Divergent Hepatic and Adipose Tissue Effects of Kupffer Cell Depletion in a Male Rat Model of Metabolic-Associated Steatohepatitis
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Model
2.2. Tissue and Serum Collection
2.3. Insulin Signaling Pathway
2.4. Systemic Biochemical Parameters
2.5. Insulin and Pyruvate Tolerance Tests
2.6. Liver Triacylglyceride Content, Tissue Sectioning, and Staining
2.7. RNA Isolation and qPCR
2.8. Protein Isolation and Western Blot
2.9. TBARS and Antioxidant Enzymes Activity
2.10. Data Analysis
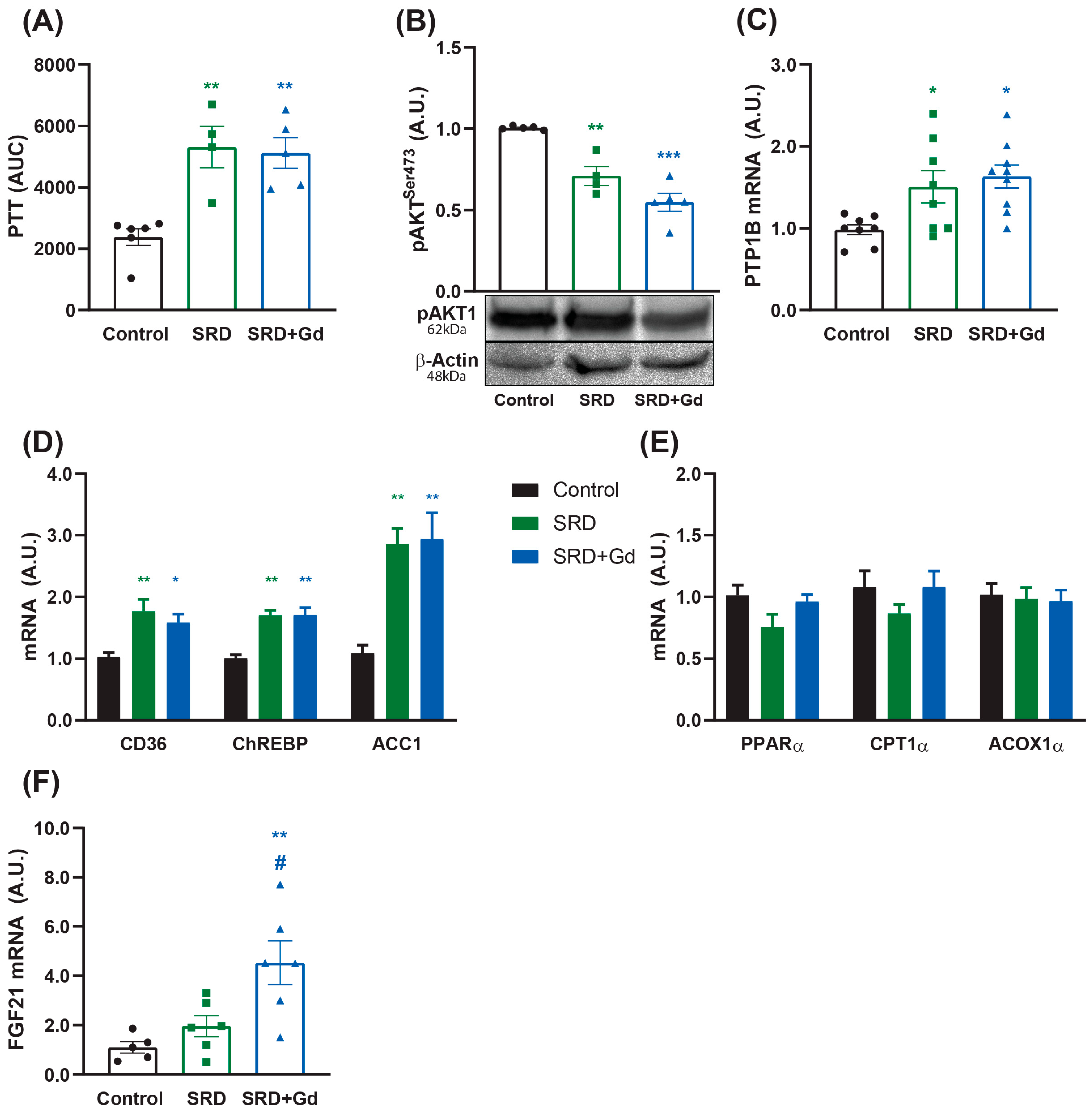
3. Results
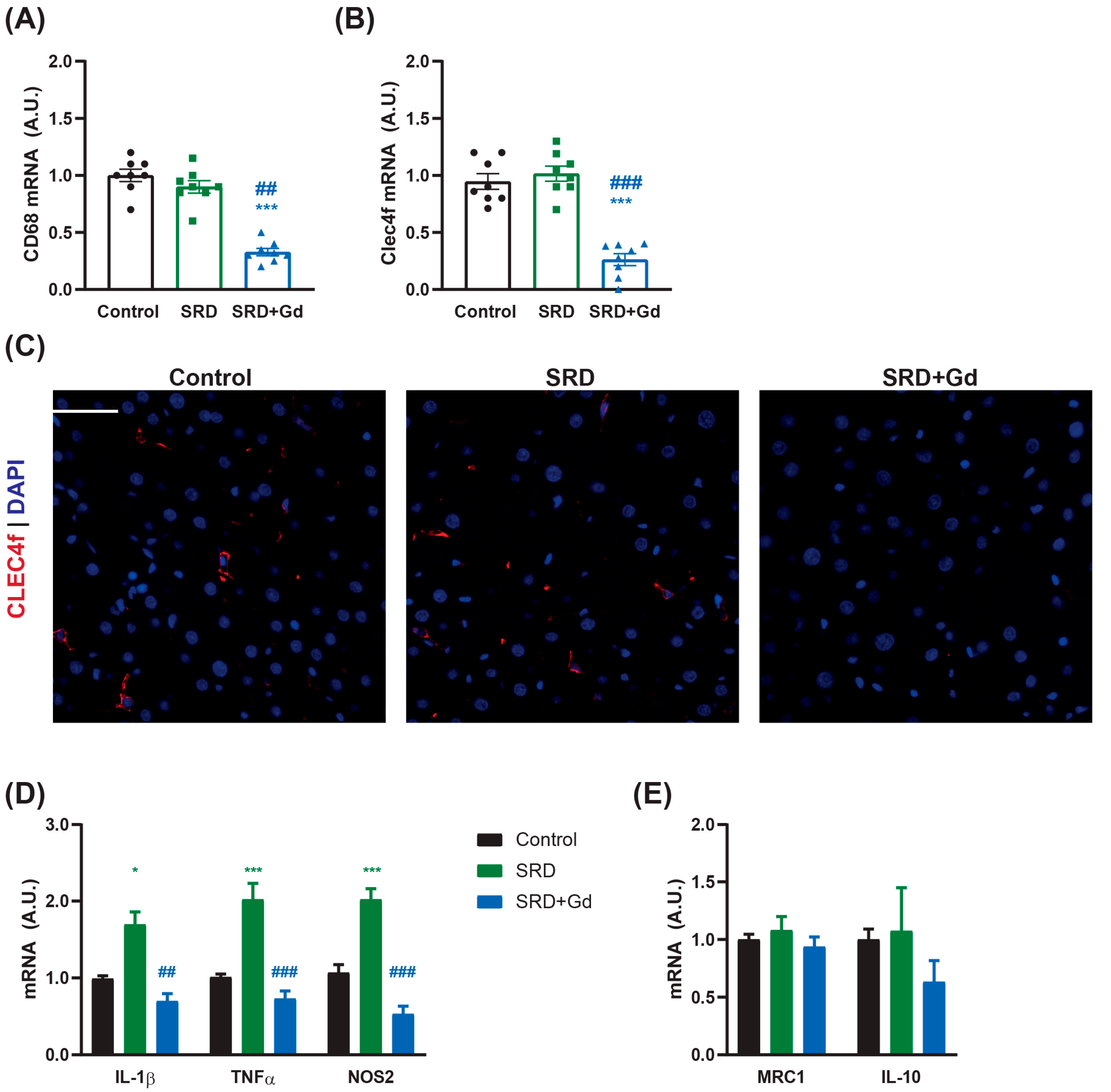
3.1. Gadolinium Chloride Depletes the Kupffer Cell Population in the Liver
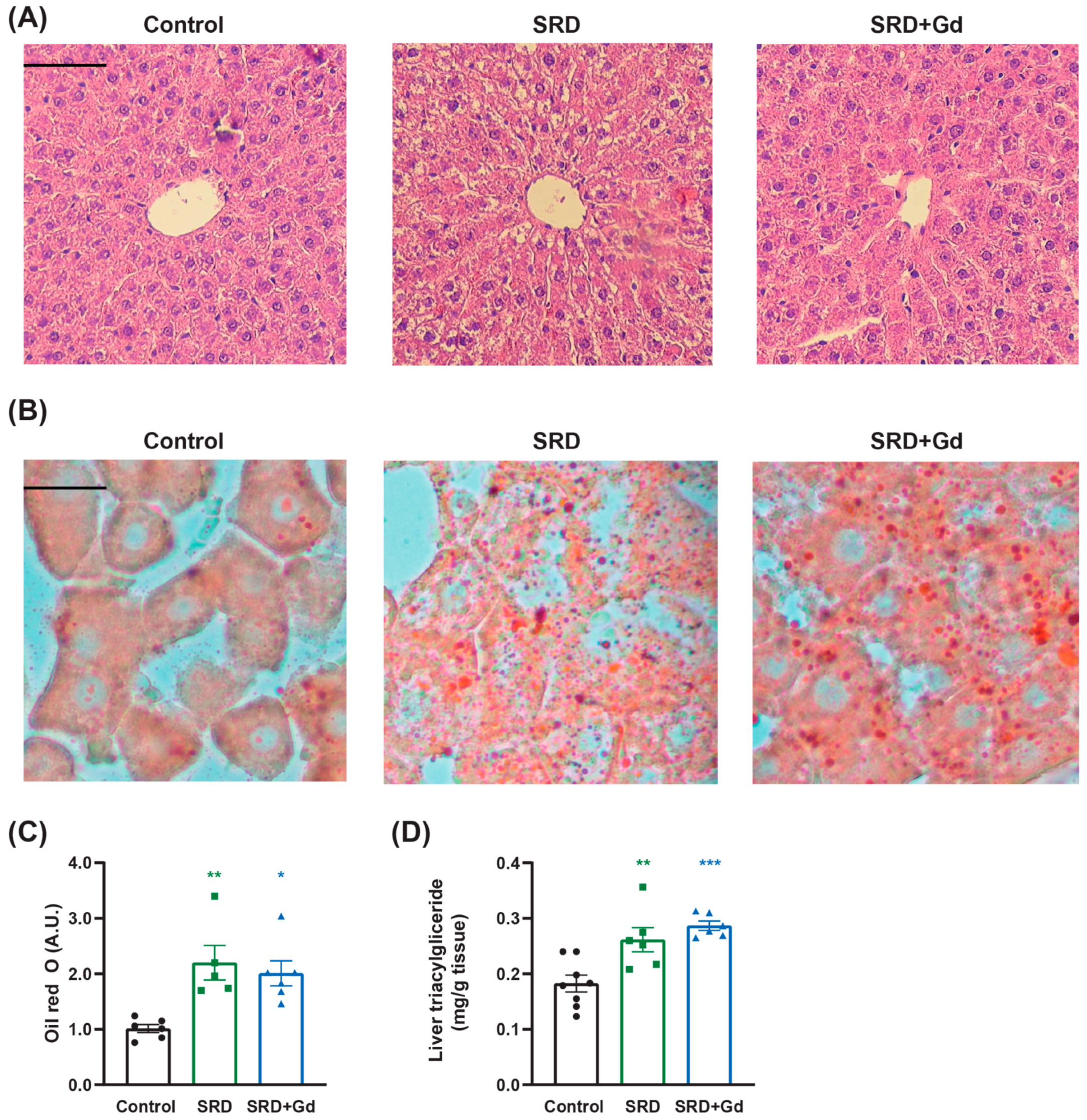
3.2. Effect of Gadolinium Administration on Liver Histological Parameters
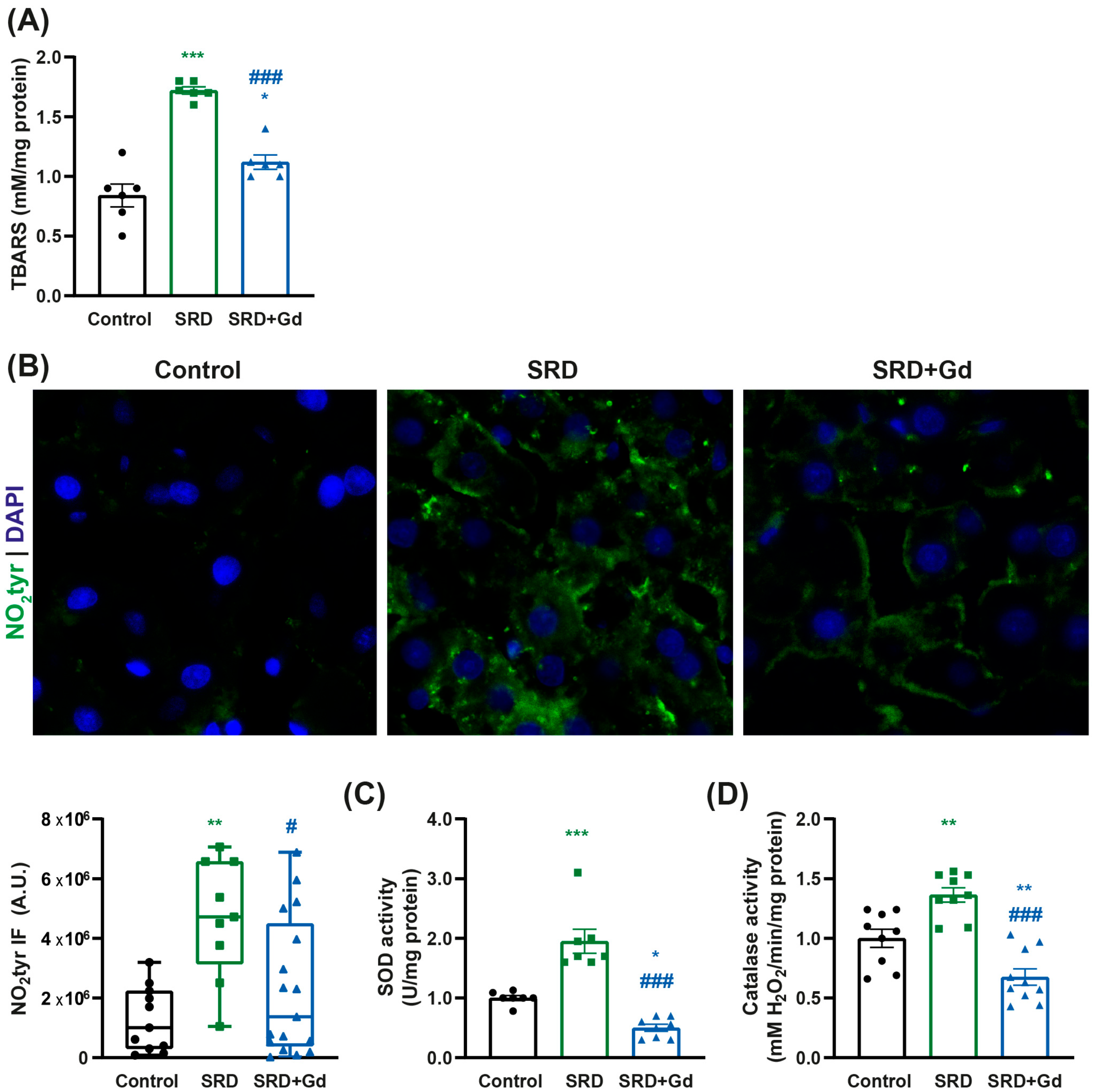
3.3. Kupffer Cell Depletion Is Associated with a Decrease in Oxidative Stress Markers
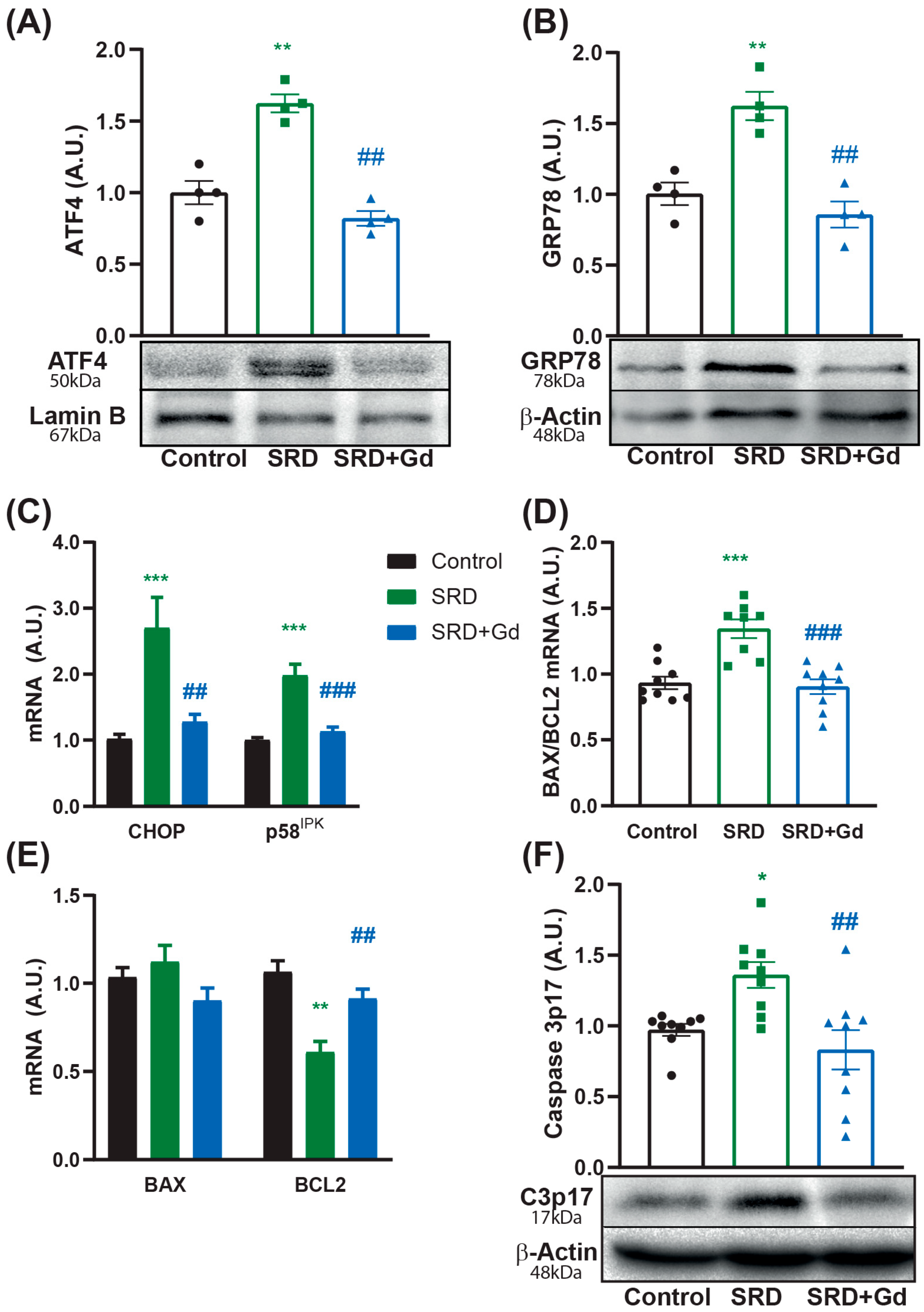
3.4. Kupffer Cell Depletion Is Associated with Reduced Cell Death
3.5. Liver and WAT Metabolic Effects Associated with Kupffer Cell Depletion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinberg, G.R.; Valvano, C.M.; De Nardo, W.; Watt, M.J. Integrative metabolism in MASLD and MASH: Pathophysiology and emerging mechanisms. J. Hepatol. 2025, 83, 584–595. [Google Scholar] [CrossRef]
- Park, S.J.; Diaz, J.G.; Um, E.; Hahn, Y.S. Major roles of kupffer cells and macrophages in NAFLD development. Front. Endocrinol. 2023, 14, 1150118. [Google Scholar] [CrossRef]
- Mundi, M.S.; Velapati, S.; Patel, J.; Kellogg, T.A.; Dayyeh, B.K.A.; Hurt, R.T. Evolution of NAFLD and Its Management. Nutr. Clin. Pract. 2020, 35, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef]
- Hagström, H.; Shang, Y.; Hegmar, H.; Nasr, P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol. Hepatol. 2024, 9, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Cho, Y.E.; Kim, S.-J.; Hwang, S. Immune-related pathogenesis and therapeutic strategies of nonalcoholic steatohepatitis. Arch. Pharm. Res. 2022, 45, 229–244. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Lara-Castor, L.; O’Hearn, M.; Cudhea, F.; Miller, V.; Shi, P.; Zhang, J.; Sharib, J.R.; Cash, S.B.; Barquera, S.; Micha, R.; et al. Burdens of Type 2 Diabetes and Cardiovascular Disease Attributable to Sugar-Sweetened Beverages in 184 Countries. Nat. Med. 2025, 31, 552–564. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef]
- Wiering, L.; Tacke, F. Treating inflammation to combat non-alcoholic fatty liver disease. J. Endocrinol. 2022, 256, e220194. [Google Scholar] [CrossRef]
- Castillo-Armengol, J.; Fajas, L.; Lopez-Mejia, I.C. Inter-organ communication: A gatekeeper for metabolic health. EMBO Rep. 2019, 20, e47903. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Leung, P.S. Fibroblast growth factor 21: A regulator of metabolic disease and health span. Am. J. Physiol.-Endocrinol. Metab. 2017, 313, E292–E302. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
- Metlakunta, A.; Huang, W.; Stefanovic-Racic, M.; Dedousis, N.; Sipula, I.; O’Doherty, R.M. Kupffer cells facilitate the acute effects of leptin on hepatic lipid metabolism. Am. J. Physiol.-Endocrinol. Metab. 2017, 312, E11–E18. [Google Scholar] [CrossRef]
- Diehl, K.L.; Vorac, J.; Hofmann, K.; Meiser, P.; Unterweger, I.; Kuerschner, L.; Weighardt, H.; Förster, I.; Thiele, C. Kupffer Cells Sense Free Fatty Acids and Regulate Hepatic Lipid Metabolism in High-Fat Diet and Inflammation. Cells 2020, 9, 2258. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Qian, S.; van Der Merwe, S.; Dhar, D.; Brenner, D.A.; Tacke, F. Immunopathogenic mechanisms and immunoregulatory therapies in MASLD. Cell Mol. Immunol. 2025, 1–19. [Google Scholar] [CrossRef]
- Wiszniewski, M.; Caldareri, L.; Mori, D.; Calejman, C.M.; Cymeryng, C.B.; Repetto, E.M. Exploring the impact of caloric restriction on molecular mechanisms of liver damage induced by sucrose intake in the drinking water. Br. J. Nutr. 2024, 132, 1562–1574. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Remmerie, A.; Martens, L.; Thoné, T.; Castoldi, A.; Seurinck, R.; Pavie, B.; Roles, J.; Vanneste, B.; De Prijck, S.; Vanhockerhout, M.; et al. Osteopontin Expression Identifies a Subset of Recruited Macrophages Distinct from Kupffer Cells in the Fatty Liver. Immunity 2020, 53, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Elchaninov, A.; Lokhonina, A.; Nikitina, M.; Vishnyakova, P.; Makarov, A.; Arutyunyan, I.; Poltavets, A.; Kananykhina, E.; Kovalchuk, S.; Karpulevich, E.; et al. Comparative Analysis of the Transcriptome, Proteome, and miRNA Profile of Kupffer Cells and Monocytes. Biomedicines 2020, 8, 627. [Google Scholar] [CrossRef]
- Bloomer, S.A.; Moyer, E.D.; Brown, K.E.; Kregel, K.C. Aging results in accumulation of M1 and M2 hepatic macrophages and a differential response to gadolinium chloride. Histochem. Cell Biol. 2020, 153, 37–48. [Google Scholar] [CrossRef]
- Pervin, M.; Karim, M.R.; Kuramochi, M.; Izawa, T.; Kuwamura, M.; Yamate, J. Macrophage Populations and Expression of Regulatory Inflammatory Factors in Hepatic Macrophage-depleted Rat Livers under Lipopolysaccharide (LPS) Treatment. Toxicol. Pathol. 2018, 46, 540–552. [Google Scholar] [CrossRef]
- Lackner, C. Hepatocellular ballooning in nonalcoholic steatohepatitis: The pathologist’s perspective. Expert. Rev. Gastroenterol. Hepatol. 2011, 5, 223–231. [Google Scholar] [CrossRef]
- Luci, C.; Bourinet, M.; Leclère, P.S.; Anty, R.; Gual, P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies. Front. Endocrinol. 2020, 11, 597648. [Google Scholar] [CrossRef]
- Alabdulaali, B.; Al-rashed, F.; Al-Onaizi, M.; Kandari, A.; Razafiarison, J.; Tonui, D.; Williams, M.R.; Blériot, C.; Ahmad, R.; Alzaid, F. Macrophages and the development and progression of non-alcoholic fatty liver disease. Front. Immunol. 2023, 14, 1195699. [Google Scholar] [CrossRef]
- Fan, J.; He, M.; Wang, C.J.; Zhang, M. Gadolinium Chloride Inhibits the Production of Liver Interleukin-27 and Mitigates Liver Injury in the CLP Mouse Model. Mediat. Inflamm. 2021, 2021, 2605973. [Google Scholar] [CrossRef]
- Zeng, T.; Liu, F.; Zhou, J.; Pan, S.; Xia, W.; Chen, L. Depletion of Kupffer cells attenuates systemic insulin resistance, inflammation and improves liver autophagy in high-fat diet fed mice. Endocr. J. 2015, 62, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Drożdż, K.; Nabrdalik, K.; Hajzler, W.; Kwiendacz, H.; Gumprecht, J.; Lip, G.Y.H. Metabolic-associated fatty liver disease (MAFLD), diabetes, and cardiovascular disease: Associations with fructose metabolism and gut microbiota. Nutrients 2022, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar] [CrossRef]
- Slevin, E.; Baiocchi, L.; Wu, N.; Ekser, B.; Sato, K.; Lin, E.; Ceci, L.; Chen, L.; Lorenzo, S.R.; Xu, W.; et al. Kupffer Cells. Am. J. Pathol. 2020, 190, 2185–2193. [Google Scholar] [CrossRef]
- Kawai, T.; Kayama, K.; Tatsumi, S.; Akter, S.; Miyawaki, N.; Okochi, Y.; Abe, M.; Sakimura, K.; Yamamoto, H.; Kihara, S.; et al. Regulation of hepatic oxidative stress by voltage-gated proton channels (Hv1/VSOP) in Kupffer cells and its potential relationship with glucose metabolism. FASEB J. 2020, 34, 15805–15821. [Google Scholar] [CrossRef]
- Rensen, S.S.; Slaats, Y.; Nijhuis, J.; Jans, A.; Bieghs, V.; Driessen, A.; Malle, E.; Greve, J.W.; Buurman, W.A. Increased Hepatic Myeloperoxidase Activity in Obese Subjects with Nonalcoholic Steatohepatitis. Am. J. Pathol. 2009, 175, 1473–1482. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Luís, A.; Gorman, A.M.; Samali, A.; Kaltenecker, D.; Moriggl, R.; Kozlov, A.V. Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine 2019, 124, 154577. [Google Scholar] [CrossRef]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.-R.; Chae, H.-J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Fernandes-da-Silva, A.; Miranda, C.S.; Santana-Oliveira, D.A.; Oliveira-Cordeiro, B.; Rangel-Azevedo, C.; Silva-Veiga, F.M.; Martins, F.F.; Souza-Mello, V. Endoplasmic reticulum stress as the basis of obesity and metabolic diseases: Focus on adipose tissue, liver, and pancreas. Eur. J. Nutr. 2021, 60, 2949–2960. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Paulmurugan, R.; Ramkumar, K.M. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014, 47, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Gong, X.; Zhang, M.; Kang, S.; Shu, B.; Wei, Z.; Huang, Z.-S.; Li, D. Upregulation of BCL-2 by acridone derivative through gene promoter i-motif for alleviating liver damage of NAFLD/NASH. Nucleic Acids Res. 2020, 48, 8255–8268. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J. Endocrinol. 2013, 218, R25–R36. [Google Scholar] [CrossRef]
- Dentin, R.; Liu, Y.; Koo, S.-H.; Hedrick, S.; Vargas, T.; Heredia, J.; Yates, J.; Montminy, M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 2007, 449, 366–369. [Google Scholar] [CrossRef]
- Lanthier, N.; Molendi-Coste, O.; Cani, P.D.; Rooijen, N.; Horsmans, Y.; Leclercq, I.A. Kupffer cell depletion prevents but has no therapeutic effect on metabolic and inflammatory changes induced by a high-fat diet. FASEB J. 2011, 25, 4301–4311. [Google Scholar] [CrossRef]
- Huang, W.; Metlakunta, A.; Dedousis, N.; Zhang, P.; Sipula, I.; Dube, J.J.; Scott, D.K.; O’Doherty, R.M. Depletion of Liver Kupffer Cells Prevents the Development of Diet-Induced Hepatic Steatosis and Insulin Resistance. Diabetes 2010, 59, 347–357. [Google Scholar] [CrossRef]
- Helsley, R.N.; Moreau, F.; Gupta, M.K.; Radulescu, A.; DeBosch, B.; Softic, S. Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr. Diab Rep. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Coronell-Tovar, A.; Pardo, J.P.; Rodríguez-Romero, A.; Sosa-Peinado, A.; Vásquez-Bochm, L.; Cano-Sánchez, P.; Álvarez-Añorve, L.I.; González-Andrade, M. Protein tyrosine phosphatase 1B (PTP1B) function, structure, and inhibition strategies to develop antidiabetic drugs. FEBS Lett. 2024, 598, 1811–1838. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, Á.; Valdecantos, M.P.; Rada, P.; Addante, A.; Barahona, I.; Rey, E.; Pardo, V.; Ruiz, L.; Laiglesia, L.M.; Moreno-Aliaga, M.J.; et al. Dual role of protein tyrosine phosphatase 1B in the progression and reversion of non-alcoholic steatohepatitis. Mol. Metab. 2018, 7, 132–146. [Google Scholar] [CrossRef]
- Szablewski, L. Changes in Cells Associated with Insulin Resistance. Int. J. Mol. Sci. 2024, 25, 2397. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Petrescu, M.; Vlaicu, S.I.; Ciumărnean, L.; Milaciu, M.V.; Mărginean, C.; Florea, M.; Vesa, Ș.C.; Popa, M. Chronic Inflammation—A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina 2022, 58, 641. [Google Scholar] [CrossRef]
- Alisi, A.; Carpino, G.; Oliveira, F.L.; Panera, N.; Nobili, V.; Gaudio, E. The Role of Tissue Macrophage-Mediated Inflammation on NAFLD Pathogenesis and Its Clinical Implications. Mediat. Inflamm. 2017, 2017, 8162421. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Cani, P.D.; Dewulf, E.M.; De Backer, F.; Bindels, L.B.; Delzenne, N.M. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochem. Biophys. Res. Commun. 2009, 385, 351–356. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, D.; He, F.; Tang, L. The Role of Kupffer Cells as Mediators of Adipose Tissue Lipolysis. J. Immunol. 2019, 203, 2689–2700. [Google Scholar] [CrossRef]
- Kaur, N.; Gare, S.R.; Shen, J.; Raja, R.; Fonseka, O.; Liu, W. Multi-organ FGF21-FGFR1 signaling in metabolic health and disease. Front. Cardiovasc. Med. 2022, 9, 962561. [Google Scholar] [CrossRef]
- De Ponti, F.F.; Liu, Z.; Scott, C.L. Understanding the complex macrophage landscape in MASLD. JHEP Rep. 2024, 6, 101196. [Google Scholar] [CrossRef]

| Target | Forward | Reverse | Accession Number |
|---|---|---|---|
| ACC1 | ATGAACACCCAGAGCATTGTCCAG | AGCAGATCCATCACCACAGCCTT | NM_022193.2 |
| ACOX1α | TCGAAGCCAGCGTTATGAGG | TTGAGGCCAACAGGTTCCAC | NM_001414015.1 |
| BAX | CCAGGACGCATCCACCAAGA | AGCTGCCACACGGAAGAAGA | NM_017059.2 |
| BCL2 | GGCTACGAGTGGGATACTGGAGAT | CTCTCAGGCTGGAAGGAGAAGATG | NM_016993.2 |
| CD36 | CACAGGCTTTCCTTCTTTGC | CTCTGACATTTGCAGGTCCA | NM_031561.2 |
| CD68 | GCAATTCACCTGGACCTGCT | AGAGAGATTGGTCACTGGCG | NM_001031638.1 |
| CHOP | CTCTGCCTTTCGCCTTTGA | GCTTTGGGAGGTGCTTGTG | NM_030186.1 |
| ChREBP | ATAGAGGAGCTCAATGCTGCCAT | AACATGTCCCGCATCTGGTC | NM_001393706.1 |
| CLEC4f | TGTCATCCTACAGCCCCAAG | GAGAGAGAAGAACACAGTCACC | NM_053753.1 |
| CPT1α | ACGAAGCCCTCAAACAGA | GGATGAAATCACACCCAC | NM_031559.2 |
| IL-10 | GTTGCCAAGCCTTGTCAGAAA | TTTCTGGGCCATGGTTCTCT | NM_012854.2 |
| IL-1β | CACCTCTCAAGCAGAAGCACAG | GGGTTCCATGGTGAAGTCAAC | NM_031512.2 |
| MRC1 | CAAGGAAGGTTGGCATTTGT | GGAACGTGTGCTCTGAGTT | NM_001106123.2 |
| NOS2 | CTTGGAGCGAGTTGTGGATTG | GGTGGGAGGGGTAGTGATGTC | NM_001429940 |
| P58IPK | ATTAAAGCATACCGAAAGTTAGCAC | AGAGGGTCTTCTCCGTCATCAAA | NM_022232.2 |
| PPARα | AAGAACCTGAGGAAGCCA | AGCCACAAAAAGGGAAATG | NM_013196.2 |
| PTP1B | ACACAGTACGGCAGTTGGAG | GACTCCAAAGTCAGGCCAGG | NM_012637.2 |
| TNFα | TGACCCCCATTACTCTGACC | GCAATCCAGGCCACTACTTC | NM_1278601 |
| β-Actin | CCACACCCGCCACCAGTTC | GACCCATTCCCACCATCACACC | NM_031144 |
| Control | SRD | SRD + Gd | |
|---|---|---|---|
| n | 12 | 12 | 12 |
| Initial weight (g) | 205 ± 5 | 210 ± 7 | 204 ± 4 |
| Final weight (g) | 523 ± 9 | 639 ± 15 *** | 612 ± 11 *** |
| Weight gain (g) | 319 ± 9 | 429 ± 14 *** | 411 ± 9 *** |
| Food consumption (g/rat/day) | 31 ± 2 | 20 ± 1 *** | 17 ± 1 *** |
|
Water/sucrose consumption
(mL/rat/day) | 68 ± 2 | 69 ± 3 | 67 ± 2 |
| Total kcal consumed | 7490 ± 360 | 10,691 ± 467 *** | 10,541 ± 419 *** |
| Control | SRD | SRD + Gd | |
|---|---|---|---|
| n | 8 | 8 | 8 |
| Ballooning | 0 (0–0) | 2 (1–2) *** | 1 (0–2) ***### |
| Steatosis | 0 (0–0) | 2 (1–3) *** | 2 (1–3) *** |
| Lobular infiltration | 1 (0–1) | 1 (0–1) | 0 (0–1) |
| Fibrosis | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| NAS | 1 (0–1) | 4 (5–3) *** | 3 (0–5) **## |
| Control | SRD | SRD + Gd | |
|---|---|---|---|
| n | 12 | 12 | 12 |
| kITT (mmol/L/min) | 6.50 ± 0.50 | 3.63 ± 0.17 *** | 5.77 ± 0.41 ## |
| Glycemia (mmol/L) | 6.77 ± 0.23 | 8.45 ± 0.23 *** | 7.41 ± 0.25 ## |
| Triacylglycerides (mmol/L) | 1.00 ± 0.08 | 2.76 ± 0.25 *** | 2.66 ± 0.18 *** |
| Total cholesterol (mmol/L) | 1.88 ± 0.08 | 1.98 ± 0.09 | 1.98 ± 0.06 |
| NEFA (mmol/L) | 0.32 ± 0.02 | 0.67 ± 0.06 *** | 0.47 ± 0.04 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiszniewski, M.; Mori, D.; Sanchez Puch, S.I.; Martinez Calejman, C.; Cymeryng, C.B.; Repetto, E.M. Divergent Hepatic and Adipose Tissue Effects of Kupffer Cell Depletion in a Male Rat Model of Metabolic-Associated Steatohepatitis. Biology 2025, 14, 1058. https://doi.org/10.3390/biology14081058
Wiszniewski M, Mori D, Sanchez Puch SI, Martinez Calejman C, Cymeryng CB, Repetto EM. Divergent Hepatic and Adipose Tissue Effects of Kupffer Cell Depletion in a Male Rat Model of Metabolic-Associated Steatohepatitis. Biology. 2025; 14(8):1058. https://doi.org/10.3390/biology14081058
Chicago/Turabian StyleWiszniewski, Morena, Diego Mori, Silvia I. Sanchez Puch, Camila Martinez Calejman, Cora B. Cymeryng, and Esteban M. Repetto. 2025. "Divergent Hepatic and Adipose Tissue Effects of Kupffer Cell Depletion in a Male Rat Model of Metabolic-Associated Steatohepatitis" Biology 14, no. 8: 1058. https://doi.org/10.3390/biology14081058
APA StyleWiszniewski, M., Mori, D., Sanchez Puch, S. I., Martinez Calejman, C., Cymeryng, C. B., & Repetto, E. M. (2025). Divergent Hepatic and Adipose Tissue Effects of Kupffer Cell Depletion in a Male Rat Model of Metabolic-Associated Steatohepatitis. Biology, 14(8), 1058. https://doi.org/10.3390/biology14081058









