Passive eDNA Sampling Characterizes Fish Community Assembly in the Lancang River of Yunnan, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Effect of Time and Density on eDNA Absorption
2.2. Fish Biodiversity Detection Capacity
2.3. Measurements of Environmental Variables
2.4. Biodiversity Assessment
2.5. Statistics
3. Results
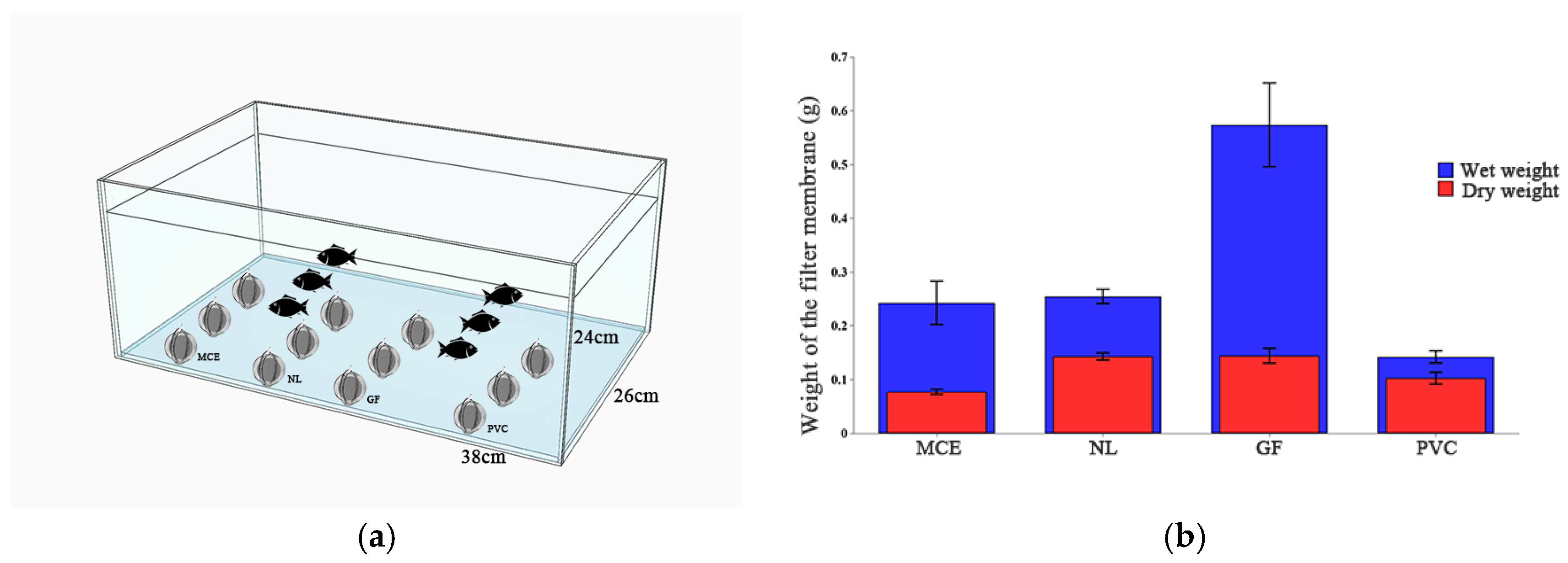
3.1. Materials Enable Passive eDNA Collection
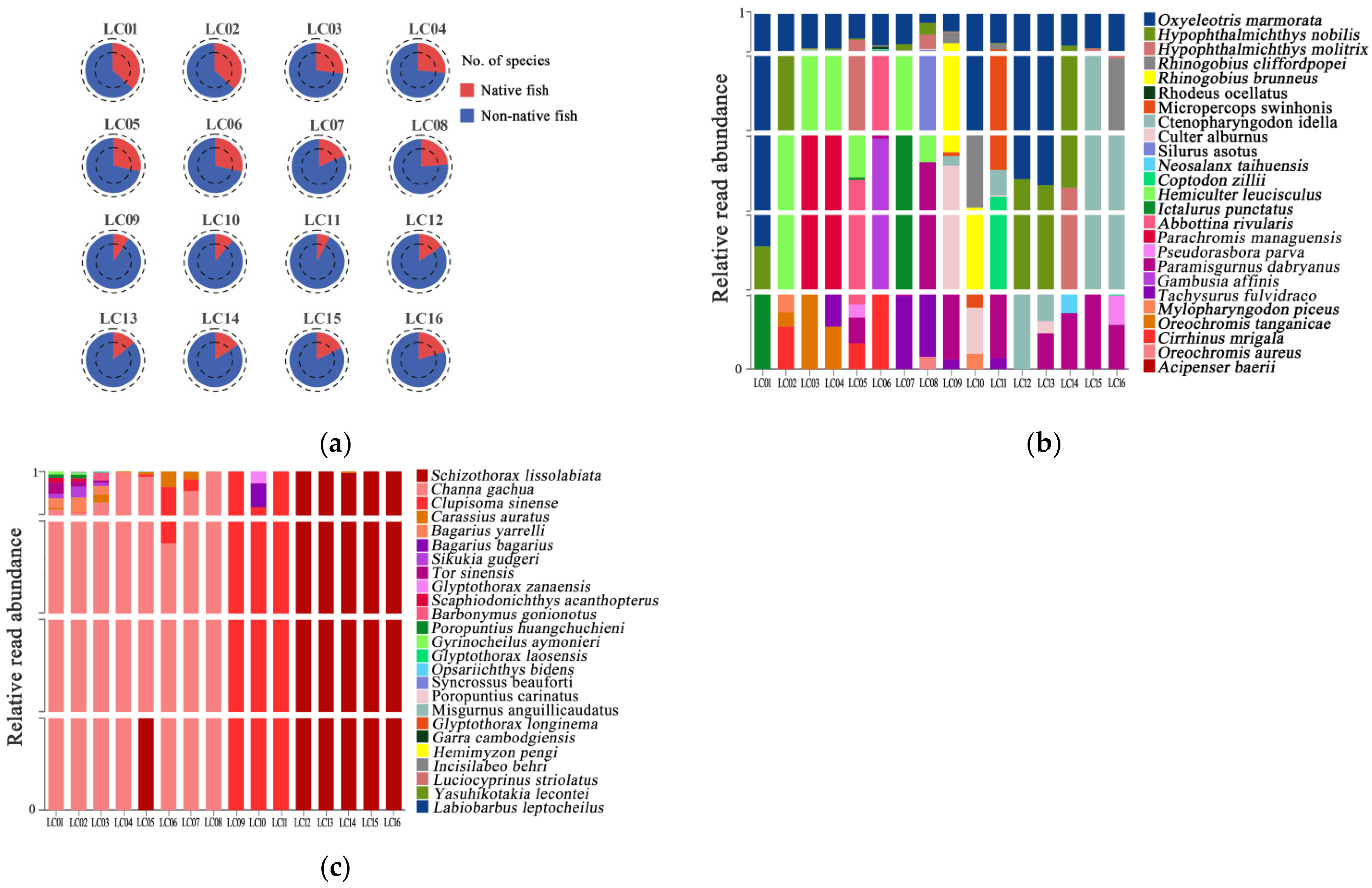
3.2. The Majority of Fish Species Were Detected with All Materials
3.3. Relationships Between Environmental Factors and Community Structures
4. Discussion
4.1. Evaluation of the Ability of Four Materials to Passively Collect Environmental DNA in Laboratory Environments
4.2. Evaluation of the Ability of Four Materials to Passively Collect Environmental DNA in the Lancang River
4.3. Relationship Between Fish Diversity Distribution and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, R.; Zhao, G.; Yang, J.; Wang, Z.; Xu, Y.; Zhang, X.; Wang, Z. eDNA Metabarcoding Revealed Differential Structures of Aquatic Communities in a Dynamic Freshwater Ecosystem Shaped by Habitat Heterogeneity. Environ. Res. 2021, 201, 111602. [Google Scholar] [CrossRef]
- Cortez, T.; Torres, A.; Guimarães, M.; Pinheiro, H.; Cabral, M.; Zielinsky, G.; Pereira, C.; de Castro, G.; Guerreiro, L.; Americo, J.; et al. Insights into the Representativeness of Biodiversity Assessment in Large Reservoir through eDNA Metabarcoding. PLoS ONE 2025, 20, e0314210. [Google Scholar] [CrossRef]
- Deiner, K.; Bik, H.M.; Mächler, E.; Seymour, M.; Lacoursière-Roussel, A.; Altermatt, F.; Creer, S.; Bista, I.; Lodge, D.M.; de Vere, N.; et al. Environmental DNA Metabarcoding: Transforming How We Survey Animal and Plant Communities. Mol. Ecol. 2017, 26, 5872–5895. [Google Scholar] [CrossRef]
- Veyñ, M.; Chalde, T.; Nardi, C. Optimisation of an Environmental DNA-Based Approach for Assessing Freshwater Fish Biodiversity in Southernmost South America. Aquat. Conserv. Mar. Freshw. Ecosyst. 2025, 35, e70112. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Lin, L.; Liu, Y.; Ye, J.; Zhang, W.; Li, H. Unraveling Fish Community Diversity and Structure in the Yellow Sea: Evidence from Environmental DNA Metabarcoding and Bottom Trawling. Animals 2025, 15, 1283. [Google Scholar] [CrossRef] [PubMed]
- Wee, A.K.S.; Salmo III, S.G.; Sivakumar, K.; Then, A.Y.-H.; Basyuni, M.; Fall, J.; Habib, K.A.; Isowa, Y.; Leopardas, V.; Peer, N.; et al. Prospects and Challenges of Environmental DNA (eDNA) Metabarcoding in Mangrove Restoration in Southeast Asia. Front. Mar. Sci. 2023, 10, 1033258. [Google Scholar] [CrossRef]
- Bista, I.; Carvalho, G.R.; Walsh, K.; Seymour, M.; Hajibabaei, M.; Lallias, D.; Christmas, M.; Creer, S. Annual Time-Series Analysis of Aqueous eDNA Reveals Ecologically Relevant Dynamics of Lake Ecosystem Biodiversity. Nat. Commun. 2017, 8, 14087. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, X.; Chen, J.; Wang, Z.; Chen, L.; Zhang, S.; Wang, K. Assessment of Fish Diversity in the Ma’an Archipelago Special Protected Area Using Environmental DNA. Biology 2022, 11, 1832. [Google Scholar] [CrossRef]
- Pope, K.L.; Goldberg, C.S.; Nelson, N.L.; Cummings, A.K.; Seaborn, T.; Piovia-Scott, J. Designing Environmental DNA Surveys in Complex Aquatic Systems: Backpack Sampling for Rare Amphibians in Sierra Nevada Meadows. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1975–1987. [Google Scholar] [CrossRef]
- Winslow, L.A.; Dugan, H.A.; Buelow, H.N.; Cronin, K.D.; Priscu, J.C.; Takacs-Vesbach, C.; Doran, P.T. Autonomous Year-Round Sampling and Sensing to Explore the Physical and Biological Habitability of Permanently Ice-Covered Antarctic Lakes. Mar. Technol. Soc. J. 2014, 48, 8–17. [Google Scholar] [CrossRef]
- Thomas, A.C.; Howard, J.; Nguyen, P.L.; Seimon, T.A.; Goldberg, C.S. eDNA Sampler: A Fully Integrated Environmental DNA Sampling System. Methods Ecol. Evol. 2018, 9, 1379–1385. [Google Scholar] [CrossRef]
- Vélez-Nicolás, M.; García-López, S.; Barbero, L.; Ruiz-Ortiz, V.; Sánchez-Bellón, Á. Applications of Unmanned Aerial Systems (UASs) in Hydrology: A Review. Remote Sens. 2021, 13, 1359. [Google Scholar] [CrossRef]
- Formel, N.; Enochs, I.C.; Sinigalliano, C.; Anderson, S.R.; Thompson, L.R. Subsurface Automated Samplers for eDNA (SASe) for Biological Monitoring and Research. HardwareX 2021, 10, e00239. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, T.; Goto, S.; Wong, M.K.-S.; Minegishi, Y.; Hyodo, S.; Makabe-Kobayashi, Y.; Sugai, Y.; Hamasaki, K. Development and Evaluation of Automated Gene Collector—ATGC-12S for Environmental DNA Sample Archive at Aquatic Environments. In Proceedings of the OCEANS 2022, Hampton Roads, Hampton Roads, VA, USA, 17–20 October 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–5. [Google Scholar] [CrossRef]
- LeviRam, I.; Gross, A.; Lintern, A.; Henry, R.; Schang, C.; Herzberg, M.; McCarthy, D. Sustainable Micropollutant Bioremediation via Stormwater Biofiltration System. Water Res. 2022, 214, 118188. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, Y.; Peng, Y.; Tang, W.; Wan, B. Experimental Study of Self-Locking Seal Structure Based on Full Ocean Depth Sediment Pressure Retaining Sampler. Appl. Ocean Res. 2023, 141, 103767. [Google Scholar] [CrossRef]
- Patin, N.V.; Goodwin, K.D. Capturing Marine Microbiomes and Environmental DNA: A Field Sampling Guide. Front. Microbiol. 2023, 13, 1026596. [Google Scholar] [CrossRef]
- Altermatt, F.; Carraro, L.; Antonetti, M.; Albouy, C.; Zhang, Y.; Lyet, A.; Zhang, X.; Pellissier, L. Quantifying Biodiversity Using eDNA from Water Bodies: General Principles and Recommendations for Sampling Designs. Environ. DNA 2023, 5, 671–682. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Zhao, J.; Yao, M. Passive eDNA Sampling Facilitates Biodiversity Monitoring and Rare Species Detection. Environ. Int. 2024, 187, 108706. [Google Scholar] [CrossRef]
- Wu, P.; Feng, J.; Ju, M.; Wu, S.; Han, W.; Wang, M.; Liao, J.; Zhao, L.; Gao, Y.; Zheng, J.; et al. Water Filter: A Rapid Water Environmental DNA Collector in the Field. Front. Environ. Sci. 2024, 12, 1415338. [Google Scholar] [CrossRef]
- Beesley, L.S.; Killerby Smith, S.; Gwinn, D.C.; Pusey, B.J.; Douglas, M.M.; Novak, P.A.; Tayer, T.C.; Keogh, C.S.; Kennard, M.J.; Canham, C.A.; et al. Modelling the Longitudinal Distribution, Abundance, and Habitat Use of the Giant Freshwater Shrimp (Macrobrachium spinipes) in a Large Intermittent, Tropical Australian River to Inform Water Resource Policy. Freshw. Biol. 2022, 68, 61–76. [Google Scholar] [CrossRef]
- Lu, S.; Zeng, H.; Xiong, F.; Yao, M.; He, S. Advances in Environmental DNA Monitoring: Standardization, Automation, and Emerging Technologies in Aquatic Ecosystems. Sci. China Life Sci. 2024, 67, 1368–1384. [Google Scholar] [CrossRef]
- Kirtane, A.; Atkinson, J.D.; Sassoubre, L. Design and Validation of Passive Environmental DNA Samplers Using Granular Activated Carbon and Montmorillonite Clay. Environ. Sci. Technol. 2020, 54, 11961–11970. [Google Scholar] [CrossRef] [PubMed]
- Bessey, C.; Neil Jarman, S.; Simpson, T.; Miller, H.; Stewart, T.; Kenneth Keesing, J.; Berry, O. Passive eDNA Collection Enhances Aquatic Biodiversity Analysis. Commun. Biol. 2021, 4, 236. [Google Scholar] [CrossRef] [PubMed]
- Maiello, G.; Talarico, L.; Carpentieri, P.; De Angelis, F.; Franceschini, S.; Harper, L.R.; Neave, E.F.; Rickards, O.; Sbrana, A.; Shum, P.; et al. Little Samplers, Big Fleet: eDNA Metabarcoding from Commercial Trawlers Enhances Ocean Monitoring. Fish. Res. 2022, 249, 106259. [Google Scholar] [CrossRef]
- Chen, X.; Kong, Y.; Zhang, S.; Zhao, J.; Li, S.; Yao, M. Comparative Evaluation of Common Materials as Passive Samplers of Environmental DNA. Environ. Sci. Technol. 2022, 56, 10798–10807. [Google Scholar] [CrossRef]
- Harrison, J.B.; Sunday, J.M.; Rogers, S.M. Predicting the Fate of eDNA in the Environment and Implications for Studying Biodiversity. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191409. [Google Scholar] [CrossRef]
- Kumar, G.; Farrell, E.; Reaume, A.M.; Eble, J.A.; Gaither, M.R. One Size Does Not Fit All: Tuning eDNA Protocols for High- and Low-turbidity Water Sampling. Environ. DNA 2021, 4, 167–180. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Zhang, H.; Gao, T.; Wang, X. Fishery Resource Monitoring of the East China Sea via Environmental DNA Approach: A Case Study Using Black Sea Bream (Acanthopagrus schlegelii). Front. Mar. Sci. 2022, 9, 848950. [Google Scholar] [CrossRef]
- Tzafesta, E.; Shokri, M. The Combined Negative Effect of Temperature, UV Radiation and Salinity on eDNA Detection: A Global Meta-Analysis on Aquatic Ecosystems. Ecol. Indic. 2025, 176, 113669. [Google Scholar] [CrossRef]
- McKee, A.M.; Spear, S.F.; Pierson, T.W. The Effect of Dilution and the Use of a Post-Extraction Nucleic Acid Purification Column on the Accuracy, Precision, and Inhibition of Environmental DNA Samples. Biol. Conserv. 2015, 183, 70–76. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Jiang, L.; Yu, X.; Mikelsons, K.; Shen, F. Global Estimation of Suspended Particulate Matter from Satellite Ocean Color Imagery. J. Geophys. Res. Ocean. 2021, 126, e2021JC017303. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Galib, S.M.; Ding, L.; Tao, J.; Ding, C.; He, D. Research Status of the Lancang-Mekong River Basin: Fish and Environmental Stressors. Rev. Fish Biol. Fish. 2023, 33, 89–109. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The Biodiversity of Species and Their Rates of Extinction, Distribution, and Protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Duarte, C.; Antão, L.H.; Magurran, A.E.; de Deus, C.P. Shifts in Fish Community Composition and Structure Linked to Seasonality in a Tropical River. Freshw. Biol. 2022, 67, 1789–1800. [Google Scholar] [CrossRef]
- Cilleros, K.; Allard, L.; Vigouroux, R.; Brosse, S. Disentangling Spatial and Environmental Determinants of Fish Species Richness and Assemblage Structure in Neotropical Rainforest Streams. Freshw. Biol. 2017, 62, 1707–1720. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, L.; Ding, C.; Chen, L.; Sun, J.; Jiang, X. Responses of Species and Phylogenetic Diversity of Fish Communities in the Lancang River to Hydropower Development and Exotic Invasions. Ecol. Indic. 2018, 90, 261–279. [Google Scholar] [CrossRef]
- Jerde, C.L.; Mahon, A.R.; Chadderton, W.L.; Lodge, D.M. “Sight-Unseen” Detection of Rare Aquatic Species Using Environmental DNA. Conserv. Lett. 2011, 4, 150–157. [Google Scholar] [CrossRef]
- Xin, N.; Li, Z.; Jiang, Y.-W.; Wang, H.; Tan, J.; Li, Y.; Sun, B.-J.; Lin, X.-L. Environmental DNA Metabarcoding Reveals Fish Diversity, Community Assembly and One Invasive Species Prevalence in a National Park of Liaohe in September. Front. Mar. Sci. 2024, 11, 1403700. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Li, S.; Li, B.; Ma, L.; Chen, X. Human Activities Strengthen the Influence of Deterministic Processes in the Mechanisms of Fish Community Assembly in Tropical Rivers of Yunnan, China. J. Environ. Manag. 2024, 368, 122131. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, J.; Cui, G.; Zhang, B.; Yan, B.; Nie, Q. Environmental DNA Metabarcoding: Current Applications and Future Prospects for Freshwater Fish Monitoring. J. Environ. Manag. 2025, 376, 124531. [Google Scholar] [CrossRef]
- Barnes, M.A.; Turner, C.R.; Jerde, C.L.; Renshaw, M.A.; Chadderton, W.L.; Lodge, D.M. Environmental Conditions Influence eDNA Persistence in Aquatic Systems. Environ. Sci. Technol. 2014, 48, 1819–1827. [Google Scholar] [CrossRef]
- Trujillo-Gonzalez, A.; Hinlo, R.; Godwin, S.; Barmuta, L.A.; Watson, A.; Turner, P.; Koch, A.; Gleeson, D. Environmental DNA Detection of the Giant Freshwater Crayfish (Astacopsis gouldi). Environ. DNA 2021, 3, 950–958. [Google Scholar] [CrossRef]
- Fu, G.Q.; Qin, T.; Chen, X.Y.; Lei, C.Y.; Li, G.H. Composition and Species Diversity of Fishes in Xishuangbanna Reach of Lancang River. Acta Ecol. Sin. 2021, 41, 9557–9573. [Google Scholar] [CrossRef]
- Bessey, C.; Gao, Y.; Truong, Y.B.; Miller, H.; Jarman, S.N.; Berry, O. Comparison of Materials for Rapid Passive Collection of Environmental DNA. Mol. Ecol. Resour. 2022, 22, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.E.; Baerwald, M.R.; Rodzen, J.; Schreier, B.M.; Mahardja, B.; Finger, A.J. Evaluating Environmental DNA Detection of a Rare Fish in Turbid Water Using Field and Experimental Approaches. PeerJ 2024, 11, e16453. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Konsowa, A.H.; Zhu, X.; Crittenden, J.C. Evaluation of an Innovative Polyvinyl Chloride (PVC) Ultrafiltration Membrane for Wastewater Treatment. Sep. Purif. Technol. 2009, 70, 71–78. [Google Scholar] [CrossRef]
- Rodriguez, L.; De Bonis, L.; McKee, J.; McKenna, J.A.; Urvois, T.; Barbaccia, E.; Dillane, E.; Lanfredi, C.; Hjellnes, H.; Jung, A.; et al. Comparing the Efficiency of DNA Extraction Protocols Across a Multinational Environmental DNA Initiative; Pensoft Publishers: Sofia, Bulgaria, 2024. [Google Scholar] [CrossRef]
- Bairoliya, S.; Koh Zhi Xiang, J.; Cao, B. Extracellular DNA in Environmental Samples: Occurrence, Extraction, Quantification, and Impact on Microbial Biodiversity Assessment. Appl. Environ. Microbiol. 2022, 88, e01845-21. [Google Scholar] [CrossRef]
- Jo, T.S.; Murakami, H.; Nakadai, R. Spatial Dispersal of Environmental DNA Particles in Lentic and Marine Ecosystems: An Overview and Synthesis. Ecol. Indic. 2025, 174, 113469. [Google Scholar] [CrossRef]
- Morris, L.; Beesley, L.S.; Stevens, E.R.; Gwinn, D.C.; Hyde, J.; Thompson, S.; Gleeson, D.B.; Douglas, M.M. Active eDNA Is More Cost-Effective Than Fyke Nets or Passive eDNA Collection When Monitoring the Invasion of an Alien Freshwater Fish. Environ. DNA 2024, 6, e70010. [Google Scholar] [CrossRef]
- Caza-Allard, I.; Laporte, M.; Côté, G.; April, J.; Bernatchez, L. Effect of Biotic and Abiotic Factors on the Production and Degradation of Fish Environmental DNA: An Experimental Evaluation. Environ. DNA 2021, 4, 453–468. [Google Scholar] [CrossRef]
- Batista, S.; Curto, M.; Veríssimo, A.; Ribeiro, F.; Alves, M.J.; Pina-Martins, F.; Santos, C.D.; Jentoft, S.; Gante, H. Evaluating Environmental DNA Efficiency in the Detection of Freshwater Species in a System with High Endemism. Biol. Life Sci. Forum 2022, 13, 116. [Google Scholar] [CrossRef]
- Miya, M. Environmental DNA Metabarcoding: A Novel Method for Biodiversity Monitoring of Marine Fish Communities. Annu. Rev. Mar. Sci. 2022, 14, 161–185. [Google Scholar] [CrossRef]
- Saunders, M.D.; Steeves, R.; MacIntyre, L.P.; Knysh, K.M.; Coffin, M.R.S.; Boudreau, M.; Pater, C.C.; van den Heuvel, M.R.; Courtenay, S.C. Monitoring Estuarine Fish Communities: Environmental DNA (eDNA) Metabarcoding as a Complement to Beach Seining. Can. J. Fish. Aquat. Sci. 2024, 81, 1344–1357. [Google Scholar] [CrossRef]
- López-Rodríguez, A.; Meerhoff, M.; D’Anatro, A.; de Ávila-Simas, S.; Silva, I.; Pais, J.; Teixeira de Mello, F.; Reynalte-Tataje, D.A.; Zaniboni-Filho, E.; González-Bergonzoni, I. Longitudinal Changes on Ecological Diversity of Neotropical Fish along a 1700 Km River Gradient Show Declines Induced by Dams. Perspect. Ecol. Conserv. 2024, 22, 186–195. [Google Scholar] [CrossRef]
- Liu, M.; Chen, D.; Duan, X.; Wang, K.; Liu, S. Ichthyofauna Composition and Distribution of Fishes in Yunnan Section of Lancang River. J. Fish. Sci. China 2013, 18, 156–170. [Google Scholar] [CrossRef]
- Trovillion, D.C.; Sauer, E.L.; Shay, G.; Crone, E.R.; Preston, D.L. Habitat Complexity, Connectivity, and Introduced Fish Drive Pond Community Structure along an Urban to Rural Gradient. Ecol. Appl. 2023, 33, e2828. [Google Scholar] [CrossRef] [PubMed]
- Crone, E.R.; Sauer, E.L.; Preston, D.L. Non-native Fish Facilitate Non-native Snails and Alter Food Web Structure in Experimental Pond Communities. Funct. Ecol. 2023, 37, 947–958. [Google Scholar] [CrossRef]
- Zhang, H.; Yoshizawa, S.; Iwasaki, W.; Xian, W. Seasonal Fish Assemblage Structure Using Environmental DNA in the Yangtze Estuary and Its Adjacent Waters. Front. Mar. Sci. 2019, 6, 00515. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhuo, X.; Gao, X.; Ma, X.; Zhang, X. Salinity-Dependent Mitigation of Naphthalene Toxicity in Migratory Takifugu Obscurus Juveniles: Implications for Survival, Oxidative Stress, and Osmoregulation. Sci. Total Environ. 2023, 896, 165248. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, X.; Zhou, T.; Cai, S.; Zhang, W.; Mao, K.; Ou, H.; Ran, L.; Yang, Q.; Wang, Y. Monitoring Chlorophyll-a Concentration Variation in Fish Ponds from 2013 to 2022 in the Guangdong-Hong Kong-Macao Greater Bay Area, China. Remote Sens. 2024, 16, 2033. [Google Scholar] [CrossRef]
- Diamond, J.; Roy, D. Patterns of Functional Diversity along Latitudinal Gradients of Species Richness in Eleven Fish Families. Glob. Ecol. Biogeogr. 2023, 32, 450–465. [Google Scholar] [CrossRef]
- Nhat, N.H.; Saito, M.; Onodera, S.; Hamada, M.; Hyodo, F.; Nagare, H. Environmental DNA Reveals the Impact of Submarine Groundwater Discharge on the Spatial Variability of Coastal Fish Diversity. Biology 2024, 13, 609. [Google Scholar] [CrossRef]
- Zhao, Q.; Van den Brink, P.J.; Xu, C.; Wang, S.; Clark, A.T.; Karakoç, C.; Sugihara, G.; Widdicombe, C.E.; Atkinson, A.; Matsuzaki, S.S.; et al. Relationships of Temperature and Biodiversity with Stability of Natural Aquatic Food Webs. Nat. Commun. 2023, 14, 3507. [Google Scholar] [CrossRef]
- Suhaimi Sulaiman, M.; Abd Rahman, M.F.; Mohd Adam, A.F. Variance of Total Dissolved Solids and Electrical Conductivity for Water Quality in Sabak Bernam. Int. J. Electr. Comput. Eng. (IJECE) 2023, 13, 2259–2269. [Google Scholar] [CrossRef]
- Nagel, C.; Mueller, M.; Pander, J.; Stoeckle, B.C.; Kuehn, R.; Geist, J. Going with the Flow: Spatio-temporal Drift Patterns of Larval Fish in a Large Alpine River. Freshw. Biol. 2021, 66, 1765–1781. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Duan, X.; Liu, M.; Chen, D.; Huang, X.; Wang, D.; Ma, B.; Fu, S.; Zhong, L. Passive eDNA Sampling Characterizes Fish Community Assembly in the Lancang River of Yunnan, China. Biology 2025, 14, 1080. https://doi.org/10.3390/biology14081080
Ding L, Duan X, Liu M, Chen D, Huang X, Wang D, Ma B, Fu S, Zhong L. Passive eDNA Sampling Characterizes Fish Community Assembly in the Lancang River of Yunnan, China. Biology. 2025; 14(8):1080. https://doi.org/10.3390/biology14081080
Chicago/Turabian StyleDing, Li, Xinbin Duan, Mingdian Liu, Daqing Chen, Xiaofeng Huang, Dengqiang Wang, Baoshan Ma, Shijian Fu, and Liqiao Zhong. 2025. "Passive eDNA Sampling Characterizes Fish Community Assembly in the Lancang River of Yunnan, China" Biology 14, no. 8: 1080. https://doi.org/10.3390/biology14081080
APA StyleDing, L., Duan, X., Liu, M., Chen, D., Huang, X., Wang, D., Ma, B., Fu, S., & Zhong, L. (2025). Passive eDNA Sampling Characterizes Fish Community Assembly in the Lancang River of Yunnan, China. Biology, 14(8), 1080. https://doi.org/10.3390/biology14081080






