Enhancing Alfalfa (Medicago sativa) Seed Yield: The Effect of Honey Bee (Apis mellifera) Supplementation and Efficiency of Other Pollinators
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Layout
2.3. Abundance of Honey Bees and Other Insect Pollinators
2.4. Foraging Behavior
2.5. Tripping Behaviour of Honey Bees and Other Pollinators
2.6. Single-Visit Seed Set Efficiency and Reproductive Success of Alfalfa
2.7. Seed Yield
2.8. Statistical Analysis
3. Results
3.1. Abundance of Insect Pollinators
3.2. Stay Time
3.3. Visitation Rate
3.4. Tripping
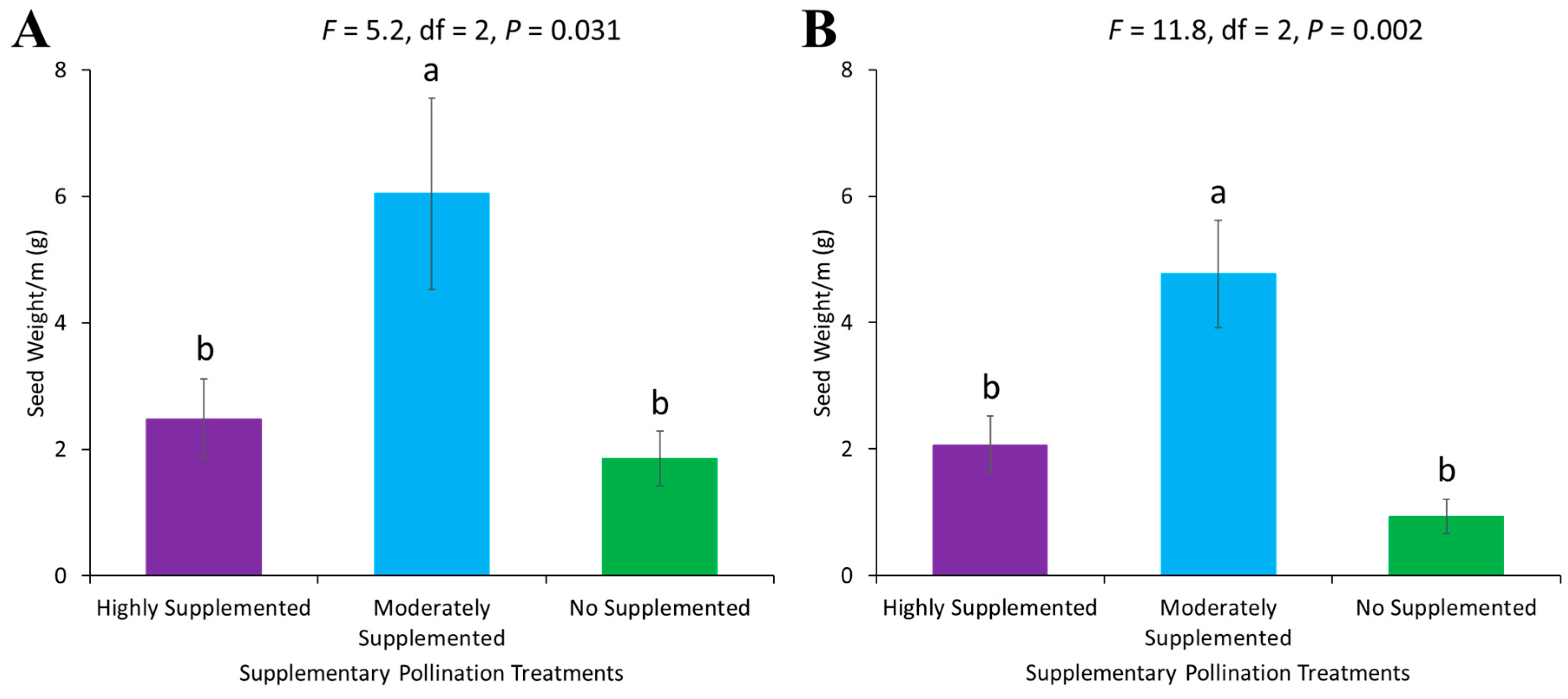
3.5. Seed Weight
3.6. Single-Visit Seed Set Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayub Agricultural Research Institute (AARI). AARI Fodder Crop Varieties; Ayub Agricultural Research Institute: Faisalabad, Pakistan, 2025.
- Horvat, D.; Viljevac Vuletić, M.; Andrić, L.; Baličević, R.; Kovačević Babić, M.; Tucak, M. Characterization of Forage Quality, Phenolic Profiles, and Antioxidant Activity in Alfalfa (Medicago sativa L.). Plants 2022, 11, 2735. [Google Scholar] [CrossRef] [PubMed]
- Ben-Laouane, R.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Oufdou, K.; Wahbi, S.; Meddich, A. Microbial Biotechnologies for Salt Tolerance in Alfalfa: Agro-Nutritional Comparison Between Local and Imported Varieties. Nitrogen 2025, 6, 27. [Google Scholar] [CrossRef]
- Bell, L.W. Relative Growth Rate, Resource Allocation and Root Morphology in the Perennial Legumes, Medicago sativa, Dorycnium rectum and D. hirsutum Grown under Controlled Conditions. Plant Soil. 2005, 270, 199–211. [Google Scholar] [CrossRef]
- Russelle, M.P. Alfalfa: After an 8,000-Year Journey, the “Queen of Forages” Stands Poised to Enjoy Renewed Popularity. Am. Sci. 2001, 89, 252–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. Advances in Basic Biology of Alfalfa (Medicago sativa L.): A Comprehensive Overview. Hortic. Res. 2025, 12, uhaf081. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Zhang, Y.; Xie, X.J.; Knapp, A. Seed Priming with Brassinolide Improves Lucerne (Medicago sativa L.) Seed Germination and Seedling Growth in Relation to Physiological Changes under Salinity Stress. Aust. J. Agric. Res. 2007, 58, 811. [Google Scholar] [CrossRef]
- Haq, I.U.; Ijaz, S. Sustainable Winter Fodder: Production, Challenges, and Prospects; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-0-367-51798-4. [Google Scholar]
- Latif, A.; Sun, Y.; Noman, A. Herbaceous Alfalfa Plant as a Multipurpose Crop and Predominant Forage Specie in Pakistan. Front. Sustain. Food Syst. 2023, 7, 1126151. [Google Scholar] [CrossRef]
- Ahmad, J.; Iqbal, A.; Mahmood, A.; Iqbal, M.A.; Khan, H.Z.; Abbas, R.N.; Akbar, N.; Maqsood, M. Effect of Cutting Management, Seeding Rates and Sowing Method on Seed Yield of Alfalfa (Medicago sativa L.). Pak. J. Bot. 2020, 52, 1449–1454. [Google Scholar] [CrossRef]
- Larkin, R.A.; Graumann, H.O. Anatomical Structure of the Alfalfa Flower and an Explanation of the Tripping Mechanism. Bot. Gaz. 1954, 116, 40–52. [Google Scholar] [CrossRef]
- Linsley, G.E. Insect Pollinators of Alfalfa in California. J. Econ. Entomol. 1946, 39, 18–29. [Google Scholar] [CrossRef]
- Bohart, G.E. Pollination of Alfalfa and Red Clover. Annu. Rev. Entomol. 1957, 2, 355–380. [Google Scholar] [CrossRef]
- Batra, S.W.T. Comparative Efficiency of Alfalfa Pollination by Nomia melanderi, Megachile rotundata, Anthidium florentinum and Pithitis smaragdula (Hymenoptera: Apoidea). J. Kans. Entomol. Soc. 1976, 49, 18–22. [Google Scholar]
- Calderone, N.W. Insect Pollinated Crops, Insect Pollinators and US Agriculture: Trend Analysis of Aggregate Data for the Period 1992–2009. PLoS ONE 2012, 7, e37235. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI Books; CABI: Wallingford, CT, USA, 2000; ISBN 978-0-85199-783-4. [Google Scholar]
- Layek, U.; Kundu, A.; Bisui, S.; Karmakar, P. Impact of Managed Stingless Bee and Western Honey Bee Colonies on Native Pollinators and Yield of Watermelon: A Comparative Study. Ann. Agric. Sci. 2021, 66, 38–45. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Sun, K.; Bao, J.; He, C.; Hou, X. Supplementary Honey Bee (Apis mellifera L.) Pollination Enhances Fruit Growth Rate and Fruit Yield in Paeonia Ostii (Family: Paeoniaceae). PLoS ONE 2022, 17, e0272921. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Vaissière, B.E.; Gemmill-Herren, B.; Hipólito, J.; Freitas, B.M.; Ngo, H.T.; Azzu, N.; Sáez, A.; Åström, J.; et al. Mutually Beneficial Pollinator Diversity and Crop Yield Outcomes in Small and Large Farms. Science 2016, 351, 388–391. [Google Scholar] [CrossRef]
- Alomar, D.; González-Estévez, M.A.; Traveset, A.; Lázaro, A. The Intertwined Effects of Natural Vegetation, Local Flower Community, and Pollinator Diversity on the Production of Almond Trees. Agric. Ecosyst. Environ. 2018, 264, 34–43. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Garratt, M.P.D.; Powney, G.D.; Shaw, R.F.; Osborne, J.L.; Soroka, J.; Lindström, S.A.M.; Stanley, D.; Ouvrard, P.; Edwards, M.E.; et al. Meta-Analysis Reveals That Pollinator Functional Diversity and Abundance Enhance Crop Pollination and Yield. Nat. Commun. 2019, 10, 1481. [Google Scholar] [CrossRef]
- Hagler, J.R.; Mueller, S.; Teuber, L.R.; Machtley, S.A.; Van Deynze, A. Foraging Range of Honey Bees, Apis mellifera, in Alfalfa Seed Production Fields. J. Insect Sci. 2011, 11, 1–12. [Google Scholar] [CrossRef]
- Gordon, D.M.; Barthell, J.F.; Page, R.E.; Kim Fondrk, M.; Thorp, R.W. Colony Performance of Selected Honey Bee (Hymenoptera: Apidae) Strains Used for Alfalfa Pollination. J. Econ. Entomol. 1995, 88, 51–57. [Google Scholar] [CrossRef]
- Rader, R.; Howlett, B.G.; Cunningham, S.A.; Westcott, D.A.; Newstrom-Lloyd, L.E.; Walker, M.K.; Teulon, D.A.J.; Edwards, W. Alternative Pollinator Taxa Are Equally Efficient but Not as Effective as the Honeybee in a Mass Flowering Crop. J. Appl. Ecol. 2009, 46, 1080–1087. [Google Scholar] [CrossRef]
- Haedo, J.P.; Martínez, L.C.; Graffigna, S.; Marrero, H.J.; Torretta, J.P. Managed and Wild Bees Contribute to Alfalfa (Medicago sativa) Pollination. Agric. Ecosyst. Environ. 2022, 324, 107711. [Google Scholar] [CrossRef]
- Nagano, Y.; Wabiko, N.; Yokoi, T. Female Solitary Bees Flexibly Change Foraging Behaviour According to Their Floral Resource Requirements and Foraging Experiences. Sci. Nat. 2023, 110, 37. [Google Scholar] [CrossRef]
- Herrera, C.M.; Núñez, A.; Valverde, J.; Alonso, C. Body Mass Decline in a MEditerranean Community of Solitary Bees Supports the Size Shrinking Effect of Climatic Warming. Ecology 2023, 104, e4128. [Google Scholar] [CrossRef]
- Melone, G.G.; Stuligross, C.; Williams, N.M. Heatwaves Increase Larval Mortality and Delay Development of a Solitary Bee. Ecol. Entomol. 2024, 49, 433–444. [Google Scholar] [CrossRef]
- Rollin, O.; Garibaldi, L.A. Impacts of Honeybee Density on Crop Yield: A Meta-Analysis. J. Appl. Ecol. 2019, 56, 1152–1163. [Google Scholar] [CrossRef]
- Rauf, A.; Saeed, S.; Ali, M.; Nadeem Tahir, M.H. Comparative Efficiency of Native Insect Pollinators in Reproductive Performance of Medicago sativa L. in Pakistan. Insects 2021, 12, 1029. [Google Scholar] [CrossRef]
- Ahmed, N.; Umer, A.; Ali, M.A.; Iqbal, J.; Mubashir, M.; Grewal, A.G.; Butt, B.; Rasheed, M.K.; Chaudhry, U.K. Micronutrients Status of Mango (Mangifera indica) Orchards in Multan Region, Punjab, Pakistan, and Relationship with Soil Properties. Open Agric. 2020, 5, 271–279. [Google Scholar] [CrossRef]
- Abbas, F. Analysis of a Historical (1981–2010) Temperature Record of the Punjab Province of Pakistan. Earth Interact. 2013, 17, 1–23. [Google Scholar] [CrossRef]
- Abbas, F.; Ahmad, A.; Safeeq, M.; Ali, S.; Saleem, F.; Hammad, H.M.; Farhad, W. Changes in Precipitation Extremes over Arid to Semiarid and Subhumid Punjab, Pakistan. Theor. Appl. Clim. 2014, 116, 671–680. [Google Scholar] [CrossRef]
- Putnam, D.H. Factors Influencing Yield and Quality in Alfalfa. In The Alfalfa Genome; Yu, L.-X., Kole, C., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2021; pp. 13–27. ISBN 978-3-030-74465-6. [Google Scholar]
- Mazeed, A.R.; Marey, R.A. Pollinators Activity on Onion Flowers and Its Effect on Seeds Yield at Sohag Governorate, Egypt. Egypt. J. Agric. Res. 2018, 96, 465–475. [Google Scholar] [CrossRef]
- Ali, M.; Saeed, S.; Sajjad, A.; Whittington, A. In Search of the Best Pollinators for Canola (Brassica napus L.) Production in Pakistan. Appl. Entomol. Zool. 2011, 46, 353–361. [Google Scholar] [CrossRef]
- Cane, J.H. Pollinating Bees (Hymenoptera: Apiformes) of U.S. Alfalfa Compared for Rates of Pod and Seed Set. J. Econ. Entomol. 2002, 95, 22–27. [Google Scholar] [CrossRef]
- Brunet, J.; Stewart, C.M. Impact of Bee Species and Plant Density on Alfalfa Pollination and Potential for Gene Flow. Psyche A J. Entomol. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Morris, W.F.; Vázquez, D.P.; Chacoff, N.P. Benefit and Cost Curves for Typical Pollination Mutualisms. Ecology 2010, 91, 1276–1285. [Google Scholar] [CrossRef]
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee Pollination Increases Yield Quantity and Quality of Cash Crops in Burkina Faso, West Africa. Sci. Rep. 2017, 7, 17691. [Google Scholar] [CrossRef] [PubMed]
- Peace, A.; Pattemore, D.; Broussard, M.; Fonseka, D.; Tomer, N.; Bosque-Pérez, N.A.; Crowder, D.; Shaw, A.K.; Jesson, L.; Howlett, B.G.; et al. Orchard Layout and Plant Traits Influence Fruit Yield More Strongly than Pollinator Behaviour and Density in a Dioecious Crop. PLoS ONE 2020, 15, e0231120. [Google Scholar] [CrossRef]
- Sáez, A.; Morales, C.L.; Ramos, L.Y.; Aizen, M.A. Extremely Frequent Bee Visits Increase Pollen Deposition but Reduce Drupelet Set in Raspberry. J. Appl. Ecol. 2014, 51, 1603–1612. [Google Scholar] [CrossRef]
- Haedo, J.P.; Graffigna, S.; Martínez, L.C.; Pérez-Méndez, N.; Torretta, J.P.; Marrero, H.J. Effectiveness Landscape of Crop Pollinator Assemblages: Implications to Pollination Service Management. Agric. Ecosyst. Environ. 2023, 348, 108417. [Google Scholar] [CrossRef]
- Blettler, D.C.; Fagúndez, G.A.; Caviglia, O.P. Contribution of Honeybees to Soybean Yield. Apidologie 2018, 49, 101–111. [Google Scholar] [CrossRef]
- Gazzoni, D.L.; Barateiro, J.V.G.R.P.; Da Rosa Santos, P. Supplemental Bee Pollination Effect on the Productivity of Soybean Grown in a Low Yield Potential Condition. J. Apic. Res. 2024, 63, 788–800. [Google Scholar] [CrossRef]
- Duque-Trujillo, D.; Hincapié, C.A.; Osorio, M.; Zartha-Sossa, J.W. Strategies for the Attraction and Conservation of Natural Pollinators in Agroecosystems: A Systematic Review. Int. J. Environ. Sci. Technol. 2023, 20, 4499–4512. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; De Groot, G.A.; Albrecht, M.; Bosch, J.; Breeze, T.D.; Fountain, M.T.; Klein, A.M.; McKerchar, M.; Park, M.; Paxton, R.J.; et al. Opportunities to Reduce Pollination Deficits and Address Production Shortfalls in an Important Insect-pollinated Crop. Ecol. Appl. 2021, 31, e02445. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.F.; Ali, M.; Khan, F.Z.A.; Awan, T.H. From Petals to Seeds: Understanding the Role of Hymenopteran and Dipteran Pollinators in the Reproductive Success of Onion (Allium cepa L.). Plant Bull. 2024, 3, 19–25. [Google Scholar] [CrossRef]
- Haider, S.; Khan, F.Z.A.; Gul, H.T.; Ali, M.; Iqbal, S. Assessing the Role of Conservation Strips in Enhancing Beneficial Fauna in the Wheat-Cotton Agricultural System in Punjab, Pakistan. Pak. J. Zool. 2024, 56, 1–9. [Google Scholar] [CrossRef]
- Mashilingi, S.K.; Zhang, H.; Garibaldi, L.A.; An, J. Honeybees Are Far Too Insufficient to Supply Optimum Pollination Services in Agricultural Systems Worldwide. Agric. Ecosyst. Environ. 2022, 335, 108003. [Google Scholar] [CrossRef]
- Gross, C.L.; Mackay, D. Honeybees Reduce Fitness in the Pioneer Shrub Melastoma affine (Melastomataceae). Biol. Conserv. 1998, 86, 169–178. [Google Scholar] [CrossRef]
- Ali, M.; Saeed, S.; Sajjad, A.; Bashir, M.A. Exploring the Best Native Pollinators for Pumpkin (Cucurbita pepo) Production in Punjab, Pakistan. Pak. J. Zool. 2014, 46, 531–539. [Google Scholar]
- Abdullah, S.; Ali, M.; Khan, F.Z.A.; Sajjad, A.; Qayyum, M.A.; Ahmad, N. Solitary Bees Are More Efficient Pollinators of Sponge Gourd than Giant Honeybees and Syrphid Flies. Sociobiology 2024, 71, e10279. [Google Scholar] [CrossRef]
- Reinhardt, J.F. Some Responses of Honey Bees to Alfalfa Flowers. Am. Nat. 1952, 86, 257–275. [Google Scholar] [CrossRef]







| Variables | Flower Tripping | Pod Weight (g) | No. Seeds | Seed Weight (g) |
|---|---|---|---|---|
| Flower tripping | ||||
| Pod weight (g) | 0.2575 (p = 0.0433) | |||
| No. Seeds | 0.3605 (p = 0.0040) | 0.4812 (p < 0.001) | ||
| Seed weight (g) | 0.3347 (p = 0.0078) | 0.5286 (p < 0.001) | 0.8907 (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejaz, K.; Ali, M.; Khan, F.Z.A.; Mozūratis, R. Enhancing Alfalfa (Medicago sativa) Seed Yield: The Effect of Honey Bee (Apis mellifera) Supplementation and Efficiency of Other Pollinators. Biology 2025, 14, 599. https://doi.org/10.3390/biology14060599
Ejaz K, Ali M, Khan FZA, Mozūratis R. Enhancing Alfalfa (Medicago sativa) Seed Yield: The Effect of Honey Bee (Apis mellifera) Supplementation and Efficiency of Other Pollinators. Biology. 2025; 14(6):599. https://doi.org/10.3390/biology14060599
Chicago/Turabian StyleEjaz, Kamran, Mudssar Ali, Fawad Zafar Ahmad Khan, and Raimondas Mozūratis. 2025. "Enhancing Alfalfa (Medicago sativa) Seed Yield: The Effect of Honey Bee (Apis mellifera) Supplementation and Efficiency of Other Pollinators" Biology 14, no. 6: 599. https://doi.org/10.3390/biology14060599
APA StyleEjaz, K., Ali, M., Khan, F. Z. A., & Mozūratis, R. (2025). Enhancing Alfalfa (Medicago sativa) Seed Yield: The Effect of Honey Bee (Apis mellifera) Supplementation and Efficiency of Other Pollinators. Biology, 14(6), 599. https://doi.org/10.3390/biology14060599







