Harnessing the Power of Microbiota: How Do Key Lactobacillus Species Aid in Clearing High-Risk Human Papilloma Virus Infection and Promoting the Regression of Cervical Dysplasia?
Simple Summary
Abstract
1. Introduction
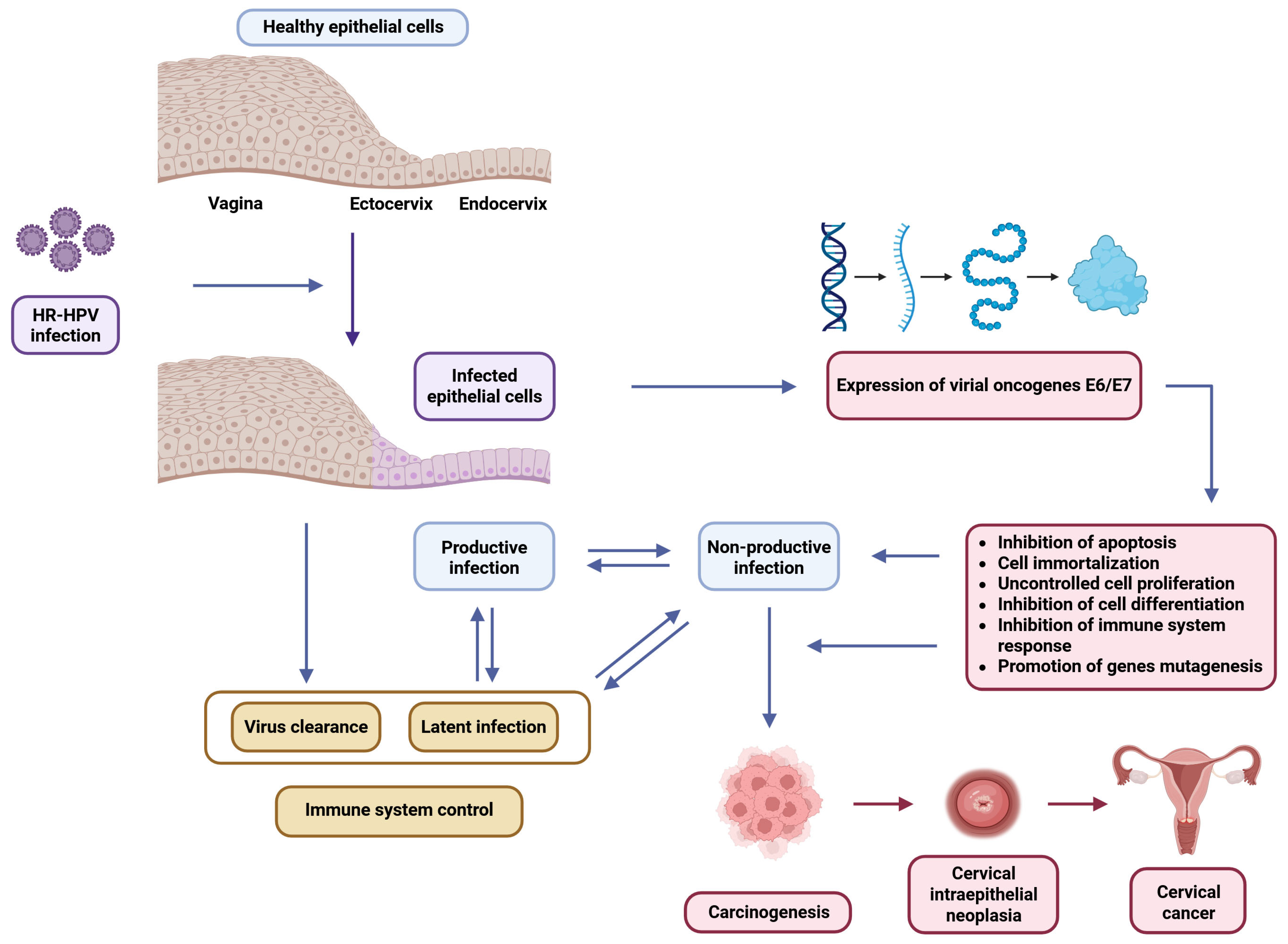
2. HR-HPV Infection and Cervical Dysplasia: Virological and Oncogenic Foundations
3. Vaginal Microbiota: Community State Types and Dysbiotic Conditions
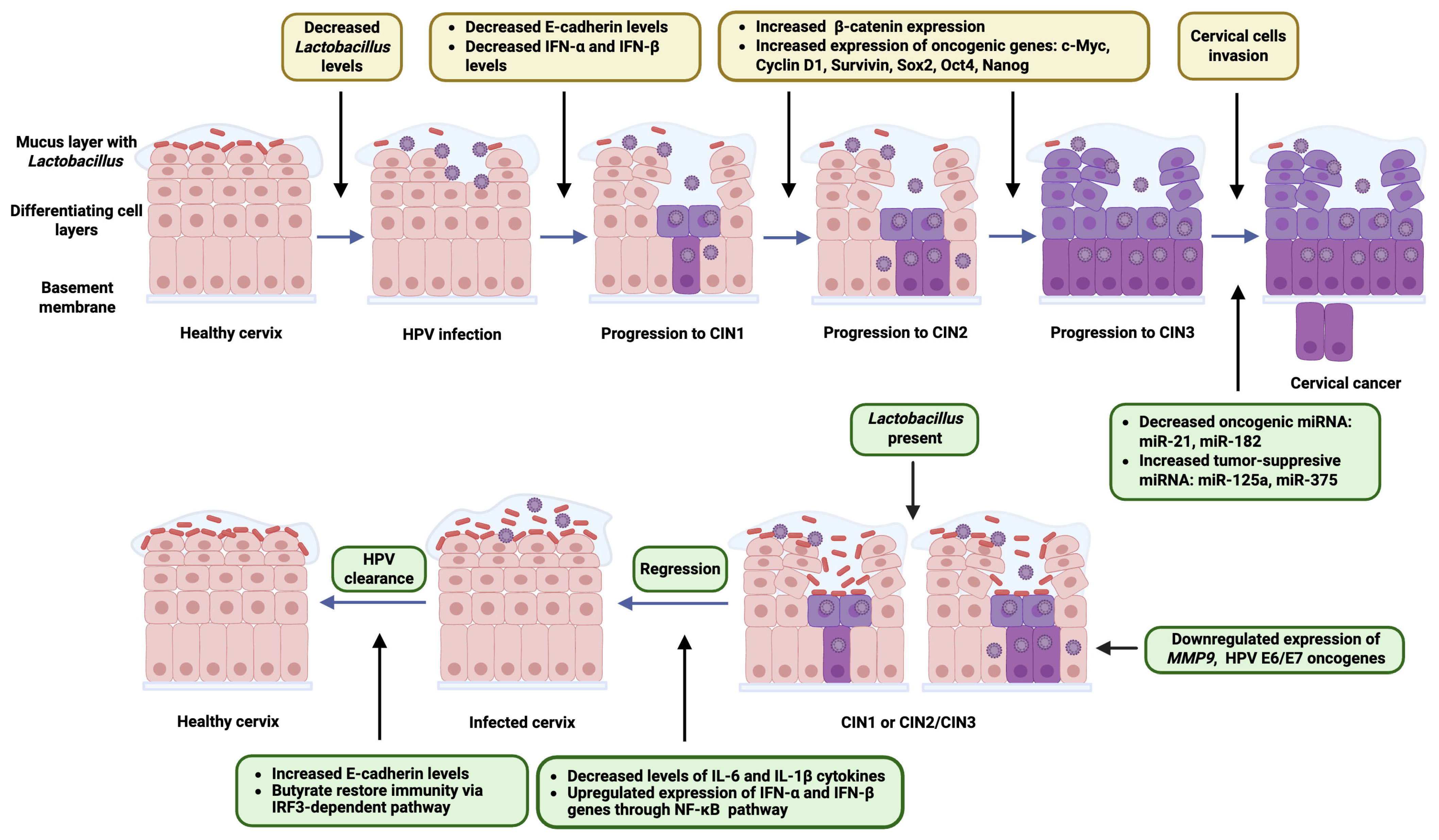
4. The Role of Lactobacillus in HR-HPV Clearance and CIN Regression
5. Current Evidence Linking Vaginal Microbiota Composition to HR-HPV Persistence and CIN Outcomes
| Author, Year; Country [Ref] | Study Aim | Groups: Cases and HPV-Negative Controls | Material and Detection Method | Results | Changes in Microbiota Abundance | Conclusions |
|---|---|---|---|---|---|---|
| Brotman et al., 2014; USA [104] | Assess temporal links between microbiota dynamics and HPV detection | 32 women, 937 self-collected vaginal samples over 16 weeks | Vaginal swabs; 16S rRNA sequencing; HPV DNA testing | CST II (L. gasseri) linked to HPV clearance; CST IV-B (anaerobes) linked to persistence; the most abundant Lactobacillus species in the control and clearance group | L. gasseri enriched in clearance; Atopobium in slow clearance states | Microbiota state changes HPV detection and outcomes; longitudinal sampling is key |
| Di Paola et al., 2017; Italy [6] | Characterize cervicovaginal microbiota in persistent HR-HPV | 55 HPV-positive (from 1029 screened), 17 HPV-negative controls | Cervicovaginal swabs; 16S rRNA pyrosequencing | CST IV more frequent in persistent HPV (72.7%) vs. controls (16.6%) | Decreased Lactobacillus, increased Gardnerella, and Atopobium in the persistent group | Lactobacillus-dominated microbiota linked to HPV clearance; dysbiosis favors persistence |
| Mitra et al., 2020; UK [31] | Study how vaginal microbiota influences CIN2 lesion regression | 87 women (16–26 years) with CIN2, followed-up for 24 months | Vaginal swabs; 16S rRNA sequencing | Lactobacillus-dominant microbiota associated with lesion regression | Increased Gardnerella, Prevotella, and Megasphaera in the persistent group | Vaginal microbiota could predict CIN2 outcome and serve as a therapeutic target |
| Zeng et al., 2023; China [103] | Explore vaginal microbiota’s role in HPV infection, persistence, and clearance | 90 HPV-positive (persistent vs. cleared), 45 HPV-negative controls | Vaginal swabs; 16S rRNA sequencing | Higher alpha diversity in persistent HPV; L. iners prevalent in infections | Increased Sneathia amnii, Bacteroidaceae in persistence; L. crispatus in clearance | Microbiota composition affects HPV outcomes; L. crispatus dominance may promote clearance |
6. Therapeutic Perspectives
| Number of Clinical Trial | Study Period, Time Frame (Months); and Location | Intervention/Treatment | Inclusion Criteria | Exclusion Criteria | Study Design and Masking | Sample Size | Study Group | Control Group | Primary Outcome Measures | Secondary Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT01097356 | 2010–2011 (6); Belgium | Probiotic drink vs. no intervention | Women; age 18–65; LSIL+ HPV+ in the Papanicolaou test | Age >65; immuno-compromised | Randomized, parallel; single-blind (participant) | 60 | Probiotic drink daily (6 months) | No intervention (6-months observation) | HPV positivity; LSIL regression | No data |
| NCT01599416 | 2011–2013 (12); Taiwan | Oral U-relax (L. rhamnosus GR-1 + L. reuteri RC-14) vs. placebo | Women; age 30–65; HPV+ 6 months after conization; negative for intraepithelial lesion or malignancy in the Papanicolaou test | CIN before conization, cervical cancer, genital infection issues, long-term antibiotics | Randomized, parallel; triple-blind (participant, care provider, investigator) | 80 | U-relax: 2 caps/day → 1/day until day 360 | Placebo (identical schedule) | Vaginal health status | HPV DNA index change |
| NCT03372395 | 2015–2016 (9); Italy | Vaginal L. rhamnosus BMX 54 + standard treatment | Age >18, bacterial vaginosis/yeast vaginitis + HPV; (ASCUS, LSIL, CIN1, HPV DNA+) | Pregnancy; CIN2/3, cancer, immuno-deficiency, corticosteroids | Randomized, sequential (pilot); open label | 117 (60 short, 57 long) | Long-term probiotics (6 months) | Short-term probiotics (3 months) | Infection symptom resolution | Adverse events (CTCAE v4.0) |
| NCT05109533 | 2018–2021 (12); Italy | Vaginal + oral probiotics: L. rhamnosus BMX 54, L. reuteri RC-14, L. rhamnosus GR-1 + standard treatment | Age >18, positive vaginal infection swabs, HPV+ | Pregnancy or breast-feeding, malignancies, immunological diseases, comorbidities, corticosteroids | Randomized, parallel; open label | 483 (252 probiotic, 231 controls) | Standard treatment + 9-months probiotics | Standard treatment | HPV clearance; vaginal infection resolution | No data |
| NCT06802809 | 2022–2024 (4); Italy | Oral L. crispatus M247 (Crispact®) vs. placebo | Women; age 18–69, HR-HPV+, ASCUS or LSIL; negative colposcopy (biopsy) | Prior HPV vaccination, HSIL, immunotherapy, neoplasia, pregnancy, allergy | Randomized, parallel; placebo-controlled, single-blind (participant) | 66 | L. crispatus M247, 20 billion CFU/day for 4 months | Placebo, 1 stick/day for 4 months | HR-HPV clearance | Microbiota change, cytology normalization, side effects |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HR | High risk |
| HPV | Human papillomavirus |
| CIN | Cervical intraepithelial neoplasia |
| LR | Low risk |
| pRB | Retinoblastoma protein |
| CST | Community state type |
| BV | Bacterial vaginosis |
| AV | Aerobic vaginitis |
| IFN | Interferon |
| IL | Interleukin |
| TLR | Toll-like receptor |
| IRF | Interferon regulatory factor |
| miRNA | microRNA |
| DC | Dendritic cell |
References
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.; Reis, R.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.; et al. Cervical Cancer in Low and Middle-income Countries (Review). Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Zhang, H.; Shen, J.; Chen, J.; Hong, J.; Xu, Y.; Qian, C. Prophylactic and Therapeutic HPV Vaccines: Current Scenario and Perspectives. Front. Cell. Infect. Microbiol. 2022, 12, 909223. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, S.; Shehzadi, A.; Ahmad, M.; Asrar, A.; Ahmed, I.; Iqbal, H.M.N.; Hussen Bule, M. Recent Advances in HPV Biotechnology: Understanding Host-Virus Interactions and Cancer Progression—A Review. Int. J. Surg. 2024, 110, 8025–8036. [Google Scholar] [CrossRef]
- Molina, M.A.; Coenen, B.A.; Leenders, W.P.J.; Andralojc, K.M.; Huynen, M.A.; Melchers, W.J.G. Assessing the Cervicovaginal Microbiota in the Context of hrHPV Infections: Temporal Dynamics and Therapeutic Strategies. mBio 2022, 13, e01619-22. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Schellekens, H.C.J.; Schmidt, L.M.S.; Morré, S.A.; Van Esch, E.M.G.; De Vos Van Steenwijk, P.J. Vaginal Microbiota and Local Immunity in HPV-Induced High-Grade Cervical Dysplasia: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3954. [Google Scholar] [CrossRef]
- Dwiyanto, J.; Hussain, M.H.; Reidpath, D.; Ong, K.S.; Qasim, A.; Lee, S.W.H.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Ethnicity Influences the Gut Microbiota of Individuals Sharing a Geographical Location: A Cross-Sectional Study from a Middle-Income Country. Sci. Rep. 2021, 11, 2618. [Google Scholar] [CrossRef] [PubMed]
- Onywera, H.; Mbulawa, Z.Z.A.; Brink, A.; Williamson, A.-L.; Mwapagha, L.M. Unravelling the Biological Interplay Between Genital HPV Infection and Cervicovaginal Microbiota in Sub-Saharan Africa: Implications for Cervical (Pre)Cancer Prevention. Venereology 2024, 3, 211–231. [Google Scholar] [CrossRef]
- Głowienka-Stodolak, M.; Bagińska-Drabiuk, K.; Szubert, S.; Hennig, E.E.; Horala, A.; Dąbrowska, M.; Micek, M.; Ciebiera, M.; Zeber-Lubecka, N. Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies. Cancers 2024, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses; International Agency for Research on Cancer: Lyon, France, 2007. [Google Scholar]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Glinska, P.; Macios, A.; Jaworski, R.; Bobinski, M.; Pruski, D.; Przybylski, M.; Zielinska, A.; Sawicki, W.; Nowakowski, A. Baseline Data on Distribution of Human Papillomavirus (HPV) Genotypes in Cervical Samples of Gynecological Patients before Implementation of Population-Based HPV Vaccination Program in Poland. Ginekol. Pol. 2024, 95, 870–878. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated Genomic and Molecular Characterization of Cervical Cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Wei, F.; Georges, D.; Man, I.; Baussano, I.; Clifford, G.M. Causal Attribution of Human Papillomavirus Genotypes to Invasive Cervical Cancer Worldwide: A Systematic Analysis of the Global Literature. Lancet 2024, 404, 435–444. [Google Scholar] [CrossRef]
- Kajitani, N.; Satsuka, A.; Kawate, A.; Sakai, H. Productive Lifecycle of Human Papillomaviruses That Depends Upon Squamous Epithelial Differentiation. Front. Microbiol. 2012, 3, 152. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human Papillomavirus Molecular Biology. Mutat. Res. Mutat. Res. 2017, 772, 3–12. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef]
- Hong, S.; Laimins, L.A. Regulation of the Life Cycle of HPVs by Differentiation and the DNA Damage Response. Future Microbiol. 2013, 8, 1547–1557. [Google Scholar] [CrossRef]
- Haręża, D.A.; Wilczyński, J.R.; Paradowska, E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022, 23, 1818. [Google Scholar] [CrossRef] [PubMed]
- Scarth, J.A.; Patterson, M.R.; Morgan, E.L.; Macdonald, A. The Human Papillomavirus Oncoproteins: A Review of the Host Pathways Targeted on the Road to Transformation. J. Gen. Virol. 2021, 102, 001540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, K.; Ren, J.; Zhao, Y.; Cheng, P. Roles of Human Papillomavirus in Cancers: Oncogenic Mechanisms and Clinical Use. Signal Transduct. Target. Ther. 2025, 10, 44. [Google Scholar] [CrossRef]
- Sivakumar, N.; Narwal, A.; Kumar, S.; Kamboj, M.; Devi, A.; Pandiar, D.; Bhardwaj, R. Application of the Bethesda System of Reporting for Cervical Cytology to Evaluate Human Papilloma Virus Induced Changes in Oral Leukoplakia, Oral Squamous Cell Carcinoma, and Oropharyngeal Squamous Cell Carcinoma: A Cytomorphological and Genetic Study. Diagn. Cytopathol. 2021, 49, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M. Cervical Pre-Cancers: Biopsy and Immunohistochemistry. Cytojournal 2022, 19, 38. [Google Scholar] [CrossRef]
- Alrajjal, A.; Pansare, V.; Choudhury, M.S.R.; Khan, M.Y.A.; Shidham, V.B. Squamous Intraepithelial Lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System. Cytojournal 2021, 18, 16. [Google Scholar] [CrossRef]
- Bentley, J.; Executive Council of the Society of Canadian Colposcopists; Special contributors. Colposcopic Management of Abnormal Cervical Cytology and Histology. J. Obstet. Gynaecol. Can. 2012, 34, 1188–1202. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Xiong, J.; Chen, P.; Zhang, D.; Li, Q.; Zhu, P. Biomarkers Differentiating Regression from Progression among Untreated Cervical Intraepithelial Neoplasia Grade 2 Lesions. J. Adv. Res. 2024, 74, 391–402. [Google Scholar] [CrossRef]
- Loopik, D.L.; Bentley, H.A.; Eijgenraam, M.N.; IntHout, J.; Bekkers, R.L.M.; Bentley, J.R. The Natural History of Cervical Intraepithelial Neoplasia Grades 1, 2, and 3: A Systematic Review and Meta-Analysis. J. Low. Genit. Tract Dis. 2021, 25, 221–231. [Google Scholar] [CrossRef]
- Hatano, Y.; Ideta, T.; Hirata, A.; Hatano, K.; Tomita, H.; Okada, H.; Shimizu, M.; Tanaka, T.; Hara, A. Virus-Driven Carcinogenesis. Cancers 2021, 13, 2625. [Google Scholar] [CrossRef] [PubMed]
- Koeneman, M.M.; Hendriks, N.; Kooreman, L.F.; Winkens, B.; Kruitwagen, R.F.; Kruse, A.J. Prognostic Factors for Spontaneous Regression of High-Risk Human Papillomavirus-Positive Cervical Intra-Epithelial Neoplasia Grade 2. Int. J. Gynecol. Cancer 2019, 29, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.-B.; Kyrgiou, M. The Vaginal Microbiota Associates with the Regression of Untreated Cervical Intraepithelial Neoplasia 2 Lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef]
- Wang, K.; Muñoz, K.J.; Tan, M.; Sütterlin, C. Chlamydia and HPV Induce Centrosome Amplification in the Host Cell through Additive Mechanisms. Cell. Microbiol. 2021, 23, e13397. [Google Scholar] [CrossRef]
- Akbari, E.; Milani, A.; Seyedinkhorasani, M.; Bolhassani, A. HPV Co-infections with Other Pathogens in Cancer Development: A Comprehensive Review. J. Med. Virol. 2023, 95, e29236. [Google Scholar] [CrossRef]
- Cao, Y. EBV Based Cancer Prevention and Therapy in Nasopharyngeal Carcinoma. npj Precis. Oncol. 2017, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Latency and Lytic Replication in Epstein–Barr Virus-Associated Oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Scairati, R.; del Vecchio, G.; Liccardi, A.; Verde, N.; Pirchio, R.; Pivonello, R.; Ercolini, D.; Colao, A. The Vaginal Microbiome: A Long Urogenital Colonization Throughout Woman Life. Front. Cell. Infect. Microbiol. 2021, 11, 686167. [Google Scholar] [CrossRef] [PubMed]
- Heczko, P.B.; Giemza, M.; Ponikiewska, W.; Strus, M. Importance of Lactobacilli for Human Health. Microorganisms 2024, 12, 2382. [Google Scholar] [CrossRef]
- Miko, E.; Barakonyi, A. The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health. Antioxidants 2023, 12, 1055. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and Potential Use as Antimicrobials. J. Clin. Lab. Anal. 2021, 36, e24093. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Siddiqui, A.J.; Hamadou, W.S.; Surti, M.; Awadelkareem, A.M.; Ashraf, S.A.; Alreshidi, M.; Snoussi, M.; Rizvi, S.M.D.; Bardakci, F.; et al. Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus Rhamnosus with Its Physicochemical and Functional Properties. Antibiotics 2021, 10, 1546. [Google Scholar] [CrossRef]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial Peptides’ Immune Modulation Role in Intracellular Bacterial Infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Chen, T. Role of Vaginal Microbiota Dysbiosis in Gynecological Diseases and the Potential Interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef]
- Graziottin, A. Maintaining Vulvar, Vaginal and Perineal Health: Clinical Considerations. Womens Health 2024, 20, 17455057231223716. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus Iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Colonna, C.; Steelman, M. Amsel Criteria. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Simoes, J.A.; Discacciati, M.G.; Brolazo, E.M.; Portugal, P.M.; Dini, D.V.; Dantas, M.C.M. Clinical Diagnosis of Bacterial Vaginosis. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2006, 94, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Ly, C.; Hammoud, A.; Iwaza, R.; Mediannikov, O.; Bretelle, F.; Fenollar, F. Relationship between Bacterial Vaginosis and Sexually Transmitted Infections: Coincidence, Consequence or Co-Transmission? Microorganisms 2023, 11, 2470. [Google Scholar] [CrossRef] [PubMed]
- Valeriano, V.D.; Lahtinen, E.; Hwang, I.-C.; Zhang, Y.; Du, J.; Schuppe-Koistinen, I. Vaginal Dysbiosis and the Potential of Vaginal Microbiome-Directed Therapeutics. Front. Microbiomes 2024, 3, 1363089. [Google Scholar] [CrossRef]
- Fan, A.; Yue, Y.; Geng, N.; Zhang, H.; Wang, Y.; Xue, F. Aerobic Vaginitis and Mixed Infections: Comparison of Clinical and Laboratory Findings. Arch. Gynecol. Obstet. 2013, 287, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Maduta, C.S.; McCormick, J.K.; Dufresne, K. Vaginal Community State Types (CSTs) Alter Environmental Cues and Production of the Staphylococcus Aureus Toxic Shock Syndrome Toxin-1 (TSST-1). J. Bacteriol. 2024, 206, e00447-23. [Google Scholar] [CrossRef]
- De Seta, F.; Campisciano, G.; Zanotta, N.; Ricci, G.; Comar, M. The Vaginal Community State Types Microbiome-Immune Network as Key Factor for Bacterial Vaginosis and Aerobic Vaginitis. Front. Microbiol. 2019, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, K.Y.; Hong, H.; Jin, C.H.; Shim, E.; Kim, S.H.; Kim, B.-Y. Community State Types of Vaginal Microbiota and Four Types of Abnormal Vaginal Microbiota in Pregnant Korean Women. Front. Public Health 2020, 8, 507024. [Google Scholar] [CrossRef]
- Cocomazzi, G.; De Stefani, S.; Del Pup, L.; Palini, S.; Buccheri, M.; Primiterra, M.; Sciannamè, N.; Faioli, R.; Maglione, A.; Baldini, G.M.; et al. The Impact of the Female Genital Microbiota on the Outcome of Assisted Reproduction Treatments. Microorganisms 2023, 11, 1443. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Mitra, A.; Moscicki, A.-B. Does the Vaginal Microbiota Play a Role in the Development of Cervical Cancer? Transl. Res. J. Lab. Clin. Med. 2017, 179, 168–182. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Moscicki, A.-B. Vaginal Microbiome and Cervical Cancer. Semin. Cancer Biol. 2022, 86, 189–198. [Google Scholar] [CrossRef]
- Loonen, A.J.M.; Verhagen, F.; Luijten-de Vrije, I.; Lentjes-Beer, M.; Huijsmans, C.J.; van den Brule, A.J.C. Vaginal Dysbiosis Seems Associated with hrHPV Infection in Women Attending the Dutch Cervical Cancer Screening Program. Front. Cell. Infect. Microbiol. 2024, 14, 1330844. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the Complex Interplay between Microbiota, HPV, Inflammation and Cancer through Cervicovaginal Metabolic Profiling. eBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef]
- Avitabile, E.; Menotti, L.; Croatti, V.; Giordani, B.; Parolin, C.; Vitali, B. Protective Mechanisms of Vaginal Lactobacilli against Sexually Transmitted Viral Infections. Int. J. Mol. Sci. 2024, 25, 9168. [Google Scholar] [CrossRef]
- Qian, G.; Zang, H.; Tang, J.; Zhang, H.; Yu, J.; Jia, H.; Zhang, X.; Zhou, J. Lactobacillus Gasseri ATCC33323 Affects the Intestinal Mucosal Barrier to Ameliorate DSS-Induced Colitis through the NR1I3-Mediated Regulation of E-Cadherin. PLoS Pathog. 2024, 20, e1012541. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, R.; Mao, B.; Tang, X.; Zhao, J.; Zhang, Q.; Cui, S. Lactobacillus Crispatus CCFM1339 Inhibits Vaginal Epithelial Barrier Injury Induced by Gardnerella Vaginalis in Mice. Biomolecules 2024, 14, 240. [Google Scholar] [CrossRef]
- Gutierrez-Merino, J.; Isla, B.; Combes, T.; Martinez-Estrada, F.; Maluquer De Motes, C. Beneficial Bacteria Activate Type-I Interferon Production via the Intracellular Cytosolic Sensors STING and MAVS. Gut Microbes 2020, 11, 771–788. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, M.; Kim, J.H.; Son, J.E.; Lee, J.J.Y.; Park, S.; Lee, J.; Kim, M.; Oh, J.-W.; Park, M.S.; et al. Lactobacillus Salivarius HHuMin-U Activates Innate Immune Defense against Norovirus Infection through TBK1-IRF3 and NF-κB Signaling Pathways. Research 2022, 2022, 0007. [Google Scholar] [CrossRef]
- Xia, Q.; Pierson, S. HPV Infection and Oral Microbiota: Interactions and Future Implications. Int. J. Mol. Sci. 2025, 26, 1424. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Fan, T.; Luo, S.; Zheng, J.; Zhang, L.; Cao, L.; Zhang, Z.; Li, L.; Huang, Z.; Zhang, H.; et al. Lactobacillus Gasseri LGV03 Isolated from the Cervico-Vagina of HPV-Cleared Women Modulates Epithelial Innate Immune Responses and Suppresses the Growth of HPV-Positive Human Cervical Cancer Cells. Transl. Oncol. 2023, 35, 101714. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus Casei and Lactobacillus Rhamnosus GG Decrease Colon Cancer Cell Invasion in Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Zhou, X.; Yi, R.; Yang, Z.; Zhao, X. Lactobacillus Fermentum ZS09 Mediates Epithelial–Mesenchymal Transition (EMT) by Regulating the Transcriptional Activity of the Wnt/β-Catenin Signalling Pathway to Inhibit Colon Cancer Activity. J. Inflamm. Res. 2021, 14, 7281–7293. [Google Scholar] [CrossRef]
- Shiri Aghbash, P.; Hemmat, N.; Baradaran, B.; Mokhtarzadeh, A.; Poortahmasebi, V.; Ahangar Oskuee, M.; Bannazadeh Baghi, H. The Effect of Wnt/β-Catenin Signaling on PD-1/PDL-1 Axis in HPV-Related Cervical Cancer. Oncol. Res. 2022, 30, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Lin, J.; Wang, W.; Yan, K.; Liang, H.; Liang, J.; Yu, H.; Ling, B. Wnt3a/β-Catenin/CBP Activation in the Progression of Cervical Intraepithelial Neoplasia. Pathol. Oncol. Res. 2021, 27, 609620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Sun, D.; Liu, J.; Wang, Z.; Li, A. Lactobacillus GG Regulates the Wnt/β-Catenin Pathway to Reinforce Intestinal Barrier Function and Alleviate Necrotizing Enterocolitis. J. Funct. Foods 2022, 97, 105243. [Google Scholar] [CrossRef]
- Sepehr, A.; Aghamohammad, S.; Ghanavati, R.; Bavandpour, A.K.; Talebi, M.; Rohani, M.; Pourshafie, M.R. The Inhibitory Effects of the Novel Lactobacillus Cocktail on Colorectal Cancer Development through Modulating BMP Signaling Pathway: In Vitro and in Vivo Study. Heliyon 2024, 10, e36554. [Google Scholar] [CrossRef]
- Abu-Elfotuh, K.; Selim, H.M.R.M.; Riad, O.K.M.; Hamdan, A.M.E.; Hassanin, S.O.; Sharif, A.F.; Moustafa, N.M.; Gowifel, A.M.H.; Mohamed, M.Y.A.; Atwa, A.M.; et al. The Protective Effects of Sesamol and/or the Probiotic, Lactobacillus Rhamnosus, against Aluminum Chloride-Induced Neurotoxicity and Hepatotoxicity in Rats: Modulation of Wnt/β-Catenin/GSK-3β, JAK-2/STAT-3, PPAR-γ, Inflammatory, and Apoptotic Pathways. Front. Pharmacol. 2023, 14, 1208252. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Hussein, R.M.; Gaber, Y.; Hammam, O.A.; Kandeil, M.A. Modulation of JNK-1/β-Catenin Signaling by Lactobacillus casei, Inulin and Their Combination in 1,2-Dimethylhydrazine-Induced Colon Cancer in Mice. RSC Adv. 2019, 9, 29368–29383. [Google Scholar] [CrossRef] [PubMed]
- Staedel, C.; Darfeuille, F. MicroRNAs and Bacterial Infection. Cell. Microbiol. 2013, 15, 1496–1507. [Google Scholar] [CrossRef]
- Khodaii, Z.; Mehrabani Natanzi, M.; Khalighfard, S.; Ghandian Zanjan, M.; Gharghi, M.; Khori, V.; Amiriani, T.; Rahimkhani, M.; Alizadeh, A.M. Novel Targets in Rectal Cancer by Considering lncRNA–miRNA–mRNA Network in Response to Lactobacillus Acidophilus Consumption: A Randomized Clinical Trial. Sci. Rep. 2022, 12, 9168. [Google Scholar] [CrossRef]
- Şahin, T.Ö.; Yılmaz, B.; Yeşilyurt, N.; Cicia, D.; Szymanowska, A.; Amero, P.; Ağagündüz, D.; Capasso, R. Recent Insights into the Nutritional Immunomodulation of Cancer-related microRNAs. Phytother. Res. 2023, 37, 4375–4397. [Google Scholar] [CrossRef]
- Bi, K.; Zhang, X.; Chen, W.; Diao, H. MicroRNAs Regulate Intestinal Immunity and Gut Microbiota for Gastrointestinal Health: A Comprehensive Review. Genes 2020, 11, 1075. [Google Scholar] [CrossRef]
- Runtsch, M.C.; Hu, R.; Alexander, M.; Wallace, J.; Kagele, D.; Petersen, C.; Valentine, J.F.; Welker, N.C.; Bronner, M.P.; Chen, X.; et al. MicroRNA-146a Constrains Multiple Parameters of Intestinal Immunity and Increases Susceptibility to DSS Colitis. Oncotarget 2015, 6, 28556–28572. [Google Scholar] [CrossRef]
- Belcheva, A. MicroRNAs at the Epicenter of Intestinal Homeostasis. BioEssays 2017, 39, 1600200. [Google Scholar] [CrossRef]
- Alshahrani, S.H.; Al-Hadeithi, Z.S.M.; Almalki, S.G.; Malviya, J.; Hjazi, A.; Mustafa, Y.F.; Alawady, A.H.R.; Alsaalamy, A.H.; Joshi, S.K.; Alkhafaji, A.T. LncRNA-miRNA Interaction Is Involved in Colorectal Cancer Pathogenesis by Modulating Diverse Signaling Pathways. Pathol.-Res. Pract. 2023, 251, 154898. [Google Scholar] [CrossRef] [PubMed]
- Nikolaieva, N.; Sevcikova, A.; Omelka, R.; Martiniakova, M.; Mego, M.; Ciernikova, S. Gut Microbiota–MicroRNA Interactions in Intestinal Homeostasis and Cancer Development. Microorganisms 2022, 11, 107. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, T.; Yu, Q.; Nie, S.; Gong, D.; Xiong, T.; Xie, M. Exopolysaccharides from Lactobacillus Plantarum NCU116 Induce C-Jun Dependent Fas/Fasl-Mediated Apoptosis via TLR2 in Mouse Intestinal Epithelial Cancer Cells. Sci. Rep. 2017, 7, 14247. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides Produced by Lactobacillus Strains Suppress HT-29 Cell Growth via Induction of G0/G1 Cell Cycle Arrest and Apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef]
- Cheng, L.; Kaźmierczak, D.; Norenhag, J.; Hamsten, M.; Fransson, E.; Schuppe-Koistinen, I.; Olovsson, M.; Engstrand, L.; Hydbring, P.; Du, J. A MicroRNA Gene Panel Predicts the Vaginal Microbiota Composition. mSystems 2021, 6, e00175-21. [Google Scholar] [CrossRef]
- Senba, M.; Mori, N. Mechanisms of Virus Immune Evasion Lead to Development from Chronic Inflammation to Cancer Formation Associated with Human Papillomavirus Infection. Oncol. Rev. 2012, 6, e17. [Google Scholar] [CrossRef]
- A. Rahman, N.A.; Balasubramaniam, V.R.M.T.; Yap, W.B. Potential of Interleukin (IL)-12 Group as Antivirals: Severe Viral Disease Prevention and Management. Int. J. Mol. Sci. 2023, 24, 7350. [Google Scholar] [CrossRef] [PubMed]
- Zanotta, S.; Galati, D.; De Filippi, R.; Pinto, A. Enhancing Dendritic Cell Cancer Vaccination: The Synergy of Immune Checkpoint Inhibitors in Combined Therapies. Int. J. Mol. Sci. 2024, 25, 7509. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Tang, Y.-J.; Chen, Y.; Zheng, M.; Tang, Y.; Liang, X. The Double-Edged Sword—How Human Papillomaviruses Interact With Immunity in Head and Neck Cancer. Front. Immunol. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, B. The Role of Tumor-Associated Macrophages in HPV Induced Cervical Cancer. Front. Immunol. 2025, 16, 1586806. [Google Scholar] [CrossRef]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef]
- Lorusso, M.; D’aMbrosio, M.; Nesta, D.; Triggiano, F.; Diella, G.; Veneziani, P.; Santacroce, L. Assessing the Relationship between Lactobacilli and HPV: A Decade of Research. Biocell 2025, 49, 199–220. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Castro, J.; França, A.; Almeida, C.; Muzny, C.A.; Cerca, N. A New PNA-FISH Probe Targeting Fannyhessea Vaginae. Front. Cell. Infect. Microbiol. 2021, 11, 779376. [Google Scholar] [CrossRef] [PubMed]
- Frąszczak, K.; Barczyński, B.; Kondracka, A. Does Lactobacillus Exert a Protective Effect on the Development of Cervical and Endometrial Cancer in Women? Cancers 2022, 14, 4909. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Hayon, I.L.; Haupt, Y. The Regulation of P53 Growth Suppression. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Hu, S.; Hao, Y.; Zhang, X.; Yang, Y.; Liu, M.; Wang, N.; Zhang, T.-C.; He, H. Lacticaseibacillus Casei LH23 Suppressed HPV Gene Expression and Inhibited Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2023, 15, 443–450. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus Casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Lee, Y.Z.; Cheng, S.-H.; Lin, Y.-F.; Wu, C.-C.; Tsai, Y.-C. The Beneficial Effects of Lacticaseibacillus Paracasei Subsp. Paracasei DSM 27449 in a Letrozole-Induced Polycystic Ovary Syndrome Rat Model. Int. J. Mol. Sci. 2024, 25, 8706. [Google Scholar] [CrossRef]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The Vaginal Microbiota, Human Papillomavirus and Cervical Dysplasia: A Systematic Review and Network Meta-Analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 171–180. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Li, R.; Chen, X.; Wan, L.; Zhao, W. Associations of Cervicovaginal Lactobacilli With High-Risk Human Papillomavirus Infection, Cervical Intraepithelial Neoplasia, and Cancer: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2019, 220, 1243–1254. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, H.; Yuan, L.; Chen, X.; Huang, X.; Wang, K.; Li, Z. Vaginal Microbiota and HPV Clearance: A Longitudinal Study. Front. Oncol. 2022, 12, 955150. [Google Scholar] [CrossRef]
- Zeng, M.; Li, X.; Jiao, X.; Cai, X.; Yao, F.; Xu, S.; Huang, X.; Zhang, Q.; Chen, J. Roles of Vaginal Flora in Human Papillomavirus Infection, Virus Persistence and Clearance. Front. Cell. Infect. Microbiol. 2023, 12, 1036869. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of Vaginal Probiotics Lactobacillus crispatus Chen-01 in Women with High-Risk HPV Infection: A Prospective Controlled Pilot Study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef]
- Giovannetti, O.; Tomalty, D.; Velikonja, L.; Gray, G.; Boev, N.; Gilmore, S.; Oladipo, J.; Sjaarda, C.; Sheth, P.M.; Adams, M.A. Pre- and Post-LEEP: Analysis of the Female Urogenital Tract Microenvironment and Its Association with Sexual Dysfunction. Sex. Med. 2023, 11, qfad039. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Liu, C.; Deng, J.; Li, Z.; Zhou, Y.; Li, Z. Probiotics: A New Approach for the Prevention and Treatment of Cervical Cancer. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus Crispatus M247 Oral Administration: Is It Really an Effective Strategy in the Management of Papillomavirus-Infected Women? Infect. Agent. Cancer 2022, 17, 53. [Google Scholar] [CrossRef]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-Term Lactobacillus Rhamnosus BMX 54 Application to Restore a Balanced Vaginal Ecosystem: A Promising Solution against HPV-Infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Fu, H.-C.; Tseng, C.-W.; Wu, C.-H.; Tsai, C.-C.; Lin, H. The Influence of Probiotics on Genital High-Risk Human Papilloma Virus Clearance and Quality of Cervical Smear: A Randomized Placebo-Controlled Trial. BMC Womens Health 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, P.C.; Sanches, J.M.; Sparvolli, L.G.; Amaral, R.; Migliorini, I.; Gil, C.D.; Taddei, C.R.; Witkin, S.S.; Discacciati, M.G. Relationship between Papillomavirus Vaccine, Vaginal Microbiome, and Local Cytokine Response: An Exploratory Research. Braz. J. Microbiol. 2021, 52, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Taghinezhad-S, S.; Keyvani, H.; Bermúdez-Humarán, L.G.; Donders, G.G.G.; Fu, X.; Mohseni, A.H. Twenty Years of Research on HPV Vaccines Based on Genetically Modified Lactic Acid Bacteria: An Overview on the Gut-Vagina Axis. Cell. Mol. Life Sci. 2021, 78, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kęczkowska, E.; Wrotyńska-Barczyńska, J.; Bałabas, A.; Piątkowska, M.; Dąbrowska, M.; Czarnowski, P.; Hennig, E.E.; Brązert, M.; Olcha, P.; Ciebiera, M.; et al. Harnessing the Power of Microbiota: How Do Key Lactobacillus Species Aid in Clearing High-Risk Human Papilloma Virus Infection and Promoting the Regression of Cervical Dysplasia? Biology 2025, 14, 1081. https://doi.org/10.3390/biology14081081
Kęczkowska E, Wrotyńska-Barczyńska J, Bałabas A, Piątkowska M, Dąbrowska M, Czarnowski P, Hennig EE, Brązert M, Olcha P, Ciebiera M, et al. Harnessing the Power of Microbiota: How Do Key Lactobacillus Species Aid in Clearing High-Risk Human Papilloma Virus Infection and Promoting the Regression of Cervical Dysplasia? Biology. 2025; 14(8):1081. https://doi.org/10.3390/biology14081081
Chicago/Turabian StyleKęczkowska, Edyta, Joanna Wrotyńska-Barczyńska, Aneta Bałabas, Magdalena Piątkowska, Michalina Dąbrowska, Paweł Czarnowski, Ewa E. Hennig, Maciej Brązert, Piotr Olcha, Michał Ciebiera, and et al. 2025. "Harnessing the Power of Microbiota: How Do Key Lactobacillus Species Aid in Clearing High-Risk Human Papilloma Virus Infection and Promoting the Regression of Cervical Dysplasia?" Biology 14, no. 8: 1081. https://doi.org/10.3390/biology14081081
APA StyleKęczkowska, E., Wrotyńska-Barczyńska, J., Bałabas, A., Piątkowska, M., Dąbrowska, M., Czarnowski, P., Hennig, E. E., Brązert, M., Olcha, P., Ciebiera, M., & Zeber-Lubecka, N. (2025). Harnessing the Power of Microbiota: How Do Key Lactobacillus Species Aid in Clearing High-Risk Human Papilloma Virus Infection and Promoting the Regression of Cervical Dysplasia? Biology, 14(8), 1081. https://doi.org/10.3390/biology14081081







