Cellular and Matrix Organisation of the Human Aortic Valve Interleaflet Triangles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Histological Staining
2.1.1. Elastic Van Gieson Staining for Extracellular Matrix
2.1.2. Alcian Blue/Sirius Red Staining for Extracellular Matrix
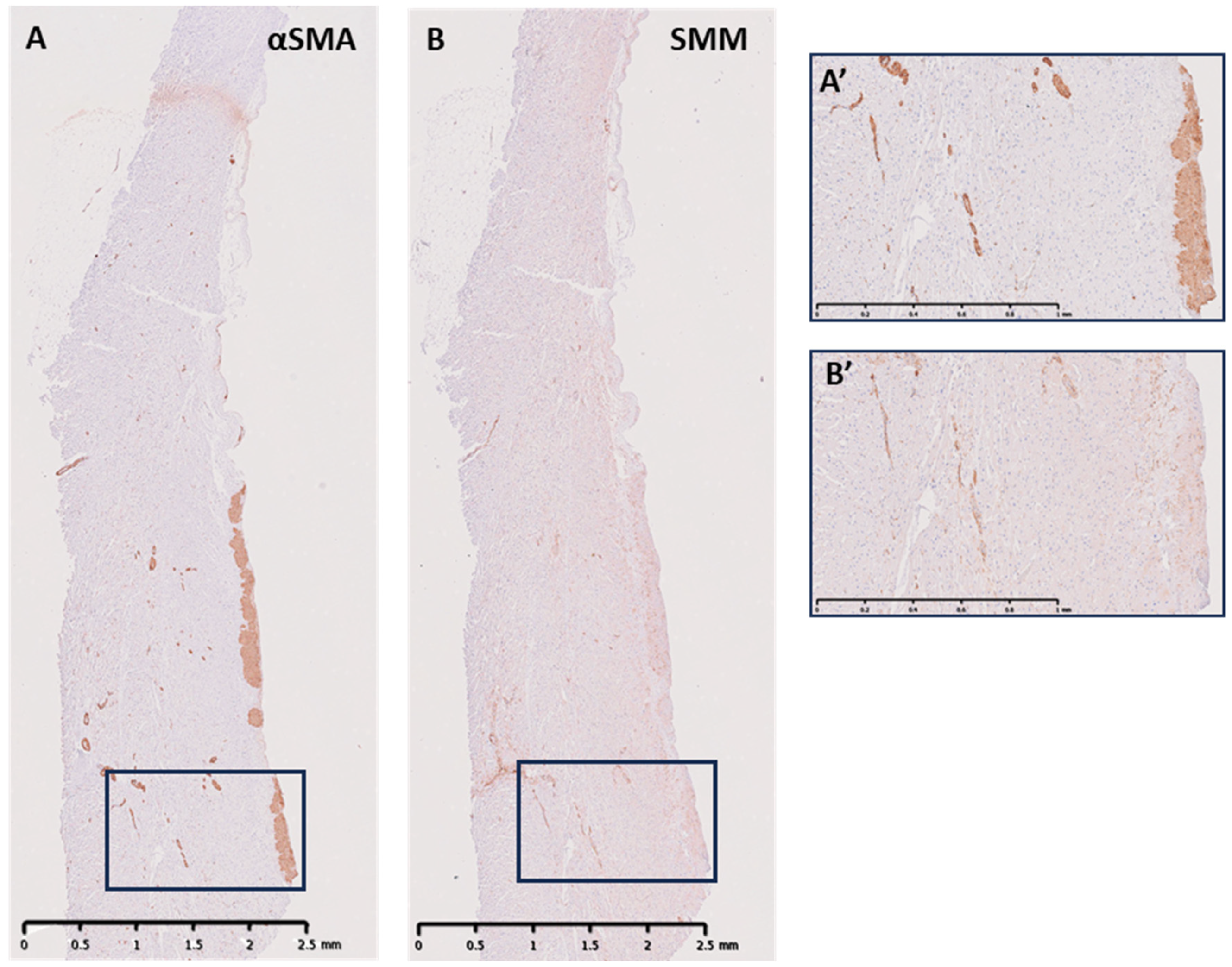
2.1.3. Immunohistochemistry
2.2. Transmission Electron Microscopy (TEM)
2.3. Scanning Electron Microscopy (SEM)
2.4. Statistics
3. Results
3.1. Structural Characteristics of the ILTs
3.2. Cellular Components of the ILTs
3.3. ECM Elements in the ILTs
3.4. Neuronal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| αSMA | Smooth muscle α-actin |
| ACH | Acetylcholine transferase |
| BSA | Bovine serum albumin |
| ILT | Interleaflet triangle |
| L-N | left-to-non-interleaflet triangle |
| L-R | left-to-right interleaflet triangle |
| N-R | non-to-right interleaflet triangle |
| NF | Neurofilament |
| SMM | Smooth muscle myosin |
| TH | Tyrosine hydroxylase |
| VIP | Vasoactive intestinal peptide |
References
- Yacoub, M.H.; Kilner, P.J.; Birks, E.J.; Misfeld, M. The aortic outflow and root: A tale of dynamism and crosstalk. Ann. Thorac. Surg. 1999, 68 (Suppl. S3), S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Higashidate, M.; Tamiya, K.; Beppu, T.; Imai, Y. Regulation of the aortic valve opening. In vivo dynamic measurement of aortic valve orifice area. J. Thorac. Cardiovasc. Surg. 1995, 110, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Dagum, P.; Green, G.R.; Nistal, F.J.; Daughters, G.T.; Timek, T.A.; Foppiano, L.E.; Bolger, A.F.; Ingels, N.B., Jr.; Miller, D.C. Deformational dynamics of the aortic root: Modes and physiologic determinants. Circulation 1999, 100 (Suppl. S2), II54–II62. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, A.G.; Von Bernuth, G.; Rastelli, G.C.; Bourgeois, M.J.; Titus, J.L.; Wood, E.H. Size and motion of the mitral valve annulus in anesthetized intact dogs. J. Appl. Physiol. 1971, 30, 611–618. [Google Scholar] [CrossRef]
- El-Nashar, H.; Sabry, M.; Tseng, Y.-T.; Francis, N.; Latif, N.; Parker, K.H.; Moore, J.E., Jr.; Yacoub, M.H. Multiscale structure and function of the aortic valve apparatus. Physiol. Rev. 2024, 104, 1487–1532. [Google Scholar] [CrossRef]
- Sutton, J.P., 3rd; Ho, S.Y.; Anderson, R.H. The forgotten interleaflet triangles: A review of the surgical anatomy of the aortic valve. Ann. Thorac. Surg. 1995, 59, 419–427. [Google Scholar] [CrossRef]
- Chester, A.H.; El-Hamamsy, I.; Butcher, J.T.; Latif, N.; Bertazzo, S.; Yacoub, M.H. The living aortic valve: From molecules to function. Glob. Cardiol. Sci. Pract. 2014, 2014, 52–77. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Eryigit, Z.; Stevens, L.-M.; Sarang, Z.; George, R.; Clark, L.; Melina, G.; Takkenberg, J.J.M.; Yacoub, M.H. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: A randomised controlled trial. Lancet 2010, 376, 524–531. [Google Scholar] [CrossRef]
- Latif, N.; Quillon, A.; Sarathchandra, P.; McCormack, A.; Lozanoski, A.; Yacoub, M.H.; Chester, A.H. Modulation of human valve interstitial cell phenotype and function using a fibroblast growth factor 2 formulation. PLoS ONE 2015, 10, e0127844. [Google Scholar] [CrossRef]
- Latif, N.; Sarathchandra, P.; Chester, A.H.; Yacoub, M.H. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur. Heart J. 2015, 36, 1335–1345. [Google Scholar] [CrossRef]
- Misfeld, M.; Chester, A.H.; Sievers, H.H.; Yacoub, M.H. Biological mechanisms influencing the function of the aortic root. J. Card. Surg. 2002, 17, 363–368. [Google Scholar] [CrossRef]
- Yacoub, M.H.; Tsang, V.; Sarathchandra, P.; Jensen, H.; Hughes, S.; Latif, N. Long-term adaptive versus maladaptive remodelling of the pulmonary autograft after the Ross operation. Eur. J. Cardiothorac. Surg. 2020, 57, 977–985. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Balachandran, K.; Yacoub, M.H.; Stevens, L.M.; Sarathchandra, P.; Taylor, P.M.; Yoganathan, A.P.; Chester, A.H. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J. Am. Coll. Cardiol. 2009, 53, 1448–1455. [Google Scholar] [CrossRef]
- Chester, A.H.; Kershaw, J.D.B.; Sarathchandra, P.; Yacoub, M.H. Localisation and function of nerves in the aortic root. J. Mol. Cell. Cardiol. 2008, 44, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Misfeld, M.; Yacoub, M.H. Receptor-mediated contraction of aortic valve leaflets. J. Heart Valve Dis. 2000, 9, 250–254; discussion 254–255. [Google Scholar] [PubMed]
- Izawa, Y.; Mori, S.; Tretter, J.T.; Quintessenza, J.A.; Toh, H.; Toba, T.; Watanabe, Y.; Kono, A.K.; Okada, K.; Hirata, K.-I. Normative Aortic Valvar Measurements in Adults Using Cardiac Computed Tomography―A Potential Guide to Further Sophisticate Aortic Valve-Sparing Surgery. Circ. J. 2021, 85, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Contino, M.; Mangini, A.; Lemma, M.G.; Romagnoni, C.; Zerbi, P.; Gelpi, G.; Antona, C. A geometric approach to aortic root surgical anatomy. Eur. J. Cardiothorac. Surg. 2016, 49, 93–100. [Google Scholar] [CrossRef]
- Khelil, N.; Sleilaty, G.; Palladino, M.; Fouda, M.; Escande, R.; Debauchez, M.; Di Centa, I.; Lansac, E. Surgical anatomy of the aortic annulus: Landmarks for external annuloplasty in aortic valve repair. Ann. Thorac. Surg. 2015, 99, 1220–1226. [Google Scholar] [CrossRef]
- Grande, K.J.; Cochran, R.P.; Reinhall, P.G. Stress variations in the human aortic root and valve: The role of anatomic asymmetry. Ann. Biomed. Eng. 1998, 26, 534–545. [Google Scholar] [CrossRef]
- David, T.E.; Feindel, C.M.; Bos, J. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J. Thorac. Cardiovasc. Surg. 1995, 109, 345–351; discussion 351–352. [Google Scholar] [CrossRef]
- Richardson, R.; Eley, L.; Donald-Wilson, C.; Davis, J.; Curley, N.; Alqahtani, A.; Murphy, L.; Anderson, R.H.; Henderson, D.J.; Chaudhry, B. Development and maturation of the fibrous components of the arterial roots in the mouse heart. J. Anat. 2018, 232, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Odelin, G.; Faure, E.; Coulpier, F.; Di Bonito, M.; Bajolle, F.; Studer, M.; Avierinos, J.-F.; Charnay, P.; Topilko, P.; Zaffran, S. Krox20 defines a subpopulation of cardiac neural crest cells contributing to arterial valves and bicuspid aortic valve. Development 2018, 145, dev151944. [Google Scholar] [CrossRef] [PubMed]
- Bechsgaard, T.; Lindskow, T.; Lading, T.; Hasenkam, J.M.; Røpcke, D.M.; Nygaard, H.; Johansen, P.; Nielsen, S.L. Biomechanical characterization of the native porcine aortic root. J. Biomech. 2018, 74, 156–162. [Google Scholar] [CrossRef] [PubMed]
- de Heer, L.M.; Budde, R.P.J.; Mali, W.P.T.M.; de Vos, A.M.; van Herwerden, L.A.; Kluin, J. Aortic root dimension changes during systole and diastole: Evaluation with ECG-gated multidetector row computed tomography. Int. J. Cardiovasc. Imaging 2011, 27, 1195–1204. [Google Scholar] [CrossRef]
- Suchá, D.; Tuncay, V.; Prakken, N.H.J.; Leiner, T.; van Ooijen, P.M.A.; Oudkerk, M.; Budde, R.P.J. Does the aortic annulus undergo conformational change throughout the cardiac cycle? A systematic review. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1307–1317. [Google Scholar] [CrossRef]
- Balachandran, K.; Sucosky, P.; Jo, H.; Yoganathan, A.P. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: Implications for degenerative aortic valve disease. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H756–H764. [Google Scholar] [CrossRef]
- Kershaw, J.D.; Misfeld, M.; Sievers, H.H.; Yacoub, M.H.; Chester, A.H. Specific regional and directional contractile responses of aortic cusp tissue. J. Heart Valve Dis. 2004, 13, 798–803. [Google Scholar]
- Fedele, L.; Brand, T. The Intrinsic Cardiac Nervous System and Its Role in Cardiac Pacemaking and Conduction. J. Cardiovasc. Dev. Dis. 2020, 7, 54. [Google Scholar] [CrossRef]
- Lansakara, M.; Unai, S. An overview of aortic valve anatomy: The current understanding. Indian J. Thorac. Cardiovasc. Surg. 2023, 39 (Suppl. S2), 246–252. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latif, N.; Sarathchandra, P.; Al-Holy, A.; Vaz, S.; Chester, A.H.; Yacoub, M.H. Cellular and Matrix Organisation of the Human Aortic Valve Interleaflet Triangles. Biology 2025, 14, 863. https://doi.org/10.3390/biology14070863
Latif N, Sarathchandra P, Al-Holy A, Vaz S, Chester AH, Yacoub MH. Cellular and Matrix Organisation of the Human Aortic Valve Interleaflet Triangles. Biology. 2025; 14(7):863. https://doi.org/10.3390/biology14070863
Chicago/Turabian StyleLatif, Najma, Padmini Sarathchandra, Albaraa Al-Holy, Sanida Vaz, Adrian H. Chester, and Magdi H. Yacoub. 2025. "Cellular and Matrix Organisation of the Human Aortic Valve Interleaflet Triangles" Biology 14, no. 7: 863. https://doi.org/10.3390/biology14070863
APA StyleLatif, N., Sarathchandra, P., Al-Holy, A., Vaz, S., Chester, A. H., & Yacoub, M. H. (2025). Cellular and Matrix Organisation of the Human Aortic Valve Interleaflet Triangles. Biology, 14(7), 863. https://doi.org/10.3390/biology14070863






