms of bacterial biofilm and natural drug intervention. Zhongguo Zhong Yao Za Zhi 2021, 46, 3560–3565. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.G.; Xie, M.; Dong, Y.; Wu, H.; He, D.D.; Hu, G.Z.; Xu, E.P. Antimicrobial mechanisms of traditional Chinese medicine and reversal of drug resistance: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Wei, S.; Zhou, X.; Xiao, X. Antimicrobial Effects of Chemical Compounds Isolated from Traditional Chinese Herbal Medicine (TCHM) Against Drug-Resistant Bacteria: A Review Paper. Mini Rev. Med. Chem. 2019, 19, 125–137. [Google Scholar] [CrossRef]
- Wang, D.; Huang, L.; Chen, S. Senecio scandens Buch.-Ham.: A review on its ethnopharmacology, phytochemistry, pharmacology, and toxicity. J. Ethnopharmacol. 2013, 149, 1–23. [Google Scholar] [CrossRef]
- Ngai, H.L.; Yang, X.; Chu, A.J.; Harper, R.; Jacobsen, A.; Lau, D.T.; Yu, H.Y.; Lee, H.K.; Shaw, P.C. Multi-methodological approach for the Quality assessment of Senecionis scandentis Herba (Qianliguang) in the herbal market. PLoS ONE 2022, 17, e0267143. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, X.Y.; Sun, R. New Progress in the Study of the Pharmacological Effects and Toxicity of Senecio scandens. Chin. J. Pharmacovigil. 2014, 11, 151–153+157. [Google Scholar] [CrossRef]
- Li, H.; Nie, F.H.; Chen, J.D.; He, S.Y.; Yu, Z.J.; Lin, H.Y.; Chen, J.J. Study on the Chemical Components of the Antibacterial Effective Part of Senecio scandens and Its Acute Toxicity. J. Tradit. Chin. Vet. Med. 2008, 1, 7–9. [Google Scholar] [CrossRef]
- Liu, X.; An, L.; Zhou, Y.; Peng, W.; Huang, C. Antibacterial Mechanism of Patrinia scabiosaefolia Against Methicillin Resistant Staphylococcus epidermidis. Infect. Drug Resist. 2023, 16, 1345–1355. [Google Scholar] [CrossRef]
- Ao, D.S.; Qian, G.; Luo, S.Y.; Wang, A.L. Comparison of the Bacteriostatic Effects of the Decoction of Plants in the Senecio scandens. Lishizhen Med. Mater. Medica Res. 2009, 20, 3051–3052. [Google Scholar]
- Trimble, M.J.; Mlynarcik, P.; Kolar, M.; Hancock, R.E. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Liu, H.; Zhang, L.; Guo, Y.; Yu, S.; Wozniak, D.J.; Ma, L.Z. The exopolysaccharide Psl-eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Env. Environ. Microbiol. Rep. 2015, 7, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, L.; Cao, X.; Gu, Z.; Zhao, G.; Ran, M.; Yan, Y.; Yan, J.; Xu, L.; Gao, C.; et al. The Origin, Function, Distribution, Quantification, and Research Advances of Extracellular DNA. Int. J. Mol. Sci. 2022, 23, 3690. [Google Scholar] [CrossRef]
- Ding, W.Y.; Zheng, S.D.; Qin, Y.; Yu, F.; Bai, J.W.; Cui, W.Q.; Yu, T.; Chen, X.R.; Bello-Onaghise, G.; Li, Y.H. Chitosan Grafted with beta-Cyclodextrin: Synthesis, Characterization, Antimicrobial Activity, and Role as Absorbefacient and Solubilizer. Front. Chem. 2018, 6, 657. [Google Scholar] [CrossRef]
- Liao, J.; Qi, Q.; Kuang, L.; Zhou, Y.; Xiao, Q.; Liu, T.; Wang, X.; Guo, L.; Jiang, Y. Chloramphenicol Binding Sites of Acinetobacter baumannii Chloramphenicol Acetyltransferase CatB8. ACS Infect. Dis. 2024, 10, 870–878. [Google Scholar] [CrossRef]
- Gonzalez-Zorn, B.; Teshager, T.; Casas, M.; Porrero, M.C.; Moreno, M.A.; Courvalin, P.; Dominguez, L. armA and aminoglycoside resistance in Escherichia coli. Emerg. Infect. Dis. 2005, 11, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Jouybari, M.A.; Ahanjan, M.; Mirzaei, B.; Goli, H.R. Role of aminoglycoside-modifying enzymes and 16S rRNA methylase (ArmA) in resistance of Acinetobacter baumannii clinical isolates against aminoglycosides. Rev. Soc. Bras. Med. Trop. 2021, 54, e05992020. [Google Scholar] [CrossRef]
- Labrador-Herrera, G.; Perez-Pulido, A.J.; Alvarez-Marin, R.; Casimiro-Soriguer, C.S.; Cebrero-Cangueiro, T.; Moran-Barrio, J.; Pachon, J.; Viale, A.M.; Pachon-Ibanez, M.E. Virulence role of the outer membrane protein CarO in carbapenem-resistant Acinetobacter baumannii. Virulence 2020, 11, 1727–1737. [Google Scholar] [CrossRef]
- Sariyer, E. The role of Acinetobacter baumannii CarO outer membrane protein in carbapenems influx. Res. Microbiol. 2022, 173, 103966. [Google Scholar] [CrossRef]
- Zhu, L.J.; Chen, X.Y.; Hou, P.F. Mutation of CarO participates in drug resistance in imipenem-resistant Acinetobacter baumannii. J. Clin. Lab. Anal. 2019, 33, e22976. [Google Scholar] [CrossRef]
- Bojkovic, J.; Richie, D.L.; Six, D.A.; Rath, C.M.; Sawyer, W.S.; Hu, Q.; Dean, C.R. Characterization of an Acinetobacter baumannii lptD Deletion Strain: Permeability Defects and Response to Inhibition of Lipopolysaccharide and Fatty Acid Biosynthesis. J. Bacteriol. 2015, 198, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Frisinger, F.S.; Jana, B.; Ortiz-Marquez, J.C.; van Opijnen, T.; Donadio, S.; Guardabassi, L. LptD depletion disrupts morphological homeostasis and upregulates carbohydrate metabolism in Escherichia coli. FEMS Microbes 2023, 4, xtad013. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Li, C.; Li, W.; Ye, Z.; Pan, J. LptD is a promising vaccine antigen and potential immunotherapeutic target for protection against Vibrio species infection. Sci. Rep. 2016, 6, 38577. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.K.; Miu, A.; Oh, A.; Reichelt, M.; Ho, H.; Chalouni, C.; Labadie, S.; Wang, L.; Liang, J.; Nickerson, N.N.; et al. Disrupting Gram-Negative Bacterial Outer Membrane Biosynthesis through Inhibition of the Lipopolysaccharide Transporter MsbA. Antimicrob. Agents Chemother. 2018, 62, e01142-18. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Miu, A.; Alexander, M.K.; Garcia, N.K.; Oh, A.; Zilberleyb, I.; Reichelt, M.; Austin, C.D.; Tam, C.; Shriver, S.; et al. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 2018, 557, 196–201. [Google Scholar] [CrossRef]
- Rajangam, S.L.; Narasimhan, M.K. Current treatment strategies for targeting virulence factors and biofilm formation in Acinetobacter baumannii. Future Microbiol. 2024, 19, 941–961. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, M.; Nigro, S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 2019, 5, e000306. [Google Scholar] [CrossRef] [PubMed]
- Nigro, S.J.; Hall, R.M. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J. Antimicrob. Chemother. 2016, 71, 1135–1147. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Liu, X.; Cheng, P.; Li, L.; Chai, Y.; Cao, M.; Yang, Y. Aspirin enhances the antibacterial activity of colistin against multidrug-resistant Pseudomonas aeruginosa. Eur. J. Pharmacol. 2025, 997, 177480. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Stansfeld, P.J.; Zeng, Y.; Dong, H.; Wang, W.; Dong, C. Lipopolysaccharide is inserted into the outer membrane through an intramembrane hole, a lumen gate, and the lateral opening of LptD. Structure 2015, 23, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; Van der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.; et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 2010, 327, 1010–1013. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, L.; Zhang, F.; Liu, Y.; Wang, Y.; Meng, F.; Li, S.; Cheng, X.; Bi, Y. Preparation and pharmacokinetics in vivo of linarin solid dispersion and liposome. Chin. Herb. Med. 2022, 14, 310–316. [Google Scholar] [CrossRef]
- Li, Y.; Guang, C.; Zhao, N.; Feng, X.; Qiu, F. LC-MS/MS Method for Simultaneous Determination of Linarin and Its Metabolites in Rat Plasma and Liver Tissue Samples: Application to Pharmacokinetic and Liver Tissue Distribution Study After Oral Administration of Linarin. Molecules 2019, 24, 3342. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Taghrir, H.; Boveiri Dehsheikh, A.; Zomorodian, K.; Irajie, C.; Mahmoodi Sourestani, M.; Iraji, A. Linarin, a Glycosylated Flavonoid, with Potential Therapeutic Attributes: A Comprehensive Review. Pharmaceuticals 2021, 14, 1104. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, F.; Feng, W.; Qiu, X.; Liu, Y.; Yang, B.; Chen, Y.; Xia, P. Hyperoside inhibits biofilm formation of Pseudomonas aeruginosa. Exp. Ther. Med. 2017, 14, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef] [PubMed]



| Identification | No. | Gender | Department | Infection Source | Clinical Diagnosis |
|---|---|---|---|---|---|
| Acinetobacter baumannii | 55282 | Male | ICU | Sputum | Severe Pneumonia |
| Acinetobacter baumannii | 61759 | Female | ICU | Blood | Diabetic foot |
| Acinetobacter baumannii | 64295 | Male | Geriatrics | Secretion | Pressure ulcer |
| Acinetobacter baumannii | 63440 | Male | Respiratory medicine | Sputum | Severe Pneumonia |
| Acinetobacter baumannii | 65912 | Male | Hematology | Sputum | Lymphadenectasis |
| Acinetobacter baumannii | 58195 | Female | Orthopedics department | Drainage fluid | Multiple myeloma |
| Acinetobacter baumannii | 55149 | Female | ICU | Sputum | Renal insufficiency |
| Acinetobacter baumannii | 55547 | Male | ICU | Sputum | Severe Pneumonia |
| Acinetobacter baumannii | 61412 | Female | Geriatrics | Blood | Gastrointestinal hemorrhage |
| Acinetobacter baumannii | 55731 | Male | ICU | Sputum | Severe Pneumonia |
| Gene | Primer 5′-3′ |
|---|---|
| lptD | Forward: ACACCAGCAGCCTTTGTAATTCC |
| Reverse: GAACCGCCGTCTAAACCTTGAG | |
| msbA | Forward: TTTATCGGGTGGTCAACGTCAAC |
| Reverse: CGCACTTGTCGCCTCATCC | |
| 16S rDNA | Forward: ACGACTTCACCCCAGTCATC |
| Reverse: CACACCATGGGAGTTTGTTG |
| Type | Antibacterial Drug | Number of Cases (n) | Resistance Rate (%) |
|---|---|---|---|
| β-lactamase | Ampicillin/Sulbactam | 6 | 60 |
| Ceftriaxone | 8 | 80 | |
| Piperacillin/tazobactam | 2 | 20 | |
| Ceftazidime | 8 | 80 | |
| Cefepime | 7 | 70 | |
| Cefazolin | 5 | 50 | |
| Imipenem | 8 | 80 | |
| Meropenem (K-B method) | 5 | 50 | |
| Quinolones | Ciprofloxacin | 6 | 60 |
| Levofloxacin | 7 | 70 | |
| Aminoglycosides | Gentamicin | 5 | 50 |
| Tobramycin | 6 | 60 | |
| Amikacin (K-B method) | 8 | 80 |
| Antibacterial Drug | MIC (μg/mL) | Determination Result | Antibacterial Drug (K-B Method) | Zone of Inhibition (mm) | Determination Result |
|---|---|---|---|---|---|
| Ceftazidime | >256 | R | Amikacin | 6 | R |
| Meropenem/Vaborbactam | >256 | R | Imipenem | 9 | R |
| Imipenem/Relebactam | >32 | R | Ampicillin/Sulbactam | 6 | R |
| Meropenem | ≥32 | R | Cefoperazone/Sulbactam | 18 | I |
| Tigecycline | 0.25 | S | Minocycline | 16 | S |
| Colistin | 0.25 | S | Tigecycline | 16 | S |
| Cefoxitin | 256 | R | |||
| Compound trimethoprim | ≥320 | R | |||
| Gentamicin | ≥16 | R | |||
| Tobramycin | ≥16 | R |
| Strains | Colistin | Senecio scandens | ||
|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| 55282 | 0.25 | 0.5 | 640 | 1280 |
| 61759 | 2 | 4 | 640 | 1280 |
| 64295 | 2 | 4 | 640 | 1280 |
| 63440 | 2 | 4 | 640 | 1280 |
| 65912 | 1 | 2 | 640 | 1280 |
| 58195 | 2 | 4 | 640 | 1280 |
| 55149 | 2 | 4 | 640 | 640 |
| 55547 | 0.5 | 1 | 640 | 640 |
| 61412 | 1 | 2 | 640 | 1280 |
| 55731 | 2 | 4 | 640 | 1280 |
| ATCC 19606 | 0.25 | 0.25 | 640 | 1280 |
| PDB ID | Protein ID | Gene Name | Fold Change of Senecio scandens/Control | t Test p Value |
|---|---|---|---|---|
| 8J40 | Q5D169 | catB8 | 0.1761 | 0.00008 |
| 1T36 | A0A009HU80 | carA | 0.1056 | 0.00005 |
| 9CSI | A0A009IKL5 | msbA | 0.0863 | 0.0003 |
| 4Q35 | A0A219CCC6 | lptD | 0.1592 | 0.0075 |
| 3FZG | A7U830 | armA | 0.3681 | 0.0298 |
| 4FUV | Q4A209 | carO | 0.1511 | 0.0269 |
| 5M2Y | A0A059ZI91 | tssK | 0.2426 | 0.007 |
| Compound Name | Gene Name | Docking Score (kcal/mol) | Hydrogen Bond |
|---|---|---|---|
| linarin | lptD | −10.1 | GLU-235 |
| ASN-297 | |||
| ARG-319 | |||
| ASN-293 | |||
| ARG-267 | |||
| ASP-264 | |||
| msbA | −9.9 | ALA-209 | |
| ASN-415 | |||
| GLN-417 | |||
| ARG-478 | |||
| GLN-469 | |||
| hyperoside | lptD | −8.4 | TYR-320 |
| ASN-293 | |||
| THR-236 | |||
| ARG-266 | |||
| ARG-267 | |||
| msbA | −8.1 | GLN-477 | |
| LYS-352 | |||
| ARG-480 |
| Bioactive Components | Molecular Weight (g/mol) | Chemical Formula | MIC | MBC | ||
|---|---|---|---|---|---|---|
| μmol/L | g/L | μmol/L | g/L | |||
| Emodin | 270.23 | C15H10O5 | 5000 | 1.35 | >5000 | >1.35 |
| Fisetin | 286.23 | C15H10O6 | 5000 | 1.43 | >5000 | >1.43 |
| Hyperoside | 464.38 | C21H20O12 | 312.5 | 0.15 | 625 | 0.29 |
| Linarin | 592.55 | C25H32O14 | 312.5 | 0.19 | 625 | 0.37 |
| Quercetin | 302.23 | C15H10O7 | 5000 | 1.51 | >5000 | >1.51 |
| β-carotene | 536.88 | C40H56 | 5000 | 2.68 | >5000 | >2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, X.; Chen, Y.; Zhang, Y.; Li, L.; Chai, Y.; Pu, X.; Liu, X. Linarin and Hyperoside Inhibit lptD/msbA to Disrupt Membranes of Multidrug-Resistant Acinetobacter baumannii. Biology 2025, 14, 1087. https://doi.org/10.3390/biology14081087
Yang Y, Li X, Chen Y, Zhang Y, Li L, Chai Y, Pu X, Liu X. Linarin and Hyperoside Inhibit lptD/msbA to Disrupt Membranes of Multidrug-Resistant Acinetobacter baumannii. Biology. 2025; 14(8):1087. https://doi.org/10.3390/biology14081087
Chicago/Turabian StyleYang, Yuqi, Xue Li, Yunshi Chen, Yan Zhang, Lailai Li, Yihui Chai, Xiang Pu, and Xin Liu. 2025. "Linarin and Hyperoside Inhibit lptD/msbA to Disrupt Membranes of Multidrug-Resistant Acinetobacter baumannii" Biology 14, no. 8: 1087. https://doi.org/10.3390/biology14081087
APA StyleYang, Y., Li, X., Chen, Y., Zhang, Y., Li, L., Chai, Y., Pu, X., & Liu, X. (2025). Linarin and Hyperoside Inhibit lptD/msbA to Disrupt Membranes of Multidrug-Resistant Acinetobacter baumannii. Biology, 14(8), 1087. https://doi.org/10.3390/biology14081087






