Facial Bone Defects Associated with Lateral Facial Clefts Tessier Type 6, 7 and 8 in Syndromic Neurocristopathies: A Detailed Micro-CT Analysis on Historical Museum Specimens
Simple Summary
Abstract
1. Introduction
Facial Development
2. Materials and Methods
3. Results
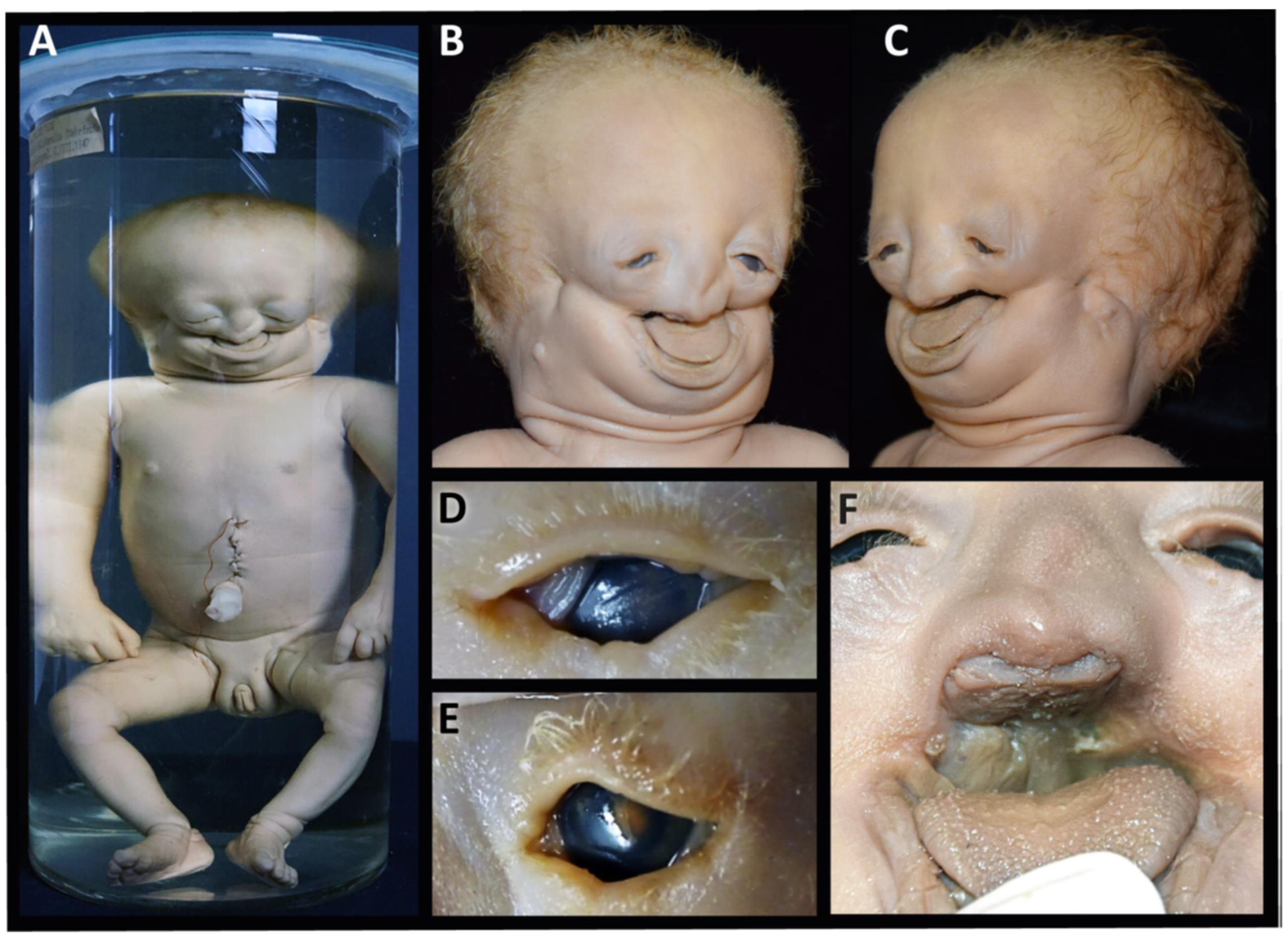
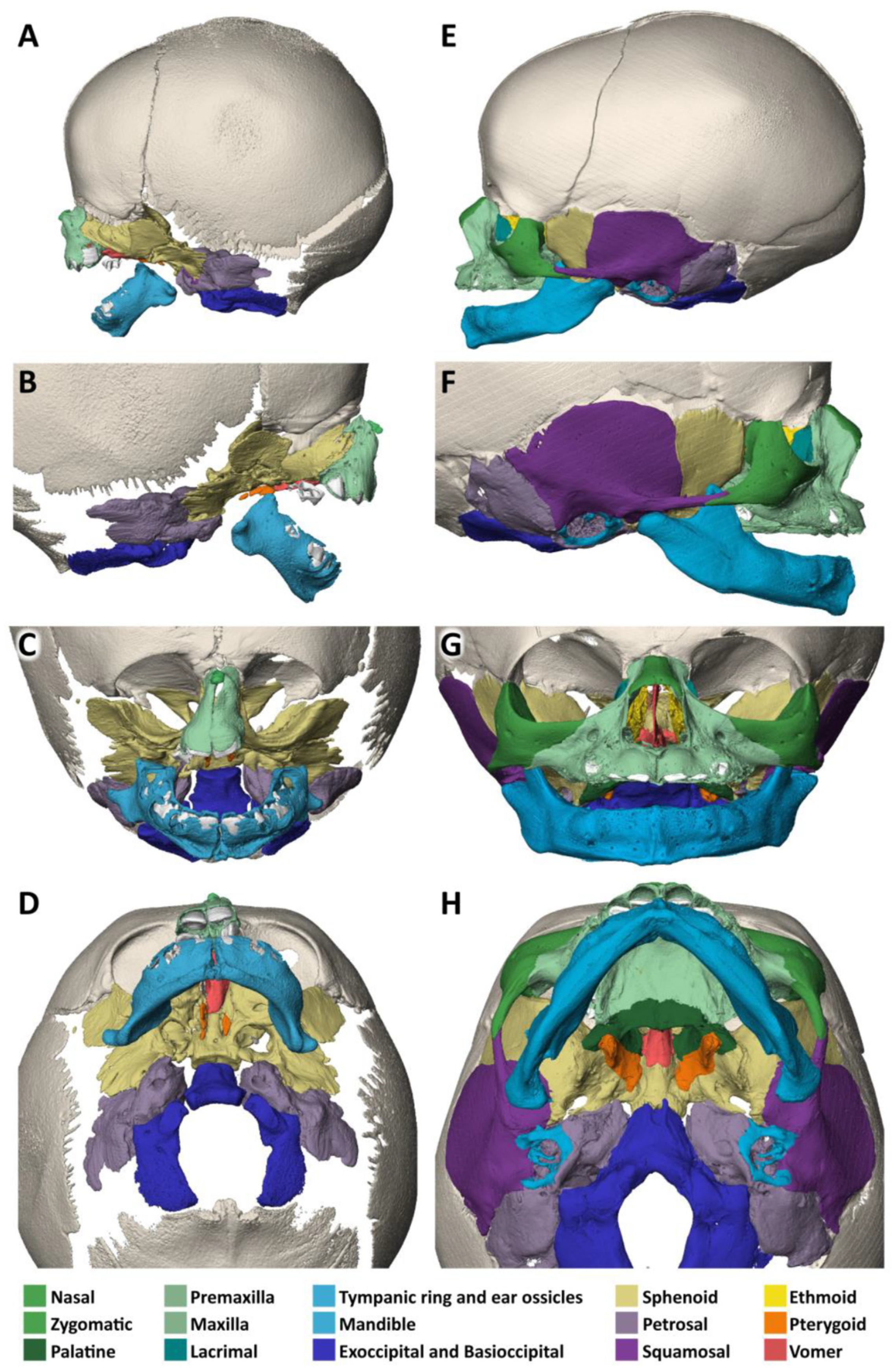
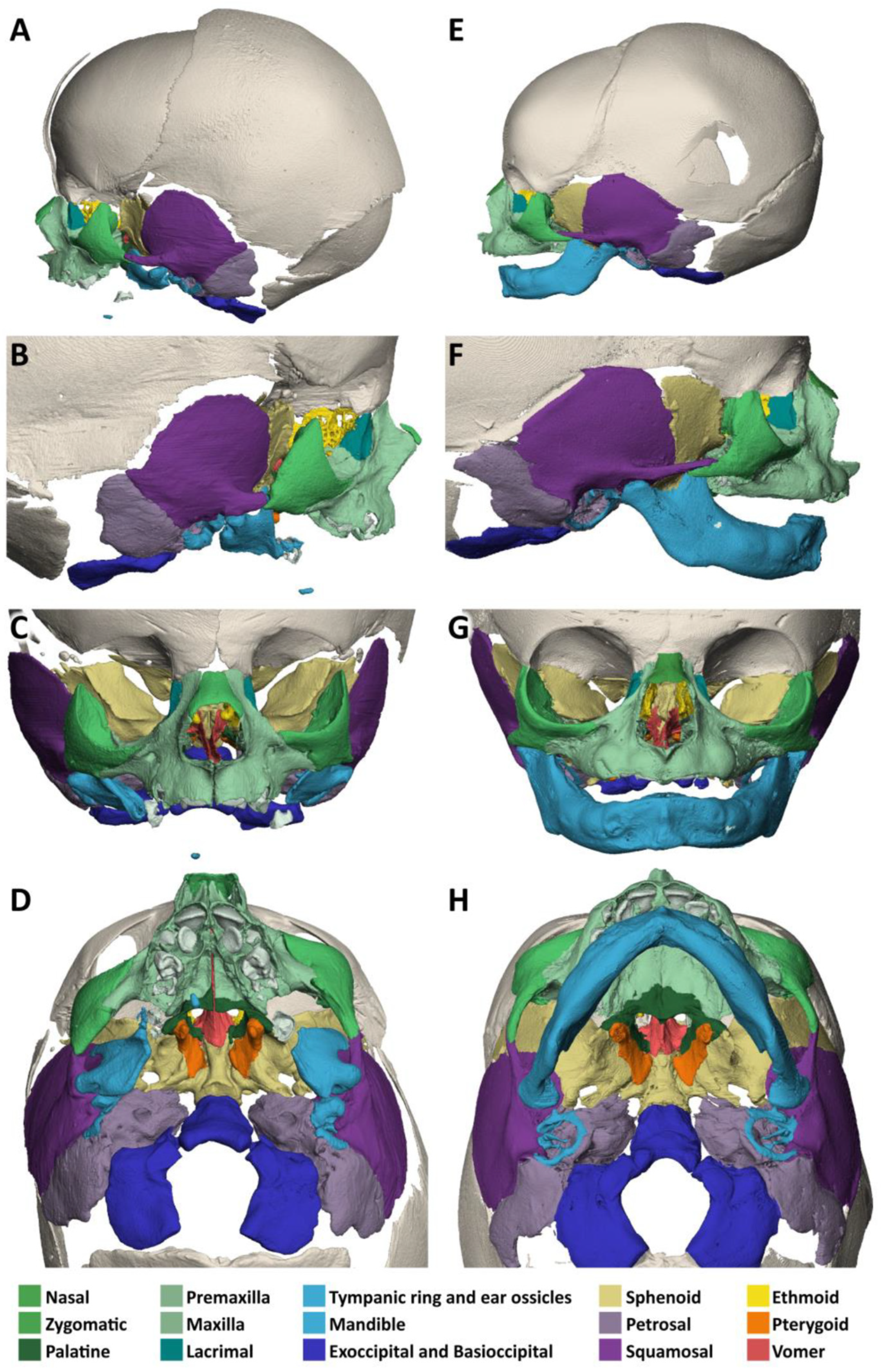
3.1. Specimen 1 (MN.18.560/1836)
- The nasal bone (dark green) is almost absent except for a very small cranial segment. A nasal septum is absent. The vomer (red) appears markedly broadened, frontally shortened and with hypoplastic dorsal wings. The two lamellae of the vomer are not fused cranially and an ossified attachment to a nasal septum is missing.
- The upper jaw (light green) is missing its lateral parts. A narrow median segment, representing the premaxilla, is displaced upwards. It encloses four deciduous incisors, which are developed according to the indicated age of approximately 40 weeks of gestation (±1 weeks) [23]. There are no canines. The cusps of the first right and left deciduous molar and a rudimentary second right deciduous molar are located in the soft tissue near the premaxillary bone and are not encased by a bony crypt. A median furrow of the premaxilla widens into a gap towards the rudimentary nasal bone, possibly representing the osseous nasal orifice and suggesting choanal stenosis. The premaxilla forms a primary palate, while the palatal shelves of the bilateral maxillary bones which should form the secondary palate are absent. The upper roof of the palate consists of a widened vomer and laterally of a bony conglomerate. There are no incisive, palatal or infraorbital foramina. There is a bifid uvula.
- The lower jaw (light blue) is shortened and shows a latero-caudal bend instead of a cranial bend. The ramus of the mandible and the coronoid process is hypoplastic and the condylar process is deformed and close to the angle of the mandible. A temporomandibular joint is bilaterally absent. All the teeth of the mandible are enclosed in osseous crypts and are within the normal range for age. The mandibular canals are recognizable as grooves. There is no midline fusion of the two halves of the mandible. The mental foramina are present, as are the mandibular canals and foramina located at the back of the deformed mandibular rami.
- The zygomatic bones are missing. The sphenoid, ethmoid, lacrimal, a rudimentary squamosal part of the temporal bones and possibly parts of the lateral maxillary bones appear to be irregularly fused into a single bony structure with no apparent boundaries (beige). The zygomatic and styloid processes are absent, as are the tympanic portions of the temporal bones (light violet). Foramina rotunda and ovalia of the skull base are not identifiable. The orbits are formed medially by the fused bony mass, and laterally and superiorly by the frontal bones. The inferior orbital ridges are lacking.
- The ears show absent auricles, with a residual skin tag. The length of auditory canals is 6 mm on the right and 11 mm on the left side. The tympanic membranes and middle ear cavities are lacking, except for a rudimentary intraosseous vesicular structure without ossicles, from which the air-filled Eustachian tubes branch off, although their course cannot be traced with certainty. Both inner ears show a hypoplastic and clumped cochlea without foramina but with obvious coils. The labyrinth has one thick semicircular canal on the right and two on the left. The remaining semicircular canals appear as rudimentary nodular projections (Figure 4A,C).
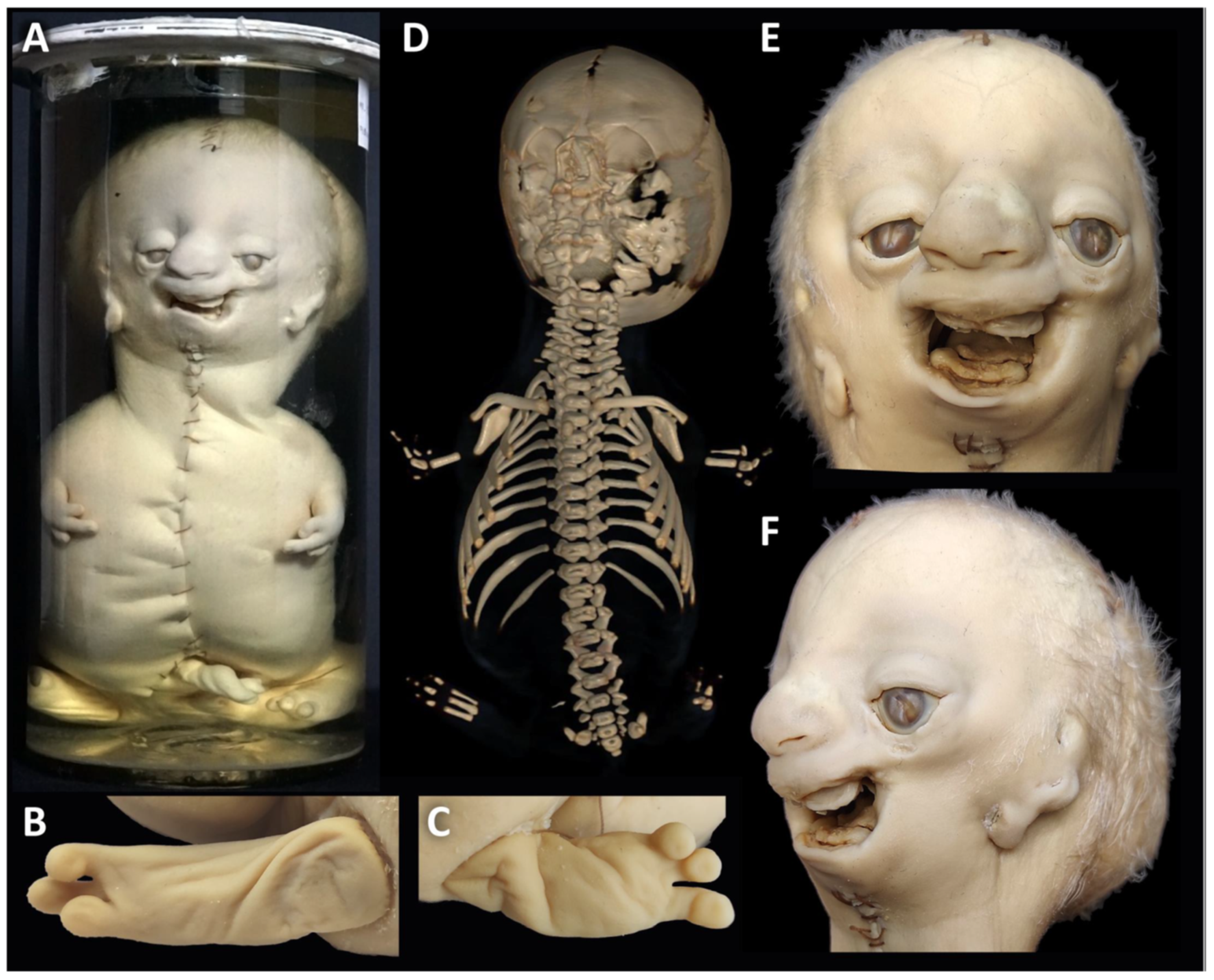
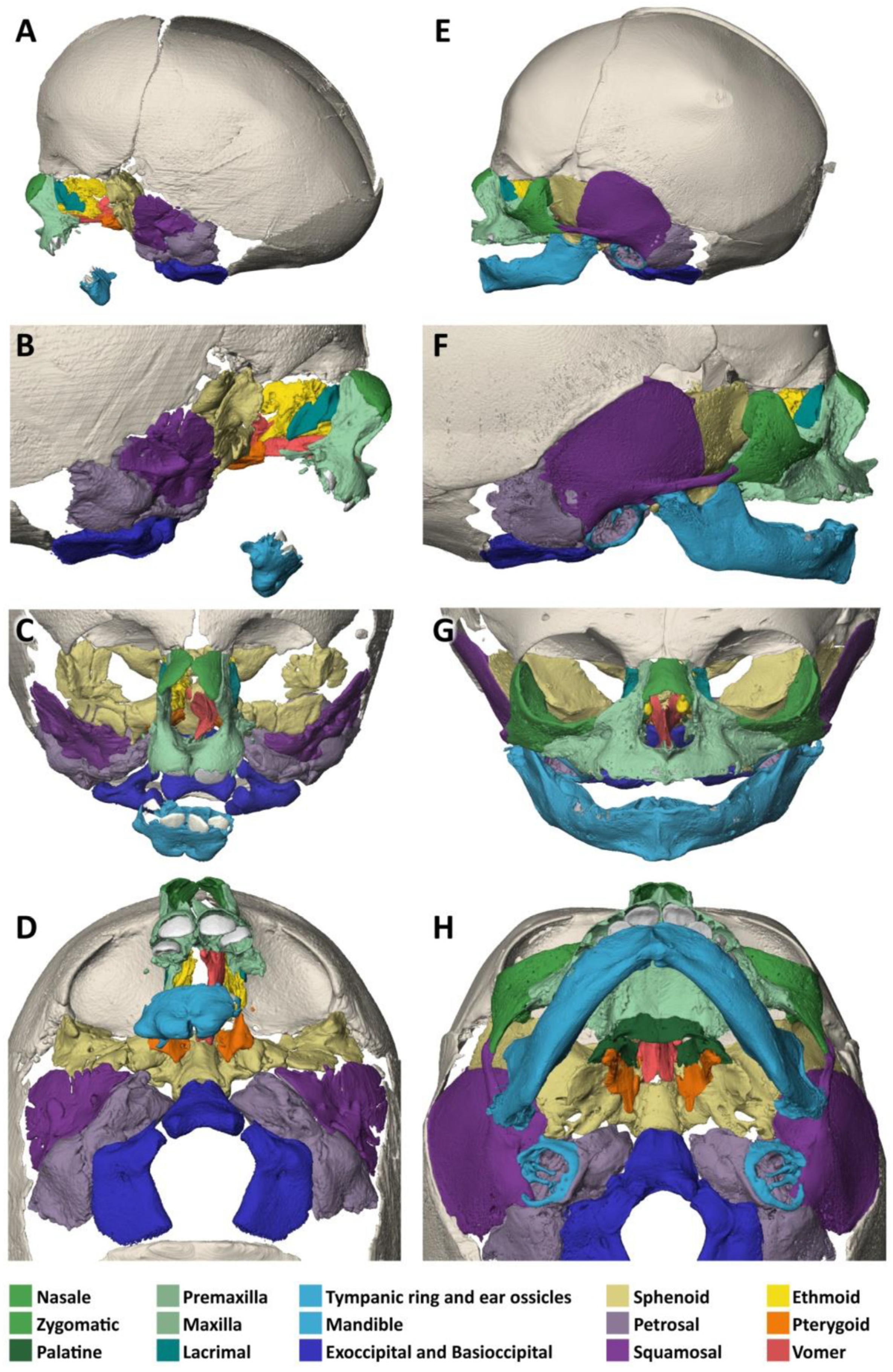
3.2. Specimen 2 (MN.32.808/40)
- The nasal bone (dark green) is not yet fused in the midline and has a caudal and rostral cleft. The vomer (red) is strongly curved to the left. The upper lamellae are not yet fused, the base is rounded, and a basal plate is missing in the absence of a secondary palate. The cartilaginous part of the nasal septum is also bent to the left and narrows the left nasal passage.
- The upper jaw (light green) is missing most of its lateral parts, including the osseous crypts of the canines and molars, orbital segments, infraorbital, incisive and palatine foramina and the zygomatic processes. Some rudimentary fragments of the lateral maxilla are attached to the lateral edges of the premaxilla, more pronounced on the left than on the right side. The alveolar ridge of the premaxilla contains four primary incisors within incompletely developed bony crypts. The nasal part shows no significant changes, but the primary palate including the incisive foramen is absent. The secondary palate including the palatal bones (dark green) is completely missing. There is also no soft palate. There is a direct connection between the oral cavity and the nasal conchae.
- The lower jaw (light blue) is completely missing its lateral parts, including the nerve canals and the mandibular and mental foramina. A small central part is incompletely fused in the lower and dorsal midline and, like the maxilla, has an underdeveloped alveolar ridge. The upper edge of the alveolar bone ends halfway up the teeth. There is one right and two left primary incisors. The second primary incisor on the right side is slightly distant from the first incisor and not enclosed in an ossified crypt, corresponding to the position of a canine. There are no molars.
- The zygomatic bones are completely absent. The greater wings of the sphenoid bones (beige) look severely dysplastic and fragmented. But the basal sphenoids, the presphenoids and orbitosphenoids appear relatively normal in shape. Fragmentation tends to affect more those parts of the sphenoid that are formed by intramembranous ossification. The squamosal parts of the temporal bones (deep violet) appear hypoplastic with wide open temporosphenoidal sutures and only rudimentary right and left zygomatic processes. The petrosal and tympanic part of the temporal bones (light violet) are present. The lacrimal bones (turquoise) are greatly enlarged or fused with the hypoplastic ethmoid bones (yellow). The orbital roofs, formed by the frontal bones, are of normal shape. The medial walls are formed only by the lacrimal bones and parts of the ethmoid bones, as the corresponding parts of the maxilla are missing. The orbital floor and lateral walls are completely absent. These are normally formed by the maxilla, zygomatic, and sphenoidal bones. Foramina rotunda and ovalia of the skull base are not identifiable.
- The ears are lacking the upper part of the auricles. The length of the auditory canals is 2.2 mm on the right and 6.5 mm on the left side. In the middle ears, behind membranous structures without identifiable tympanic rings, possibly representing the tympanic membrane, there are very small, non-air-filled tympanic cavities, each containing a deformed stapes but lacking the malleus and incus. Rudimentary Eustachian tubes branch off, but their further course cannot be determined with certainty. They have connections to the pharynx. The inner ears are lying in close proximity to the middle ears and look completely normal, with well-developed cochlea and labyrinth (Figure 4D). Foramina rotunda and ovalia are present.
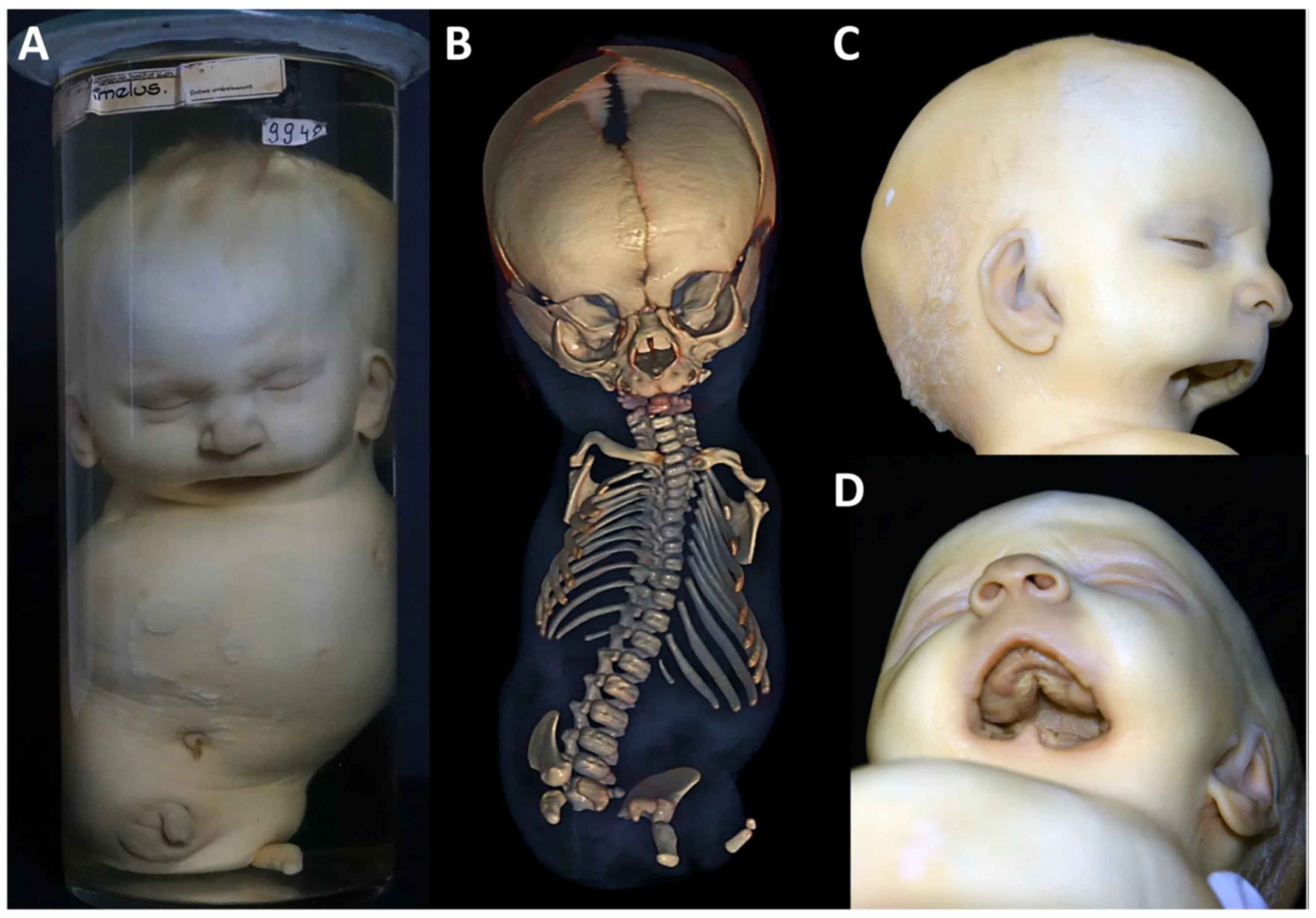
3.3. Specimen 3 (MN.9948)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFD1 | Nager syndrome |
| AFD-Rod | Acrofacial dysostosis of Rodriguez |
| AGOTC | Agnathia otocephaly complex |
| CHL/CRL/HC | Crown-heel length/crown-rump length/head circumference |
| DW/GW | Developmental week/gestational week |
| EFTUD2 | Elongation factor Tu GTP-binding domain-containing 2 |
| LGR | Leucine-rich repeat-containing G protein-coupled receptor |
| MC | Meckel’s cartilage |
| MFD | Mandibulofacial dysostosis |
| MFDM | Mandibulofacial dysostosis with microcephaly |
| MRN complex | Accumulation of Mre11, Rad50 and Nbs1 at sites of double-strand DNA breaks |
| OTX | Orthodenticle homeobox X1 |
| POLR | Polymerase |
| PRRX | Paired-related homeobox |
| RNF | Ring finger protein |
| RSPO | R-Spondin |
| SNAI | Snail family transcriptional repressor |
| SOX | SRY-BOX |
| SF3B4 | Splicing factor3 subunit 4 |
| TCOF1 | Treacle ribosome biogenesis factor |
| TC | Tessier cleft |
| TCS | Treacher Collins syndrome |
| TETAMS | Tetra-amelia syndrome |
| tfap2e | Transcription factor AP2-epsilon |
| TGF | Transforming growth factor |
| WNT | Wingless-related integration site |
| ZNRF’ | Zinc finger and ring finger protein |
References
- Fearon, J.A. Rare craniofacial clefts: A surgical classification. J. Craniofac. Surg. 2008, 19, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Hormozi, A.; Abbaszadeh-Kasbi, A.; Goravanchi, F.; Davai, N.R. Prevalence of Rare Craniofacial Clefts. J. Craniofac. Surg. 2017, 28, e467–e470. [Google Scholar] [CrossRef] [PubMed]
- Bütow, K.W.; Botha, A. A classification and construction of congenital lateral facial clefts. J. Craniomaxillofac. Surg. 2010, 38, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, K.; Dixon, J.; Trainor, P.A.; Dixon, M.J. Rare syndromes of the head and facemandibulofacial and acrofacial dysostoses. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vincent, M.; Geneviève, D.; Ostertag, A.; Marlin, S.; Lacombe, D.; Martin-Coignard, D.; Coubes, C.; David, A.; Lyonnet, S.; Vilain, C.; et al. Treacher Collins syndrome: A clinical and molecular study based on a large series of patients. Genet. Med. 2016, 18, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Drivas, T.G.; Taylor, J.A.; Zackai, E.H. The final demise of Rodriguez lethal acrofacial dysostosis: A case report and review of the literature. Am. J. Med. Genet. Part A 2019, 179, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.B.; Pina, R.; Ramos, L.; Pereira, N.; Krahn, M.; Borozdin, W.; Kohlhase, J.; Amorim, M.; Gonnet, K.; Lévy, N.; et al. Tetra-amelia and lung hypo/aplasia syndrome: New case report and review. Am. J. Med. Genet. Part A 2008, 146, 2799–2803. [Google Scholar] [CrossRef] [PubMed]
- Tessier, P. Anatomical classification facial, cranio-facial and latero-facial clefts. J. Maxillofac. Surg. 1976, 4, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Hidayatullah; Hayat, W.; Khattak, D.A.; Khan, A.; Hayat, N.; Amjad, Q.; Khan, R. Rare craniofacial clefts: Surgical management protocols. J. Plast. Reconstr. Aesthet. Surg. 2024, 97, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Versnel, S.L.; van den Elzen, M.E.; Wolvius, E.B.; Biesmeijer, C.S.; Vaandrager, J.M.; van der Meulen, J.C.; Mathijssen, I.M. Long-term results after 40 years experience with treatment of rare facial clefts: Part 1—Oblique and paramedian clefts. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Basart, H.; Suttie, M.; Ibrahim, A.; Ferretti, P.; van der Horst, C.M.A.M.; Hennekam, R.C.; Hammond, P. Objectifying Micrognathia Using Three-Dimensional Photogrammetric Analysis. J. Craniofac. Surg. 2018, 29, 2106–2109. [Google Scholar] [CrossRef] [PubMed]
- Boer, L.L.; Kircher, S.G.; Rehder, H.; Behunova, J.; Winter, E.; Ringl, H.; Scharrer, A.; de Boer, E.; Oostra, R.J. History and highlights of the teratological collection in the Narrenturm, Vienna (Austria). Am. J. Med. Genet. Part A 2023, 191, 1301–1324. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.; Teschler-Nicola, M.; Macfelda, K.; Vohland, K. The pathological anatomical collection of the Natural History Museum Vienna. Wien. Med. Wochenschr. 2024, 174, 265–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Compagnucci, C.; Martinus, K.; Griffin, J.; Depew, M.J. Programmed Cell Death Not as Sledgehammer but as Chisel: Apoptosis in Normal and Abnormal Craniofacial Patterning and Development. Front. Cell Dev. Biol. 2021, 9, 717404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoenwolf, G.C.; Bleyl, S.B.; Brauer, P.R.; Francis-West, P.H. Larsen’s Human Embryology, 4th ed.; Churchill Livingstone: London, UK, 2009; 687p. [Google Scholar]
- Marchini, M.; Keller, G.; Khan, N.; Shah, R.; Saliceti Galarza, A.; Starr, K.B.; Apostopoulos, A.; Sanger, T.J. Sonic hedgehog and fibroblast growth factor 8 regulate the evolution of amniote facial proportions. Commun. Biol. 2025, 8, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Percival, C.J.; Green, R.; Roseman, C.C.; Gatti, D.M.; Morgan, J.L.; Murray, S.A.; Donahue, L.R.; Mayeux, J.M.; Pollard, K.M.; Hua, K.; et al. Developmental constraint through negative pleiotropy in the zygomatic arch. Evodevo 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lisson, J.A.; Kjaer, I. Location of alveolar clefts relative to the incisive fissure. Cleft Palate Craniofac. J. 1997, 34, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Helwany, M.; Arbor, T.C.; Tadi, P. Embryology, Ear. 2023 Aug 8. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Anthwal, N.; Thompson, H. The development of the mammalian outer and middle ear. J. Anat. 2016, 228, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology—E-Book: Development, Structure, and Function; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; p. 507. [Google Scholar] [PubMed Central]
- Radlanski, R.J. Oral Structure & Biology; Quintessence Publishing Co Inc.: Batavia, IL, USA, 2018; ISBN 9780867157468. [Google Scholar]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 2010, 142, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, G. 2. Osteometrie. a) Kraniometrie. In Anthropologie. Handbuch der Vergleichenden Biologie des Menschen Band 1; Knußmann, R., Ed.; Gustav Fischer Verlag: New York, NY, USA, 1988; pp. 160–192. [Google Scholar]
- Allanson, J.E.; Cunniff, C.; Hoyme, H.E.; McGaughran, J.; Muenke, M.; Neri, G. Elements of morphology: Standard terminology for the head and face. Am. J. Med. Genet. Part A 2009, 149A, 6–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, J.G.; Allanson, J.E.; Gripp, K.W.; Slavotinek, A.M. Handbook of Physical Measurements; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Lu, K.; Ye, W.; Zhou, L.; Collins, L.B.; Chen, X.; Gold, A.; Ball, L.M.; Swenberg, J.A. Structural characterization of formaldehyde-induced cross-links between amino acids and deoxynucleosides and their oligomers. J. Am. Chem. Soc. 2010, 132, 3388–3399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dauwerse, J.G.; Dixon, J.; Seland, S.; Ruivenkamp, C.A.; van Haeringen, A.; Hoefsloot, L.H.; Peters, D.J.; Boers, A.C.; Daumer-Haas, C.; Maiwald, R.; et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat. Genet. 2011, 43, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Laplace-Builhé, B.; Mau-Them, F.T.; Richard, E.; Goldenberg, A.; Toler, T.L.; Guignard, T.; Gatinois, V.; Vincent, M.; Blanchet, C.; et al. POLR1B and neural crest cell anomalies in Treacher Collins syndrome type 4. Genet. Med. 2020, 22, 547–556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadakia, S.; Helman, S.N.; Badhey, A.K.; Saman, M.; Ducic, Y. Treacher Collins Syndrome: The genetics of a craniofacial disease. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.; Collet, C.; Genevieve, D.; Vincent, M.; Lohmann, D.R.; Sanchez, E.; Bolender, C.; Eliot, M.M.; Nürnberg, G.; Passos-Bueno, M.R.; et al. Autosomal recessive POLR1D mutation with decrease of TCOF1 mRNA is responsible for Treacher Collins syndrome. Genet. Med. 2014, 16, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, B.; Yang, H.; Henning, D.; Valdez, B.C. Cloning and functional characterization of the Xenopus orthologue of the Treacher Collins syndrome (TCOF1) gene product. Gene 2005, 359, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Noack Watt, K.E.; Achilleos, A.; Neben, C.L.; Merrill, A.E.; Trainor, P.A. The Roles of RNA Polymerase I and III Subunits Polr1c and Polr1d in Craniofacial Development and in Zebrafish Models of Treacher Collins Syndrome. PLoS Genet. 2016, 12, e1006187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, P.; Pan, B.; Jiang, H.; Yang, Q.; He, L.; Lin, L. Prevention methods for Treacher Collins syndrome: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 134, 110062. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.C.; Lynn, M.L.; Gaudenz, K.; Sakai, D.; Aoto, K.; Rey, J.P.; Glynn, E.F.; Ellington, L.; Du, C.; Dixon, J.; et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat. Med. 2008, 14, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Dixon, J.; Achilleos, A.; Dixon, M.; Trainor, P.A. Prevention of Treacher Collins syndrome craniofacial anomalies in mouse models via maternal antioxidant supplementation. Nat. Commun. 2016, 7, 10328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weaver, K.N.; Watt, K.E.; Hufnagel, R.B.; Navajas Acedo, J.; Linscott, L.L.; Sund, K.L.; Bender, P.L.; König, R.; Lourenco, C.M.; Hehr, U.; et al. Acrofacial Dysostosis, Cincinnati Type, a Mandibulofacial Dysostosis Syndrome with Limb Anomalies, Is Caused by POLR1A Dysfunction. Am. J. Hum. Genet. 2015, 96, 765–774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marques, F.; Tenney, J.; Duran, I.; Martin, J.; Nevarez, L.; Pogue, R.; Krakow, D.; Cohn, D.H.; Li, B. Altered mRNA Splicing, Chondrocyte Gene Expression and Abnormal Skeletal Development due to SF3B4 Mutations in Rodriguez Acrofacial Dysostosis. PLoS Genet. 2016, 12, e1006307, Erratum in PLoS Genet. 2016, 12, e1006502. https://doi.org/10.1371/journal.pgen.1006502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McPherson, E.; Zaleski, C.; Ye, Z.; Lin, S. Rodriguez syndrome with SF3B4 mutation: A severe form of Nager syndrome? Am. J. Med. Genet. Part A 2014, 164A, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Bernier, F.P.; Caluseriu, O.; Ng, S.; Schwartzentruber, J.; Buckingham, K.J.; Innes, A.M.; Jabs, E.W.; Innis, J.W.; Schuette, J.L.; Gorski, J.L.; et al. Haploinsufficiency of SF3B4, a component of the pre-mRNA spliceosomal complex, causes Nager syndrome. Am. J. Hum. Genet. 2012, 90, 925–933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, J.I.; Palacios, J.; Urioste, M. New acrofacial dysostosis syndrome in 3 sibs. Am. J. Med. Genet. 1990, 35, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Lines, M.A.; Huang, L.; Schwartzentruber, J.; Douglas, S.L.; Lynch, D.C.; Beaulieu, C.; Guion-Almeida, M.L.; Zechi-Ceide, R.M.; Gener, B.; Gillessen-Kaesbach, G.; et al. Haploinsufficiency of a spliceosomal GTPase encoded by EFTUD2 causes mandibulofacial dysostosis with microcephaly. Am. J. Hum. Genet. 2012, 90, 369–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffin, C.; Saint-Jeannet, J.P. Spliceosomopathies: Diseases and mechanisms. Dev. Dyn. 2020, 249, 1038–1046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, B.Y.; Tachi-Duprat, M.; Ihewulezi, C.; Devotta, A.; Saint-Jeannet, J.P. The Core Splicing Factors EFTUD2, SNRPB and TXNL4A Are Essential for Neural Crest and Craniofacial Development. J. Dev. Biol. 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beauchamp, M.C.; Djedid, A.; Bareke, E.; Merkuri, F.; Aber, R.; Tam, A.S.; Lines, M.A.; Boycott, K.M.; Stirling, P.C.; Fish, J.L.; et al. Mutation in Eftud2 causes craniofacial defects in mice via mis-splicing of Mdm2 and increased P53. Hum. Mol. Genet. 2021, 30, 739–757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wood, K.A.; Rowlands, C.F.; Qureshi, W.M.S.; Thomas, H.B.; Buczek, W.A.; Briggs, T.A.; Hubbard, S.J.; Hentges, K.E.; Newman, W.G.; O’Keefe, R.T. Disease modeling of core pre-mRNA splicing factor haploinsufficiency. Hum. Mol. Genet. 2019, 28, 3704–3723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maharana, S.K.; Saint-Jeannet, J.P. Molecular mechanisms of hearing loss in Nager syndrome. Dev. Biol. 2021, 476, 200–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ades, L.C.; Sillence, D.O. Agnathia-holoprosencephaly with tetramelia. Clin. Dysmorphol. 1992, 1, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Falcon, O.; Coteron, J.J.; Ocon, L.; Zubiria, A.; Garcia, J.A. A case of agnathia, tetramelia and diaphragmatic hernia at 18 weeks’ gestation. Ultrasound Obstet. Gynecol. 2004, 23, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S.; Sonoda, T.; Ohba, K. Natural history and postmortem anatomy of a patient with tetra-amelia, ectodermal dysplasia, peculiar face, and developmental retardation (MIM 273390). J. Med. Genet. 1994, 31, 980–981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Ragavan, M.; Reddy, S.; Kumar, C. Tetra-amelia with lung hypoplasia and facial clefts, Roberts-SC syndrome: Report of two cases. Pediatr. Surg. Int. 2010, 26, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Schwickert, A.; Dame, C.; Akanbi, S.; Spielmann, M.; Schönborn, I.; Henrich, W. Pränatale Diagnostik und postnatale Komplikationen im Fall einer extrem seltenen Tetraamelie [Prenatal Diagnostics and Postnatal Complications in a Case of Extremely Rare Tetra-Amelia]. Z. Geburtshilfe Neonatol. 2021, 225, 279–282. (In German) [Google Scholar] [CrossRef] [PubMed]
- Horn, M.P.; Khaja, M.S.; Stucken, E.Z.; Saad, W.E. Collaborative Establishment of Difficult Vascular Access for General Anesthetic Management of an Adult With Tetra-Amelia: A Case Report. A&A Pract. 2018, 11, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Nieman, S.; Zhao, C.; Pascu, F.; Stahl, U.; Aulepp, U.; Niswander, L.; Weber, J.L.; Müller, U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am. J. Hum. Genet. 2004, 74, 558–563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szenker-Ravi, E.; Altunoglu, U.; Leushacke, M.; Bosso-Lefèvre, C.; Khatoo, M.; Thi Tran, H.; Naert, T.; Noelanders, R.; Hajamohideen, A.; Beneteau, C.; et al. RSPO2 inhibition of RNF43 and ZNRF3 governs limb development independently of LGR4/5/6. Nature 2018, 557, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Weikard, R.; Schulze, C.; Wohlsein, P.; Kühn, C. A 50-kb deletion disrupting the rspo2 gene is associated with tetradysmelia in Holstein Friesian cattle. Genet. Sel. Evol. 2020, 52, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Svandova, E.; Anthwal, N.; Tucker, A.S.; Matalova, E. Diverse Fate of an Enigmatic Structure: 200 Years of Meckel’s Cartilage. Front. Cell Dev. Biol. 2020, 8, 821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alexander, N.L.; Chandy, B.; Barton, G.; Liu, Y.C. A case of rare isolated agnathia and literature review. Am. J. Med. Genet. Part A 2020, 182, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Patat, O.; van Ravenswaaij-Arts, C.M.; Tantau, J.; Corsten-Janssen, N.; van Tintelen, J.P.; Dijkhuizen, T.; Kaplan, J.; Chassaing, N. Otocephaly-Dysgnathia Complex: Description of Four Cases and Confirmation of the Role of OTX2. Mol. Syndromol. 2013, 4, 302–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lunt, R.C.; Law, D.B. A review of the chronology of eruption of deciduous teeth. J. Am. Dent. Assoc. 1974, 89, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Schlump, J.U.; Stein, A.; Hehr, U.; Karen, T.; Möller-Hartmann, C.; Elcioglu, N.H.; Bogdanova, N.; Woike, H.F.; Lohmann, D.R.; Felder-hoff-Mueser, U.; et al. Treacher Collins syndrome: Clinical implications for the paediatrician—A new mutation in a severely affected newborn and comparison with three further patients with the same mutation, and re-view of the literature. Eur. J. Pediatr. 2012, 171, 1611–1618. [Google Scholar] [CrossRef] [PubMed]


| Skeletal Features | Specimen 1 | TCS | Specimen 2 | AFD-Rod | Specimen 3 | TETAMS |
|---|---|---|---|---|---|---|
| CLEFT TYPE | TC-6, 7 and 8 | TC-6, 7, 8 (X-ray) (eyelid coloboma and macrostomia) | TC-6, 7 and 8 | TC-6 ? (defect of lower eyelashes) | TC-7 | TC-7 (1 patient) |
| MAXILLA | ||||||
| Lateral maxilla | A | hypoplastic | rudimentary | hypoplastic | NN | N |
| Premaxilla | narrow, severely hypopl., displaced upwards | midline hypoplasia | narrow, hypopl. | short philtrum | narrow, hypopl. | n.d. |
| Primary palate | hypoplastic | n.d. | hypoplastic | n.d. | hypoplastic | n.d. |
| Secondary palate | A | cleft, high arched | A | cleft, high arched | narrow, cleft, high arched, | cleft lip and palate |
| Tongue | N | glossoptosis | glossoptosis | n.d. | hypoplastic | Ankyloglossia |
| Dentition | defective | defective | defective | n.d. | N crowded | n.d. |
| Choanae | stenosis | atresia/stenosis | atresia | stenosis/atresia | N | Atresia |
| MANDIBLE | ||||||
| Corpus | shortened, sev. micrognathia | hypoplastic, sev. micrognathia | defective, sev. micrognathia | hypoplastic, sev. micrognathia, microstomia | A | micrognathia, agnathia in 2 cases, no medial fusion |
| Rami/angles | hypoplastic | hypoplastic | A | n.d. | rudimentary | n.d. |
| Condylus | deformed | n.d. | A | n.d. | deformed | n.d. |
| Coronoid proc. | hypoplastic | n.d. | A | n.d. | deformed | n.d. |
| Dentition | N | defective | defective | n.d. | A | n.d. |
| OTHER CRANIAL BONES | ||||||
| Zygomatic bone | A | A/ hypoplastic | A | malar hypoplasia | N | NN |
| Zygomatic arch | A | hypoplastic | A | hypoplastic | shortened | NN |
| Sphenoid bone | hypoplastic/ fused | hypoplastic | hypoplastic/ fragmented | hypoplastic | N | NN |
| Nasal bone | rudimentary | hypoplastic | caudal and rostral cleft | prominent nose | N | NN |
| Ethmoidal bone | hypoplastic/ fused | hypoplastic | hypoplastic/ fragmented | hypoplastic | N | lacr. duct abnomal |
| Temporal bone | hypoplastic/ fused | hypoplastic | hypoplastic/ fragmented | hypoplastic | NN | NN |
| Orbits | A (lower rim) | hypoplastic (upper rim) | A (lateral and lower rim) | hypoplastic (upper rim) | elongated and distorted downward | hypertelorism and deep-set eyes |
| EAR STRUCTURES | ||||||
| Inner ear | malformed cochlea and labyrinth | n.d. | N | n.d. | N | N |
| Middle ear | rudimentary | conductive, hearing loss | rudimentary | n.d. | N | N |
| Ossicles | A | n.d. | A (incus and malleolus) | n.d. | N | N |
| Eustachian tube | N air-filled | n.d. | rudimentary, non-air-filled | n.d. | N non-air-filled | n.d. |
| Tympanic membrane | A | n.d. | A | n.d. | not identifiable | N |
| Auditory canal | blind fistulas | blind fistulas | stenotic | blind fistulas | stenotic | N |
| Auricles | Aear tag | hypo-/dysplastic, and ear tags | absent upper auricles and 3-lobed lower tags | low set and hypo-/dysplastic | N, low set and | low set, mildly hypoplastic |
| MO grade IV | MO grade I–III | MO grade III | MO grade I–III | macrotia | MO grade I | |
| EXTRAFACIAL SKELETON | N | N | tetraphocomelia absent pelvis and hypopl.scapulae | phocomelia, hypoplast. pelvis and scapulae | tetra-amelia, hypoplastic pelvis | tetra-amelia/ tetradysmelia, hypoplastic pelvis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behunova, J.; Rehder, H.; Dobsak, A.; Kircher, S.G.; Boer, L.L.; Mueller, A.A.; Patsch, J.M.; Winter, E.; Oostra, R.-J.; Piehslinger, E.; et al. Facial Bone Defects Associated with Lateral Facial Clefts Tessier Type 6, 7 and 8 in Syndromic Neurocristopathies: A Detailed Micro-CT Analysis on Historical Museum Specimens. Biology 2025, 14, 872. https://doi.org/10.3390/biology14070872
Behunova J, Rehder H, Dobsak A, Kircher SG, Boer LL, Mueller AA, Patsch JM, Winter E, Oostra R-J, Piehslinger E, et al. Facial Bone Defects Associated with Lateral Facial Clefts Tessier Type 6, 7 and 8 in Syndromic Neurocristopathies: A Detailed Micro-CT Analysis on Historical Museum Specimens. Biology. 2025; 14(7):872. https://doi.org/10.3390/biology14070872
Chicago/Turabian StyleBehunova, Jana, Helga Rehder, Anton Dobsak, Susanne G. Kircher, Lucas L. Boer, Andreas A. Mueller, Janina M. Patsch, Eduard Winter, Roelof-Jan Oostra, Eva Piehslinger, and et al. 2025. "Facial Bone Defects Associated with Lateral Facial Clefts Tessier Type 6, 7 and 8 in Syndromic Neurocristopathies: A Detailed Micro-CT Analysis on Historical Museum Specimens" Biology 14, no. 7: 872. https://doi.org/10.3390/biology14070872
APA StyleBehunova, J., Rehder, H., Dobsak, A., Kircher, S. G., Boer, L. L., Mueller, A. A., Patsch, J. M., Winter, E., Oostra, R.-J., Piehslinger, E., & Reich, K. M. (2025). Facial Bone Defects Associated with Lateral Facial Clefts Tessier Type 6, 7 and 8 in Syndromic Neurocristopathies: A Detailed Micro-CT Analysis on Historical Museum Specimens. Biology, 14(7), 872. https://doi.org/10.3390/biology14070872







