Responses of Fish Zeta Diversity (ζ) to Human Pressure and Cumulative Effects: A Feasibility Study of Fishing Ban Measures in the Pearl River Basin, China
Simple Summary
Abstract
1. Introduction
2. Methods
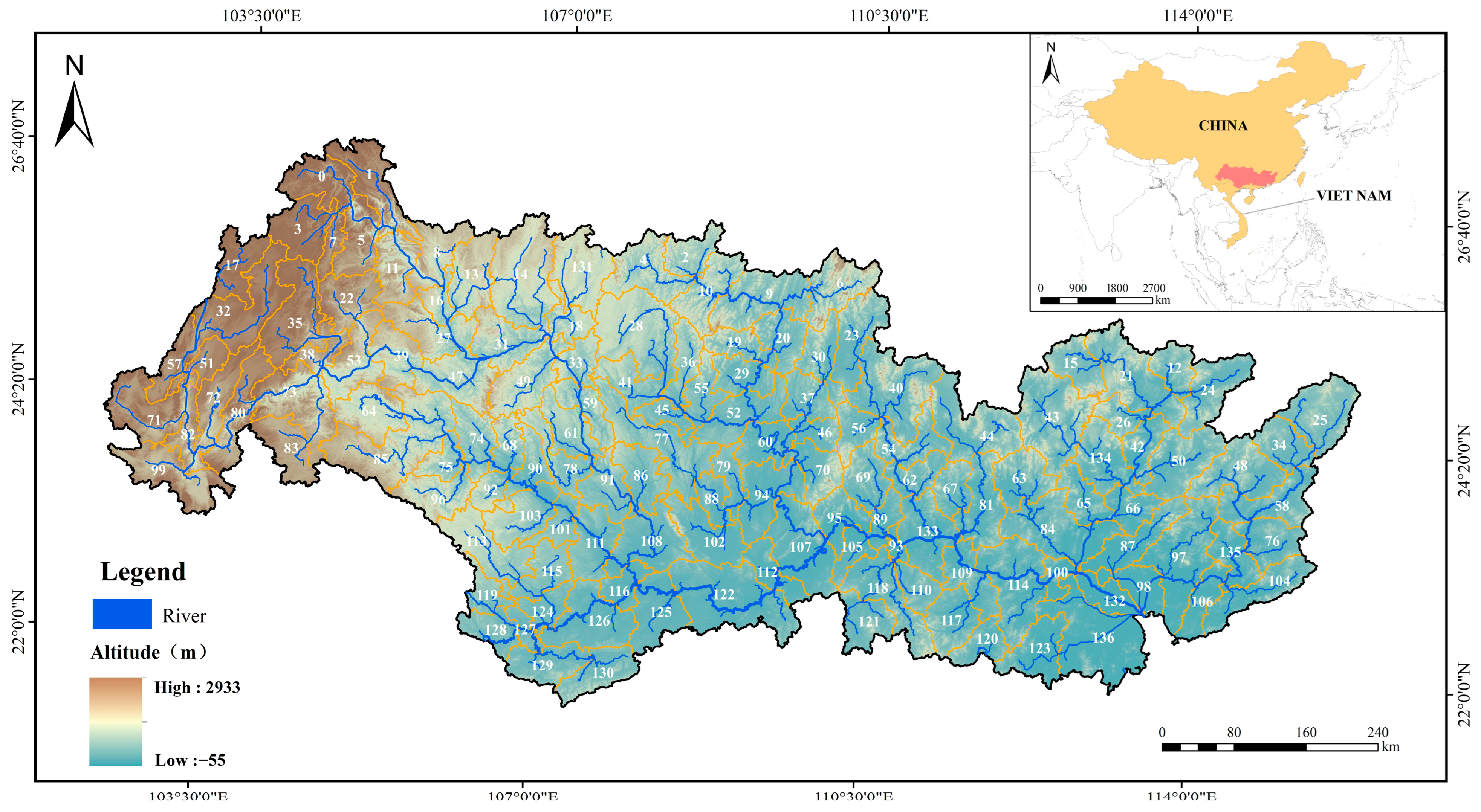
2.1. Study Area
2.2. Fish Occurrence Records
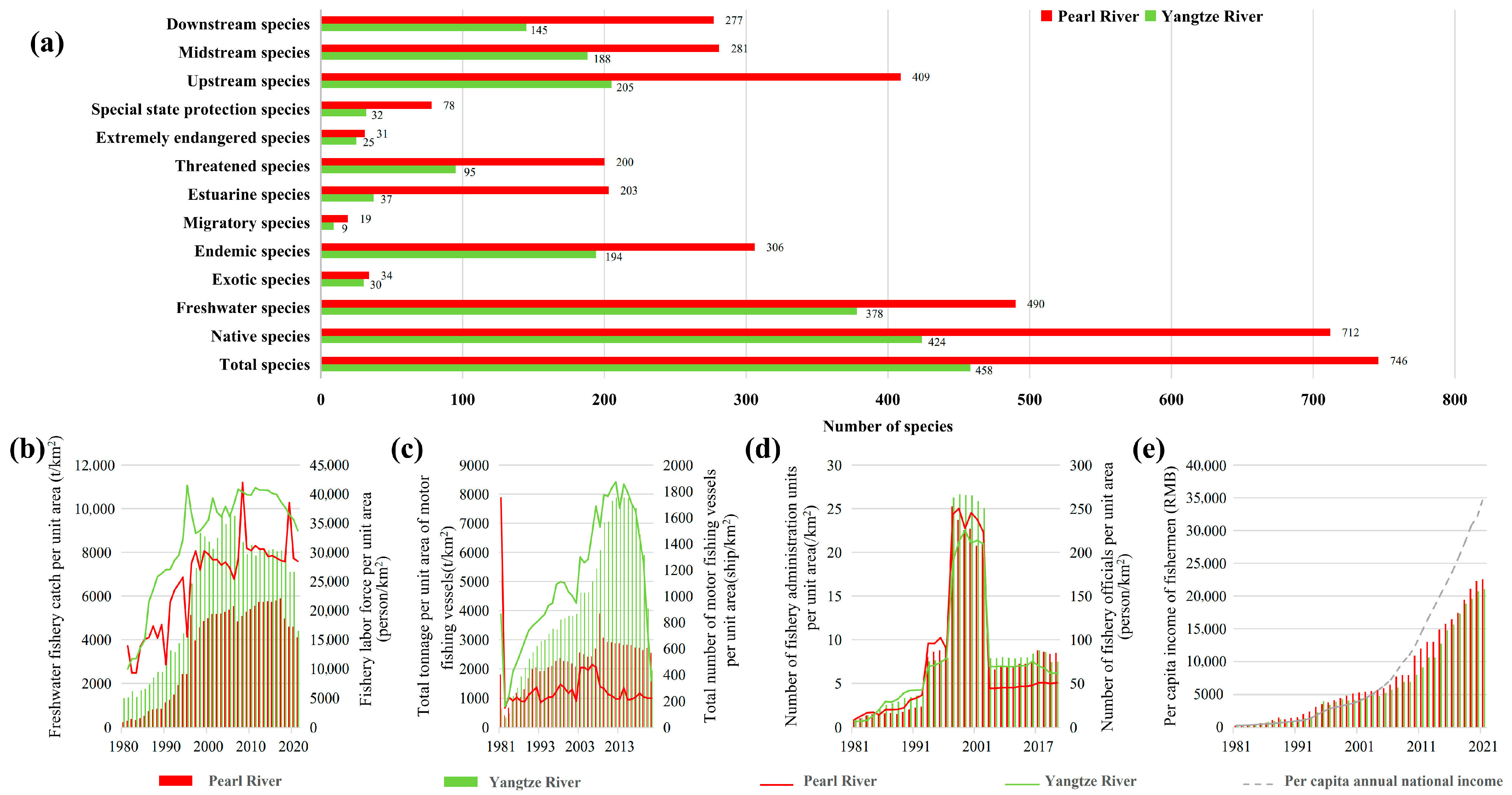
2.3. Variable Collection and Processing
2.4. Sub-Basin Level Analyses
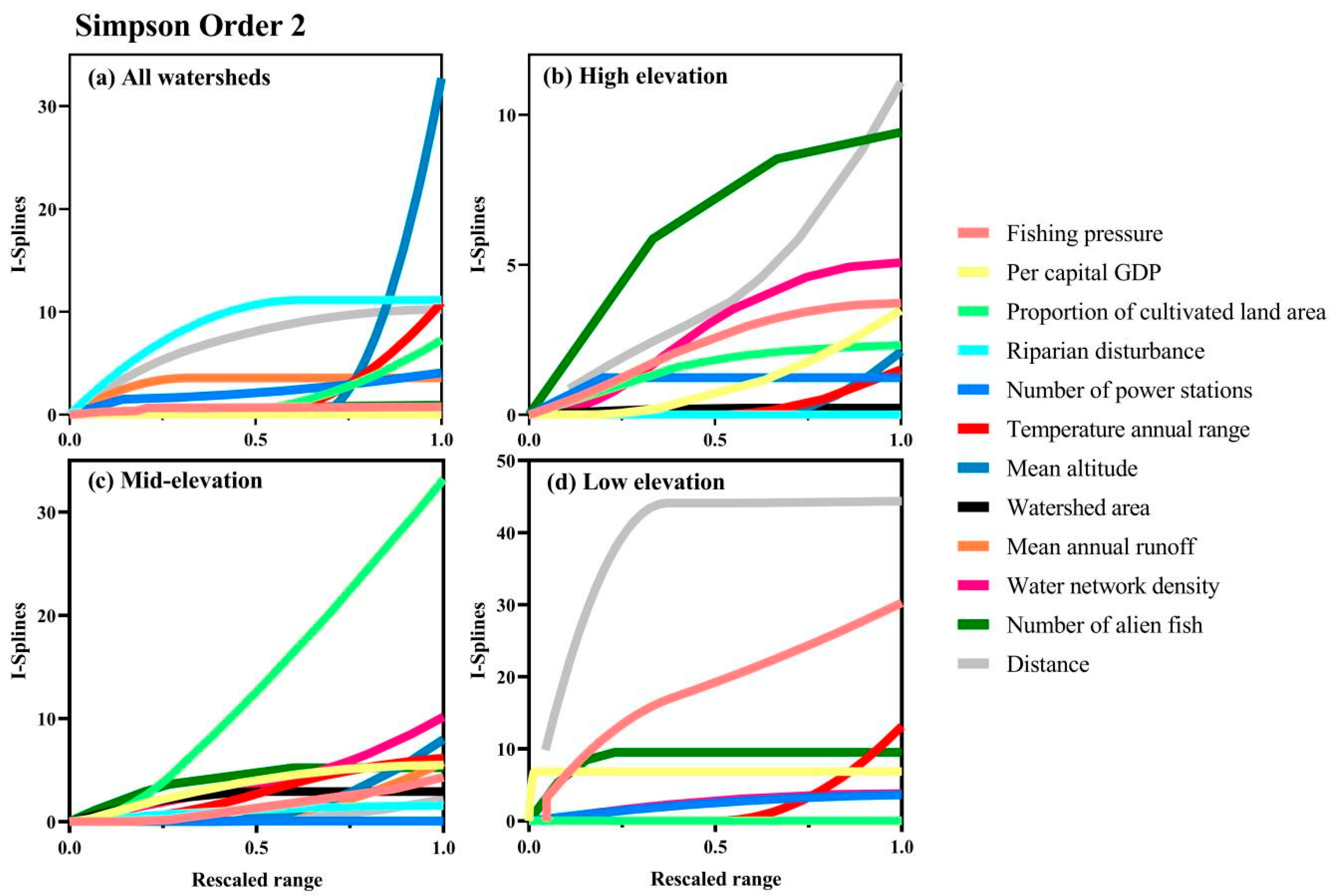
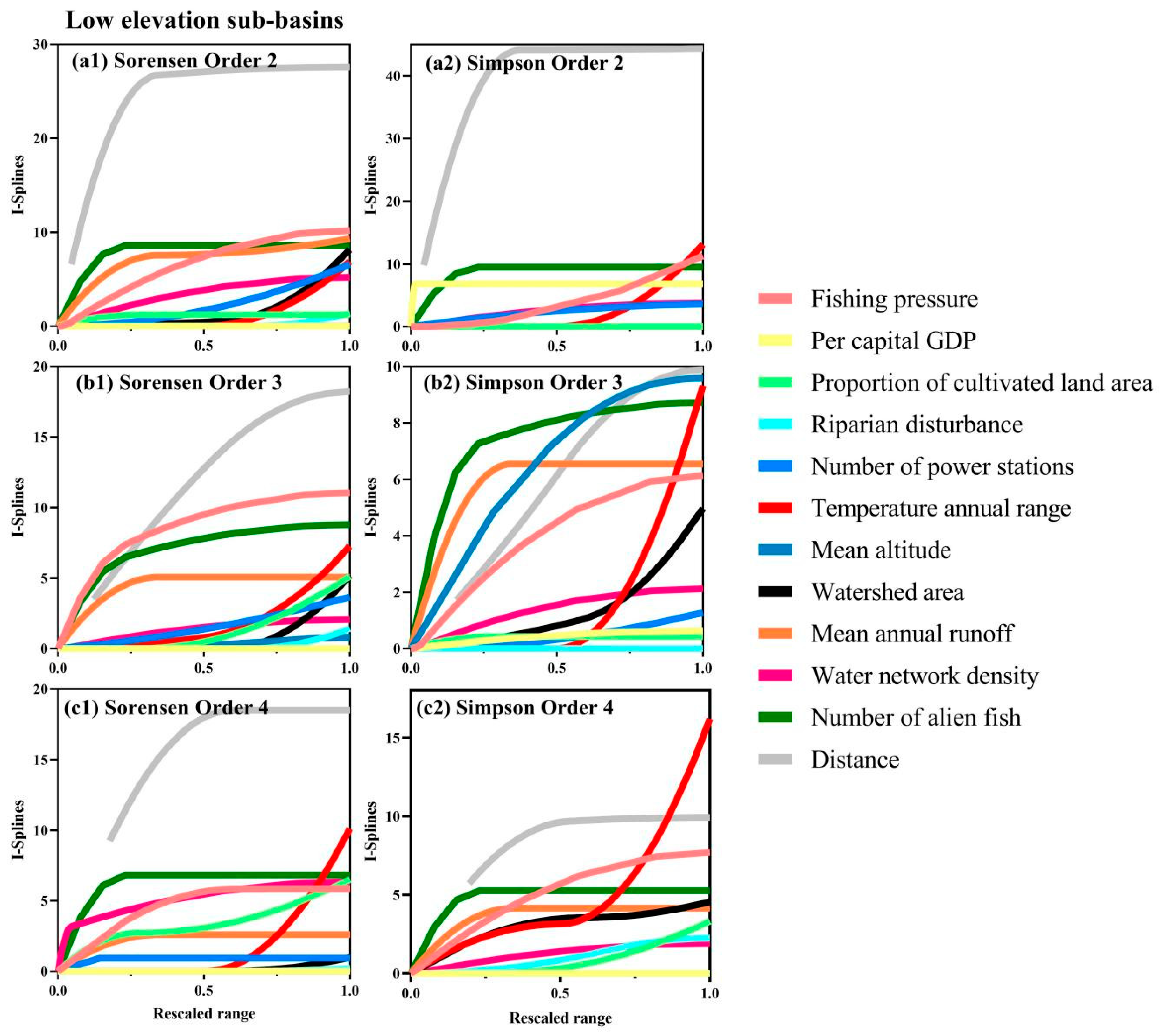
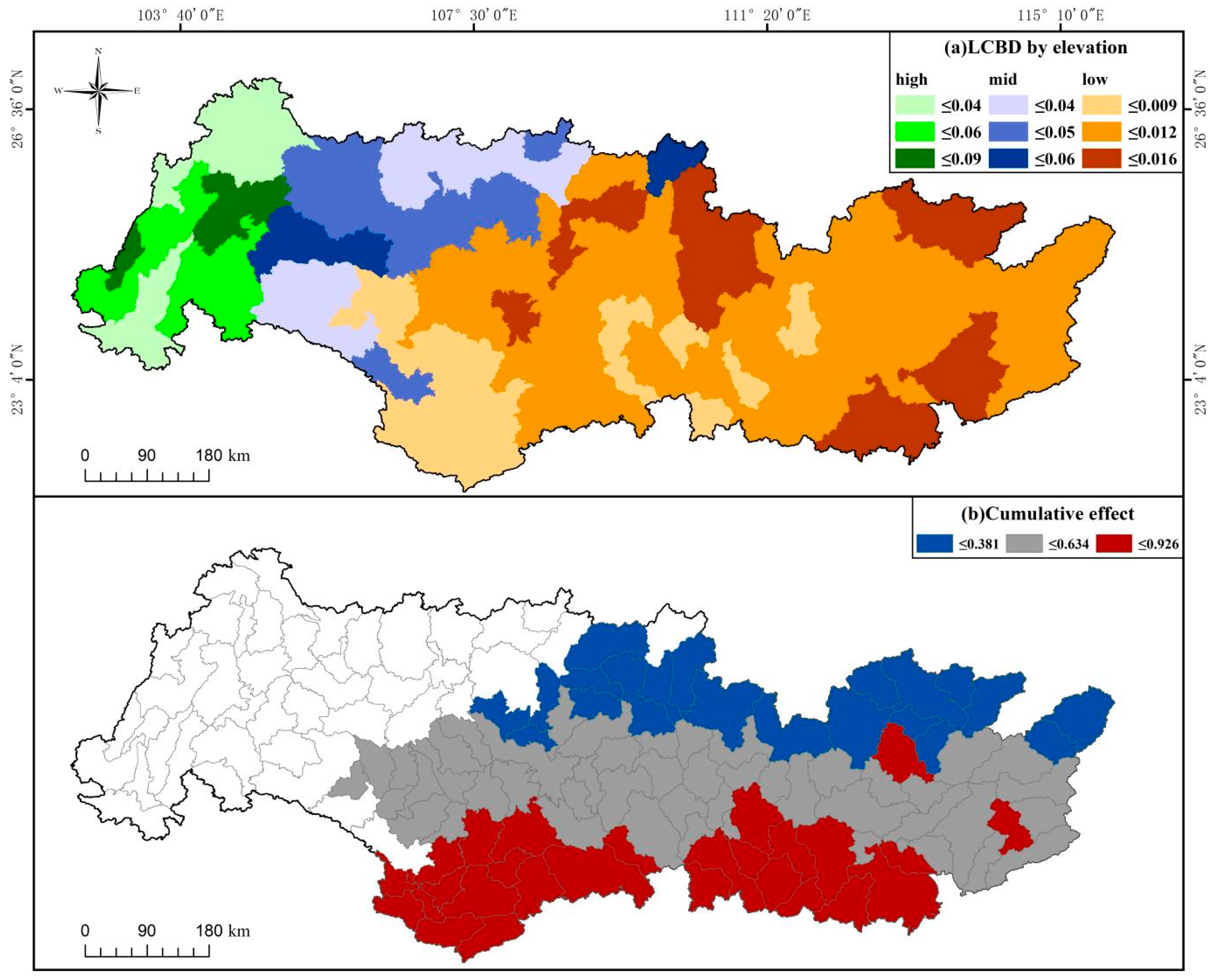
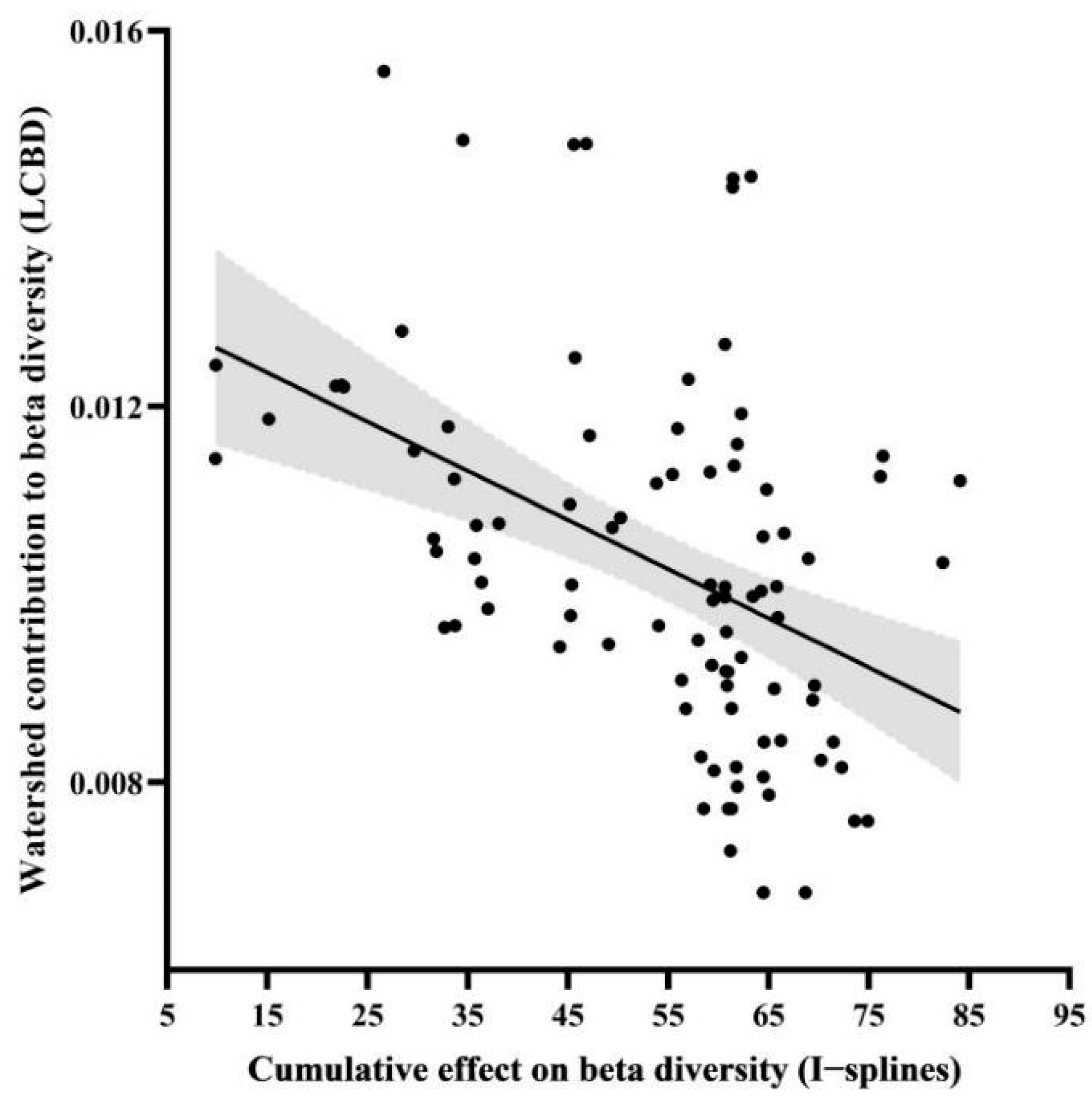
3. Results
4. Discussion
- (i)
- Effective restoration of aquatic life and fisheries may be achieved through the strategic adjustment of human-induced pressures and stressors, in conjunction with stringent fishery regulations [14]. This approach necessitates the establishment of an extensive monitoring framework for the Pearl River ecosystem, the enhancement of current fishing restrictions in both duration and coverage, rigorous enforcement of said regulations, and the initiation of large-scale ecological rehabilitation initiatives. Such measures are crucial for the revival of the Pearl River Basin, home to China’s richest freshwater fish diversity and fisheries. This model is also pertinent to developing regions in Africa, Southeast Asia, and South America, where similar patterns of economic advancement and population growth are accompanied by the ecological deterioration of aquatic habitats (Chen et al., 2020) [14].
- (ii)
- To promote the recovery and replenishment of aquatic species, a 10-year moratorium on fishing is proposed to be implemented in the waters of Guangdong–Hong Kong–Macao (GH-MO) Greater Bay Area (Pearl River Delta) in view of the high cumulative effects of human pressures (e.g., fishing pressure) and species rarity in the low-elevation Pearl River Delta. This conservation strategy, which is inextricably linked to the National Development Agenda, aims to raise the ecological quality and governance of the Guangdong–Hong Kong–Macao Greater Bay Area to global standards and implement the most stringent environmental protection protocols.
- (iii)
- A compensatory framework is suggested for the fishing prohibition in the Guangdong–Hong Kong–Macao Greater Bay Area, which would steer the Pearl River Basin Delta’s fishing community toward alternative livelihoods, expediting their shift from fishing to other forms of production. This transition should be supported by comprehensive policies addressing employment and social security for the fishermen transitioning out of the industry.
- (iv)
- Special consideration is warranted for the conservation of aquatic ecosystems, including groundwater, within the karst regions of the Pearl River Basin. Intensified monitoring and protection of freshwater fish biodiversity in these areas are imperative. A substantial body of fundamental research is required to ascertain the endangered status and categorize the risk levels of fish species inhabiting these karst environments.
- (v)
- By implementing successful fisheries regulation and mitigating anthropogenic impacts, China can enhance freshwater management while leading by example for developing nations. Future management should integrate advanced technologies, including remote sensing, environmental DNA (eDNA) monitoring, and real-time water quality sensors, for dynamic, adaptive management. Satellite-based habitat monitoring combined with machine learning algorithms can provide early warning systems for biodiversity loss. Establishing international collaborative platforms for sharing monitoring data, management protocols, and technological innovations will enhance the global freshwater biodiversity conservation capacity, representing next-generation conservation strategies that move beyond static protection toward responsive ecosystem management.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ban, N.C.; Gurney, G.G.; Marshall, N.A.; Whitney, C.K.; Mills, M.; Gelcich, S.; Bennett, N.J.; Meehan, M.C.; Butler, C.; Ban, S.; et al. Well-being outcomes of marine protected areas. Nat. Sustain. 2019, 2, 524–532. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Atkinson, P.W.; Butchart, S.H.M.; Capaja, M.; Dicks, L.V.; Fleishman, E.; Gaston, K.J.; Hails, R.S.; Hughes, A.C.; Le Anstey, B.; et al. A horizon scan of global biological conservation issues for 2022. Trends. Ecol. Evol. 2022, 37, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.J.; Elliott, V.; Phang, S.C.; Claussen, J.E.; Harrison, I.; Murchie, K.J.; Steel, E.A.; Stokes, G.L. Inland fish and fisheries integral to achieving the Sustainable Development Goals. Nat. Sustain. 2020, 3, 579–587. [Google Scholar] [CrossRef]
- Maasri, A.; Jahnig, S.C.; Adamescu, M.C. A global agenda for advancing freshwater biodiversity research. Ecol. Lett. 2022, 25, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Ding, C.Z.; Chen, J.N.; Ding, L.Y.; Brosse, S.; Heino, J.; Hermoso, V.; Wu, R.D.; Wang, Z.W.; Hu, J.X.; et al. Boosting freshwater fish conservation with high-resolution distribution mapping across a large territory. Conserv. Biol. 2023, 37, e14036. [Google Scholar] [CrossRef]
- Chen, Y.S. Construction: Limit China’s sand mining. Nature 2017, 550, 457. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Troell, M. Fishing for prawn larvae in Bangladesh: An important coastal livelihood causing negative effects on the environment. Ambio 2010, 39, 20–29. [Google Scholar] [CrossRef]
- Campos-Silva, J.V.; Hawes, J.E.; Andrade, P.C.; Peres, C.A. Unintended multispecies co-benefits of an Amazonian community-based conservation programme. Nat. Sustain. 2018, 1, 650–656. [Google Scholar] [CrossRef]
- Amali Infantina, J.; Priya, P.; Deepak Samuel, V.K.; Abhilash, K.R.; Purvaja, R.; Ramesh, R. Sustainability at a cost: An inceptive analysis of ‘extended’ fishing ban on the livelihoods of fishers of Tamil Nadu. Fish. Manag. Ecol. 2020, 27, 336–344. [Google Scholar] [CrossRef]
- He, Y.; Chen, T. Does the 10-Year Fishing Ban Compensation Policy in the Yangtze River Basin Improve the Livelihoods of Fishing Households? Evidence from Ma’anshan City, China. Agriculture 2022, 12, 2088. [Google Scholar] [CrossRef]
- Heilpern, S.A.; Sethi, S.A.; Barthem, R.B.; Batista, V.D.; Doria, C.R.C.; Duponchelle, F.; Vasquez, A.G.; Goulding, M.; Isaac, V.; Naeem, S.; et al. Biodiversity underpins fisheries resilience to exploitation in the Amazon river basin. Proc. R. Soc. B-Biol. Sci. 2022, 289, 20220726. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, M.; Hanson, J.O.; Wang, J.; Locke, H.; Watson, J.E.; Ellis, E.C.; Li, S.; Ma, K. Countries’ differentiated responsibilities to fulfill area-based conservation targets of the Kunming-Montreal Global Biodiversity Framework. One Earth 2023, 6, 548–559. [Google Scholar] [CrossRef]
- Chen, Y.S.; Qu, X.; Xiong, F.Y.; Lu, Y.; Wang, L.Z.; Hughes, R.M. Challenges to saving China’s freshwater biodiversity: Fishery exploitation and landscape pressures. Ambio 2020, 49, 926–938. [Google Scholar] [CrossRef]
- Klein, C.J.; Kuempel, C.D.; Watson, R.A.; Teneva, L.; Coll, M.; Mora, C. Global fishing between jurisdictions with unequal fisheries management. Environ. Res. Lett. 2022, 17, 114004. [Google Scholar] [CrossRef]
- Wang, Y. Actual Costs of Uneconomic Growth of Marine Fisheries in China’s Pearl River Delta. Nat. Resour. 2018, 9, 297–312. [Google Scholar] [CrossRef][Green Version]
- Mei, Z.; Cheng, P.; Wang, K.; Wei, Q.; Barlow, J.; Wang, D. A first step for the Yangtze. Science 2020, 367, 1314. [Google Scholar] [CrossRef]
- Qiu, Q.; Dai, L.; Van Rijswick, H.F.; Tu, G. Improving the water quality monitoring system in the Yangtze River basin-Legal suggestions to the implementation of the Yangtze River protection law. Laws 2021, 10, 25. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, C.; Fan, E.; Zhao, Y. Freshwater Fishes of China: Species Richness, Endemism, Threatened Species and Conservation. Divers. Distrib. 2015, 22, 358–370. [Google Scholar] [CrossRef]
- Turnhout, E.; Dewulf, A.; Hulme, M. What does policy-relevant global environmental knowledge do? The cases of climate and biodiversity. Curr. Opin. Environ. Sustain. 2016, 18, 65–72. [Google Scholar] [CrossRef]
- Scherer, L.; Svenning, J.C.; Huang, J.; Seymour, C.L.; Sandel, B.; Mueller, N.; Kummu, M.; Bekunda, M.; Bruelheide, H.; Hochman, Z.; et al. Global priorities of environmental issues to combat food insecurity and biodiversity loss. Sci. Total Environ. 2020, 730, 139096. [Google Scholar] [CrossRef]
- Xu, H.G.; Cao, Y.; Yu, D.D.; Cao, M.C.; He, Y.X.; Gill, M.; Pereira, H.M. Ensuring effective implementation of the post-2020 global biodiversity targets. Nat. Ecol. Evol. 2021, 5, 411–418. [Google Scholar] [CrossRef]
- Joly, C.A. The Kunming-Montréal Global Biodiversity Framework. Biota. Neotrop. 2023, 22, e2022e001. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Iacarella, J.C.; Adamczyk, E.; Bowen, D.; Chalifour, L.; Eger, A.; Heath, W.; Helms, S.; Hessing-Lewis, M.; Hunt, B.P.V.; MacInnis, A.; et al. Anthropogenic disturbance homogenizes seagrass fish communities. Glob. Change Biol. 2018, 24, 1904–1918. [Google Scholar] [CrossRef]
- Hill, M.J.; White, J.C.; Biggs, J. Local contributions to beta diversity in urban pond networks: Implications for biodiversity conservation and management. Divers. Distrib. 2021, 27, 887–900. [Google Scholar] [CrossRef]
- Iacarella, J.C. Fish zeta diversity responses to human pressures and cumulative effects across a freshwater basin. Divers. Distrib. 2022, 28, 830–843. [Google Scholar] [CrossRef]
- Latombe, G.; Hui, C.; McGeoch, M.A. Multi-site generalised dissimilarity modelling: Using zeta diversity to differentiate drivers of turnover in rare and widespread species. Methods Ecol. Evol. 2017, 8, 431–442. [Google Scholar] [CrossRef]
- Hui, C.; McGeoch, M.A. Zeta diversity as a concept and metric that unifies incidence-based biodiversity patterns. Am. Nat. 2014, 184, 684–694. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhang, Z.; Xu, D.; Wang, L.; Liu, Y. Zeta Diversity Reveals the Small Wetland Complex Promotes Biodiversity. Water 2024, 16, 476. [Google Scholar] [CrossRef]
- Dai, B.; Chen, S.; Wang, C.; Jiang, Z. Functional zeta diversity can better reveal fish metacommunity assembly process in a transitional floodplain. Ecol. Indic. 2024, 160, 111831. [Google Scholar] [CrossRef]
- Hodgson, E.E.; Halpern, B.S. Investigating cumulative effects across ecological scales. Conserv. Biol. 2019, 33, 22–32. [Google Scholar] [CrossRef]
- Seitz, N.E.; Westbrook, C.J.; Noble, B.F. Bringing science into river systems cumulative effects assessment practice. Environ. Impact. Asses. 2011, 31, 172–179. [Google Scholar] [CrossRef]
- Wen, Z.X.; Cai, T.L.; Wu, Y.J.; Fejio, A.; Xia, L.; Cheng, J.L.; Peng, X.W.; Zhang, Q.; Zhang, Z.J.; Ran, J.H.; et al. Environmental drivers of sympatric mammalian species compositional turnover in giant panda nature reserves: Implications for conservation. Sci. Total Environ. 2022, 806, 150944. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, H.Y.; Yang, Y.X. Uncovering the determinants of biodiversity hotspots in China: Evidence from the drivers of multiple diversity metrics on insect assemblages and implications for conservation. Sci. Total Environ. 2023, 880, 163287. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, G.J.; Tian, P.; Mu, X.M.; Tian, X.J.; Feng, J.H.; Bai, Y.P. Runoff changes in the major river basins of China and their responses to potential driving forces. J. Hydrol. 2022, 607, 127536. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.Y.; Duan, L.J.; Liu, Y. Fishery policy exploration in the Pearl River Estuary based on an Ecosim model. Ecol. Model. 2012, 230, 34–43. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Gozlan, R.E.; Qu, X.; Xia, W.; Cheng, F.; Wang, L.; Paukert, C.P.; Olden, J.D.; Xie, S. Fish diversity reduction and assemblage structure homogenization in lakes:A case study on unselective fishing in China. Water Biol. Secur. 2022, 1, 43–50. [Google Scholar] [CrossRef]
- Wang, J.; Ding, C.; Heino, J.; Ding, L.; Chen, J.; He, D.; Tao, J. Analysing spatio- temporal patterns of non- native fish in a biodiversity hotspot across decades. Divers. Distrib. 2023, 62, 1364–1377. [Google Scholar] [CrossRef]
- Radinger, J.; Wolter, C. Patterns and predictors of fish dispersal in rivers. Fish Fish. 2014, 15, 456–473. [Google Scholar] [CrossRef]
- Brosse, S.; Charpin, N.; Su, G.; Toussaint, A.; Herrera, R.G.A.; Tedesco, P.A.; Villéger, S. FISHMORPH: A global database on morphological traits of freshwater fishes. Glob. Ecol. Biogeogr. 2021, 30, 2330–2336. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Web Electronic Publication Version (10/2022). 2022. Available online: https://www.fishbase.org/search.php (accessed on 18 June 2023).
- Lehner, B.; Grill, G. Global river hydrography and network routing: Baseline data and new approaches to study the world’s large river systems. Hydrol. Process. 2013, 27, 2171–2186. [Google Scholar] [CrossRef]
- Page, M.J.; Boutron, I.; Tetzlaff, J.M.; Chou, R.; Lalu, M.M.; Mayo-Wilson, E.; Stewart, L.A.; Welch, V.A. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 10, 372. [Google Scholar] [CrossRef]
- Ascensão, F.; Latombe, G.; Anadón, J.D.; Abellán, P.; Cardador, L.; Carrete, M.; Tella, J.L.; Capinha, C. Drivers of compositional dissimilarity for native and alien birds: The relative roles of human activity and environmental suitability. Biol. Invasions 2020, 22, 1447–1460. [Google Scholar] [CrossRef]
- Li, Z.; Heino, J.; Liu, Z.; Meng, X.; Chen, X.; Ge, Y.; Xie, Z. The drivers of multiple dimensions of stream macroinvertebrate beta diversity across a large montane landscape. Limnol. Oceanogr. 2021, 66, 226–236. [Google Scholar] [CrossRef]
- Chen, K.; Olden, J.D. Threshold responses of riverine fish communities to land use conversion across regions of the world. Glob. Change Biol. 2020, 26, 4952–4965. [Google Scholar] [CrossRef]
- Xu, M.; Li, S.P.; Dick, J.T.A.; Gu, D.G.; Fang, M.; Yang, Y.X.; Hu, Y.C.; Mu, X.D. Exotic fishes that are phylogenetically close but functionally distant to native fishes are more likely to establish. Glob. Change Biol. 2022, 28, 5683–5694. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Global. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How should beta-diversity inform biodiversity conservation? Trends. Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef]
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Madi, N. adespatial: Multivariate Multiscale Spatial Analysis. R Package Version 0.3-7. 2019. Available online: https://cran.r-project.org/web/packages/adespatial/index.html (accessed on 28 June 2023).
- Latombe, G.; McGeoch, M.; Nipperess, D.; Hui, C. zetadiv: Functions to Compute Compositional Turnover Using Zeta Diversity. R Package version 1.2.0. 2020. Available online: https://rdrr.io/cran/zetadiv/ (accessed on 1 March 2025).
- Latombe, G.; Roura-Pascual, N.; Hui, C. Similar compositional turnover but distinct insular environmental and geographical drivers of native and exotic ants in two oceans. J. Biogeogr. 2019, 46, 2299–2310. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Latombe, G.; Andrew, N.R.; Nakagawa, S.; Nipperess, D.A.; Roigé, M.; Marzinelli, E.M.; Campbell, A.H.; Vergés, A.; Thomas, T.; et al. Measuring continuous compositional change using decline and decay in zeta diversity. Ecology 2019, 100, e02832. [Google Scholar] [CrossRef]
- Latombe, G.; Richardson, D.M.; Pyšek, P.; Kučera, T.; Hui, C. Drivers of species turnover vary with species commonness for native and alien plants with different residence times. Ecology 2018, 99, 2763–2775. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends. Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.M.; Gorfine, H.; Shan, X.J.; Shen, L.; Yang, H.L.; Du, H.; Li, J.Y.; Wang, C.Y.; Zhou, Q.; et al. Inland fisheries development versus aquatic biodiversity conservation in China and its global implications. Rev. Fish. Biol. Fish. 2020, 30, 637–655. [Google Scholar] [CrossRef]
- Oberdorff, T.; Lek, S.; Guegan, J.F. Patterns of endemism in riverine fish of the Northern Hemisphere. Ecol. Lett. 1999, 2, 75–81. [Google Scholar] [CrossRef]
- He, J.; Wu, Z.; Huang, L.; Gao, M.; Liu, H.; Sun, Y.; Rad, S.; Du, L. Diversity, Distribution, and Biogeography of Freshwater Fishes in Guangxi, China. Animals 2022, 12, 1626. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, C.; Yang, Z.; Chen, X.J. Study on the biological integrity of Damming River -Take Hongshui River of Pearl River Basin as a case study. J. Hydroecol. 2022, 2, 1–14. [Google Scholar] [CrossRef]
| Sub-Basins Group | Order of Zeta | Exponential Coefficient | Power-Law Coefficient | ||||
|---|---|---|---|---|---|---|---|
| b | p | AIC | c | p | AIC | ||
| All | 1–3 | −0.11 | 0.15 | −8.16 | −0.49 | 0.06 | −13.98 |
| High | 1–3 | −0.08 | 0.22 | −8.66 | −0.34 | 0.11 | −12.44 |
| 1–4 | −0.05 | 0.09 | −11.18 | −0.29 | 0.03 | −15.90 | |
| Mid | 1–3 | −0.11 | 0.13 | −9.22 | −0.47 | 0.04 | −16.98 |
| Low | 1–3 | −0.09 | 0.16 | −9.31 | −0.38 | 0.06 | −14.71 |
| 1–4 | −0.07 | 0.06 | −11.65 | −0.35 | 0.01 | −18.49 | |
| Sub-Basin Groups | Order of Zeta | Sorensen, r | Simpson, r |
|---|---|---|---|
| All | 2 | 65.40% ± 5.95% | 62.19% ± 6.67% |
| High | 2 | 57.18% ± 8.04% | 56.16% ± 5.14% |
| Mid | 2 | 55.87% ± 5.25% | 50.98% ± 5.61% |
| Low | 2 | 55.56% ± 9.64% | 54.79% ± 7.36% |
| 3 | 63.19% ± 6.63% | 63.14% ± 6.12% | |
| 4 | 66.99% ± 5.99% | 65.61% ± 9.49% | |
| 5 | 68.18% ± 7.86% | 64.86% ± 6.62% | |
| 6 | 67.17% ± 5.97% | 63.39% ± 5.96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Liu, H.; Bi, X.; Wu, Z. Responses of Fish Zeta Diversity (ζ) to Human Pressure and Cumulative Effects: A Feasibility Study of Fishing Ban Measures in the Pearl River Basin, China. Biology 2025, 14, 796. https://doi.org/10.3390/biology14070796
He J, Liu H, Bi X, Wu Z. Responses of Fish Zeta Diversity (ζ) to Human Pressure and Cumulative Effects: A Feasibility Study of Fishing Ban Measures in the Pearl River Basin, China. Biology. 2025; 14(7):796. https://doi.org/10.3390/biology14070796
Chicago/Turabian StyleHe, Jiayang, Hao Liu, Xianda Bi, and Zhiqiang Wu. 2025. "Responses of Fish Zeta Diversity (ζ) to Human Pressure and Cumulative Effects: A Feasibility Study of Fishing Ban Measures in the Pearl River Basin, China" Biology 14, no. 7: 796. https://doi.org/10.3390/biology14070796
APA StyleHe, J., Liu, H., Bi, X., & Wu, Z. (2025). Responses of Fish Zeta Diversity (ζ) to Human Pressure and Cumulative Effects: A Feasibility Study of Fishing Ban Measures in the Pearl River Basin, China. Biology, 14(7), 796. https://doi.org/10.3390/biology14070796







