Metabolomics Analysis Reveals the Influence Mechanism of Different Growth Years on the Growth, Metabolism and Accumulation of Medicinal Components of Bupleurum scorzonerifolium Willd. (Apiaceae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Instruments and Reagents
2.2. Plant Source
2.3. Determination of the Saikosaponins Content
2.4. Elemental Analysis
2.5. GC-MS Analysis
2.6. Multivariate Statistical Analysis
3. Results
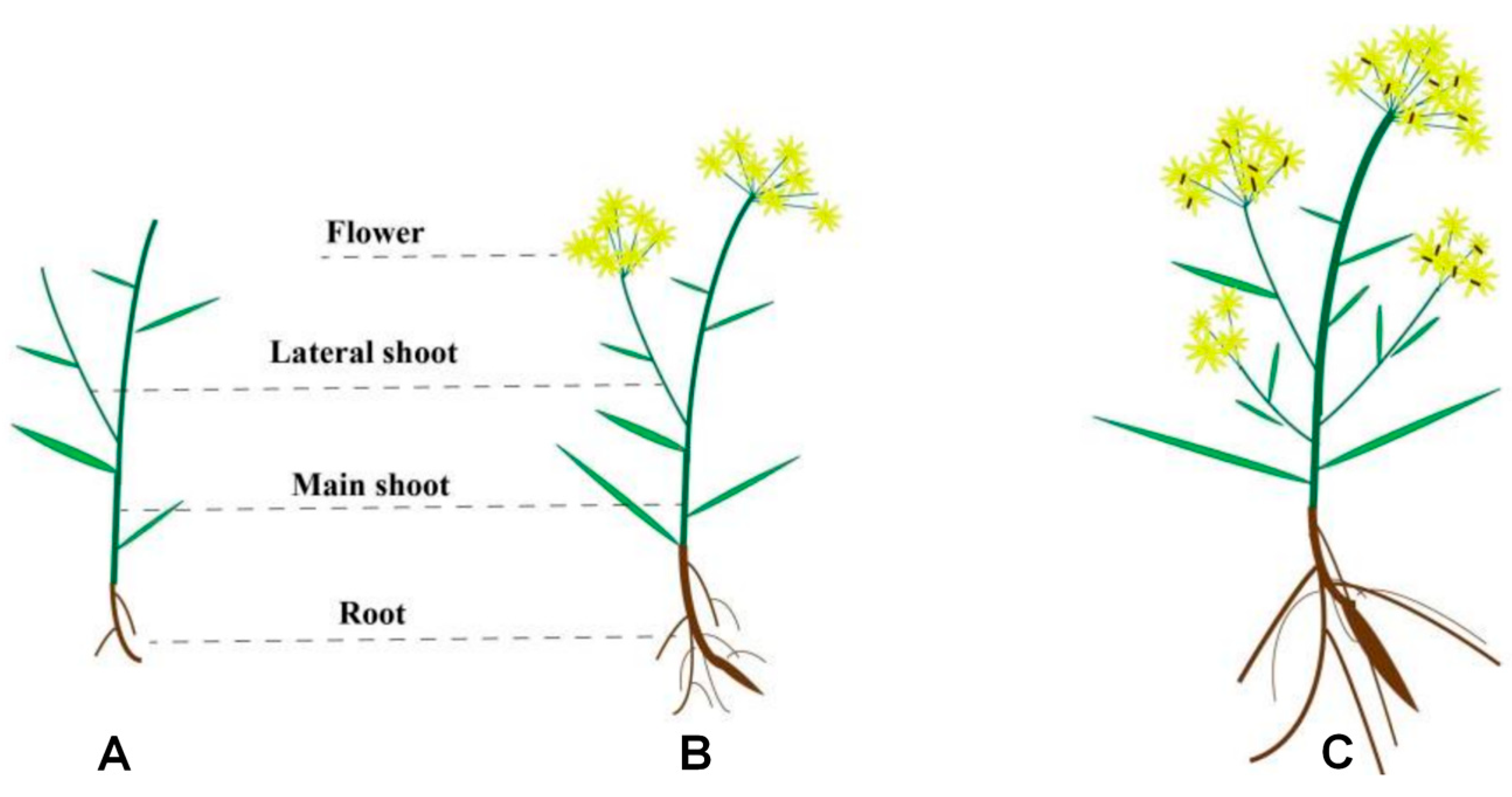
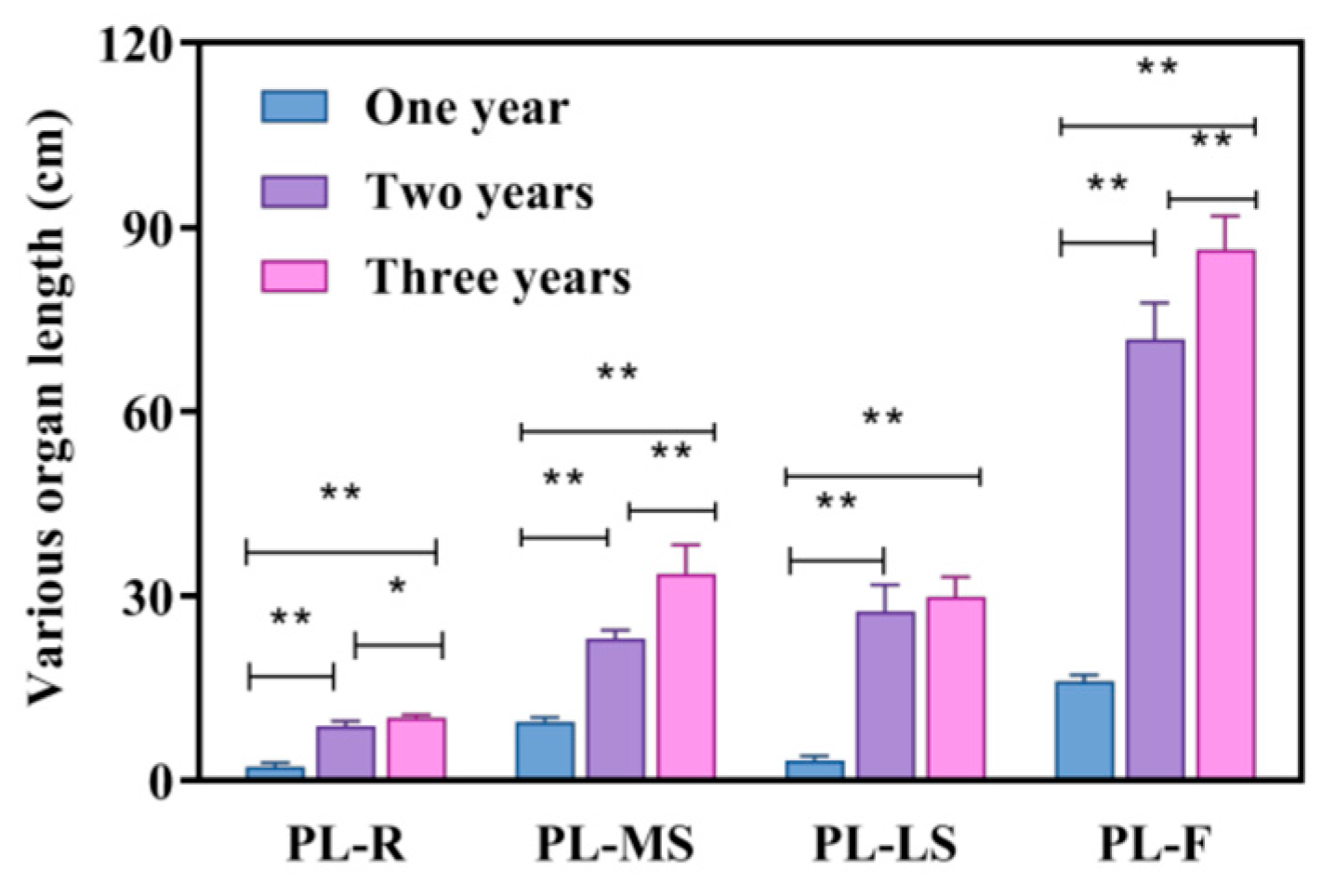
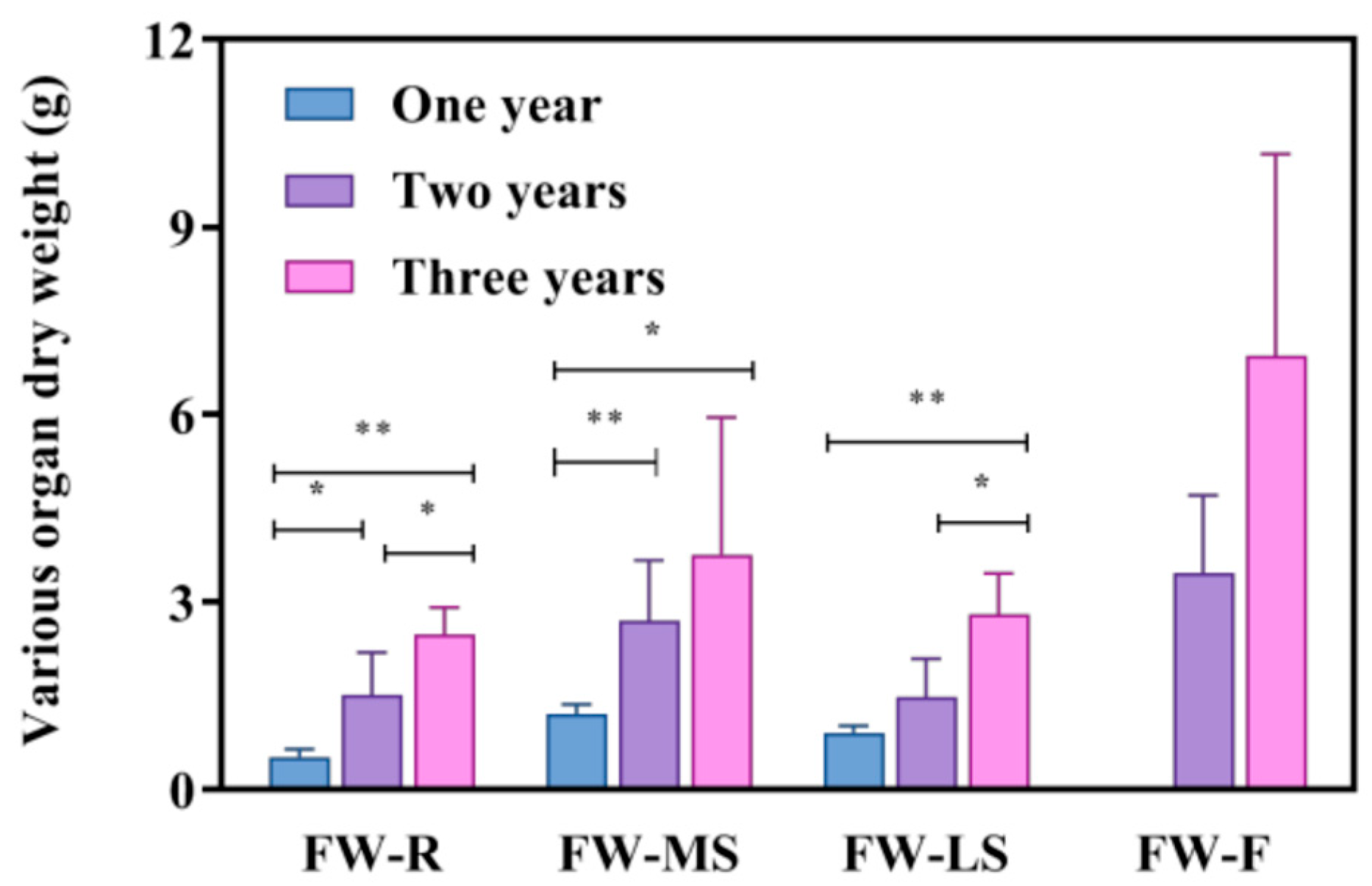
3.1. Comparative Analysis of Quality and Traits of Bupleurum Under Different Growth Years
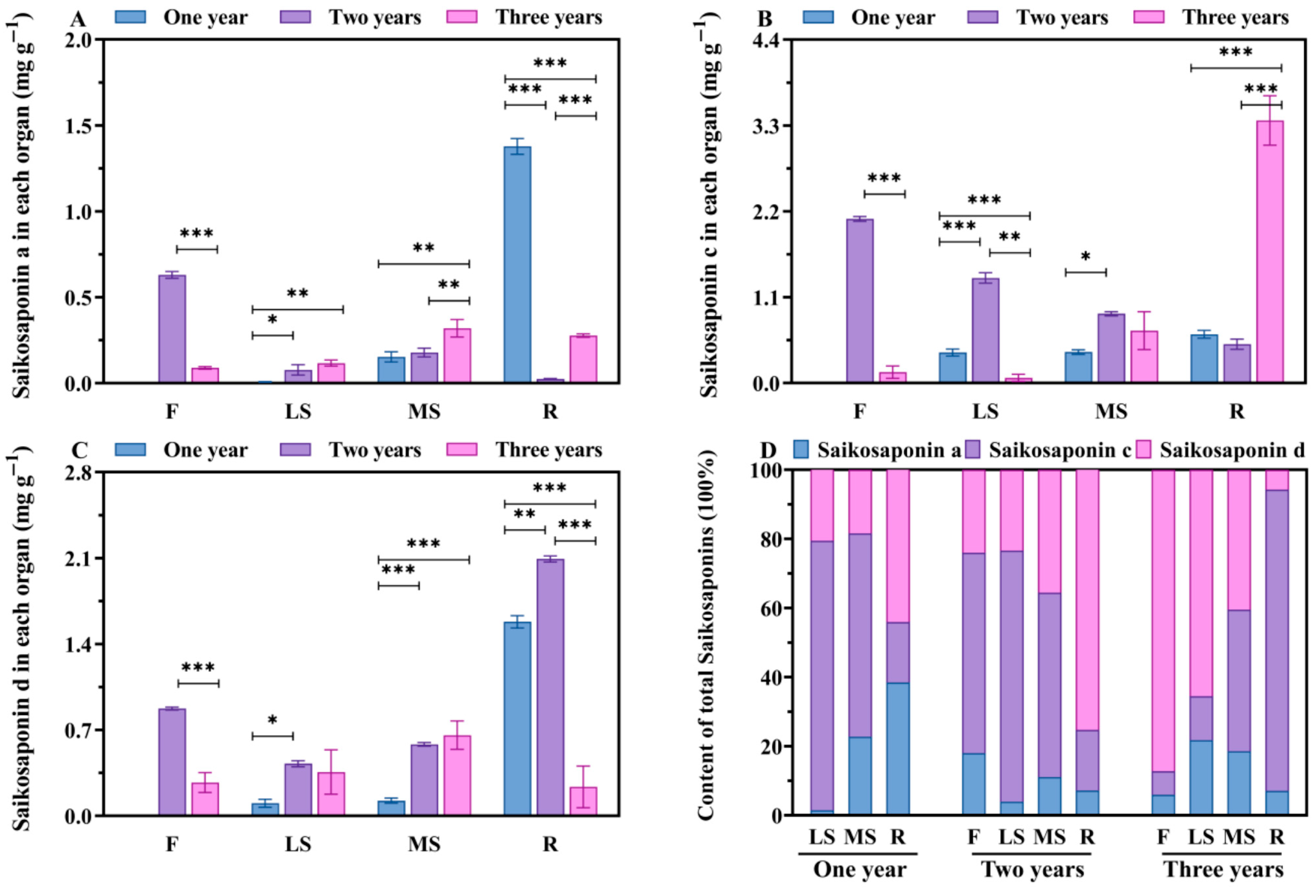
3.2. Changes in Saikosaponins Content in Different Growth Years
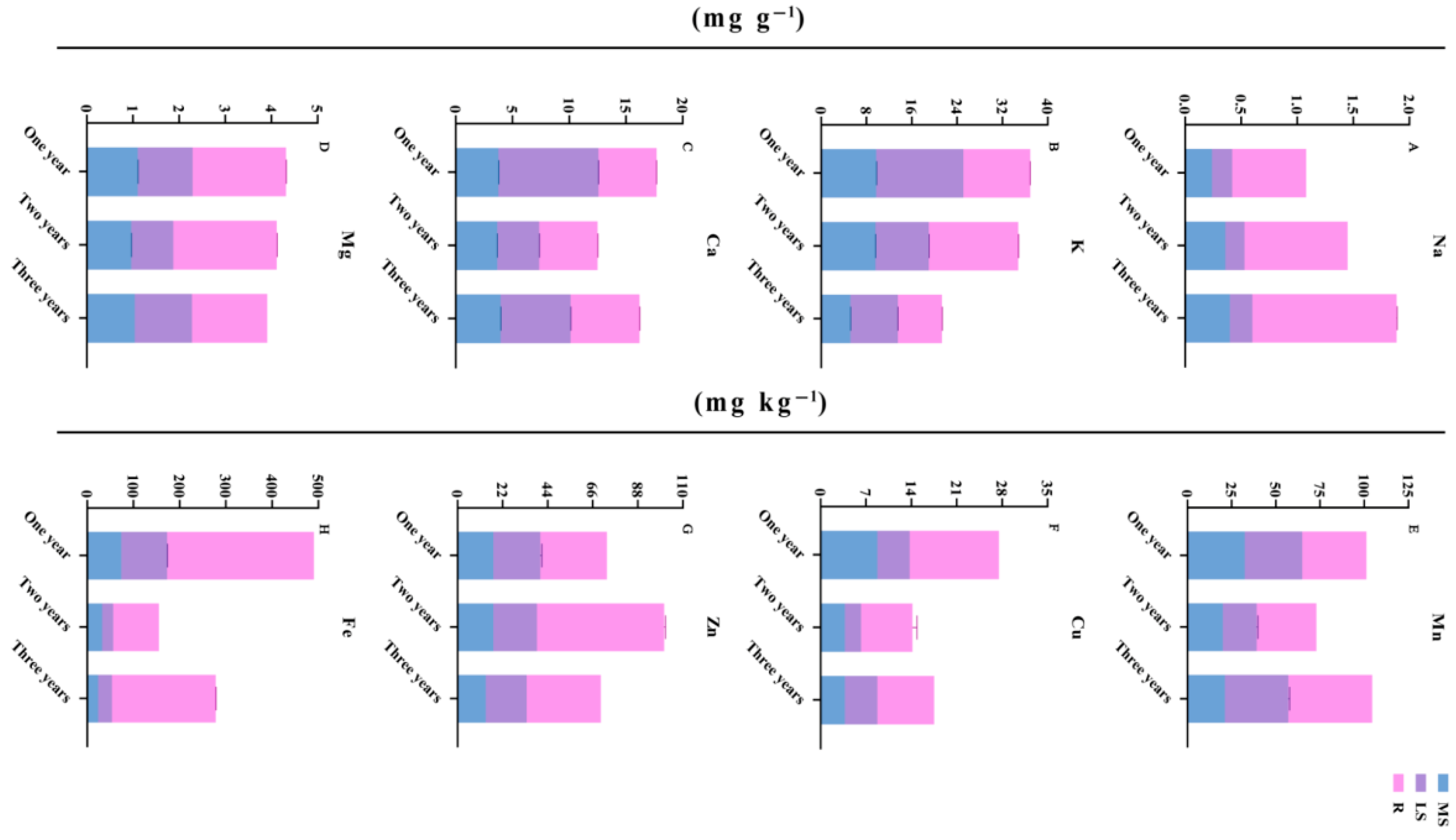
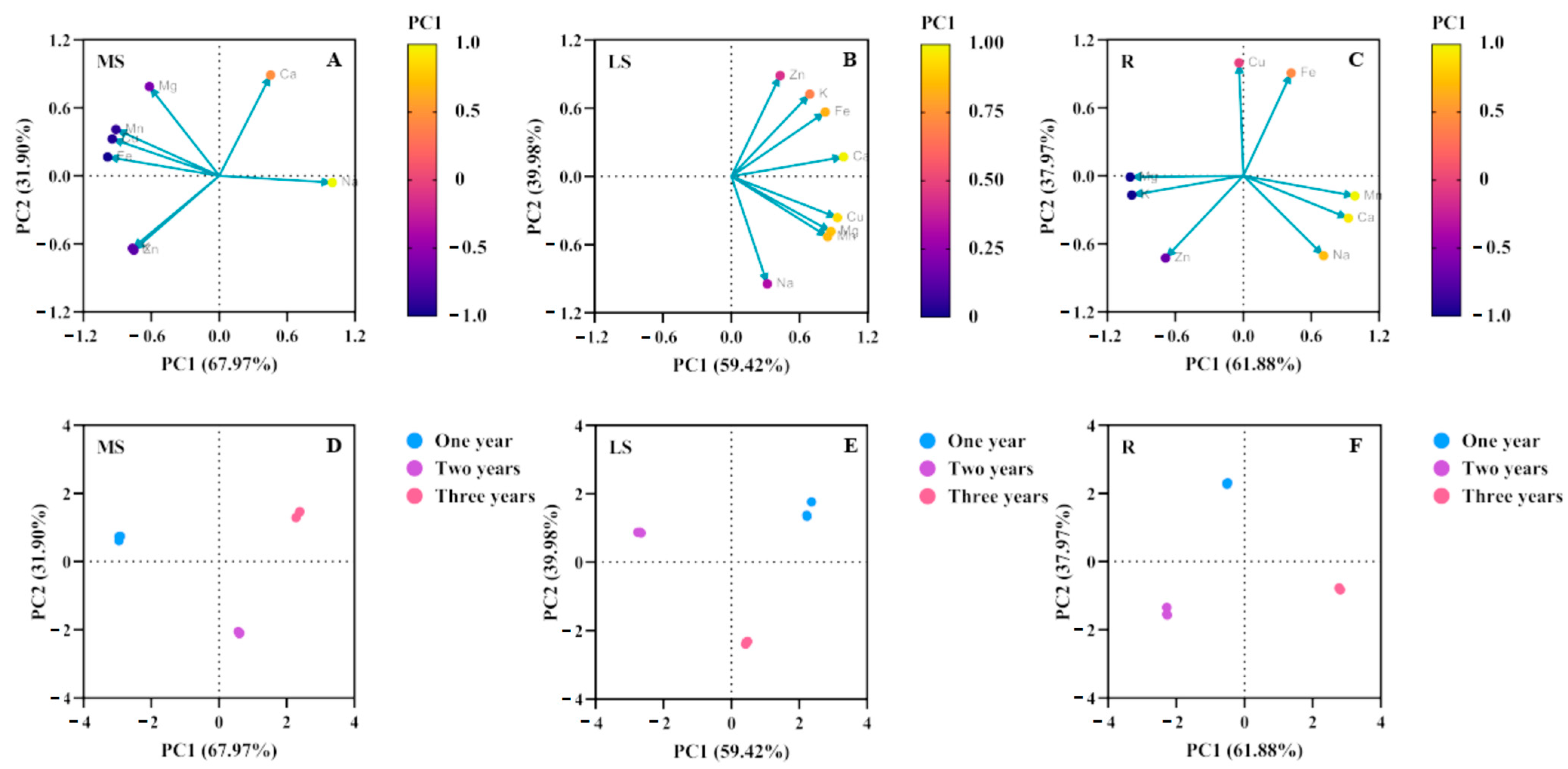
3.3. Changes in Element Accumulation in Different Growth Years
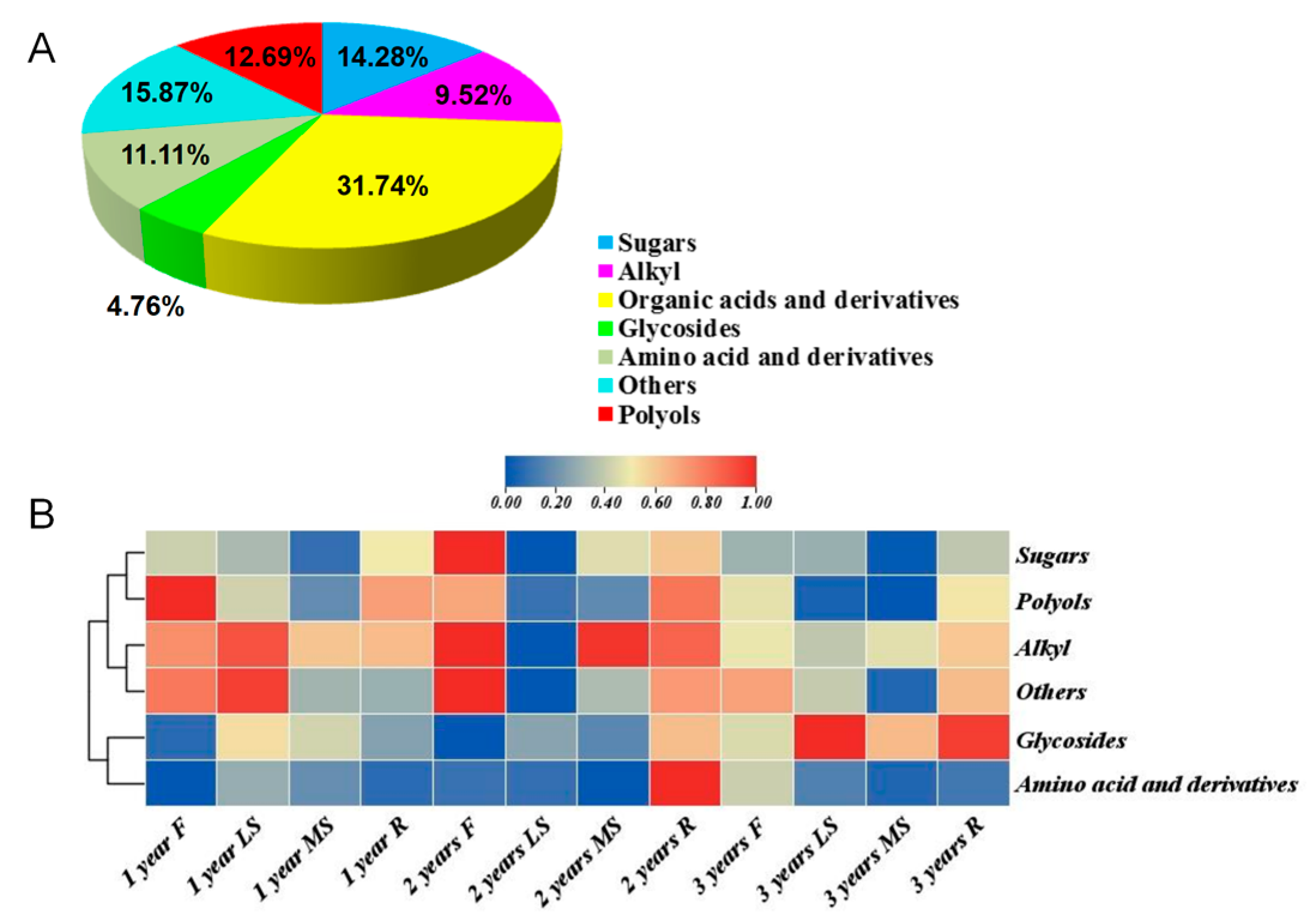
3.4. GC-MS Analysis of Bupleurum in Different Growth Years
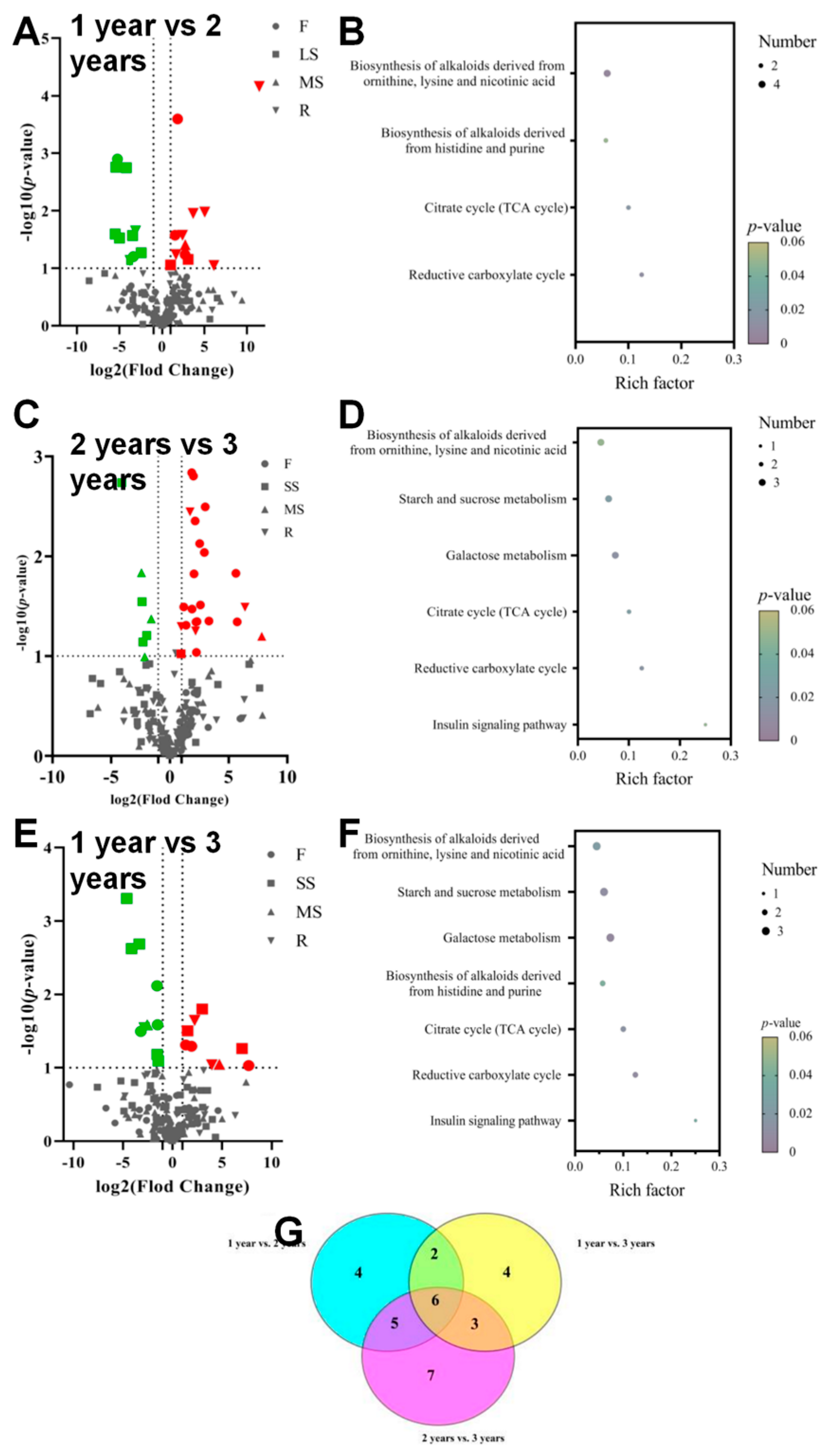
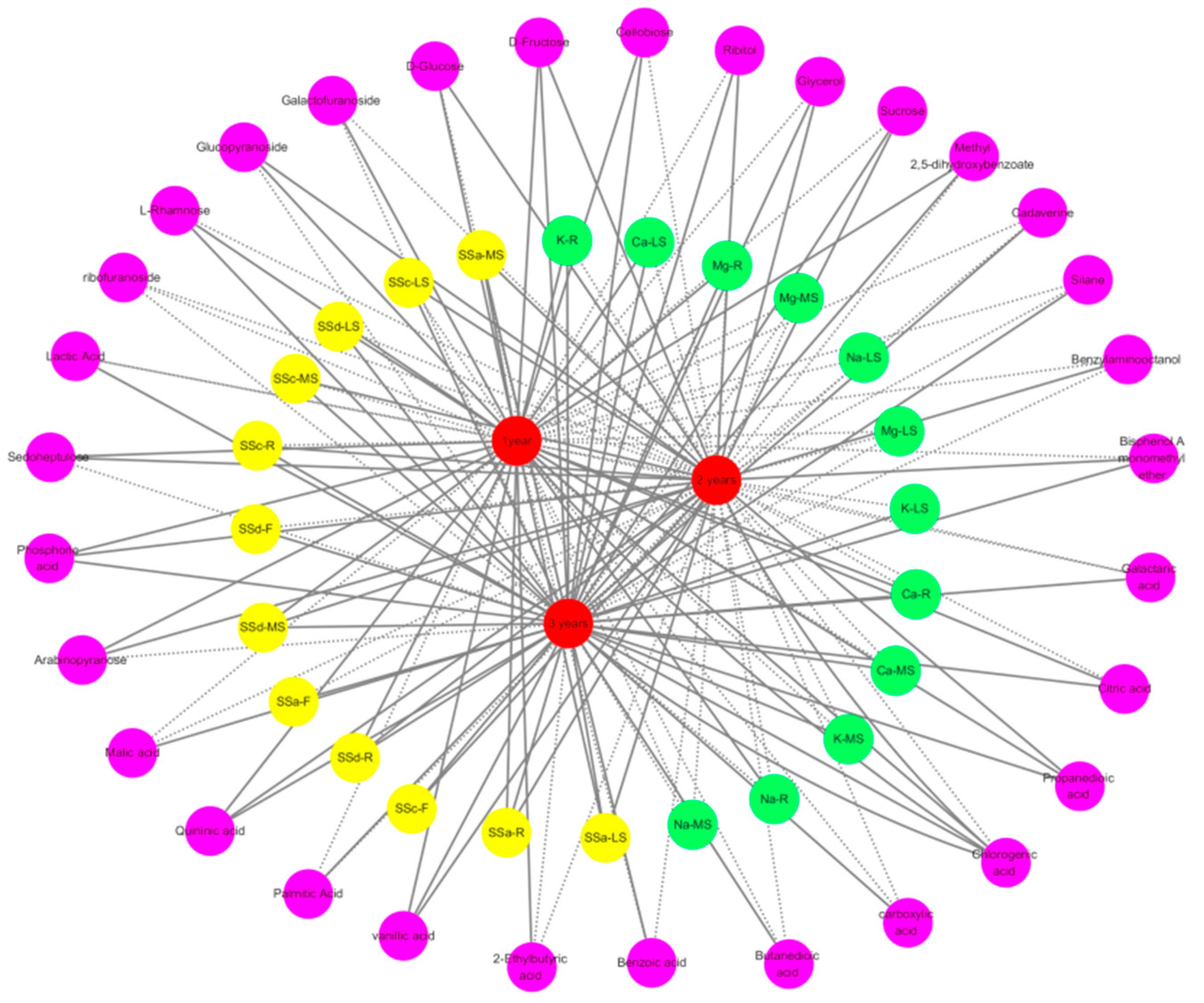
3.5. Metabolite Profiling of Bupleurum at Growth Cycles in Volcanic Map
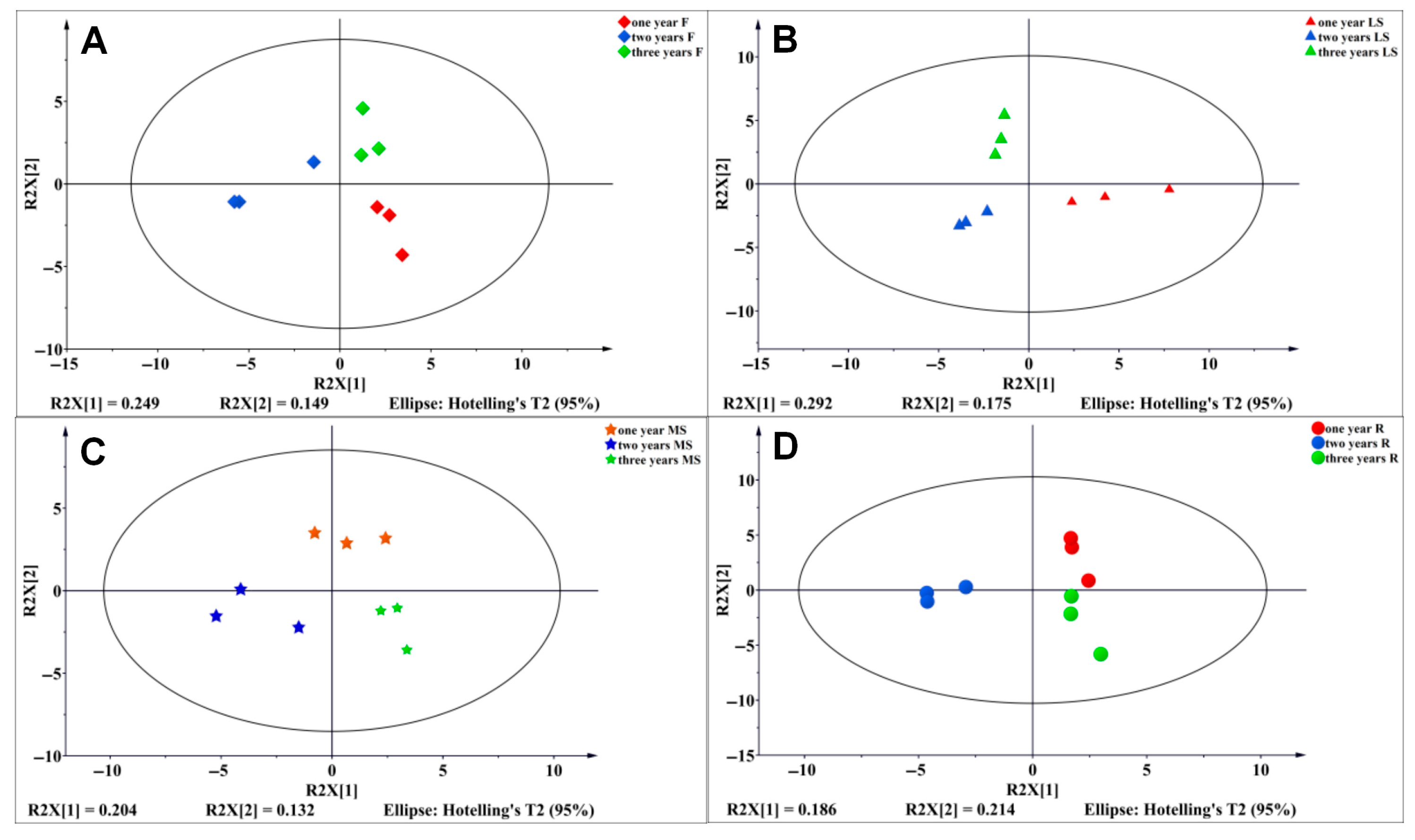
3.6. Establishment of PLS-DA Model of Primary Metabolites of Bupleurum in Different Growth Cycles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Teng, L.; Guo, X.; Ma, Y.; Xu, L.; Wei, J.; Xiao, P. A comprehensive review on traditional and modern research of the genus Bupleurum (Bupleurum L., Apiaceae) in recent 10 years. J. Ethnopharmacol. 2023, 306, 116129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Bing, Y.F.; Wu, S.; Luo, L.T.; Sun, Z.W.; Li, W.L.; Qu, Z.Y.; Zou, X. Discovery and verification of anti-inflammatory-related quality markers in the aerial part of Bupleurum scorzonerifolium by UPLC-Q-TOF-MS/MS and in RAW 264.7 cells and a zebrafish model. Phytochem. Anal. 2024, 35, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Liang, J.; Zhang, Y.; Kuang, H.X.; Xia, Y.G. Effects of various processed methods on chemical constituents and anti-hepatitis activity of Bupleurum scorzonerifolium Willd. by metabonomics and molecular docking. J. Pharm. Biomed. Anal. 2022, 218, 114837. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Liu, X.; Tong, L.; Wang, H.; Feng, M.; Xi, X.; He, P.; Qin, X. Comparison of chemical constituents of Bupleurum marginatum var. stenophyllum and Bupleurum chinense DC. using UHPLC-Q-TOF-MS based on a metabonomics approach. Biomed. Chromatogr. 2021, 35, e5133. [Google Scholar] [CrossRef] [PubMed]
- Jialin, S.; Weinan, L.; Ye, Z.; Yun, G.; Zejia, D.; Zhonghua, T.; Ann, A. Metabolomics Analysis Reveals Potential Mechanisms in Bupleurum L. (Apiaceae) Induced by Three Levels of Nitrogen Fertilization. Agronomy 2021, 11, 2291. [Google Scholar] [CrossRef]
- Bi, Y.; Li, M.; Wang, Y.; Yao, J.; Wang, Y.; Wang, S.; Zhuang, L.; Liu, S.; Li, Z.; Hao, Z.; et al. Saikosaponins from Bupleurum scorzonerifolium Willd. alleviates microglial pyroptosis in depression by binding and inhibiting P2X7 expression. Phytomedicine 2025, 136, 156240. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zheng, Y.; Chen, C.; Liu, J.; Wang, Y.; Wang, J.; Li, Y. Research progress on the molecular mechanisms of Saikosaponin D in various diseases (Review). Int. J. Mol. Med. 2025, 55, 37. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Pi, D.; Cui, J.; Liang, Y.; Wu, P.; Ouyang, M.; Zuo, Q. Saikosaponin A induces cellular senescence in triple-negative breast cancer by inhibiting the PI3K/Akt signalling pathway. Front. Pharmacol. 2025, 16, 1532579. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, J.; Shen, J.; Zhang, H.; Liu, Y.; Sun, J.; Zheng, Y.; Jiang, X. Saikosaponin A targets HDAC6 to inhibit Mycobacterium tuberculosis-induced macrophage Pyroptosis via autophagy-mediated NLRP3 inflammasome inactivation. Phytomedicine 2025, 142, 156693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liang, Z.T.; Yi, T.; Ma, Y.; Zhao, Z.Z.; Guo, B.L.; Zhang, J.Y.; Chen, H.B. Comparison of chemical profiles between the root and aerial parts from three Bupleurum species based on a UHPLC-QTOF-MS metabolomics approach. BMC Complement. Altern. Med. 2017, 17, 305. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Zheng, W.; Guo, J.Q.; Fang, W.; Ma, B.P.; Guo, B.L.; Peng, Y.X.; Zhang, D.J. Comparative analysis on chemical constituents in Bupleurum chinense, B. marginatum, B. marginatum var. stenophyllum and B. smithii var. parvifolium. Zhongguo Zhong Yao Za Zhi 2019, 44, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Ji, H.; Rong, M.; Liu, Y.; Jiang, J.; Guo, X.; Gao, Z.; Xu, Y.; Wei, J. Integrated metabolomics and transcriptomics reveal differences in terpenoids and the molecular basis among the roots of three Bupleurum species. BMC Plant Biol. 2025, 25, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cao, Q.; Ke, I.; Qin, X. Analysis of correlation between commercial traits and chemical characteristics and absolute growth years of Astragali Radix. Chin. Herb. Med. 2020, 51, 451–460. [Google Scholar]
- Hu, T. Accumulative dynamic variation of active ingredients in Codonopsis tangshen Oliv. from different growing ages and harvesting time. Pharm. Res. 2017, 36, 571–574. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Luo, C.; Huang, J. Accumulative dynamic variation of active ingredients in Polygonati Odorati Rhizoma from different growing ages and harvesting time. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 30–34. [Google Scholar]
- Bai, C.; Yang, J.; Cao, B.; Xue, Y.; Gao, P.; Liang, H.; Li, G. Growth years and post-harvest processing methods have critical roles on the contents of medicinal active ingredients of Scutellaria baicalensis. Ind. Crops Prod. 2020, 158, 112985. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Fu, X.; Shao, J.; Tang, Y.; Yu, M.; Li, L.; Huang, L.; Tang, K. Transcriptional regulatory network of high-value active ingredients in medicinal plants. Trends Plant Sci. 2023, 28, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lei, S.; Gong, H.; Liu, Z.; Zhang, Y.; Ouyang, Z. Field performance of sweet sorghum in salt-affected soils in China: A quantitative synthesis. Environ. Res. 2023, 222, 115362. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture--status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Bibi, F.; Saleem, I.; Ehsan, S.; Jamil, S.; Ullah, H.; Mubashir, M.; Kiran, S.; Ahmad, I.; Irshad, I.; Saleem, M.; et al. Effect of various application rates of phosphorus combined with different zinc rates and time of zinc application on phytic acid concentration and zinc bioavailability in wheat. Agric. Nat. Resour. 2020, 54, 265–272. [Google Scholar] [CrossRef]
- Farhat, N.; Rabhi, M.; Krol, M.; Barhoumi, Z.; Ivanov, A.G.; McCarthy, A.; Abdelly, C.; Smaoui, A.; Hüner, N.P.A. Starch and sugar accumulation in Sulla carnosa leaves upon Mg2+ starvation. Acta Physiol. Plant. 2014, 36, 2157–2165. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Duy, D.; Philippar, K. Chloroplast Iron Transport Proteins—Function and Impact on Plant Physiology. Front. Plant Sci. 2016, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Pyšek, P.; van Kleunen, M.; Kinlock, N.L.; Lučanová, M.; Leitch, I.J.; Pierce, S.; Dawson, W.; Essl, F.; Kreft, H.; et al. Plant invasion and naturalization are influenced by genome size, ecology and economic use globally. Nat. Commun. 2024, 15, 1330. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Pei, J.; Zhao, L.; Ahmad, B.; Huang, L.F. Fighting climate change: Soil bacteria communities and topography play a role in plant colonization of desert areas. Environ. Microbiol. 2021, 23, 6876–6894. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, J.; Nasr, A.; Tang, Z.; Liu, W.; Liu, X.; Ma, W. Metabonomics Analysis Reveals the Influence Mechanism of Three Potassium Levels on the Growth, Metabolism and Accumulation of Medicinal Components of Bupleurum scorzonerifolium Willd. (Apiaceae). Biology 2025, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, L.; Wang, H.; Zhang, L.; Li, H.; Li, Y.; Niu, P.; Tian, G.; Yang, Y.; Mei, X.; et al. The Impact of Growth Years on the Medicinal Material Characteristics and Metabolites of Stellaria dichotoma L. var. lanceolata Bge. Reveals the Optimal Harvest Age. Plants 2023, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Wang, Y.; Abozeid, A.; Zu, Y.G.; Tang, Z.H. The integration of GC-MS and LC-MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue- and species-specific connectivity of primary metabolites and ginsenosides accumulation. J. Pharm. Biomed. Anal. 2017, 135, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC–MS combined with chemometrics. J. Pharm. Biomed. Anal. 2015, 102, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-F.; Qu, W.-J.; Jia, Z.; Han, T.; Liu, M.-N.; Bai, Y.-Y.; Wang, M.; Lin, R.-C.; Hua, Q.; Li, X.-R. Effect of cultivation ages on anti-inflammatory activity of a new type of red ginseng. Biomed. Pharmacother. 2021, 136, 111280. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-L.; Qiao, M.-D.; Yu, P.; Zheng, F.; Yue, H.; Liu, S.-Y. Comparing eight types of ginsenosides in ginseng of different plant ages and regions using RRLC-Q-TOF MS/MS. J. Ginseng Res. 2020, 44, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.; Ogunsina, M.O.; Novak, A.; Joshi, A.; Kokai, J.; Rocha Ida, C.; de Santayana, M.P. Comparative study of the in vitro bioactivities of Bupleurum rigidum and B. fruticescens. Nat. Prod. Commun. 2012, 7, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Lee, S.Y.; Hyun, S.H.; Kim, D.Y.; Marriott, P.J.; Choi, H.K. Gas chromatography/mass spectrometry-based metabolic profiling and differentiation of ginseng roots according to cultivation age using variable selection. J. AOAC Int. 2013, 96, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Seo, S.H.; Kim, E.J.; Park, D.H.; Park, K.M.; Cho, S.S.; Son, H.S. Metabolomic Approach for Discrimination of Cultivation Age and Ripening Stage in Ginseng Berry Using Gas Chromatography-Mass Spectrometry. Molecules 2019, 24, 3837. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Dijkstra, F.A.; Zhang, K.; Tan, H.; Zhao, Y.; Li, X. Influence of life form, taxonomy, climate, and soil properties on shoot and root concentrations of 11 elements in herbaceous plants in a temperate desert. Plant Soil 2016, 398, 339–350. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.; White, P. Functions of Macronutrients; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 135–189. [Google Scholar]
- Chatzav, M.; Peleg, Z.; Ozturk, L.; Yazici, A.; Fahima, T.; Cakmak, I.; Saranga, Y. Genetic diversity for grain nutrients in wild emmer wheat: Potential for wheat improvement. Ann. Bot. 2010, 105, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.R.; Haegele, J.W.; Below, F.E. Nutrient Uptake, Partitioning, and Remobilization in Modern Soybean Varieties. Agron. J. 2015, 107, 563–573. [Google Scholar] [CrossRef]
- Chu, Q.; Watanabe, T.; Shinano, T.; Nakamura, T.; Oka, N.; Osaki, M.; Sha, Z. The dynamic state of the ionome in roots, nodules, and shoots of soybean under different nitrogen status and at different growth stages. J. Plant Nutr. Soil Sci. 2016, 179, 488–498. [Google Scholar] [CrossRef]
- Andrekowisk Fioravanti, M.I.; Peixoto Pizano, F.; Rebellato, A.P.; Milani, R.F.; Morgano, M.A.; Pavesi Arisseto Bragotto, A. Turmeric products: Evaluation of curcumin and trace elements. Food Res. Int. 2024, 196, 115028. [Google Scholar] [CrossRef] [PubMed]
- Ru, L.; Ma, Y.; Xiaoyan, S.; XingWang, Y.; Dabin, H. The Content Analyses on Total Flavonoids and Trace Elements of Three Kinds of Bupleurum L. from Different Produced-area. Hubei Agric. Sci. 2016, 55, 670–672+677. [Google Scholar] [CrossRef]
- Xue, W.; Liu, C.; Qi, W. Correlation Analysis Between Mineral Elements and Active Ingredients in Bupleuri Radix. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 45–49. [Google Scholar] [CrossRef]
- Cui, X.S.; Dong, X.H.; Guo, Y.H. Determination of mineral elements in Bupleurum based on ICP-AES. Guang Pu Xue Yu Guang Pu Fen Xi 2012, 32, 529–531. [Google Scholar] [PubMed]
- Cao, H.L.; Wei, J.H.; He, C.E.; Yang, C.M.; Yu, J. Comparison of the contents of mineral elements in the roots of four germplasms Bupleurum L. planted in different soils. Guang Pu Xue Yu Guang Pu Fen Xi 2010, 30, 1105–1108. [Google Scholar] [PubMed]
| Time | Acetonitrile (%) | Water (%) |
|---|---|---|
| 0~10 | 25~38 | 75~62 |
| 11~20 | 38~51 | 62~49 |
| 21~30 | 51~64 | 49~36 |
| 31~40 | 64~77 | 36~23 |
| 41~55 | 77~90 | 23~10 |
| PL-R (cm) | PL-MS (cm) | PL-LS (cm) | PL-F (cm) | RW (cm) | NB | BL | |
|---|---|---|---|---|---|---|---|
| One year | 2.189 ± 0.63 | 9.538 ± 0.68 | 3.48 ± 0.51 | 16.068 ± 0.87 | 0.19 ± 0.01 | 3 ± 1 | 1–2 |
| Two years | 8.8 ± 0.84 | 23.1 ± 1.34 | 27.4 ± 4.39 | 71.8 ± 5.39 | 0.44 ± 0.05 | 7 ± 1 | 2–3 |
| Three years | 10.16 ± 0.50 | 33.6 ± 4.72 | 29.8 ± 3.27 | 86.4 ± 5.41 | 0.86 ± 0.18 | 13 ± 3 | 4–5 |
| FW-R (g) | FW-MS (g) | FW-LS (g) | FW-F (g) | DR | DSR | |
|---|---|---|---|---|---|---|
| One year | 0.51 ± 0.13 | 1.196 ± 0.17 | 0.9 ± 0.11 | - | 63.81% ± 9.71% | 19.6% ± 4% |
| Two years | 1.506 ± 0.68 | 2.692 ± 0.97 | 1.466 ± 0.62 | 3.456 ± 1.25 | 66.61% ± 13.25% | 16.52% ± 3% |
| Three years | 2.47 ± 0.44 | 3.754 ± 2.19 | 2.788 ± 0.66 | 7.87 ± 1.4 | 66.09% ± 12.2% | 14.63% ± 10.3% |
| Saikosaponin A (mg·g−1) | Saikosaponin C (mg·g−1) | Saikosaponin D (mg·g−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| One Year | Two Years | Three Years | One Year | Two Years | Three Years | One Year | Two Years | Three Years | |
| F | - | 0.629 ± 0.02 | 0.089 ± 0.01 | - | 2.104 ± 0.03 | 0.140 ± 0.08 | - | 0.874 ± 0.01 | 0.271 ± 0.08 |
| LS | 0.007 ± 0.01 | 0.078 ± 0.03 | 0.117 ± 0.02 | 0.391 ± 0.05 | 1.347 ± 0.07 | 0.067 ± 0.05 | 0.103 ± 0.03 | 0.425 ± 0.03 | 0.356 ± 0.18 |
| MS | 0.156 ± 0.03 | 0.178 ± 0.02 | 0.319 ± 0.05 | 0.398 ± 0.03 | 0.888 ± 0.026 | 0.673 ± 0.242 | 0.124 ± 0.02 | 0.582 ± 0.02 | 0.657 ± 0.116 |
| R | 1.378 ± 0.01 | 0.024 ± 0.01 | 0.278 ± 0.01 | 0.625 ± 0.05 | 0.491 ± 0.06 | 3.364 ± 0.32 | 1.581 ± 0.05 | 2.092 ± 0.02 | 0.235 ± 0.17 |
| Element | Treatment | MS | LS | R | RDR |
|---|---|---|---|---|---|
| Na (mg/g−1) | One year | 0.24 ± 0.0007 | 0.18 ± 0.0007 | 0.66 ± 0.0031 | 61.13% |
| Two years | 0.36 ± 0.0007 | 0.17 ± 0.0000 | 0.92 ± 0.0019 | 63.51% | |
| Three years | 0.40 ± 0.0013 | 0.20 ± 0.0007 | 1.28 ± 0.0029 | 68.27% | |
| K (mg/g−1) | One year | 9.72 ± 0.0610 | 15.39 ± 0.0087 | 11.80 ± 0.0102 | 31.96% |
| Two years | 9.62 ± 0.0323 | 9.39 ± 0.0477 | 15.80 ± 0.0824 | 45.40% | |
| Three years | 5.18 ± 0.0425 | 8.37 ± 0.0170 | 7.78 ± 0.0454 | 36.47% | |
| Ca (mg/g−1) | One year | 3.77 ± 0.0335 | 8.81 ± 0.0118 | 5.12 ± 0.0025 | 28.89% |
| Two years | 3.66 ± 0.0188 | 3.70 ± 0.0207 | 5.15 ± 0.0293 | 41.17% | |
| Three years | 3.96 ± 0.0325 | 6.17 ± 0.0254 | 6.07 ± 0.0333 | 37.48% | |
| Mg (mg/g−1) | One year | 1.10 ± 0.0038 | 1.19 ± 0.0014 | 2.02 ± 0.0056 | 46.84% |
| Two years | 0.96 ± 0.0022 | 0.91 ± 0.0012 | 2.24 ± 0.0094 | 54.54% | |
| Three years | 1.04 ± 0.0014 | 1.24 ± 0.0036 | 1.63 ± 0.0007 | 41.68% | |
| Mn (mg/g−1) | One year | 32.50 ± 0.0000 | 32.50 ± 0.0000 | 36.25 ± 0.0000 | 35.80% |
| Two years | 20.00 ± 0.0000 | 19.17 ± 0.7217 | 33.75 ± 0.0000 | 46.29% | |
| Three years | 21.25 ± 0.0000 | 35.83 ± 0.7217 | 47.50 ± 0.0000 | 45.42% | |
| Cu (mg/g−1) | One year | 8.75 ± 0.0000 | 5.00 ± 0.0000 | 13.75 ± 0.0000 | 50.00% |
| Two years | 3.75 ± 0.0000 | 2.50 ± 0.0000 | 7.92 ± 0.7217 | 55.88% | |
| Three years | 3.75 ± 0.0000 | 5.00 ± 0.0000 | 8.75 ± 0.0000 | 50.00% | |
| Zn (mg/g−1) | One year | 17.50 ± 0.0000 | 22.92 ± 0.7217 | 32.50 ± 0.0000 | 44.57% |
| Two years | 17.50 ± 0.0000 | 21.25 ± 0.0000 | 62.08 ± 0.7217 | 61.57% | |
| Three years | 13.75 ± 0.0000 | 20.00 ± 0.0000 | 36.25 ± 0.0000 | 51.79% | |
| Fe (mg/g−1) | One year | 73.75 ± 0.0000 | 99.17 ± 0.7217 | 317.50 ± 0.0000 | 64.74% |
| Two years | 32.50 ± 0.0000 | 23.75 ± 0.0000 | 98.75 ± 0.0000 | 63.71% | |
| Three years | 23.75 ± 0.0000 | 30.00 ± 0.0000 | 224.17 ± 0.7217 | 80.66% |
| MS | PC1 | PC2 | LS | PC1 | PC2 | R | PC1 | PC2 |
|---|---|---|---|---|---|---|---|---|
| Na | 0.998 | −0.060 | Na | 0.316 | −0.945 | Na | 0.498 | −0.843 |
| K | −0.767 | −0.640 | K | 0.688 | 0.722 | K | 0.884 | −0.391 |
| Ca | 0.453 | 0.889 | Ca | 0.985 | 0.171 | Ca | 0.696 | 0.664 |
| Mg | −0.617 | 0.787 | Mg | 0.874 | −0.485 | Mg | 0.973 | −0.132 |
| Mn | −0.912 | 0.409 | Mn | 0.846 | −0.531 | Mn | 0.573 | 0.798 |
| Cu | −0.946 | 0.325 | Cu | 0.931 | −0.364 | Cu | 0.952 | −0.251 |
| Zn | −0.754 | −0.656 | Zn | 0.429 | 0.886 | Zn | 0.998 | −0.019 |
| Fe | −0.986 | 0.166 | Fe | 0.824 | 0.565 | Fe | 0.266 | 0.873 |
| Total variation explained | 67.97% | 31.90% | 59.42% | 39.98% | 61.88% | 37.97% |
| Classification | Metabolites | Classification | Metabolites |
|---|---|---|---|
| Acids and derivatives (18) | 2-Propenoic acid | Sugars (9) | D-Mannose |
| Aminobutanoic acid | D-Cellobiose | ||
| Aminocyclopentanecarboxylic acid | Arabinofuranose | ||
| Benzoic acid | D-Fructose | ||
| Butanedioic acid | D-Glucose | ||
| Butyric acid | L-Rhamnose | ||
| Carboxylic acid | α-D-Lactose | ||
| Chlorogenic acid | Sedoheptulose | ||
| Citric acid | Sucrose | ||
| Lactic acid | Polyols (8) | D-Mannitol | |
| Galactaric acid | Glycerol | ||
| Palmitic acid | Muco-Inositol | ||
| Pentanedioic acid | Cuminyl alcohol | ||
| Benzenediol | |||
| Pipecolic acid | Benzylaminooctanol | ||
| Propanedioic acid | Ribitol | ||
| Quininic acid | Phenol | ||
| β-D-Glucofuranuronic acid | Alkyl (4) | Heptane | |
| Malic acid | |||
| Glycosides (3) | α-D-Galactopyranoside | ||
| α-D-Glucopyranoside | Nonane | ||
| α-D-Ribofuranoside | Sulfone | ||
| Others (10) | Ether | Decane | |
| Galactose oxime | |||
| Glycerol monostearate | Amino acid and derivatives (7) | L-Alanine | |
| Methyl benzoate | L-Valine | ||
| Heptabarbital | Urea | ||
| Isoquinolinium | Amide | ||
| Androst | Ethanolamine | ||
| Carbamate | Cadaverine | ||
| Cyclohexene | Diaziridine | ||
| Monopalmitin |
| R2X (cum) | R2Y (cum) | Q2 (cum) | R2Y-Q2 | |
|---|---|---|---|---|
| PLS-DA(F) | 0.397 | 0.814 | 0.134 | 0.680 |
| PLS-DA(LS) | 0.467 | 0.893 | 0.518 | 0.375 |
| PLS-DA(MS) | 0.337 | 0.850 | 0.019 | 0.831 |
| PLS-DA(R) | 0.751 | 0.999 | 0.900 | 0.099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wu, J.; Li, W.; Liu, X.; Ma, W. Metabolomics Analysis Reveals the Influence Mechanism of Different Growth Years on the Growth, Metabolism and Accumulation of Medicinal Components of Bupleurum scorzonerifolium Willd. (Apiaceae). Biology 2025, 14, 864. https://doi.org/10.3390/biology14070864
Sun J, Wu J, Li W, Liu X, Ma W. Metabolomics Analysis Reveals the Influence Mechanism of Different Growth Years on the Growth, Metabolism and Accumulation of Medicinal Components of Bupleurum scorzonerifolium Willd. (Apiaceae). Biology. 2025; 14(7):864. https://doi.org/10.3390/biology14070864
Chicago/Turabian StyleSun, Jialin, Jianhao Wu, Weinan Li, Xiubo Liu, and Wei Ma. 2025. "Metabolomics Analysis Reveals the Influence Mechanism of Different Growth Years on the Growth, Metabolism and Accumulation of Medicinal Components of Bupleurum scorzonerifolium Willd. (Apiaceae)" Biology 14, no. 7: 864. https://doi.org/10.3390/biology14070864
APA StyleSun, J., Wu, J., Li, W., Liu, X., & Ma, W. (2025). Metabolomics Analysis Reveals the Influence Mechanism of Different Growth Years on the Growth, Metabolism and Accumulation of Medicinal Components of Bupleurum scorzonerifolium Willd. (Apiaceae). Biology, 14(7), 864. https://doi.org/10.3390/biology14070864





