Novel Insights into T-Cell Exhaustion and Cancer Biomarkers in PDAC Using ScRNA-Seq
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. ScRNA-Seq Dataset Retrieval
2.2. Data Preprocessing and Clustering
2.3. Cell Type Annotation
2.4. Molecular Subtypes Classification of PDAC Samples
2.5. Gene Expression Profiling Across Conditions
2.6. Pathways Enrichment Analysis, Protein–Protein Interaction (PPI) Analysis, and Hub-Genes Identification
2.7. Aberrant Gene Expression Validation and Survival Rates in PDAC Patients
3. Results
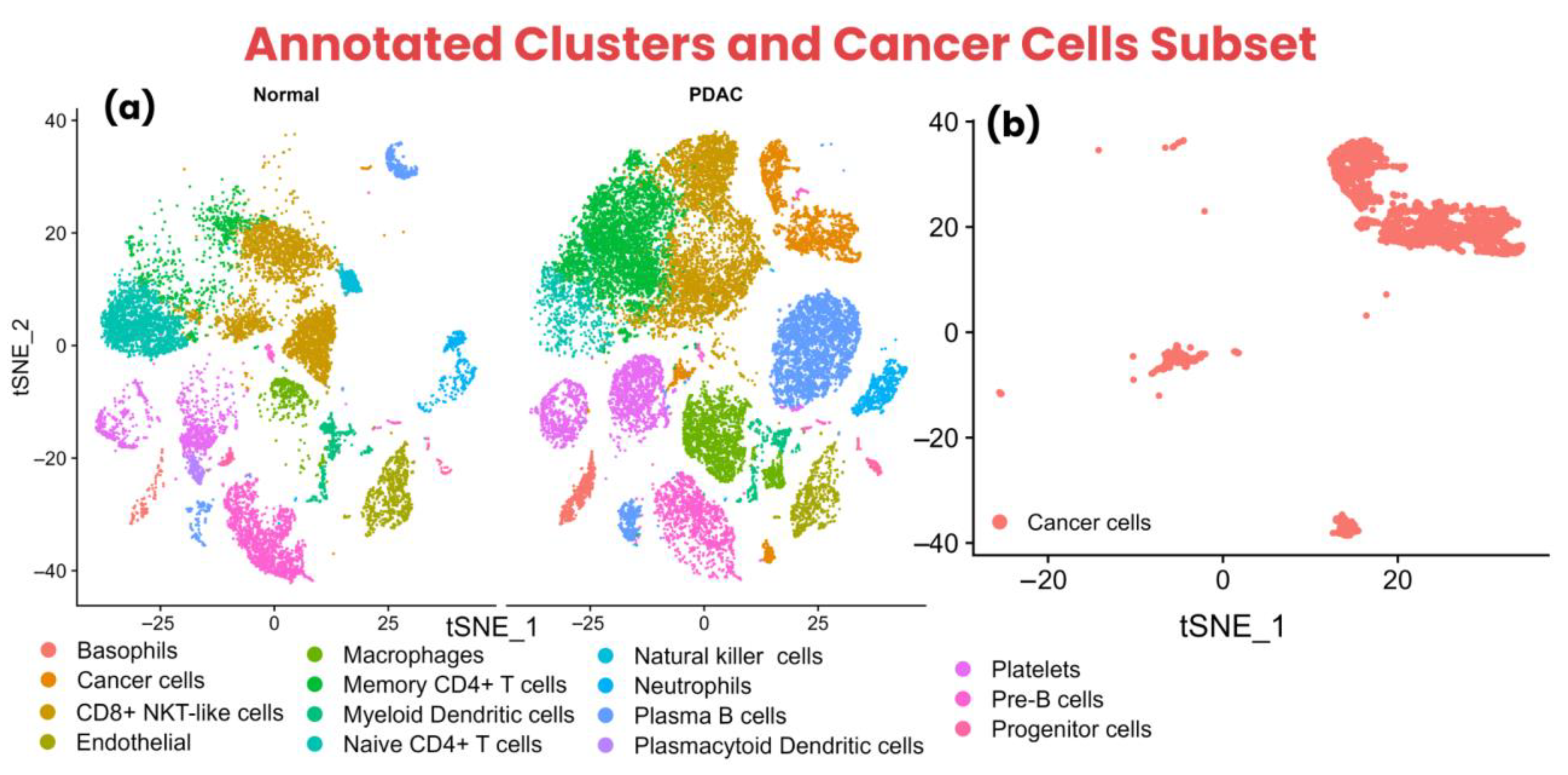
3.1. Data Preprocessing and Cell Type Annotation
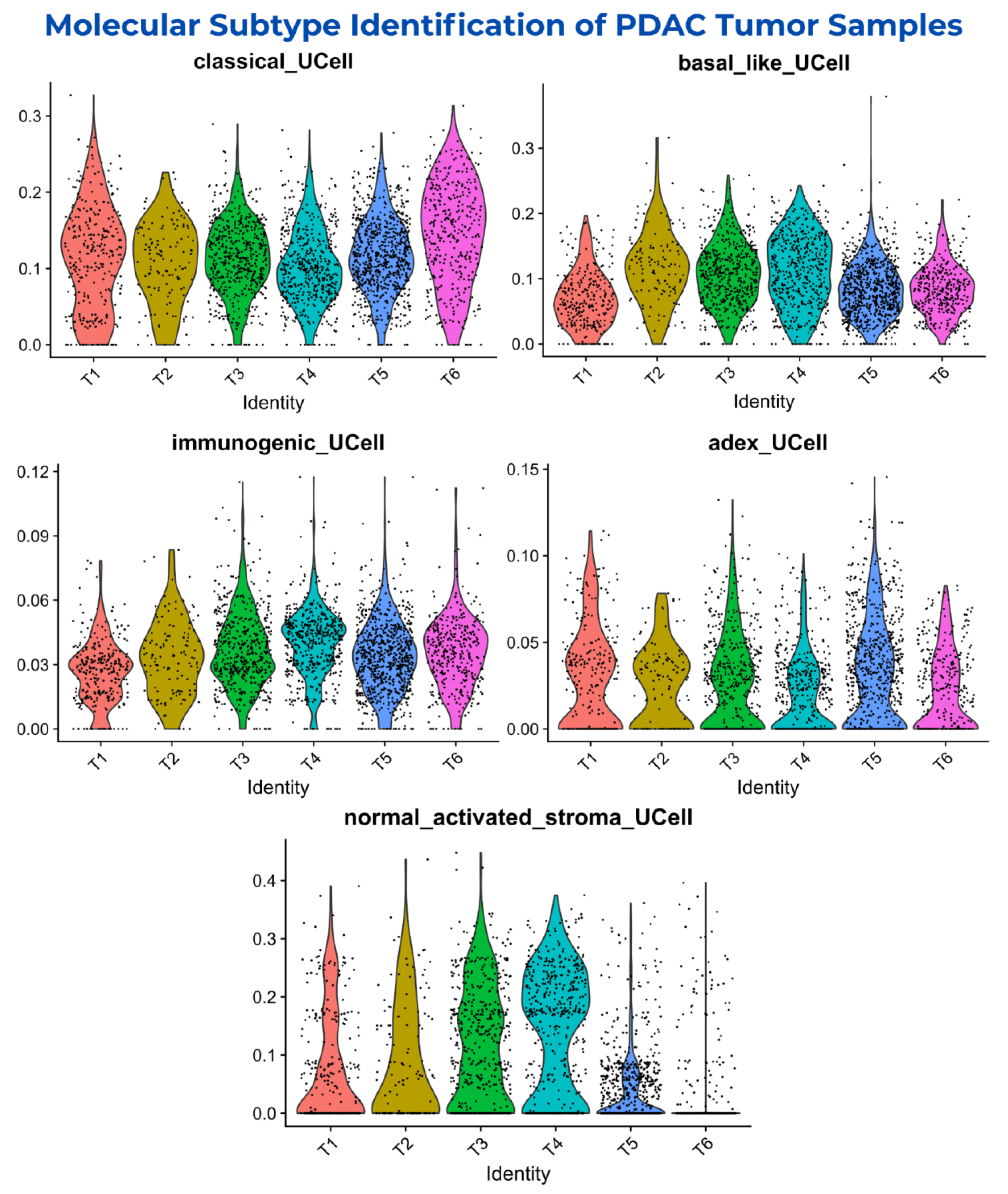
3.2. Molecular Subtypes Classification of PDAC Samples
3.3. Aberrant Markers of Cancer Cells, CD8+ NKT-like Cells, Memory CD4+ T Cells, and Naive CD4+ T Cells
3.4. Pathways Dysregulation Leading to T-Cell Exhaustion and PDAC Progression
3.5. Key Candidate Proteins Associated with T-Cell Exhaustion Within PDAC TIME
3.6. Expression Profiling and Survival Correlation of Key Hub-Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic Ductal Adenocarcinoma |
| scRNA-seq | single-cell RNA sequencing |
| TIME | Tumor Immune Microenvironment |
| TAAs | Tumor-Associated Antigens |
| APCs | Antigen-Presenting Cells |
| GEO | Gene Expression Omnibus |
| NCBI | National Center for Biotechnology Information |
| vst | Variance Stabilizing Transformation |
| PCA | Principal Component Analysis |
| ADEX | Aberrantly Differentiated Endocrine Exocrine |
| PPI | Protein–Protein Interaction |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| GEPIA2 | Gene Expression Profiling Interactive Analysis 2 |
| TISCH2 | Tumor Immune Single Cell Hub 2 |
| TCGA | The Cancer Genome Atlas |
References
- Schawkat, K.; Manning, M.A.; Glickman, J.N.; Mortele, K.J. Pancreatic Ductal Adenocarcinoma and Its Variants: Pearls and Perils. RadioGraphics 2020, 40, 1219–1239. [Google Scholar] [CrossRef] [PubMed]
- Mukund, A.; Afridi, M.A.; Karolak, A.; Park, M.A.; Permuth, J.B.; Rasool, G. Pancreatic Ductal Adenocarcinoma (PDAC): A Review of Recent Advancements Enabled by Artificial Intelligence. Cancers 2024, 16, 2240. [Google Scholar] [CrossRef]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Carvalho, T.M.A.; Di Molfetta, D.; Greco, M.R.; Koltai, T.; Alfarouk, K.O.; Reshkin, S.J.; Cardone, R.A. Tumor Microenvironment Features and Chemoresistance in Pancreatic Ductal Adenocarcinoma: Insights into Targeting Physicochemical Barriers and Metabolism as Therapeutic Approaches. Cancers 2021, 13, 6135. [Google Scholar] [CrossRef]
- Taherian, M.; Wang, H.; Wang, H. Pancreatic Ductal Adenocarcinoma: Molecular Pathology and Predictive Biomarkers. Cells 2022, 11, 3068. [Google Scholar] [CrossRef]
- Espiau-Romera, P.; Courtois, S.; Parejo-Alonso, B.; Sancho, P. Molecular and Metabolic Subtypes Correspondence for Pancreatic Ductal Adenocarcinoma Classification. J. Clin. Med. 2020, 9, 4128. [Google Scholar] [CrossRef]
- Nakaoka, K.; Ohno, E.; Kawabe, N.; Kuzuya, T.; Funsaka, K.; Nakagawa, Y.; Nagasaka, M.; Ishikawa, T.; Watanabe, A.; Tochio, T.; et al. Current Status of the Diagnosis of Early-Stage Pancreatic Ductal Adenocarcinoma. Diagnostics 2023, 13, 215. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Seeber, A.; Zimmer, K.; Kocher, F.; Puccini, A.; Xiu, J.; Nabhan, C.; Elliott, A.; Goldberg, R.M.; Grothey, A.; Shields, A.F.; et al. Molecular characteristics of BRCA1/2 and PALB2 mutations in pancreatic ductal adenocarcinoma. ESMO Open 2020, 5, e000942. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Anderson, E.M.; Thomassian, S.; Gong, J.; Hendifar, A.; Osipov, A. Advances in Pancreatic Ductal Adenocarcinoma Treatment. Cancers 2021, 13, 5510. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, G.; Volta, F.; Garajova, I.; Balsano, R.; Cavazzoni, A. Biological Hallmarks and New Therapeutic Approaches for the Treatment of PDAC. Life 2021, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Kunk, P.R.; Bauer, T.W.; Slingluff, C.L.; Rahma, O.E. From bench to bedside: A comprehensive review of pancreatic cancer immunotherapy. J. Immunother. Cancer 2016, 4, 14. [Google Scholar] [CrossRef]
- Chouari, T.; Costa, F.S.L.; Merali, N.; Jessel, M.-D.; Sivakumar, S.; Annels, N.; Frampton, A.E. Advances in Immunotherapeutics in Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 4265. [Google Scholar] [CrossRef]
- Saka, D.; Gökalp, M.; Piyade, B.; Cevik, N.C.; Arik Sever, E.; Unutmaz, D.; Ceyhan, G.O.; Demir, I.E.; Asimgil, H. Mechanisms of T-Cell Exhaustion in Pancreatic Cancer. Cancers 2020, 12, 2274. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Johnson, I.I.I.B.A.; Yarchoan, M.; Lee, V.; Laheru, D.A.; Jaffee, E.M. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clin. Cancer Res. 2017, 23, 1656–1669. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Łuksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; Sendabaoglu, Y.; et al. Identification of unique neoantigen qualities in long term pancreatic cancer survivors. Nature 2017, 551, 512–516. [Google Scholar] [CrossRef]
- Goulart, M.R.; Stasinos, K.; Fincham, R.E.A.; Delvecchio, F.R.; Kocher, H.M. T cells in pancreatic cancer stroma. World J. Gastroenterol. 2021, 27, 7956–7968. [Google Scholar] [CrossRef]
- Sivakumar, S.; Abu-Shah, E.; Ahern, D.J.; Arbe-Barnes, E.H.; Jainarayanan, A.K.; Mangal, N.; Reddy, S.; Rendek, A.; Easton, A.; Kurz, E.; et al. Activated Regulatory T-Cells, Dysfunctional and Senescent T-Cells Hinder the Immunity in Pancreatic Cancer. Cancers 2021, 13, 1776. [Google Scholar] [CrossRef]
- Werba, G.; Werba, G.; Weissinger, D.; Kawaler, E.A.; Zhao, E.; Kalfakakou, D.; Dhara, S.; Wang, L.; Lim, H.B.; Oh, G.; et al. Single-cell RNA sequencing reveals the effects of chemotherapy on human pancreatic adenocarcinoma and its tumor microenvironment. Nat. Commun. 2023, 14, 797. [Google Scholar] [CrossRef]
- Clough, E.; Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; et al. NCBI GEO: Archive for gene expression and epigenomics data sets: 23-year update. Nucleic Acids Res. 2024, 52, D138–D144. [Google Scholar] [CrossRef]
- Nader, K.; Tasci, M.; Ianevski, A.; Erickson, A.; Verschuren, E.W.; Aittokallio, T.; Miihkinen, M. ScType enables fast and accurate cell type identification from spatial transcriptomics data. Bioinformatics 2024, 40, btae426. [Google Scholar] [CrossRef]
- García-Moreno, A.; López-Domínguez, R.; Ramírez-Mena, A.; Pascual-Montano, A.; Aparicio-Puerta, E.; Hackenberg, M.; Carmona-Sáez, P. GeneCodis 4: Expanding the modular enrichment analysis to regulatory elements. biorXiv 2021. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2022, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Dong, X.; Sun, D.; Liu, Z.; Yue, J.; Wang, H.; Li, T.; Wang, C. TISCH2: Expanded datasets and new tools for single-cell transcriptome analyses of the tumor microenvironment. Nucleic Acids Res. 2023, 51, D1425–D1431. [Google Scholar] [CrossRef]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Tasiheng, Y.; Tasiheng, Y.; Lin, X.; Wang, X.; Zou, X.; Chen, Y.; Yan, Y.; Ma, M.; Dai, Z.; Wang, X.; et al. DNA hypo-methylation and expression of GBP4 induces T cell exhaustion in pancreatic cancer. Cancer Immunol. Immunother. 2024, 73, 208. [Google Scholar] [CrossRef] [PubMed]
- Encarnación-Rosado, J.; Kimmelman, A.C. Harnessing metabolic dependencies in pancreatic cancers. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Schneeweis, C.; Hassan, Z.; Schick, M.; Keller, U.; Schneider, G. The SUMO pathway in pancreatic cancer: Insights and inhibition. Br. J. Cancer 2021, 124, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, C.; Zheng, M.; Zhang, J.; Wu, H.; He, Q.; Ding, L.; Yang, B. Insights into the role of derailed endocytic trafficking pathway in cancer: From the perspective of cancer hallmarks. Pharmacol. Res. 2024, 201, 107084. [Google Scholar] [CrossRef]
- Stoof, J.; Harrold, E.; Mariottino, S.; Lowery, M.A.; Walsh, N. DNA Damage Repair Deficiency in Pancreatic Ductal Adenocarcinoma: Preclinical Models and Clinical Perspectives. Front. Cell Dev. Biol. 2021, 9, 749490. [Google Scholar] [CrossRef]
- Venkat, S.; Alahmari, A.A.; Feigin, M.E. Drivers of gene expression dysregulation in pancreatic cancer. Trends Cancer 2021, 7, 594–605. [Google Scholar] [CrossRef]
- Ali, A.; Chianese, U.; Papulino, C.; Toraldo, A.; Abakar, M.E.A.; Passaro, E.; Cennamo, R.; Del Gaudio, N.; Altucci, L.; Benedetti, R. Metabolic Pathways as a Novel Landscape in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 3799. [Google Scholar] [CrossRef]
- Im, K.; Choi, Y.J.; Kim, D.H.; Kim, D.-S.; Ban, K.; Ji, W.; Baek, I.-J.; Choi, C.-M.; Lee, J.C.; Rho, J.K. AXL receptor tyrosine kinase inhibition improves the anti-tumor effects of CD8+ T cells by inducing CD103+ dendritic cell-mediated T cell priming. Biochem. Biophys. Res. Commun. 2023, 680, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xue, W.; Zheng, Y.; Geng, Q.; Wang, L.; Fan, Z.; Wang, W.; Yue, Y.; Zhai, Y.; Li, L.; et al. Molecular mechanism study of HGF/c-MET pathway activation and immune regulation for a tumor diagnosis model. Cancer Cell Int. 2021, 21, 374. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.E.; Vijver, S.V.; Schumacher, T.N. Modulation of the tumor micro-environment by CD8+ T cell-derived cytokines. Curr. Opin. Immunol. 2021, 69, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Emmons, T.R.; Giridharan, T.; Singel, K.L.; Khan, A.N.; Ricciuti, J.; Howard, K.; Silva-Del Toro, S.L.; Debreceni, I.L.; Aarts, C.E.M.; Brouwer, M.C.; et al. Mechanisms driving neutrophil-induced T-cell immunoparalysis in ovarian cancer. Cancer Immunol. Res. 2021, 9, 790–810. [Google Scholar] [CrossRef]
- Hu, D.; Ansari, D.; Zhou, Q.; Sasor, A.; Hilmersson, K.S.; Andersson, R. Stromal fibronectin expression in patients with resected pancreatic ductal adenocarcinoma. World J. Surg. Oncol. 2019, 17, 29. [Google Scholar] [CrossRef]
- Mansouri, V.; Arjmand, B.; Hamzeloo-Moghadam, M.; Razzaghi, Z.; Ahmadzadeh, A.; Ehsani Ardakani, M.J.; Robati, R.M. Extracellular matrix is the main targeted environment in early stage of pancreatic ductal adenocarcinoma. Gastroenterol. Hepatol. Bed Bench. 2023, 16, 401–407. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, J.; Jensen, C.; I Nissen, N.; Cox, T.R.; Kalluri, R.; Karsdal, M.; Willumsen, N. The collagen landscape in cancer: Profiling collagens in tumors and in circulation reveals novel markers of cancer-associated fibroblast subtypes. J. Pathol. 2024, 262, 22–36. [Google Scholar] [CrossRef]
- Maneshi, P.; Mason, J.; Dongre, M.; Öhlund, D. Targeting Tumor-Stromal Interactions in Pancreatic Cancer: Impact of Collagens and Mechanical Traits. Front. Cell Dev. Biol. 2021, 9, 787485. [Google Scholar] [CrossRef]
- Pan, S.; Chen, R.; Tamura, Y.; Crispin, D.A.; Lai, L.A.; May, D.H.; McIntosh, M.W.; Goodlett, D.R.; Brentnall, T.A. Quantitative Glycoproteomics Analysis Reveals Changes in N-Glycosylation Level Associated with Pancreatic Ductal Adenocarcinoma. J. Proteome Res. 2014, 13, 1293–1306. [Google Scholar] [CrossRef]
- Papalazarou, V.; Drew, J.; Juin, A.; Spence, H.J.; Whitelaw, J.; Nixon, C.; Machesky, L.M. Collagen VI expression is negatively mechanosensitive in pancreatic cancer cells and supports the metastatic niche. J. Cell Sci. 2022, 135, jcs259978. [Google Scholar] [CrossRef]
- Luan, H.; Zhang, C.; Zhang, T.; He, Y.; Su, Y.; Zhou, L. Identification of Key Prognostic Biomarker and Its Correlation with Immune Infiltrates in Pancreatic Ductal Adenocarcinoma. Dis. Markers 2020, 2020, 8825997. [Google Scholar] [CrossRef]
- Pacchiana, R.; Mullappilly, N.; Pinto, A.; Bova, S.; Forciniti, S.; Cullia, G.; Dalla Pozza, E.; Bottani, E.; Decimo, I.; Dando, I.; et al. 3-Bromo-Isoxazoline Derivatives Inhibit GAPDH Enzyme in PDAC Cells Triggering Autophagy and Apoptotic Cell Death. Cancers 2022, 14, 3153. [Google Scholar] [CrossRef]
- Qi, D.; Song, X.; Xue, C.; Yao, W.; Shen, P.; Yu, H.; Zhang, Z. AKT1/FOXP3 axis-mediated expression of CerS6 promotes p53 mutant pancreatic tumorigenesis. Cancer Lett. 2021, 522, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Handra-Luca, A.; Hammel, P.; Sauvanet, A.; Lesty, C.; Ruszniewski, P.; Couvelard, A. EGFR expression in pancreatic adenocarcinoma. Relationship to tumour morphology and cell adhesion proteins. J. Clin. Pathol. 2014, 67, 295–300. [Google Scholar] [CrossRef]
- Peruta, M.D.; Giagulli, C.; Laudanna, C.; Scarpa, A.; Sorio, C. RHOA and PRKCZ control different aspects of cell motility in pancreatic cancer metastatic clones. Mol. Cancer 2010, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Miura, N.; Kato, S.; Masuda, T.; Ohashi, R.; Matsushita, A.; Matsuda, F.; Ohtsuki, S.; Katakura, A.; Honda, K. Identification of TPI1 as a potential therapeutic target in pancreatic cancer with dependency of mutation using multi-omics analysis. Cancer Sci. 2024, 115, 3622–3635. [Google Scholar] [CrossRef]
- Frances, A.; Lumeau, A.; Bery, N.; Gayral, M.; Stuani, L.; Sorbara, M.; Saland, E.; Pagan, D.; Hanoun, N.; Torrisani, J.; et al. Cytidine deaminase-dependent mitochondrial biogenesis as a potential vulnerability in pancreatic cancer cells. Commun. Biol. 2024, 7, 1065. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martinez, O.; Cobo-Prieto, D.; De Leon-Oliva, D.; Boaru, D.L.; De Castro-Martinez, P.; Pekarek, L.; Gragera, R.; Hernández-Fernández, M.; Guijarro, L.G.; et al. Abnormal Histopathological Expression of Klotho, Ferroptosis, and Circadian Clock Regulators in Pancreatic Ductal Adenocarcinoma: Prognostic Implications and Correlation Analyses. Biomolecules 2024, 14, 947. [Google Scholar] [CrossRef]
- Bian, Y.; Yu, Y.; Wang, S.; Li, L. Up-regulation of fatty acid synthase induced by EGFR/ERK activation promotes tumor growth in pancreatic cancer. Biochem. Biophys. Res. Commun. 2015, 463, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Guo, Y.; Zhang, Y.; Duo, A.; Jia, Y.; Liu, C.; Li, B. PAFAH1B2 is a HIF1a target gene and promotes metastasis in pancreatic cancer. Biochem. Biophys. Res. Commun. 2018, 501, 654–660. [Google Scholar] [CrossRef]
- Zheng, L.; Li, M.; Wei, J.; Chen, S.; Xue, C.; Duan, Y.; Tang, F.; Li, G.; Xiong, W.; She, K.; et al. NOP2/Sun RNA methyltransferase 2 is a potential pan-cancer prognostic biomarker and is related to immunity. PLoS ONE 2023, 18, e0292212. [Google Scholar] [CrossRef]
- Kretz, A.-L.; Schaum, M.; Richter, J.; Kitzig, E.F.; Engler, C.C.; Leithäuser, F.; Henne-Bruns, D.; Knippschild, U.; Lemke, J. CDK9 is a prognostic marker and therapeutic target in pancreatic cancer. Tumor Biol. 2017, 39, 1010428317694304. [Google Scholar] [CrossRef] [PubMed]
- Trilla-Fuertes, L.; Gámez-Pozo, A.; Lumbreras-Herrera, M.I.; López-Vacas, R.; Heredia-Soto, V.; Ghanem, I.; López-Camacho, E.; Zapater-Moros, A.; Miguel, M.; Peña-Burgos, E.M.; et al. Identification of Carcinogenesis and Tumor Progression Processes in Pancreatic Ductal Adenocarcinoma Using High-Throughput Proteomics. Cancers 2022, 14, 2414. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, J.; Zhang, M.; Li, Y.; Bai, J.; Liu, P.; Yan, J.; Wang, C. Identification of RFC4 as a potential biomarker for pan-cancer involving prognosis, tumour immune microenvironment and drugs. J. Cell. Mol. Med. 2024, 28, e18478. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, X.; Sun, R.; Ma, P.; Zhang, E.; Wang, Z.; Fan, Y.; Zhou, G.; Mao, R. Ubiquitin-specific protease 7 is a druggable target that is essential for pancreatic cancer growth and chemoresistance. Investig. New Drugs 2020, 38, 1707–1716. [Google Scholar] [CrossRef]
- Wu, S.; Sun, X.; Hua, R.; Hu, C.; Qin, L. DDX21 functions as a potential novel oncopromoter in pancreatic ductal adenocarcinoma: A comprehensive analysis of the DExD box family. Discov. Oncol. 2024, 15, 333. [Google Scholar] [CrossRef]
- Sodir, N.M.; Kortlever, R.M.; Barthet, V.J.A.; Campos, T.; Pellegrinet, L.; Kupczak, S.; Anastasiou, P.; Brown Swigart, L.; Soucek, L.; Arends, M.J.; et al. MYC Instructs and Maintains Pancreatic Adenocarcinoma Phenotype. Cancer Discov. 2020, 10, 588–607. [Google Scholar] [CrossRef]
- Goebel, L.; Grage-Griebenow, E.; Gorys, A.; Helm, O.; Genrich, G.; Lenk, L.; Sebens, S. CD4+ T cells potently induce epithelial-mesenchymal-transition in premalignant and malignant pancreatic ductal epithelial cells–novel implications of CD4+ T cells in pancreatic cancer development. Oncoimmunology 2015, 4, e1000083. [Google Scholar] [CrossRef]
- Ali, L.R.; Lenehan, P.J.; Cardot-Ruffino, V.; Costa, A.D.; Katz, M.H.G.; Bauer, T.W.; Nowak, J.A.; Wolpin, B.M.; Abrams, T.A.; Patel, A.; et al. PD-1 Blockade Induces Reactivation of Nonproductive T-Cell Responses Characterized by NF-κB Signaling in Patients with Pancreatic Cancer. Clin. Cancer Res. 2024, 30, 542–553. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Q.; Liu, X.; Tian, X.; Dong, A.; Yang, Y. Immune profiling and prognostic model of pancreatic cancer using quantitative pathology and single-cell RNA sequencing. J. Transl. Med. 2023, 21, 210. [Google Scholar] [CrossRef]
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Veliça, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I.; et al. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 2017, 32, 669–683.e5. [Google Scholar] [CrossRef] [PubMed]
- Pearce, H.; Croft, W.; Nicol, S.M.; Margielewska-Davies, S.; Powell, R.; Cornall, R.; Davis, S.J.; Marcon, F.; Pugh, M.R.; Fennell, É.; et al. Tissue-Resident Memory T Cells in Pancreatic Ductal Adenocarcinoma Coexpress PD-1 and TIGIT and Functional Inhibition Is Reversible by Dual Antibody Blockade. Cancer Immunol. Res. 2023, 11, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, Y.; Hu, C.; Du, X.; Xue, W.; Chen, Y.; Dong, L.; Pan, J. Extracellular vesicles related gene HSPH1 exerts anti-tumor effects in prostate cancer via promoting the stress response of CD8 + T cells. Cell. Oncol. 2024, 47, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, Y.; Zhao, Y.; Jiang, A. Single-cell and bulk RNA sequencing identifies T cell marker genes score to predict the prognosis of pancreatic ductal adenocarcinoma. Sci. Rep. 2023, 13, 3684. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Hou, Y.; Wang, Q.; Long, D.; Liu, X.; Tian, X.; Yang, Y. Single cell RNA-seq reveals the CCL5/SDC1 receptor-ligand interaction between T cells and tumor cells in pancreatic cancer. Cancer Lett. 2022, 545, 215834. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.U.; Sajid, H.A.; Arshad, M.W.; Torres, A.O.R.; Shabbir, M.I.; Rai, S.K. Novel Insights into T-Cell Exhaustion and Cancer Biomarkers in PDAC Using ScRNA-Seq. Biology 2025, 14, 1015. https://doi.org/10.3390/biology14081015
Saleem MU, Sajid HA, Arshad MW, Torres AOR, Shabbir MI, Rai SK. Novel Insights into T-Cell Exhaustion and Cancer Biomarkers in PDAC Using ScRNA-Seq. Biology. 2025; 14(8):1015. https://doi.org/10.3390/biology14081015
Chicago/Turabian StyleSaleem, Muhammad Usman, Hammad Ali Sajid, Muhammad Waqar Arshad, Alejandro Omar Rivera Torres, Muhammad Imran Shabbir, and Sunil Kumar Rai. 2025. "Novel Insights into T-Cell Exhaustion and Cancer Biomarkers in PDAC Using ScRNA-Seq" Biology 14, no. 8: 1015. https://doi.org/10.3390/biology14081015
APA StyleSaleem, M. U., Sajid, H. A., Arshad, M. W., Torres, A. O. R., Shabbir, M. I., & Rai, S. K. (2025). Novel Insights into T-Cell Exhaustion and Cancer Biomarkers in PDAC Using ScRNA-Seq. Biology, 14(8), 1015. https://doi.org/10.3390/biology14081015





