Seasonal Variation in Flower Traits, Visitor Traits, and Reproductive Success of Solanum sisymbriifolium Lamarck (Solanaceae) in the Rarh Region of West Bengal, India
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plant Species
2.3. Floral Biology
2.4. Mating System
2.5. Floral Visitors
2.6. Pollinating Strategies of Visitors
2.7. Seasonal Reproductive Success
2.8. Statistical Analysis
3. Results
3.1. Floral Biology
3.2. Mating System
3.3. Floral Visitors
3.4. Seasonal Plant Reproduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fornoff, F.; Klein, A.M.; Hartig, F.; Benadi, G.; Venjakob, C.; Schaefer, H.M.; Ebeling, A. Functional flower traits and their diversity drive pollinator visitation. Oikos 2017, 126, 1020–1030. [Google Scholar] [CrossRef]
- Gillespie, M.A.; Baude, M.; Biesmeijer, J.; Boatman, N.; Budge, G.E.; Crowe, A.; Davies, N.; Evans, R.; Memmott, J.; Morton, R.D.; et al. Flowering plant communities mediate the effects of habitat composition and configuration on wild pollinator communities. Funct. Ecol. 2024, 38, 2576–2594. [Google Scholar] [CrossRef]
- Rering, C.C.; Vannette, R.L.; Schaeffer, R.N.; Beck, J.J. Microbial co-occurrence in floral nectar affects metabolites and attractiveness to a generalist pollinator. J. Chem. Ecol. 2020, 46, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Sponsler, D.; Iverson, A.; Steffan-Dewenter, I. Pollinator competition and the structure of floral resources. Ecography 2023, 2023, e06651. [Google Scholar] [CrossRef]
- Zeevaart, J.A. Physiology of Flowering: Flowering is hormonally controlled, but the nature of the hormone remains to be elucidated. Science 1962, 137, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Chailakhyan, M.K.; Timiriazev, K.A. Genetic and hormonal regulation of growth, flowering, and sex expression in plants. Am. J. Bot. 1979, 66, 717–736. [Google Scholar] [CrossRef]
- Shillo, R.; Halevy, A.H. The effect of various environmental factors on flowering of gladiolus. III. Temperature and moisture. Sci. Hortic. 1976, 4, 147–155. [Google Scholar] [CrossRef]
- Lee, Z.; Kim, S.; Choi, S.J.; Joung, E.; Kwon, M.; Park, H.J.; Shim, J.S. Regulation of flowering time by environmental factors in plants. Plants 2023, 12, 3680. [Google Scholar] [CrossRef] [PubMed]
- Layek, U.; Das, N.; Samanta, A.; Karmakar, P. Impact of seasonal atmospheric factors and photoperiod on floral biology, plant–pollinator interactions, and plant reproduction on Turnera ulmifolia L. (Passifloraceae). Biology 2025, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Gentry, A.H. Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 1974, 6, 64–68. [Google Scholar] [CrossRef]
- Frankie, G.W.; Baker, H.G.; Opler, P.A. Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J. Ecol. 1974, 62, 881–919. [Google Scholar] [CrossRef]
- Bawa, K.S. Patterns of flowering in tropical plants. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1983; pp. 394–410. [Google Scholar]
- Koptur, S. Outcrossing and pollinator limitation of fruit set: Breeding systems of neotropical Inga trees (Fabaceae: Mimosoideae). Evolution 1984, 38, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Bawa, K.S.; Kang, H.; Grayum, M.H. Relationships among time, frequency, and duration of flowering in tropical rain forest trees. Am. J. Bot. 2003, 90, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Delph, L.F.; Ashman, T.L. Trait selection in flowering plants: How does sexual selection contribute? Integr. Comp. Biol. 2006, 46, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Goodwillie, C.; Sargent, R.D.; Eckert, C.G.; Elle, E.; Geber, M.A.; Johnston, M.O.; Kalisz, S.; Moeller, D.A.; Ree, R.H.; Vallejo-Marin, M.; et al. Correlated evolution of mating system and floral display traits in flowering plants and its implications for the distribution of mating system variation. New Phytol. 2010, 185, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Price, M.V.; Waser, N.M.; Irwin, R.E.; Campbell, D.R.; Brody, A.K. Temporal and spatial variation in pollination of a montane herb: A seven-year study. Ecology 2005, 86, 2106–2116. [Google Scholar] [CrossRef]
- Layek, U.; Das, A.D.; Das, U.; Karmakar, P. Spatial and temporal variations in richness, diversity and abundance of floral visitors of curry plants (Bergera koenigii L.): Insights on plant-pollinator interactions. Insects 2024, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Spears Jr, E.E. A direct measure of pollinator effectiveness. Oecologia 1983, 57, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Layek, U.; Kundu, A.; Bisui, S.; Karmakar, P. Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: A comparative study. Ann. Agric. Sci. 2021, 66, 38–45. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- LeBuhn, G.; Luna, J.V. Pollinator decline: What do we know about the drivers of solitary bee declines? Curr. Opin. Insect Sci. 2021, 46, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.C. The effects of air humidity on flowering, fruit set, seed set and fruit growth of glasshouse sweet pepper (Capsicum annuum L.). Sci. Hortic. 1989, 40, 1–8. [Google Scholar] [CrossRef]
- Tutin, C.E.; Fernandez, M. Relationships between minimum temperature and fruit production in some tropical forest trees in Gabon. J. Trop. Ecol. 1993, 9, 241–248. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Kluge, J.; Gareca, Y.; Reichle, S.; Kessler, M. The influence of climatic seasonality on the diversity of different tropical pollinator groups. PLoS ONE 2011, 6, e27115. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.A.; Rands, S.A. The effects of rainfall on plant–pollinator interactions. Arthropod-Plant Interact. 2019, 13, 561–569. [Google Scholar] [CrossRef]
- Chakravarty, A.K.; Mukhopadhyay, S.; Saha, S.; Pakrashi, S.C. A neolignan and sterols in fruits of Solanum sisymbrifolium. Phytochemistry 1996, 41, 935–939. [Google Scholar] [CrossRef]
- Gupta, V.K.; Simlai, A.; Tiwari, M.; Bhattacharya, K.; Roy, A. Phytochemical contents, antimicrobial and antioxidative activities of Solanum sisymbriifolium. J. Appl. Pharm. Sci. 2014, 4, 75–80. [Google Scholar] [CrossRef]
- Gebrewbet, G.H.; Hndeya, A.G. Phytochemical screening and antibacterial activity studies on the crude leaf extract of Solanum sisymbriifolium: Traditional Ethiopian medicinal plant. Adv. Gut Microbiome Res. 2023, 2023, 5525606. [Google Scholar] [CrossRef]
- Hellión-lbarrola, M.C.; Montalbetti, Y.; Heinichen, O.; Alvarenga, N.; Figueredo, A.; Ferro, E.A. Isolation of hypotensive compounds from Solanum sisymbriifolium Lam. J. Ethnopharmacol. 2000, 70, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ferro, E.A.; Alvarenga, N.L.; Ibarrola, D.A.; Hellión-Ibarrola, M.D.C.; Ravelo, A.G. A new steroidal saponin from Solanum sisymbriifolium roots. Fitoterapia 2005, 76, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Perpétuo, L.S.; da Cunha, M.J.; Batista, M.T.; Conceição, I.L. Solanum linnaeanum and Solanum sisymbriifolium as a sustainable strategy for the management of Meloidogyne chitwoodi. Sci. Rep. 2021, 11, 3484. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Dutta, B.K. Reproductive biology of Solanum sisymbriifolium Lamk. (Solanaceae) in Tripura, North-east India. Int. J. Plant Reprod. Biol. 2017, 9, 59–62. [Google Scholar]
- Hill, M.P.; Hulley, P. Aspects of the phenology and ecology of the South American weed, Solanum sisymbriifolium, in the Eastern Cape province of South Africa. Afr. Plant Prot. 2000, 6, 53–59. [Google Scholar]
- Hopkins, H.C. Floral biology and pollination ecology of the neotropical species of Parkia. J. Ecol. 1984, 72, 1–23. [Google Scholar] [CrossRef]
- Layek, U.; Bhagira, N.K.; Das, A.; Kundu, A.; Karmakar, P. Dependency of crops on pollinators and pollination deficits: An approach to measure them in considering the influence of various reproductive traits. Agriculture 2023, 13, 1563. [Google Scholar] [CrossRef]
- Erdtman, G. The acetolysis method. A revised description. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Norton, D.J. Testing of plum pollen viability with tetrazolium salts. J. Am. Soc. Hortic. Sci. 1966, 89, 132–134. [Google Scholar]
- Parfitt, E.D.; Ganeshan, S. Comparison of procedures for estimating viability of Prunus pollen. HortScience 1989, 24, 354–356. [Google Scholar] [CrossRef]
- Dafni, A.; Maués, M.M. A rapid and simple procedure to determine stigma receptivity. Sex. Plant Reprod. 1998, 11, 177–180. [Google Scholar] [CrossRef]
- Raduski, A.R.; Haney, E.B.; Igic, B. The expression of self-incompatibility in angiosperms is bimodal. Evolution 2012, 66, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Layek, U.; Das, U.; Karmakar, P. The pollination efficiency of a pollinator depends on its foraging strategy, flowering phenology, and the flower characteristics of a plant species. J. Asia-Pac. Entomol. 2022, 25, 101882. [Google Scholar] [CrossRef]
- Margalef, R. Temporal succession and spatial heterogeneity in phytoplankton. In Perspectives in Marine Biology; Buzzati-Traverso, A.A., Ed.; University of California Press: Berke ley, CA, USA, 1958; pp. 323–347. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; p. 117. [Google Scholar]
- Augspurger, C.K. Reproductive synchrony of a tropical shrub (Hybanthus prunifolius): Influence on pollinator attraction and movement. Ecology 1981, 62, 774–788. [Google Scholar] [CrossRef]
- Martínez-Sánchez, J.J.; Segura, F.; Aguado, M.; Franco, J.A.; Vicente, M.J. Life history and demographic features of Astragalus nitidiflorus, a critically endangered species. Flora 2011, 206, 423–432. [Google Scholar] [CrossRef]
- Karsai, I.; Kőszegi, B.; Kovács, G.; Szűcs, P.; Mészáros, K.; Bedő, Z.; Veisz, O. Effects of temperature and light intensity on flowering of barley (Hordeum vulgare L.). Biol. Futura 2008, 59, 205–215. [Google Scholar] [CrossRef]
- Omolaja, S.S.; Aikpokpodion, P.; Oyedeji, S.; Vwioko, D.E. Rainfall and temperature effects on flowering and pollen productions in cocoa. Afr. Crop Sci. J. 2009, 17, 41–48. [Google Scholar] [CrossRef]
- Aronne, G.; Buonanno, M.; De Micco, V. Reproducing under a warming climate: Long winter flowering and extended flower longevity in the only Mediterranean and maritime Primula. Plant Biol. 2015, 17, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Torres-Díaz, C.; Gomez-Gonzalez, S.; Stotz, G.C.; Torres-Morales, P.; Paredes, B.; Pérez-Millaqueo, M.; Gianoli, E. Extremely long-lived stigmas allow extended cross-pollination opportunities in a high Andean plant. PLoS ONE 2011, 6, e19497. [Google Scholar] [CrossRef] [PubMed]
- Vesprini, J.; Pacini, E. Temperature-dependent floral longevity in two Helleborus species. Plant Syst. Evol. 2005, 252, 63–70. [Google Scholar] [CrossRef]
- Stace, H.M. Protogyny, self-incompatibility and pollination in Anthocercis gracilis (Solanaceae). Aust. J. Bot. 1995, 43, 451–459. [Google Scholar] [CrossRef]
- Mione, T.; Plourd, K.C.; Leiva González, S.; Yacher, L.; Philbrick, C.T.; Blume, D. Pollination decreases longevity of the protogynous flowers of Jaltomata sinuosa (Solanaceae). J. Biol. Nat. 2017, 7, 123–129. [Google Scholar]
- Wan JinPeng, W.J.; Zhu XingFu, Z.X.; Li QingJun, L.Q. Breeding system of protogynous Mandragora caulescens (Solanaceae). Plant Divers. Resour. 2011, 33, 565–570. [Google Scholar]
- Easlon, H.M.; Richards, J.H. Drought response in self-compatible species of tomato (Solanaceae). Am. J. Bot. 2009, 96, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Behling, W.L.; Douches, D.S. The effect of self-compatibility factors on interspecific compatibility in Solanum Section Petota. Plants 2023, 12, 1709. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Marín, M.; O’Brien, H.E. Correlated evolution of self-incompatibility and clonal reproduction in Solanum (Solanaceae). New Phytol. 2007, 173, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Kostyun, J.L. Functional gametophytic self-incompatibility in a peripheral population of Solanum peruvianum (Solanaceae). Heredity 2011, 107, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Schoen, D.J.; Morgan, M.T.; Bataillon, T. How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1281–1290. [Google Scholar]
- Pannell, J.; Barrett, S. Baker’s Law revisited: Reproductive assurance in a metapopulation. Evolution 1998, 52, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Baker, H. Self-compatibility and establishment after ‘long distance’ dispersal. Evolution 1955, 9, 347–349. [Google Scholar] [PubMed]
- Charlesworth, D. Evolution of plant breeding systems. Curr. Biol. 2006, 16, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Oyelana, O.A.; Ogunwenmo, K.O. Floral biology and the effects of plant-pollinator interaction on pollination intensity, fruit and seed set in Solanum. Afr. J. Biotechnol. 2012, 11, 14967–14981. [Google Scholar]
- Jayasinghe, U.S.R.; Silva, T.S.E.; Karunaratne, W.I.P. Buzzing wild bee visits enhance seed set in eggplant, Solanum melongena. Psyche 2017, 2017, 4624062. [Google Scholar] [CrossRef]
- Webber, S.M.; Garratt, M.P.; Lukac, M.; Bailey, A.P.; Huxley, T.; Potts, S.G. Quantifying crop pollinator-dependence and pollination deficits: The effects of experimental scale on yield and quality assessments. Agric. Ecosyst. Environ. 2020, 304, 107106. [Google Scholar] [CrossRef]
- Tayal, M.; Kariyat, R. Examining the role of buzzing time and acoustics on pollen extraction of Solanum elaeagnifolium. Plants 2021, 10, 2592. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Marín, M.; Pereira Nunes, C.E.; Russell, A.L. Anther cones increase pollen release in buzz-pollinated Solanum flowers. Evolution 2022, 76, 931–945. [Google Scholar] [CrossRef] [PubMed]
- McCall, C.; Primack, R.B. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am. J. Bot. 1992, 79, 434–442. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.M.; Schumacher, J.; Weisser, W.W.; Tscharntke, T. How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 2008, 117, 1808–1815. [Google Scholar] [CrossRef]
- Vrdoljak, S.M.; Samways, M.J.; Simaika, J.P. Pollinator conservation at the local scale: Flower density, diversity and community structure increase flower visiting insect activity to mixed floral stands. J. Insect Conserv. 2016, 20, 711–721. [Google Scholar] [CrossRef]
- Moulton, C.A.; Gough, L. Effects of soil nutrient availability on the role of sexual reproduction in an Alaskan tundra plant community. Arct. Antarct. Alp. Res. 2011, 43, 612–620. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M.; Thomas, J.F. Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant Cell Environ. 2000, 23, 719–726. [Google Scholar] [CrossRef]
- Falque, M.; Vincent, A.; Vaissiere, B.E.; Eskes, A.B. Effect of pollination intensity on fruit and seed set in cacao (Theobroma cacao L.). Sex. Plant Reprod. 1995, 8, 354–360. [Google Scholar] [CrossRef]
- Albrecht, M.; Schmid, B.; Hautier, Y.; Müller, C.B. Diverse pollinator communities enhance plant reproductive success. Proc. R. Soc. B Biol. Sci. 2012, 279, 4845–4852. [Google Scholar] [CrossRef] [PubMed]
- Strelin, M.M.; Aizen, M.A. The interplay between ovule number, pollination and resources as determinants of seed set in a modular plant. Peer J. 2018, 6, e5384. [Google Scholar] [CrossRef] [PubMed]




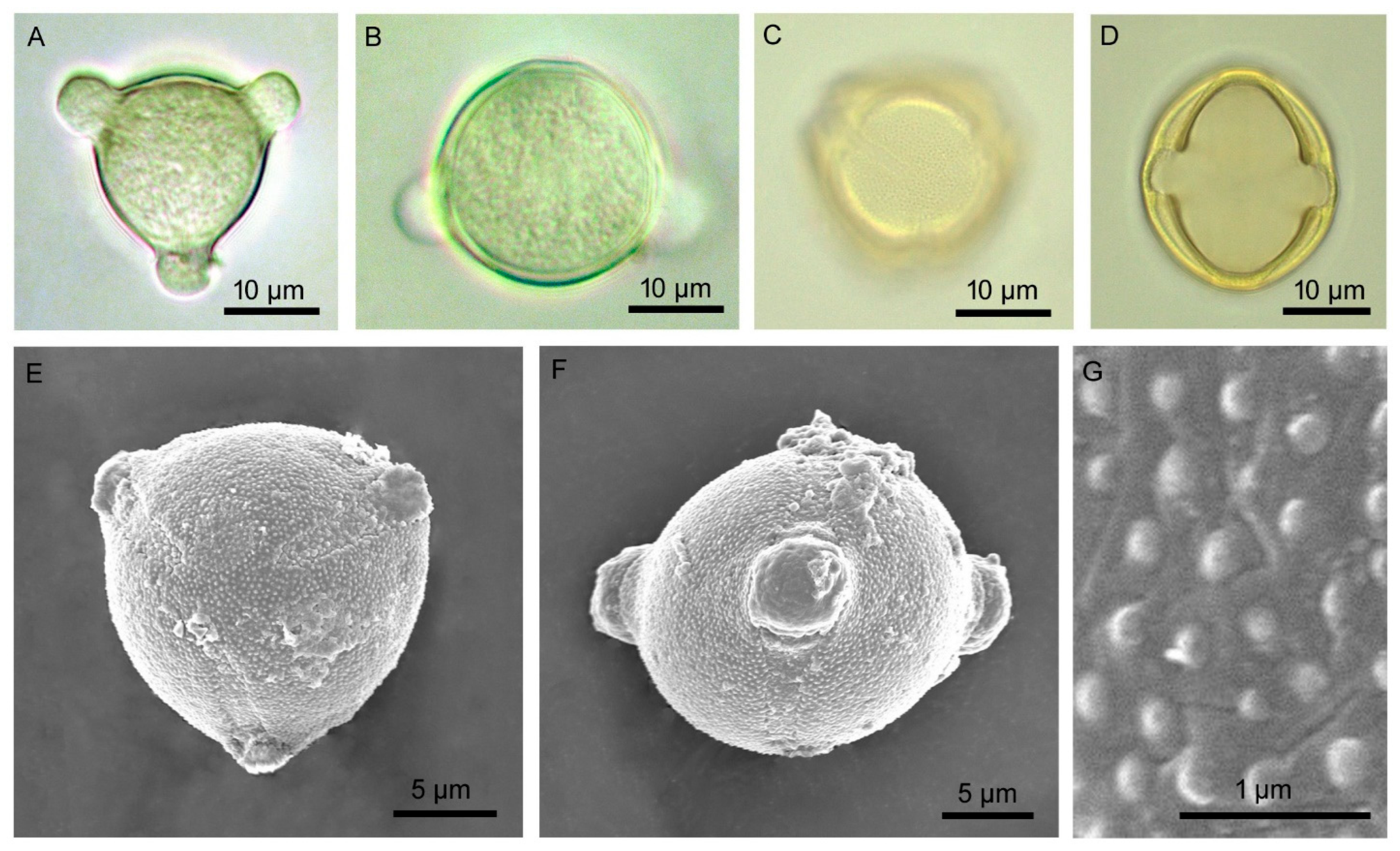
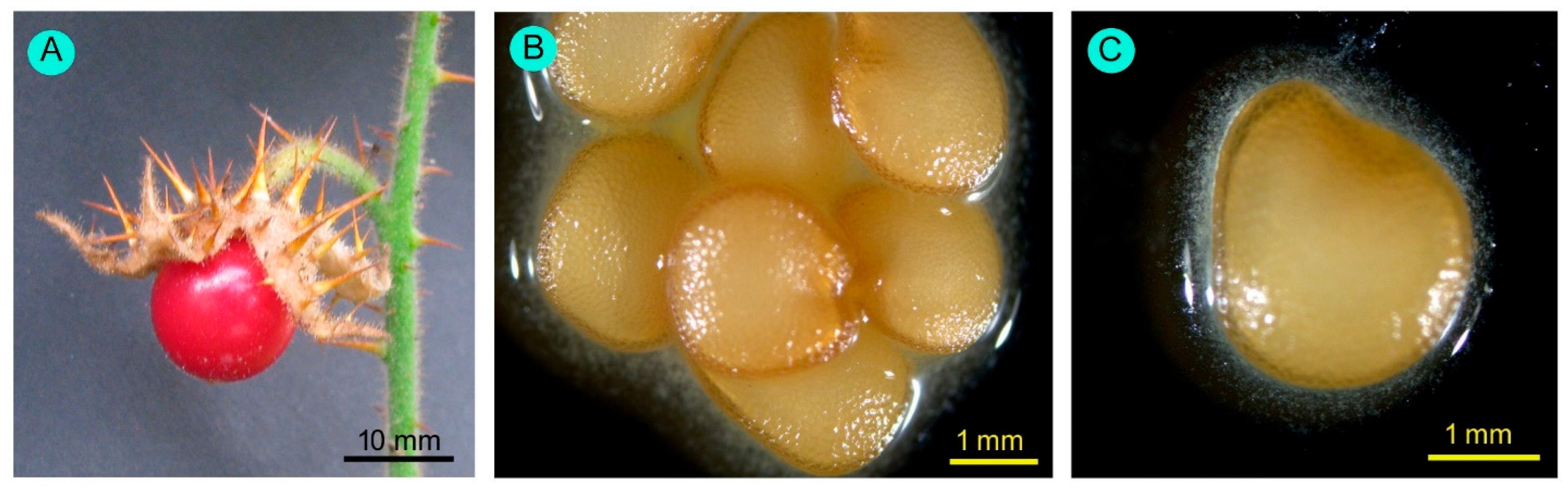



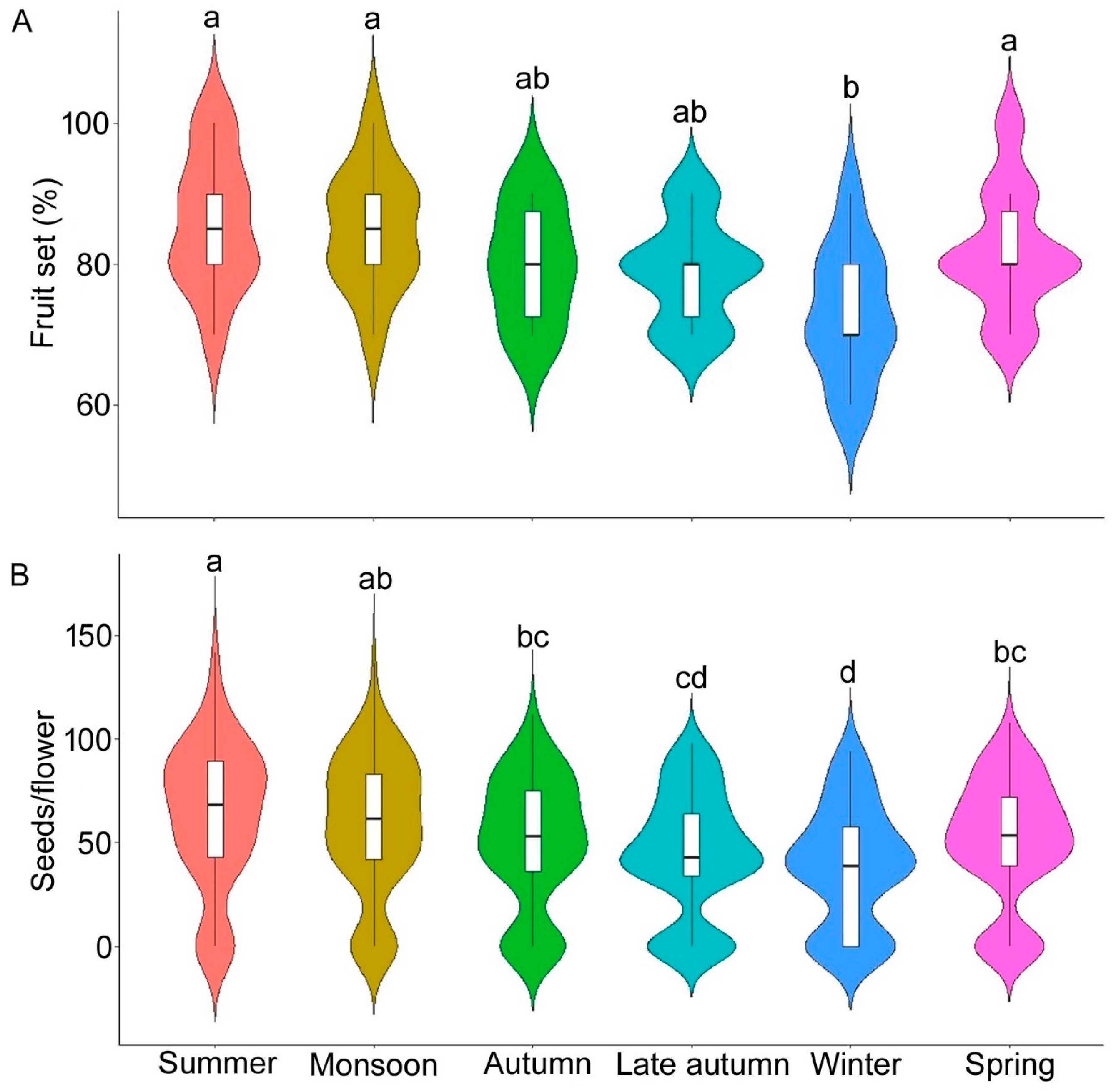
| Flower Traits | Summer | Monsoon | Autumn | Late Autumn | Winter | Spring | Statistics |
|---|---|---|---|---|---|---|---|
| Flowering intensity | 3.85 a ± 4.61 | 4.12 a ± 5.12 | 3.03 ab ± 2.97 | 2.13 bc ± 1.98 | 1.33 c ± 1.46 | 3.47 a ± 2.49 | χ2 = 39.12, *** |
| Flower display size | 7.75 a ± 9.21 | 8.18 a ± 9.73 | 6.47 ab ± 6.14 | 5.68 bc ± 5.17 | 4.13 c ± 4.58 | 7.17 a ± 4.81 | χ2 = 18.40, *** |
| Flower longevity (hours) | 32.60 d ± 1.58 | 33.10 d ± 1.81 | 33.90 d ± 1.92 | 48.05 b ± 8.98 | 55.60 a ± 1.37 | 38.10 c ± 6.55 | χ2 = 176.42, *** |
| Pollen (no. of grains flower−1) | 515,306.93 a ± 49,153.55 | 520,167.56 a ± 21,340.97 | 428,793.87 b ± 60,018.43 | 350,910.06 c ± 32,606.08 | 319,852.16 c ± 11,734.98 | 439,075.23 b ± 62,140.65 | χ2 =44.37, *** |
| Ovule (no. of ovules flower−1) | 80.25 a ± 15.01 | 83.20 a ± 13.97 | 73.38 b ± 13.40 | 68.32 bc ± 11.57 | 64.95 c ± 11.40 | 74.38 b ± 12.52 | χ2 = 50.35, *** |
| Pollen viability | 79.98 ± 7.23 | 80.73 ± 7.02 | 81.05 ± 7.11 | 81.77 ± 7.38 | 82.16 ± 7.31 | 81.54 ± 7.28 | χ2 = 1.25, p = 0.94 |
| Pollen germinability | 74.35 ± 6.42 | 74.76 ± 6.25 | 75.12 ± 6.32 | 75.86 ± 6.57 | 76.82 ± 6.54 | 75.27 ± 6.51 | χ2 = 1.23, p = 0.82 |
| Stigma receptivity (duration in hours) | 51.80 c ± 1.82 | 52.40 c ± 1.90 | 52.90 c ± 1.89 | 57.50 b ± 5.76 | 76.90 a ± 2.29 | 56.30 b ± 4.51 | χ2 = 83.74, *** |
| Flower Visitor | RA | VR | HT | Resource | Pollination Strategies | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FV | Visitation Pattern | Buzzing Activity | Pollinating Mode | Pollen Adhering Parts | AR | SR | PSi | |||||
| Coleoptera | ||||||||||||
| Epuraea luteola | 16.21 | 0.11 ± 0.03 | - | P | 0.011 | IL | No | - | H, VA, VT, L | - | - | - |
| Hycleus phalarantha | 0.19 | 0.10 ± 0.0 | - | FT | 0.001 | IL | No | - | - | - | - | - |
| Diptera | ||||||||||||
| Eristalinus megacephalus | 0.51 | 0.34 ± 0.13 | - | P | 0.003 | IL | No | - | H, L | - | - | - |
| Helophilus peregrinus | 0.33 | 0.32 ± 0.14 | - | P | 0.002 | IL | No | - | H, L | - | - | - |
| Lucilia sericata | 1.17 | 0.28 ± 0.10 | - | P | 0.002 | IL | No | - | H, L | - | - | - |
| Paragus serratus | 1.03 | 1.40 ± 0.57 | - | P | 0.003 | L | No | - | H, L, VA, VT | 1 | 0.53 | 0.002 |
| Hymenoptera | ||||||||||||
| Amegilla zonata | 6.45 | 5.13 ± 1.20 | 0.74 ± 0.23 | P | 0.096 | L | Yes | S, A | VA, VT, L | 1 | 0.92 | 0.088 |
| Apis cerana | 1.31 | 3.43 ± 1.20 | 15.54 ± 22.17 | P | 0.014 | L | No | S, A | VA, VT, L | 1 | 0.44 | 0.006 |
| Apis dorsata | 1.45 | 3.73 ± 1.38 | 14.38 ± 20.26 | P | 0.016 | L | No | S, A | VA, VT, L | 1 | 0.45 | 0.007 |
| Apis florea | 0.56 | 3.27 ± 1.07 | 16.63 ± 21.75 | P | 0.005 | L | No | S, A | VA, VT, L | 1 | 0.42 | 0.002 |
| Brounsapis mixta | 3.69 | 2.43 ± 1.13 | 12.04 ± 13.38 | P | 0.029 | L | No | S, A | VA, VT, L | 1 | 0.40 | 0.012 |
| Ceratina binghami | 7.90 | 2.87 ± 1.21 | 20.52 ± 29.81 | P | 0.068 | L | No | S, A | VA, VT, L | 1 | 0.42 | 0.029 |
| Ceratina hieroglyphica | 2.43 | 3.51 ± 1.49 | 15.85 ± 24.64 | P | 0.026 | L | No | S, A | VA, VT, L | 1 | 0.41 | 0.011 |
| Halictus (Seladonia) lucidipennis | 3.36 | 3.33 ± 1.47 | 15.73 ± 23.80 | P | 0.024 | L | No | S, A | VA, VT, L | 1 | 0.41 | 0.010 |
| Lasioglossum cavernifrons | 14.63 | 3.17 ± 1.39 | 17.68 ± 28.44 | P | 0.143 | L | Yes | S, A | VA, VT, L | 1 | 0.48 | 0.069 |
| Nomia (Curvinomia) strigata | 13.60 | 5.97 ± 1.71 | 8.18 ± 10.23 | P | 0.224 | L | Yes | S, A | VA, VT, L | 1 | 0.52 | 0.116 |
| Tetragonula pagdeni | 12.34 | 0.74 ± 0.26 | 100.78 ± 82.15 | P | 0.075 | L | No | S, A | VA, VT, L | 1 | 0.37 | 0.028 |
| Xylocopa aestuans | 4.49 | 6.53 ± 1.53 | 1.38 ± 0.50 | P | 0.088 | L | Yes | S, A | VA, VT, L | 1 | 0.94 | 0.083 |
| Xylocopa amethystina | 2.10 | 7.27 ± 1.64 | 0.78 ± 0.24 | P | 0.045 | L | Yes | S, A | VA, VT, L | 1 | 0.91 | 0.041 |
| Xylocopa fenestrata | 4.30 | 6.97 ± 1.38 | 1.29 ± 0.48 | P | 0.089 | L | Yes | S, A | VA, VT, L | 1 | 0.96 | 0.085 |
| Xylocopa latipes | 1.96 | 6.10 ± 1.63 | 1.42 ± 0.53 | P | 0.036 | L | Yes | S, A | VA, VT, L | 1 | 0.92 | 0.033 |
| Season | Abundance | Richness | Diversity |
|---|---|---|---|
| Summer | 2.40 a ± 2.05 | 0.52 a ± 0.55 | 0.38 a ± 0.38 |
| Monsoon | 2.07 ab ± 2.05 | 0.48 ab ± 0.55 | 0.34 ab ± 0.39 |
| Autumn | 1.60 cd ± 1.71 | 0.39 bc ± 0.56 | 0.26 cd ± 0.36 |
| Late autumn | 1.33 d ± 1.41 | 0.30 cd ± 0.50 | 0.19 de ± 0.31 |
| Winter | 0.88 e ± 1.13 | 0.23 e ± 0.50 | 0.13 e ± 0.28 |
| Spring | 1.91 bc ± 1.83 | 0.43 ab ± 0.54 | 0.30 bc ± 0.38 |
| Throughout year | 1.70 ± 1.80 | 0.39 ± 0.54 | 0.27 ± 0.36 |
| Statistical analysis | χ2 = 87.45, df = 5, p < 0.001 | χ2 = 51.72, df = 5, p < 0.001 | χ2 = 62.99, df = 5, p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layek, U.; Majhi, P.; Das, A.; Karmakar, P.; Kundu, A. Seasonal Variation in Flower Traits, Visitor Traits, and Reproductive Success of Solanum sisymbriifolium Lamarck (Solanaceae) in the Rarh Region of West Bengal, India. Biology 2025, 14, 865. https://doi.org/10.3390/biology14070865
Layek U, Majhi P, Das A, Karmakar P, Kundu A. Seasonal Variation in Flower Traits, Visitor Traits, and Reproductive Success of Solanum sisymbriifolium Lamarck (Solanaceae) in the Rarh Region of West Bengal, India. Biology. 2025; 14(7):865. https://doi.org/10.3390/biology14070865
Chicago/Turabian StyleLayek, Ujjwal, Pappu Majhi, Alokesh Das, Prakash Karmakar, and Arijit Kundu. 2025. "Seasonal Variation in Flower Traits, Visitor Traits, and Reproductive Success of Solanum sisymbriifolium Lamarck (Solanaceae) in the Rarh Region of West Bengal, India" Biology 14, no. 7: 865. https://doi.org/10.3390/biology14070865
APA StyleLayek, U., Majhi, P., Das, A., Karmakar, P., & Kundu, A. (2025). Seasonal Variation in Flower Traits, Visitor Traits, and Reproductive Success of Solanum sisymbriifolium Lamarck (Solanaceae) in the Rarh Region of West Bengal, India. Biology, 14(7), 865. https://doi.org/10.3390/biology14070865







