Vertebrate Community Responses to Livestock Grazing in an Ancient Mediterranean Rangeland System: Rethinking the Role of Grazing in a Biodiversity Hotspot
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
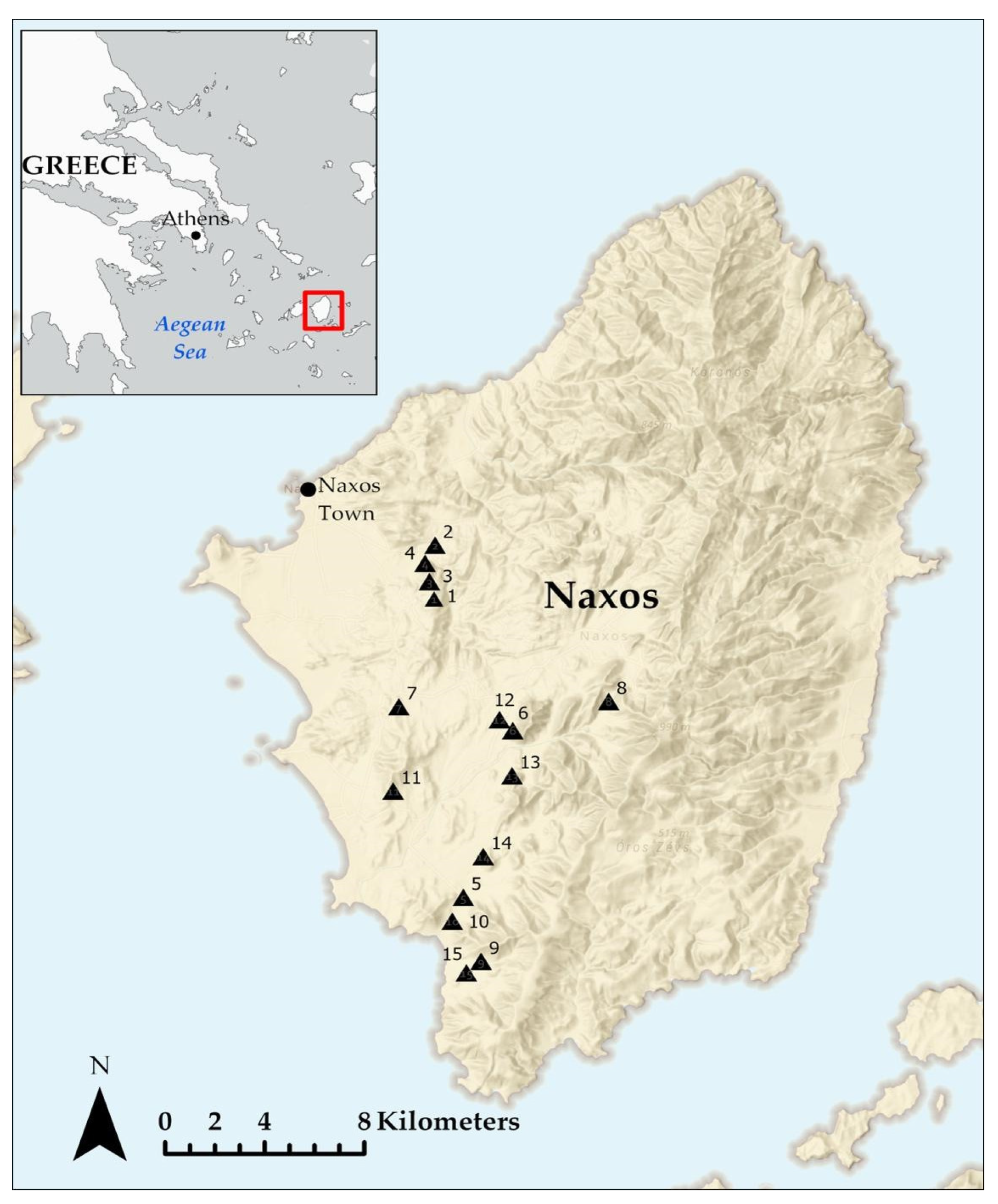
2.1. Study Area
2.2. Study Plots
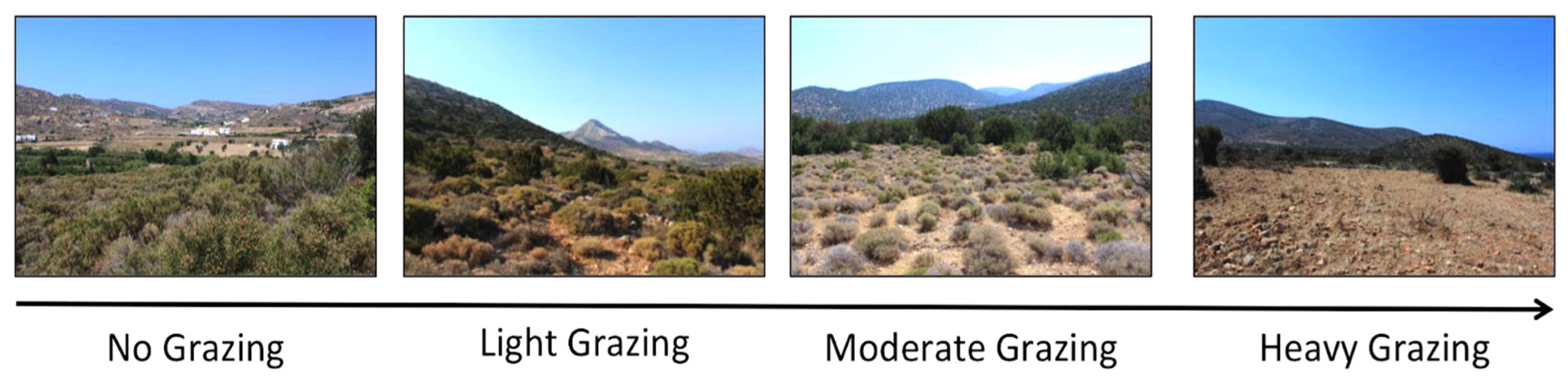
2.3. Quantification of Grazing Conditions
2.4. Vertebrate Population Measures
2.4.1. Terrestrial Vertebrates
2.4.2. Birds
2.5. Habitat Measurements
2.5.1. Vegetation Biomass (Kg/m2)
2.5.2. Vegetation Height (cm)
2.5.3. Shrub Cover
2.6. Invertebrate Population Measurements
2.7. Statistical Analyses
2.7.1. Livestock Grazing-Environment Interactions
2.7.2. Relationships Between Livestock Grazing and Vertebrate Populations
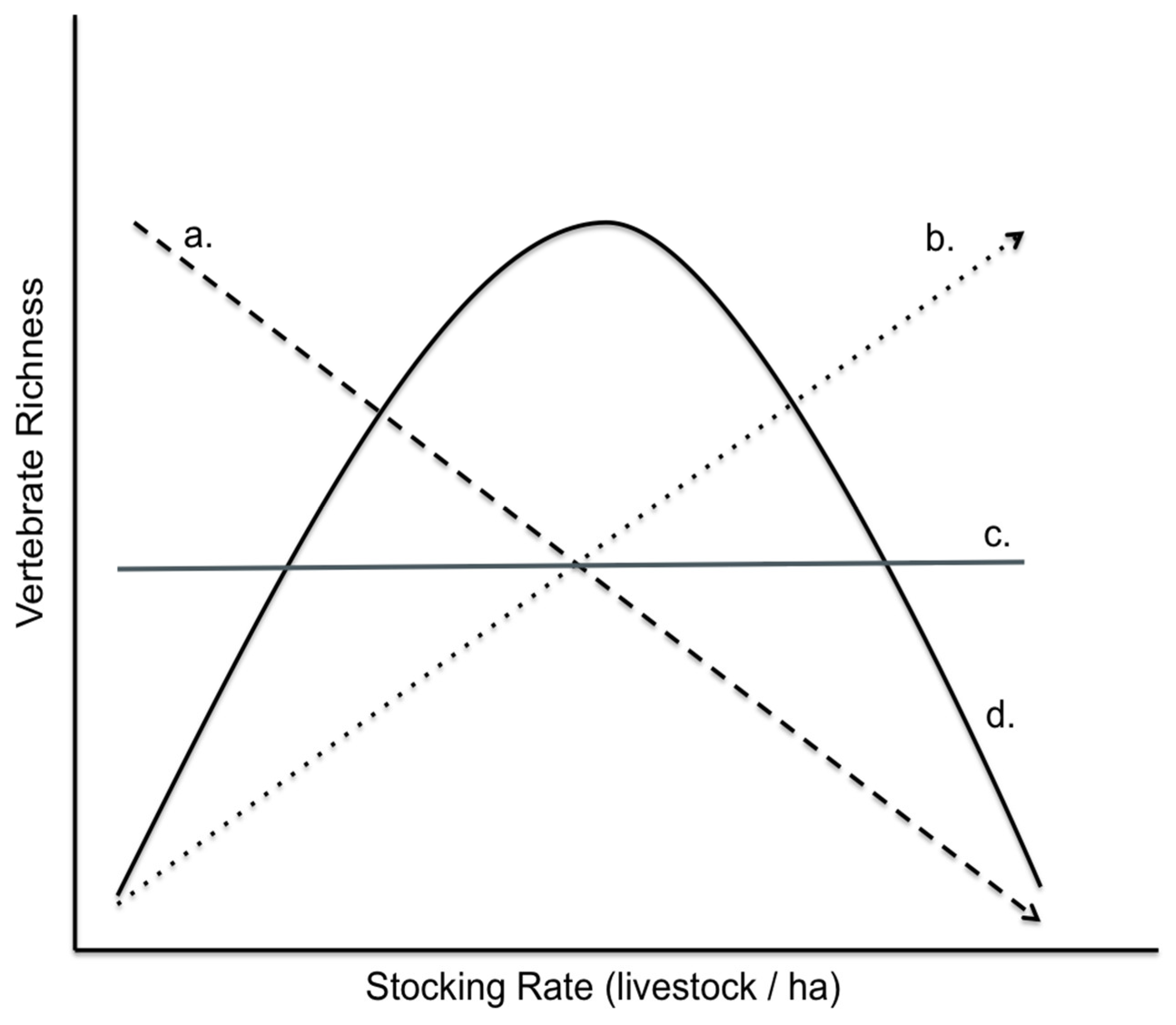
2.7.3. Mechanism
3. Results
3.1. Effects of Livestock Grazing on Vegetation Traits and Invertebrate Numbers
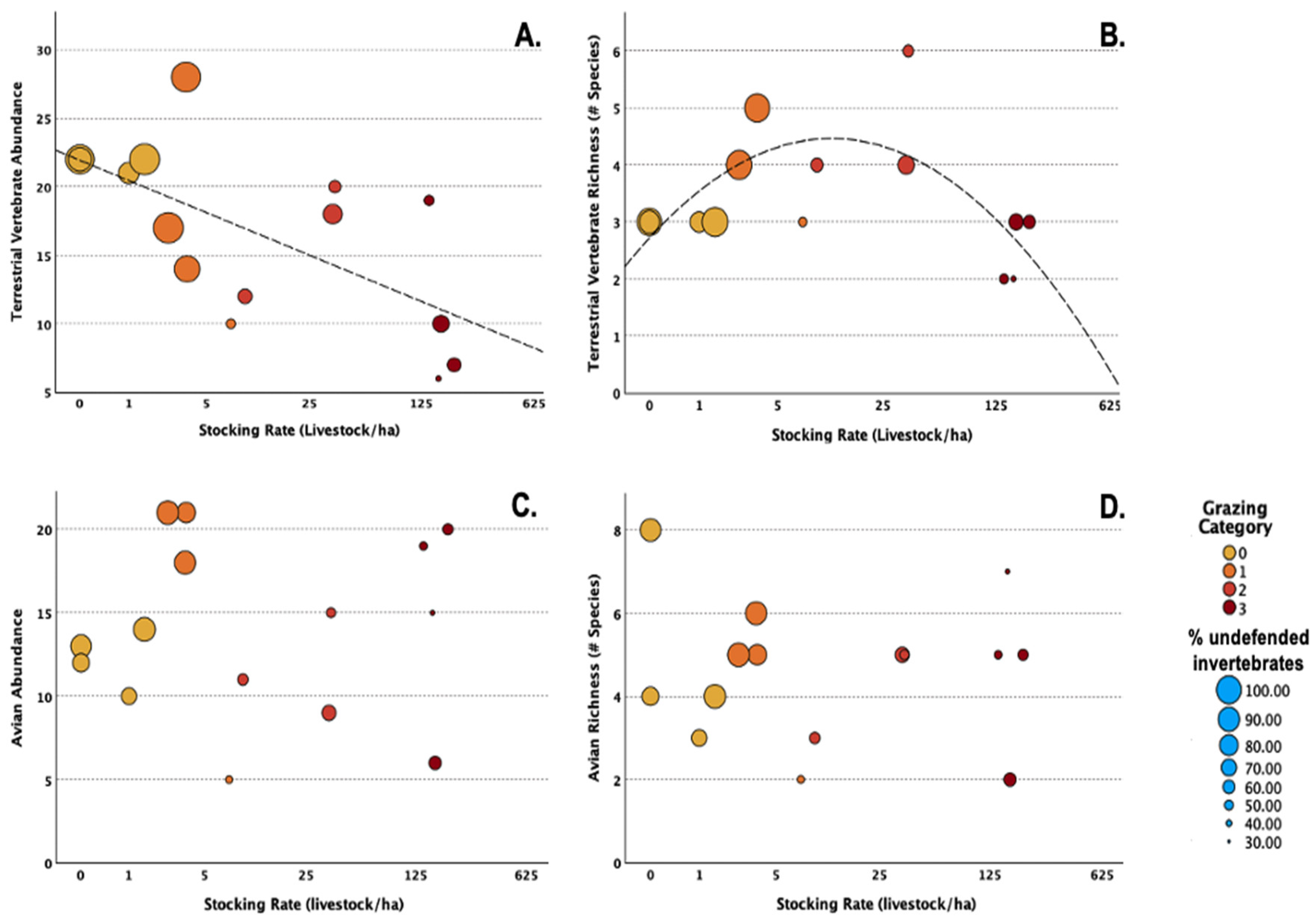
3.2. Vertebrate Populations Across Stocking Regimes and Proximate Drivers of Terrestrial Vertebrate and Avian Abundance and Species Richness
3.2.1. Terrestrial Vertebrates
3.2.2. Avian Species
4. Discussion
4.1. Response of Vertebrate Assemblages to Grazing
4.1.1. Terrestrial Vertebrates
4.1.2. Avian Species
5. Conclusions
Conservation Implications
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Site No. | Latitude | Longitude | Elevation (m) | Aspect | Primary Vegetation | Secondary Vegetation | Stocking Rate* | Livestock Dung Biomass (g/m2) |
|---|---|---|---|---|---|---|---|---|
| 1 | N37°04.463′ | E025°25.348′ | 179.53 | N | Genista/Calicotome/Cistus Phrygana | Kermes Oak | 0 | 0.00 |
| 2 | N37°04.758′ | E025°25.378′ | 66.49 | E | Cistus/Calicotome Phrygana | Pistacea | 0 | 0.00 |
| 3 | N37°04.682′ | E025°25.256′ | 150.87 | S | Cistus/Calicotome Phrygana | Kermes Oak | 1 | 0.33 |
| 4 | N37°05.023′ | E025°25.161′ | 54.86 | W | Genista Phrygana | Pistacea | 1.5 | 0.00 |
| 5 | N36°58.819′ | E025°25.999’ | 32.87 | S | Coridothymus Phrygana | Juniper | 2.5 | 3.11 |
| 6 | N37°02.026’ | E025°26.963’ | 197.39 | W | Coridothymus/Genista Phrygana | Kermes Oak/Juniper | 3.5 | 1.33 |
| 7 | N37°02.360′ | E025°24.593′ | 170.38 | E | Cistus/Calicotome Phrygana | Pistacea | 3.57 | 1.00 |
| 8 | N37°02.457′ | E025°29.137′ | 326.25 | E | Coridothymus/Genista Phrygana | Kermes Oak | 7.5 | 2.94 |
| 9 | N36°57.625′ | E025°26.374′ | 54.86 | S | Coridothymus Phrygana | Juniper | 9.38 | 5.83 |
| 10 | N36.58.375′ | E025.25.751′ | 55.42 | W | Coridothymus Phrygana | Juniper | 35 | 6.22 |
| 11 | N37°00.794′ | E025°24.470′ | 140.20 | E | Coridothymus/Genista Phrygana | Juniper | 36.1 | 2.39 |
| 12 | N37°02.067′ | E025°26.875′ | 206.65 | W | Coridothymus Phrygana | Juniper | 140 | 14.67 |
| 13 | N37°01.079′ | E025°27.044′ | 206.95 | N | Coridothymus/Genista Phrygana | None | 160 | 16.72 |
| 14 | N36°59.578′ | E025°26.421′ | 124.05 | W | Coridothymus Phrygana | Juniper | 166 | 59.33 |
| 15 | N36°57.554′ | E025°25.949′ | 61.63 | N | Sparse Coridothymus | None | 200 | 39.50 |

| Site No. | Vegetation Biomass (kg/m2) | Shrub Cover (%) | FHD | Plant Species Richness |
|---|---|---|---|---|
| 1 | 4.85 | 100 | 1.16 | 14 |
| 2 | 4.42 | 100 | 0.88 | 13 |
| 3 | 1.96 | 98 | 0.81 | 12 |
| 4 | 2.17 | 100 | 0.84 | 10 |
| 5 | 0.71 | 71 | 0.89 | 8 |
| 6 | 1.53 | 78 | 1.27 | 12 |
| 7 | 4.86 | 100 | 1.06 | 8 |
| 8 | 1.48 | 88 | 0.88 | 10 |
| 9 | 0.77 | 95 | 0.59 | 4 |
| 10 | 0.68 | 71 | 0.91 | 6 |
| 11 | 1.55 | 68 | 1.15 | 8 |
| 12 | 0.61 | 76 | 0.73 | 7 |
| 13 | 0.44 | 60 | 1.16 | 5 |
| 14 | 1.53 | 76 | 0.76 | 4 |
| 15 | 0.60 | 12 | 1.07 | 2 |
| Site No. | Invertebrate Biomass (g) | Invertebrate Abundance | Invertebrate Richness | Undefended Invertebrates (%) |
|---|---|---|---|---|
| 1 | 0.42 | 292 | 7 | 91.13 |
| 2 | 0.46 | 265 | 9 | 78.57 |
| 3 | 0.36 | 169 | 6 | 73.26 |
| 4 | 0.17 | 291 | 6 | 94.88 |
| 5 | 0.44 | 395 | 10 | 94.49 |
| 6 | 0.42 | 344 | 9 | 92.77 |
| 7 | 1.3 | 667 | 10 | 84.6 |
| 8 | 3.27 | 735 | 10 | 46.4 |
| 9 | 0.42 | 270 | 7 | 57.56 |
| 10 | 0.57 | 212 | 9 | 69.12 |
| 11 | 0.38 | 87 | 5 | 52.75 |
| 12 | 0.64 | 387 | 7 | 47.95 |
| 13 | 2.26 | 1376 | 8 | 37.17 |
| 14 | 1.29 | 612 | 10 | 63.17 |
| 15 | 1.42 | 401 | 8 | 56.47 |
| Latin Name | Common Name | Foraging Guild |
|---|---|---|
| Curruca melanocephala | Sardinian warbler | Insectivore |
| Parus major | Great tit | Insectivore/Granivore |
| Carduelis carduelis | Goldfinch | Granivore/Omnivore |
| Galerida cristata | Crested lark | Granivore/Omnivore |
| Lanius senator | Woodchat shrike | Insectivore |
| Linaria cannabina | Linnet | Granivore/Insectivore |
| Emberiza calandra | Corn bunting | Granivore/Omnivore |
| Passer domesticus | House sparrow | Granivore |
| Emberiza cirlus | Cirl bunting | Granivore/Omnivore |
| Saxicola torquata | Stonechat | Insectivore |
| Chloris chloris | Greenfinch | Granivore/Omnivore |
| Emberiza melanocephala | Black-headed bunting | Granivore/Omnivore |
| Crocidura suaveolens | Lesser white-toothed shrew | Insectivore |
| Sorex minutus | Eurasian pygmy shrew | Insectivore |
| Lacerta trilineata | Balkan green lizard | Insectivore |
| Eryx jaculus turcicus | Javelin sand boa | Carnivore/Insectivore |
| Podarcis erhardii | Aegean wall lizard | Insectivore |
| Mediodactylus kotschyi | Kotschy’s gecko | Insectivore |
| Hemidactylus turcicus | Mediterranean house gecko | Insectivore |
| Ablepharus kitaibelii | European snake-eyed skink | Insectivore |
| Vipera ammodytes meridionalis | Long-nosed viper | Carnivore/Insectivore |
| Terrestrial Species | Avian Species | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site No. | StR | R | A | Pe | Mk | Cs | Ak | Lt | Ejt | Vam | Sm | Ht | R | A | Cm | Gc | Cch | Pd | Cca | Em | Eci | St | Eca | Ls | Lc | Pm |
| 1 | 0 | 3 | 22 | x | x | x | 8 | 13 | x | x | x | x | x | x | x | x | ||||||||||
| 2 | 0 | 3 | 22 | x | x | x | 4 | 12 | x | x | x | x | ||||||||||||||
| 3 | 1 | 3 | 21 | x | x | x | 3 | 10 | x | x | x | |||||||||||||||
| 4 | 1.5 | 3 | 22 | x | x | x | 4 | 14 | x | x | x | x | ||||||||||||||
| 5 | 2.5 | 4 | 17 | x | x | x | x | 5 | 21 | x | x | x | x | x | ||||||||||||
| 6 | 3.5 | 5 | 28 | x | x | x | x | x | 6 | 18 | x | x | x | x | x | x | ||||||||||
| 7 | 3.57 | 5 | 14 | x | x | x | x | x | 5 | 21 | x | x | x | x | x | |||||||||||
| 8 | 7.5 | 3 | 10 | x | x | x | 2 | 5 | x | x | ||||||||||||||||
| 9 | 9.38 | 4 | 12 | x | x | x | x | 3 | 11 | x | x | x | ||||||||||||||
| 10 | 35 | 4 | 18 | x | x | x | x | 5 | 9 | x | x | x | x | x | ||||||||||||
| 11 | 36.1 | 6 | 20 | x | x | x | x | x | x | 5 | 15 | x | x | x | x | x | ||||||||||
| 12 | 140 | 2 | 19 | x | x | 5 | 19 | x | x | x | x | x | ||||||||||||||
| 13 | 160 | 2 | 6 | x | x | 7 | 15 | x | x | x | x | x | x | x | ||||||||||||
| 14 | 166 | 3 | 10 | x | x | x | 2 | 6 | x | x | ||||||||||||||||
| 15 | 200 | 3 | 7 | x | x | x | 5 | 20 | x | x | x | x | x | |||||||||||||
References
- Ghosh, K.; Braun, G. FAO Transforming Food and Agriculture to Achieve the SDGs; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Fetzel, T.; Petridis, P.; Noll, D.; Singh, S.J.; Fischer-Kowalski, M. Reaching a Socio-Ecological Tipping Point: Overgrazing on the Greek Island of Samothraki and the Role of European Agricultural Policies. Land Use Policy 2018, 76, 21–28. [Google Scholar] [CrossRef]
- Niu, W.; Ding, J.; Fu, B.; Zhao, W.; Eldridge, D. Global Effects of Livestock Grazing on Ecosystem Functions Vary with Grazing Management and Environment. Agric. Ecosyst. Environ. 2025, 378, 109296. [Google Scholar] [CrossRef]
- Ilea, R.C. Intensive Livestock Farming: Global Trends, Increased Environmental Concerns, and Ethical Solutions. J. Agric. Environ. Ethics 2009, 22, 153–167. [Google Scholar] [CrossRef]
- Pandey, H.O.; Upadhyay, D. Global Livestock Production Systems: Classification, Status, and Future Trends. In Emerging Issues in Climate Smart Livestock Production; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–70. ISBN 978-0-12-822265-2. [Google Scholar]
- Jongen, M.; Noll, D.; Maskalidis, G.; Domingos, T.; Fischer-Kowalski, M. Changing Pasture Management Practices on the Greek Island of Samothraki: Obstacles and Opportunities. Agric. Syst. 2024, 218, 103992. [Google Scholar] [CrossRef]
- Errea, P.; Lasanta, T.; Zabalza-Martínez, J.; Cortijos-López, M.; Nadal-Romero, E. Rethinking Extensive Livestock Grazing to Revive Mediterranean Mountain Landscapes. J. Environ. Manag. 2025, 391, 126541. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Engle, D.M. Restoring Heterogeneity on Rangelands: Ecosystem Management Based on Evolutionary Grazing Patterns. BioScience 2001, 51, 625–632. [Google Scholar] [CrossRef]
- Gao, J.; Carmel, Y. A Global Meta-Analysis of Grazing Effects on Plant Richness. Agric. Ecosyst. Environ. 2020, 302, 107072. [Google Scholar] [CrossRef]
- Grime, J.P. Control of Species Density in Herbaceous Vegetation. J. Environ. Manag. 1973, 1, 151–167. [Google Scholar]
- Huston, M. A General Hypothesis of Species Diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Shea, K.; Roxburgh, S.H.; Rauschert, E.S.J. Moving from Pattern to Process: Coexistence Mechanisms under Intermediate Disturbance Regimes. Ecol. Lett. 2004, 7, 491–508. [Google Scholar] [CrossRef]
- Fox, J.W. The Intermediate Disturbance Hypothesis Should Be Abandoned. Trends Ecol. Evol. 2013, 28, 86–92. [Google Scholar] [CrossRef]
- Randall Hughes, A.; Byrnes, J.E.; Kimbro, D.L.; Stachowicz, J.J. Reciprocal Relationships and Potential Feedbacks between Biodiversity and Disturbance. Ecol. Lett. 2007, 10, 849–864. [Google Scholar] [CrossRef]
- Mackey, R.L.; Currie, D.J. The Diversity–Disturbance Relationship: Is It Generally Strong and Peaked? Ecology 2001, 82, 3479–3492. [Google Scholar] [CrossRef]
- Rivera-Núñez, T.; Ford, A.; Barrera-Bassols, N.; Casas, A.; Fargher-Navarro, L.; Nigh, R. A Biocultural Hypothesis of Human–Environment Mediations and Biodiversity Increase. Environ. Conserv. 2025, 52, 64–70. [Google Scholar] [CrossRef]
- Pierce, S. Implications for Biodiversity Conservation of the Lack of Consensus Regarding the Humped-Back Model of Species Richness and Biomass Production. Funct. Ecol. 2014, 28, 253–257. [Google Scholar] [CrossRef]
- Oikonomou, D.; Vrahnakis, M.; Yiakoulaki, M.; Xanthopoulos, G.; Kazoglou, Y. Grazing as a Management Tool in Mediterranean Pastures: A Meta-Analysis Based on A Literature Review. Land 2023, 12, 1290. [Google Scholar] [CrossRef]
- van Wieren, S.E.; Bakker, J.P. The Impact of Browsing and Grazing Herbivores on Biodiversity. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Berlin, Heidelberg, 2008; pp. 263–292. ISBN 978-3-540-72422-3. [Google Scholar]
- Guan, H.; Zhang, S.; Huangpu, Y.; Yan, H.; Niklas, K.J.; Mipam, T.D.; Sun, S. Moderate Grazing Promotes Arthropod Species Diversity in an Alpine Meadow. Biology 2023, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Troiano, C.; Buglione, M.; Petrelli, S.; Belardinelli, S.; De Natale, A.; Svenning, J.-C.; Fulgione, D. Traditional Free-Ranging Livestock Farming as a Management Strategy for Biological and Cultural Landscape Diversity: A Case from the Southern Apennines. Land 2021, 10, 957. [Google Scholar] [CrossRef]
- Barzan, F.R.; Bellis, L.M.; Dardanelli, S. Livestock Grazing Constrains Bird Abundance and Species Richness: A Global Meta-Analysis. Basic Appl. Ecol. 2021, 56, 289–298. [Google Scholar] [CrossRef]
- Pafilis, P.; Anastasiou, I.; Sagonas, K.; Valakos, E.D. Grazing by Goats on Islands Affects the Populations of an Endemic Mediterranean Lizard. J. Zool. 2013, 290, 255–264. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Ash, A.J. Responses of Vertebrates to Pastoralism, Military Land Use and Landscape Position in an Australian Tropical Savanna. Austral Ecol. 2002, 27, 311–323. [Google Scholar] [CrossRef]
- Torre, I.; Díaz, M.; Martínez-Padilla, J.; Bonal, R.; Viñuela, J.; Fargallo, J.A. Cattle Grazing, Raptor Abundance and Small Mammal Communities in Mediterranean Grasslands. Basic Appl. Ecol. 2007, 8, 565–575. [Google Scholar] [CrossRef]
- Bock, C.E.; Bock, J.H.; Kenney, W.R.; Hawthorne, V.M. Responses of Birds, Rodents, and Vegetation to Livestock Exclosure in a Semidesert Grassland Site. J. Range Manag. 1984, 37, 239. [Google Scholar] [CrossRef]
- Dorrough, J.; Mcintyre, S.; Brown, G.; Stol, J.; Barrett, G.; Brown, A. Differential Responses of Plants, Reptiles and Birds to Grazing Management, Fertilizer and Tree Clearing. Austral Ecol. 2012, 37, 569–582. [Google Scholar] [CrossRef]
- Schieltz, J.M.; Rubenstein, D.I. Evidence Based Review: Positive versus Negative Effects of Livestock Grazing on Wildlife. What Do We Really Know? Environ. Res. Lett. 2016, 11, 113003. [Google Scholar] [CrossRef]
- Roxburgh, S.H.; Shea, K.; Wilson, J.B. The Intermediate Disturbance Hypothesis: Patch Dynamics and Mechanisms of Species Coexistence. Ecology 2004, 85, 359–371. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Abdul Malak, D.; Temple, H.; Katriya, V. The Mediterranean: A Biodiversity Hotspot under Threat. In The 2008 Review of The IUCN Red List of Threatened Species; Vié, J.-C., Hilton-Taylor, C., Stuart, S.N., Eds.; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2008; pp. 716–717. [Google Scholar]
- Lymberakis, P.; Pafilis, P.; Poulakakis, N.; Sotiropoulos, K.; Valakos, E. The Amphibians and Reptiles of the Aegean Sea. In Biogeography and Biodiversity of the Aegean: In Honour of Prof. Moysis Milonas; Sfenthourakis, S., Pafilis, P., Parmakelis, A., Poulakakis, N., Triantis, K.A., Eds.; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2018; ISBN 978-9925-563-78-4. [Google Scholar]
- Sarika, M.; Bazos, I.; Zervou, S.; Christopoulou, A. Flora and Vegetation of the European-Network “Natura 2000” Habitats of Naxos Island (GR4220014) and of Nearby Islets Mikres Kyklades (GR4220013), Central Aegean (Greece). Plant Sociol. 2015, 52, 3–56. [Google Scholar] [CrossRef]
- Hadjigeorgiou, I. Past, Present and Future of Pastoralism in Greece. Pastor. Res. Policy Pract. 2011, 1, 24. [Google Scholar] [CrossRef]
- Perevolotsky, A.; Seligman, N.G. Role of Grazing in Mediterranean Rangeland Ecosystems. BioScience 1998, 48, 1007–1017. [Google Scholar] [CrossRef]
- Sternberg, M.; Gutman, M.; Perevolotsky, A.; Ungar, E.D.; Kigel, J. Vegetation Response to Grazing Management in a Mediterranean Herbaceous Community: A Functional Group Approach. J. Appl. Ecol. 2000, 37, 224–237. [Google Scholar] [CrossRef]
- Alrababah, M.A.; Alhamad, M.A.; Suwaileh, A.; Al-Gharaibeh, M. Biodiversity of Semi-arid Mediterranean Grasslands: Impact of Grazing and Afforestation. Appl. Veg. Sci. 2007, 10, 257–264. [Google Scholar] [CrossRef]
- Lisiecki, C.; Foufopoulos, J. Profits vs. Preservation: How Can Shepherds Balance the Social and Ecological Costs of Livestock Grazing on Naxos? World Dev. Perspect. 2022, 26, 100430. [Google Scholar] [CrossRef]
- Papachristou, T.G.; Dziba, L.E.; Provenza, F.D. Foraging Ecology of Goats and Sheep on Wooded Rangelands. Small Rumin. Res. 2005, 59, 141–156. [Google Scholar] [CrossRef]
- Rogosic, J.; Pfister, J.A.; Provenza, F.D.; Grbesa, D. Sheep and Goat Preference for and Nutritional Value of Mediterranean Maquis Shrubs. Small Rumin. Res. 2006, 64, 169–179. [Google Scholar] [CrossRef]
- Delattre, L.; Debolini, M.; Paoli, J.C.; Napoleone, C.; Moulery, M.; Leonelli, L.; Santucci, P. Understanding the Relationships between Extensive Livestock Systems, Land-Cover Changes, and CAP Support in Less-Favored Mediterranean Areas. Land 2020, 9, 518. [Google Scholar] [CrossRef]
- Caraveli, H. A Comparative Analysis on Intensification and Extensification in Mediterranean Agriculture: Dilemmas for LFAs Policy. J. Rural. Stud. 2000, 16, 231–242. [Google Scholar] [CrossRef]
- Lopes, P.C.; Palmeirim, J.M.; Leal, A.I. Small Shrubby Patches Increase Bird Taxonomic and Functional Richness of Wood-Pastures. Agrofor. Syst. 2023, 97, 1511–1523. [Google Scholar] [CrossRef]
- Torre, I.; Jaime-González, C.; Díaz, M. Habitat Suitability for Small Mammals in Mediterranean Landscapes: How and Why Shrubs Matter. Sustainability 2022, 14, 1562. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal Species Diversity Driven by Habitat Heterogeneity/Diversity: The Importance of Keystone Structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Leal, A.I.; Acácio, M.; Meyer, C.F.J.; Rainho, A.; Palmeirim, J.M. Grazing Improves Habitat Suitability for Many Ground Foraging Birds in Mediterranean Wooded Grasslands. Agric. Ecosyst. Environ. 2019, 270–271, 1–8. [Google Scholar] [CrossRef]
- Nastos, P.T.; Evelpidou, N.; Vassilopoulos, A. Brief Communication: “Does Climatic Change in Precipitation Drive Erosion in Naxos Island, Greece?”. Nat. Hazards Earth Syst. Sci. 2010, 10, 379–382. [Google Scholar] [CrossRef]
- Tselepidakis, I.G.; Theoharatos, G.A. A Bioclimatic Classification of the Greek Area. Theor. Appl. Climatol. 1989, 40, 147–153. [Google Scholar] [CrossRef]
- Grove, A.T. The Nature of Mediterranean Europe: An Ecological History; Yale University Press: New Haven, CT, USA; London, UK, 2003; ISBN 978-0-300-10055-6. [Google Scholar]
- Böhling, N.B. Studien zur Landschaftsökologischen Raumgliederung auf der Mediterranen Insel Naxos (Griechenland): Unter Besonderer Berücksichtigung von Zeigerpflanzen; J. Cramer in der Gebrüder Bornträger Verlagsbuchhandlung: Stuttgart, Germany, 1994. [Google Scholar]
- Zervas, G. Quantifying and Optimizing Grazing Regimes in Greek Mountain Systems. J. Appl. Ecol. 1998, 35, 983–986. [Google Scholar] [CrossRef]
- Jasmer, G.E.; Holechek, J.L. Determining Grazing Intensity on Rangeland. J. Soil Water Conserv. 1984, 39, 32–35. [Google Scholar] [CrossRef]
- Enge, K.M. A Standardized Protocol for Drift-Fence Surveys; Florida Game and Fresh Water Fish Commission: Tallahassee, FL, USA, 1997. [Google Scholar]
- Hutto, R.L.; Pletschet, S.M.; Hendricks, P. A Fixed-Radius Point Count Method for Nonbreeding and Breeding Season Use. Auk 1986, 103, 593–602. [Google Scholar] [CrossRef]
- Gutiérrez, J.R.; Meserve, P.L. Density and Biomass Responses of Ephemeral Plants to Experimental Exclusions of Small Mammals and Their Vertebrate Predators in the Chilean Semiarid Zone. J. Arid. Environ. 2000, 45, 173–181. [Google Scholar] [CrossRef]
- Riginos, C.; Herrick, J.; Belnap, J.; Sundaresan, S.; Worden, J.; Kinnaird, M. Monitoring Rangeland Health—A Guide for Facilitators and Pastoralist Communities. Vol. Draft. Version I 2009, 1, 1–63. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Malden, MA, USA, 2004; ISBN 978-0-632-05633-0. [Google Scholar]
- Herrick, J.E.; Zee, J.W.V.; McCord, S.E.; Courtright, E.M.; Karl, J.W.; Burkett, L.M. Monitoring Manual for Grassland, Shrubland, and Savanna Ecosystems; Herrick, J.E., Jornada Experimental Range, Eds.; USDA-ARS Jordana Experimental Range; The University of Arizona Press: Las Cruces, NM, USA; Tucson, AZ, USA, 2005; Volume 2, ISBN 978-0-9755552-0-0. [Google Scholar]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized Linear and Generalized Additive Models in Studies of Species Distributions: Setting the Scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Aho, K.; Derryberry, D.; Peterson, T. Model Selection for Ecologists: The Worldviews of AIC and BIC. Ecology 2014, 95, 631–636. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Bock, C.E.; Smith, H.M.; Bock, J.H. The Effect of Livestock Grazing upon Abundance of the Lizard, Sceloporus Scalaris, in Southeastern Arizona. J. Herpetol. 1990, 24, 445–446. [Google Scholar] [CrossRef]
- Cheng, W.-C. Impact of Livestock Grazing on Ecosystem Services in a Mediterranean Ecosystem. Master’s Thesis, University of Michigan, Ann Arbor, MI, USA, 2015. [Google Scholar]
- Milchunas, D.G.; Lauenroth, W.K.; Burke, I.C. Livestock Grazing: Animal and Plant Biodiversity of Shortgrass Steppe and the Relationship to Ecosystem Function. Oikos 1998, 83, 65–74. [Google Scholar] [CrossRef]
- Cagnolo, L.; Molina, S.I.; Valladares, G.R. Diversity and Guild Structure of Insect Assemblages under Grazing and Exclusion Regimes in a Montane Grassland from Central Argentina. Biodivers. Conserv. 2002, 11, 407–420. [Google Scholar] [CrossRef]
- Reynolds, T.D.; Trost, C.H. The Response of Native Vertebrate Populations to Crested Wheatgrass Planting and Grazing by Sheep. Rangel. Ecol. Manag. /J. Range Manag. Arch. 1980, 33, 122–125. [Google Scholar] [CrossRef]
- Miller, A.D.; Roxburgh, S.H.; Shea, K. How Frequency and Intensity Shape Diversity–Disturbance Relationships. Proc. Natl. Acad. Sci. USA 2011, 108, 5643–5648. [Google Scholar] [CrossRef]
- Adler, P.; Raff, D.; Lauenroth, W. The Effect of Grazing on the Spatial Heterogeneity of Vegetation. Oecologia 2001, 128, 465–479. [Google Scholar] [CrossRef]
- Gizicki, Z.S.; Tamez, V.; Galanopoulou, A.P.; Avramidis, P.; Foufopoulos, J. Long-Term Effects of Feral Goats (Capra hircus) on Mediterranean Island Communities: Results from Whole Island Manipulations. Biol. Invasions 2018, 20, 1537–1552. [Google Scholar] [CrossRef]
- Coblentz, B.E. The Effects of Feral Goats (Capra hircus) on Island Ecosystems. Biol. Conserv. 1978, 13, 279–286. [Google Scholar] [CrossRef]
- Barnagaud, J.; Geniez, P.; Cheylan, M.; Crochet, P. Climate Overrides the Effects of Land Use on the Functional Composition and Diversity of Mediterranean Reptile Assemblages. Divers. Distrib. 2021, 27, 50–64. [Google Scholar] [CrossRef]
- Rickert, C.; Fichtner, A.; van Klink, R.; Bakker, J.P. α- and β-Diversity in Moth Communities in Salt Marshes Is Driven by Grazing Management. Biol. Conserv. 2012, 146, 24–31. [Google Scholar] [CrossRef]
| Mechanism | Model | Reference |
|---|---|---|
| Food Availability | Vertebrate Richness/Abundance~Vegetation Biomass | [26] |
| Food Availability | Vertebrate Richness/Abundance~Invertebrate Biomass | [20] |
| Shrub Cover | Vertebrate Richness/Abundance~Shrub Cover | [43,44] |
| Vegetation Structural Heterogeneity | Vertebrate Richness/Abundance~FHD | [45,46] |
| Response Variable | Spearman’s Rank Coefficient (ρ) | Significance (p-Value) |
|---|---|---|
| Vegetation measures: | ||
| Vegetation biomass | −0.699 | 0.004 * |
| Shrub cover | −0.790 | <0.001 * |
| Foliage height diversity (FHD) | −0.51 | 0.857 |
| Plant species richness | −0.890 | <0.001 * |
| Invertebrate measures: | ||
| Invertebrate biomass | 0.629 | 0.016 * |
| Invertebrate richness | 0.325 | 0.237 |
| Invertebrate abundance | 0.396 | 0.144 |
| % undefended invertebrates | −0.711 | 0.003 * |
| Log Likelihood | D | AICc | ∆AICc | wi | Evidence Ratio | |
|---|---|---|---|---|---|---|
| A1. Terrestrial Vertebrate Abundance | ||||||
| TVA~stocking_rate | −45.880 | 23.227 | 96.759 | 0.000 | 0.810 | 1.000 |
| TVA~stocking_rate + stocking_rate2 | −45.749 | 22.966 | 99.680 | 2.921 | 0.188 | 4.308 |
| TVA~intercept | −53.017 | 37.502 | 108.342 | 11.583 | 0.002 | 327.504 |
| A2. Terrestrial Vertebrate Richness | ||||||
| TVR~stocking_rate + stocking_rate2 | −18.028 | 9.717 | 48.055 | 0.000 | 0.647 | 1.000 |
| TVR~intercept | −22.540 | 17.733 | 50.079 | 2.024 | 0.235 | 2.751 |
| TVR~stocking_rate | −21.635 | 15.719 | 51.452 | 3.397 | 0.118 | 5.466 |
| B1. Avian Vertebrate Abundance | ||||||
| AA~intercept | −47.570 | 29.035 | 97.448 | 0.000 | 0.725 | 1.000 |
| AA~stocking_rate | −47.536 | 28.967 | 100.073 | 2.625 | 0.195 | 3.715 |
| AA~stocking_rate + stocking_rate2 | −46.835 | 27.564 | 101.853 | 4.405 | 0.080 | 9.048 |
| B2. Avian Vertebrate Richness | ||||||
| AR ~intercept | −28.565 | 39.600 | 62.130 | 0.000 | 0.805 | 1.000 |
| AR~stocking_rate | −28.560 | 39.572 | 65.301 | 3.171 | 0.165 | 4.882 |
| AR~stocking_rate + stocking_rate2 | −28.372 | 38.594 | 68.744 | 6.614 | 0.030 | 27.303 |
| Log Likelihood | D | AICc | ∆AICc | wi | Evidence Ratio | |
|---|---|---|---|---|---|---|
| A1. Terrestrial Vertebrate Abundance | ||||||
| TVA~invertebrate biomass | −37.376 | 10.378 | 79.843 | 0.000 | 1.000 | 1.000 |
| TVA~shrub cover | −48.291 | 28.049 | 101.581 | 21.738 | 0.000 | 52,522.661 |
| TVA~vegetation biomass | −50.797 | 33.063 | 106.595 | 26.752 | 0.000 | 644,351.717 |
| TVA~intercept | −53.017 | 37.502 | 108.342 | 28.499 | 0.000 | 154,3402.573 |
| TVA~FHD | −52.495 | 36.459 | 109.991 | 30.148 | 0.000 | 3,520,100.154 |
| A2. Terrestrial Vertebrate Richness | ||||||
| TVR~invertebrate biomass | −20.616 | 15.585 | 49.631 | 0.000 | 0.387 | 1.000 |
| TVR~intercept | −22.540 | 17.733 | 50.079 | 0.448 | 0.309 | 1.251 |
| TVR~FHD | −21.576 | 15.586 | 51.325 | 1.694 | 0.166 | 2.333 |
| TVR~vegetation biomass | −22.385 | 17.371 | 52.951 | 3.320 | 0.073 | 5.259 |
| TVR~shrub cover | −22.504 | 17.649 | 53.189 | 3.558 | 0.065 | 5.924 |
| B1. Avian Abundance | ||||||
| AA~FHD | −45.384 | 24.662 | 95.768 | 0.000 | 0.430 | 1.000 |
| AA~shrub cover | −46.190 | 26.274 | 97.380 | 1.612 | 0.192 | 2.239 |
| AA~intercept | −47.570 | 29.035 | 97.448 | 1.680 | 0.186 | 2.316 |
| AA~invertebrate biomass | −46.485 | 26.865 | 97.971 | 2.203 | 0.143 | 3.009 |
| AA~vegetation biomass | −47.562 | 29.018 | 100.124 | 4.356 | 0.049 | 8.829 |
| B2. Avian Richness | ||||||
| AR~FHD | −22.880 | 18.556 | 53.941 | 0.000 | 0.971 | 1.000 |
| AR~intercept | −28.565 | 39.600 | 62.130 | 8.189 | 0.016 | 60.009 |
| AR~invertebrate biomass | −28.274 | 38.095 | 64.730 | 10.789 | 0.004 | 220.192 |
| AR~vegetation biomass | −28.276 | 38.103 | 64.734 | 10.793 | 0.004 | 220.633 |
| AR~shrub cover | −28.300 | 38.228 | 64.783 | 10.842 | 0.004 | 226.105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Victor, E.; Brenton, S.; Pafilis, P.; Foufopoulos, J. Vertebrate Community Responses to Livestock Grazing in an Ancient Mediterranean Rangeland System: Rethinking the Role of Grazing in a Biodiversity Hotspot. Biology 2025, 14, 1057. https://doi.org/10.3390/biology14081057
Victor E, Brenton S, Pafilis P, Foufopoulos J. Vertebrate Community Responses to Livestock Grazing in an Ancient Mediterranean Rangeland System: Rethinking the Role of Grazing in a Biodiversity Hotspot. Biology. 2025; 14(8):1057. https://doi.org/10.3390/biology14081057
Chicago/Turabian StyleVictor, Erin, Scott Brenton, Panayiotis Pafilis, and Johannes Foufopoulos. 2025. "Vertebrate Community Responses to Livestock Grazing in an Ancient Mediterranean Rangeland System: Rethinking the Role of Grazing in a Biodiversity Hotspot" Biology 14, no. 8: 1057. https://doi.org/10.3390/biology14081057
APA StyleVictor, E., Brenton, S., Pafilis, P., & Foufopoulos, J. (2025). Vertebrate Community Responses to Livestock Grazing in an Ancient Mediterranean Rangeland System: Rethinking the Role of Grazing in a Biodiversity Hotspot. Biology, 14(8), 1057. https://doi.org/10.3390/biology14081057








