Functional Analysis of the Cyclin E Gene in the Reproductive Development of Rainbow Trout (Oncorhynchus mykiss)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Sample Collection
2.2. Molecular Cloning of CCNE
2.3. Bioinformatics Analysis of CCNE
2.4. Quantitative Real-Time PCR(q-PCR)
2.5. Immunohistochemistry
2.6. RNA Interference and Overexpression of CCNE
2.7. Determination of RTG2 Viability
2.8. Immunofluorescence
2.9. EDU Proliferation Test
2.10. Meiosis and Reproduction-Related Gene Testing
2.11. Statistical Analysis
3. Results
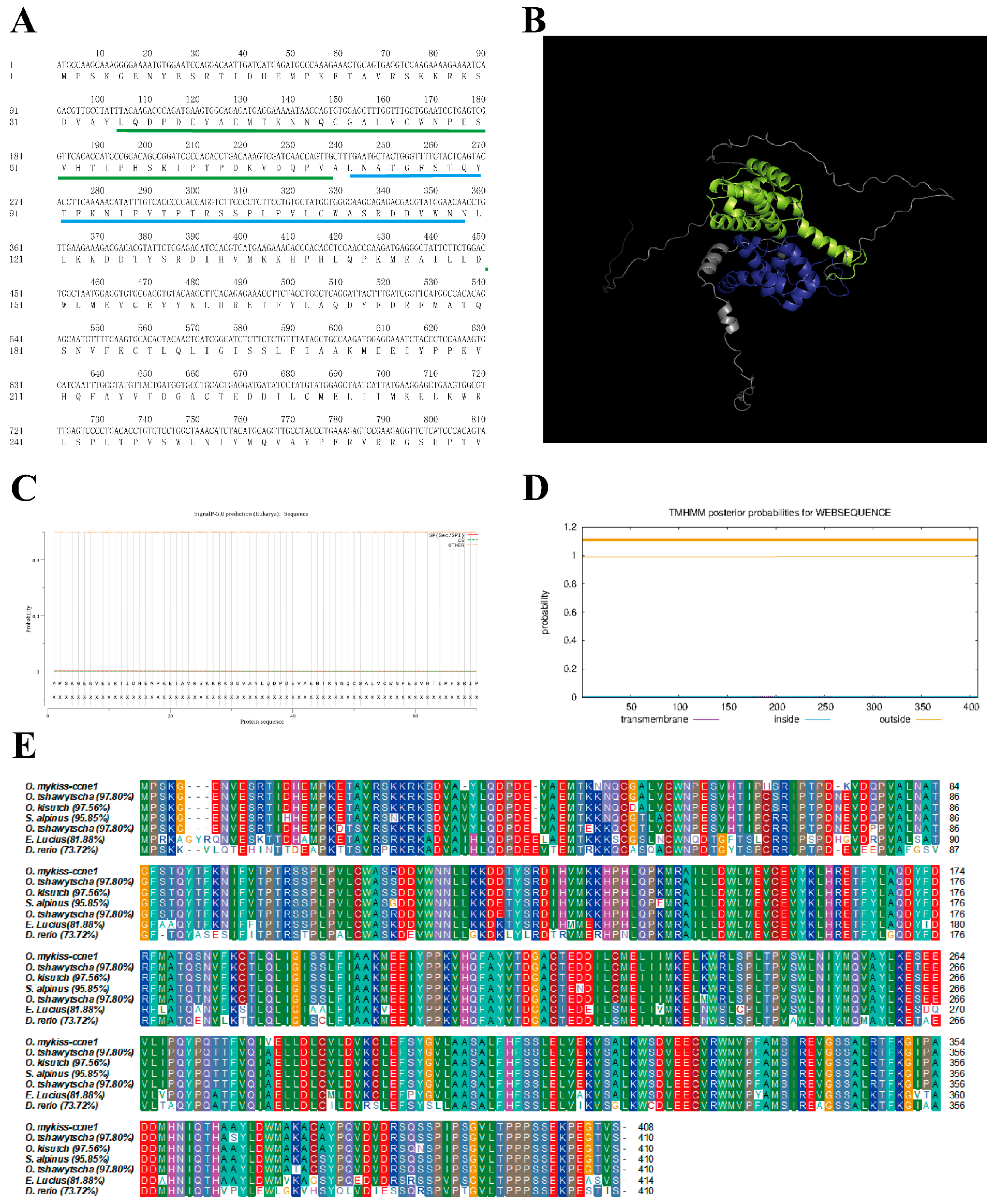
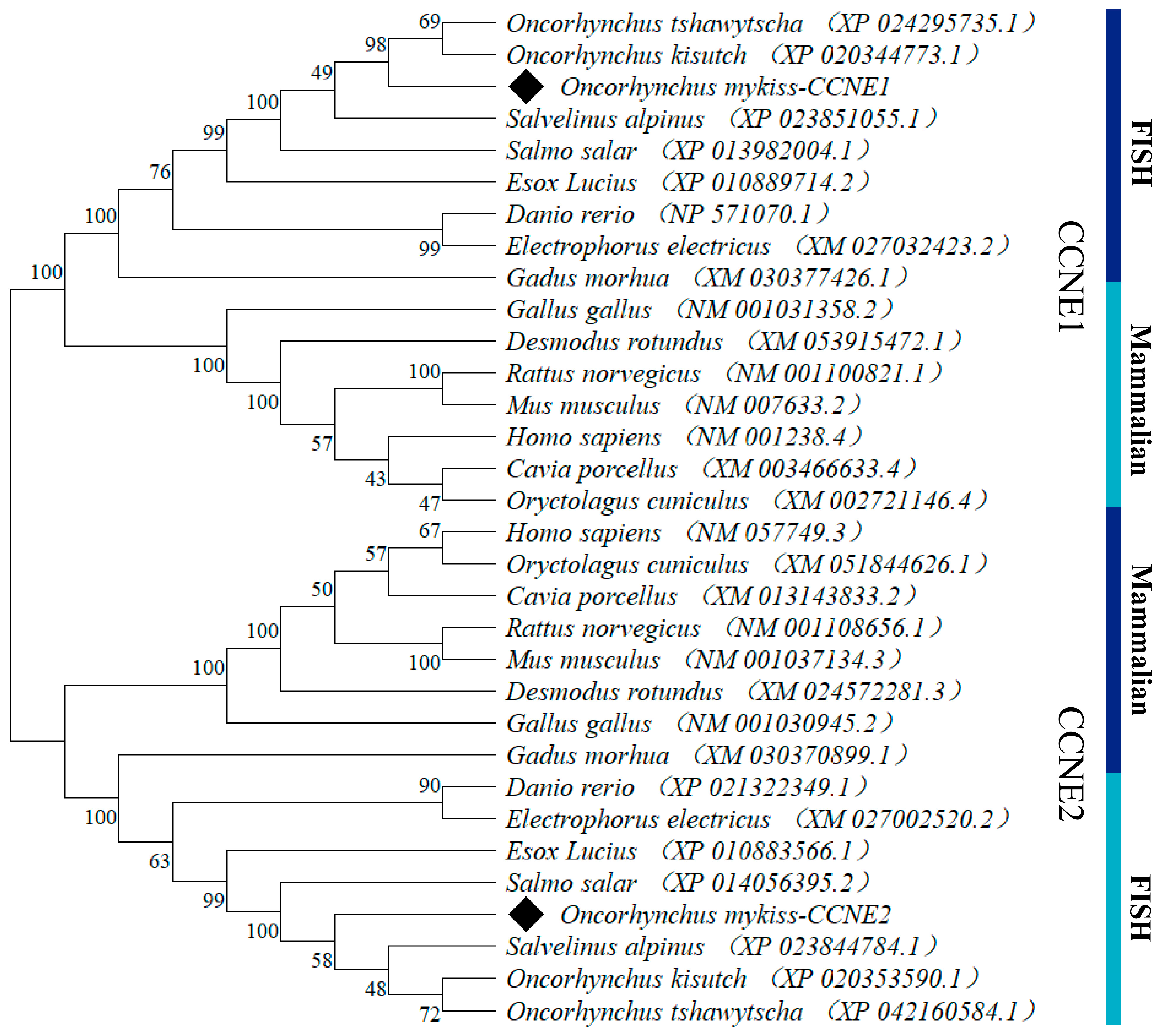
3.1. Cloning and Sequence Analysis of the CCNE1 and CCNE2
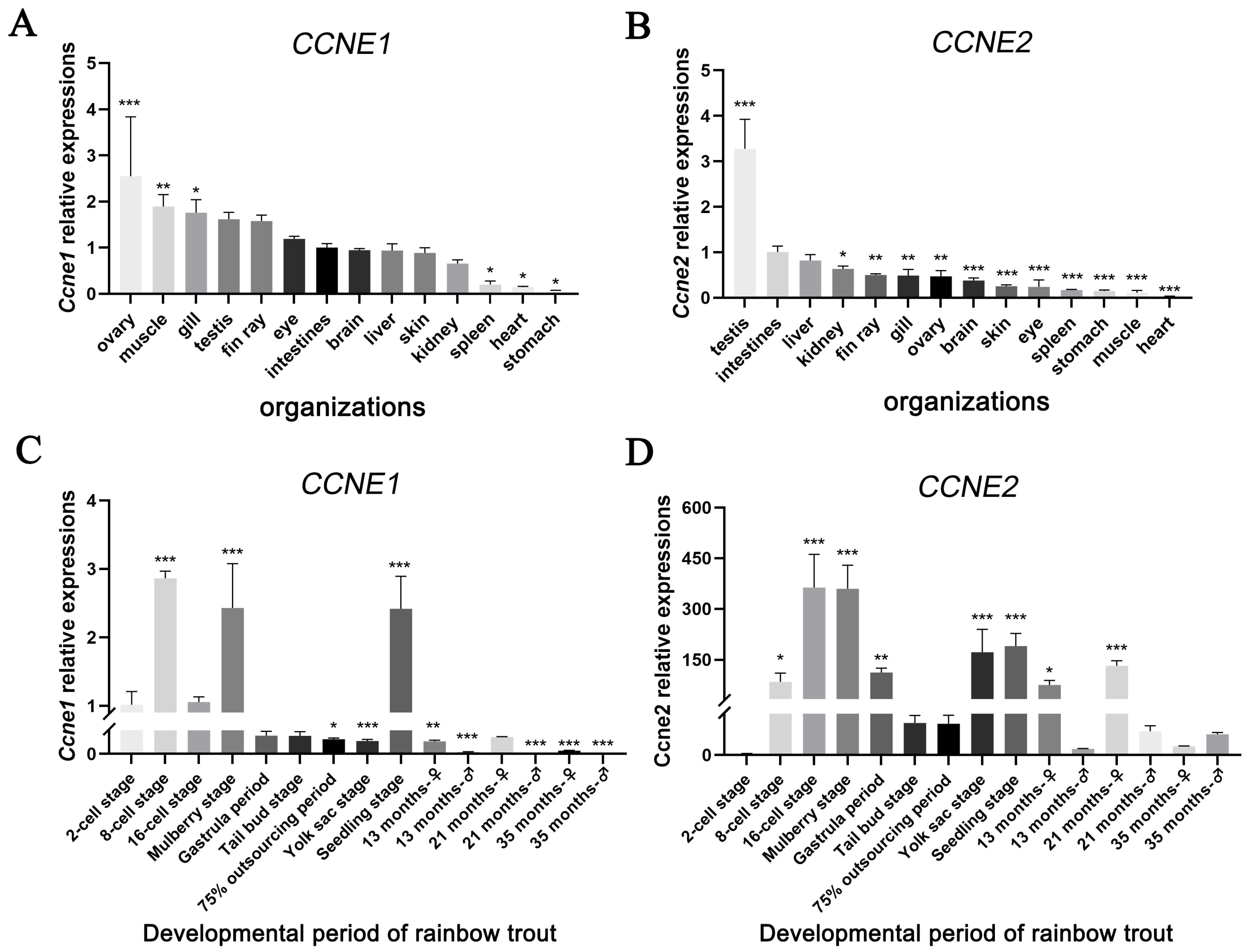
3.2. Characterization of CCNE1 and CCNE2 Expression in Different Tissues and at Different Times of Gonadal Development
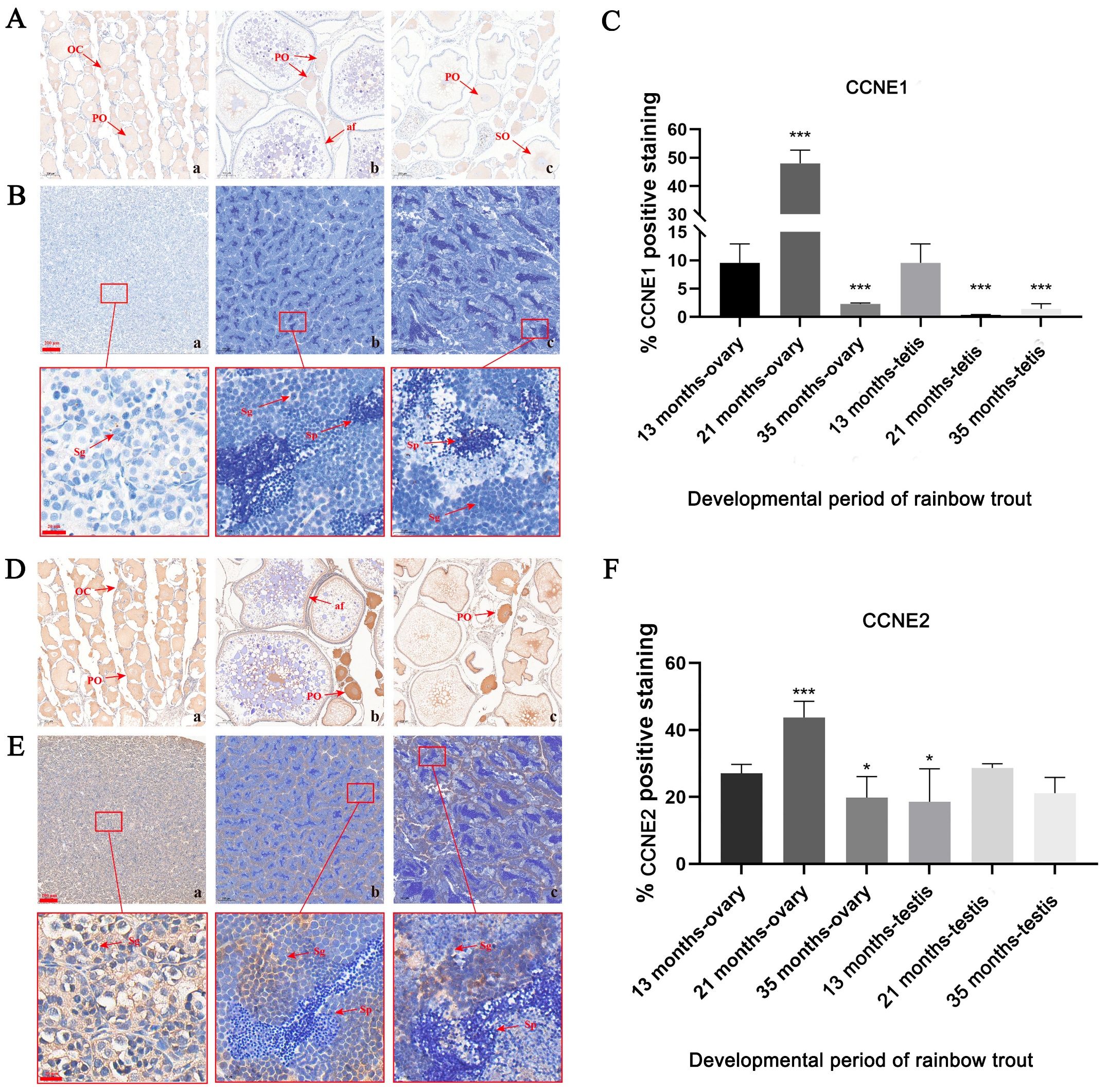
3.3. IHC Staining of the Localization of CCNE1 and CCNE2 Proteins in the Gonad
3.4. Validation of CCNE1 and CCNE2 siRNA with Overexpression
3.5. Cell Viability Assay
3.6. Localization of CCNE1 and CCNE2 Proteins in RGT2
3.7. Cell Proliferation Assay
3.8. Expression of Reproductive and Meiotic Genes
4. Discussion
4.1. Sequence Analysis of CCNE1 and CCNE2 in Rainbow Trout
4.2. Expression of CCNE1 and CCNE2 in Rainbow Trout
4.3. Immunohistochemical Analysis of Protein Expression
4.4. Biological Functional of CCNE1 and CCNE2 at the Cellular Level
4.5. Quantitative Analysis of Genes Related to Reproduction and Meiosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Barnum, K.J.; O’Connell, M.J. Cell cycle regulation by checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Wolgemuth, D.J. Function of cyclins in regulating the mitotic and meiotic cell cycles in male germ cells. Cell Cycle 2008, 7, 3509–3513. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Geng, Y.; Zhou, Y.; Sicinski, P. Cyclin E in normal physiology and disease states. Trends Cell Biol. 2021, 31, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Tanaka, H.; Yasuda, H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997, 420, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; He, Y.; Xia, J.; Pan, Y.X.; Li, S.L. The expression of Cyclin E in oral precancerous lesions and Squamous cell carcinomas and its significance. Guiyang Med. Coll. 2007, 32, 559–562. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, M. Non-dependence of cyclin E/Cdk2 kinase activity on the initiation of oocyte maturation in goldfish. Dev. Growth Differ. 2000, 42, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Josefsberg Ben-Yehoshua, L.; Beider, K.; Shimoni, A.; Ostrovsky, O.; Samookh, M.; Peled, A.; Nagler, A. Characterization of cyclin E expression in multiple myeloma and its functional role in seliciclib-induced apoptotic cell death. PLoS ONE 2012, 7, e33856. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Lowe, E.D.; Dubinina, E.; Skamnaki, V.; Cook, A.; Brown, N.R.; Johnson, L.N. The structure of cyclin E1/CDK2: Implications for CDK2 activation and CDK2-independent roles. EMBO J. 2005, 24, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.T.; Zhang, W.; Liu, Y.; Jang, H.; Nussinov, R. Binding Modalities and Phase-Specific Regulation of Cyclin/Cyclin-Dependent Kinase Complexes in the Cell Cycle. J. Phys. Chem. B 2024, 128, 9315–9326. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.T.; Fu, M.J.; Zhao, C. Molecular cloning and expression analysis of CDK2 gene from black tiger shrimps (Penaeus monodon). S. China Fish. Sci. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, H.; Li, Q. Disruption of cell division prevents gametogenesis in triploid Pacific oysters (Crassostrea gigas). Aquaculture 2022, 560, 738477. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, Z.; Gao, J.; Dai, Q.; Liu, X.; Shen, Y.; Baloch, W.A.; Noonari, S.; Wang, P.; Gao, H. Functional analysis of the cell cycle protein E gene (CCNE) in ovarian development of the white ridgetail prawn, Exopalaemon carinicauda. Aquac. Rep. 2023, 32, 101716. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, M.J.; Qiu, L.H. Molecular cloning and functional characterization of cyclin E and CDK2 from Penaeus monodon. Genet. Mol. Res. 2016, 15, 15038716. [Google Scholar] [CrossRef] [PubMed]
- Martinerie, L.; Manterola, M.; Chung, S.S.W.; Panigrahi, S.K.; Weisbach, M.; Vasileva, A.; Geng, Y.; Sicinski, P.; Wolgemuth, D.J. Mammalian E-type cyclins control chromosome pairing, telomere stability and CDK2 localization in Male meiosis. PLoS Genet. 2014, 10, e1004165. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of the People’s Republic of China. China Fisheries Statistics Yearbook; China Statistics Press: Beijing, China, 2024; pp. 45–52. [Google Scholar]
- Bao, S.; Zhuo, L.; Qi, D.; Dai, Q.; Liu, X.; Shen, Y.; Baloch, W.A.; Noonari, S.; Wang, P.; Gao, H. Comparative study on the fillet nutritional quality of diploid and triploid rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2023, 28, 101431. [Google Scholar] [CrossRef]
- Huang, T.; Gu, W.; Liu, E.; Shi, X.; Wang, B.; Wu, W.; Dong, F.; Xu, G. Comprehensive analysis of miRNA-mRNA/lncRNA during gonadal development of triploid female rainbow trout (Oncorhynchus mykiss). Genomics 2021, 113, 3533–3543. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Comparative study on gonad and gamete development and haemocytes of different ploid rainbow trout (Oncorhynchus mykiss). Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2008; pp. 14–16. [Google Scholar] [CrossRef]
- Putta, S.; Villegas, C.A.; Rubin, S.M. Differences in Binding Affinity Among Cell-cycle CDK and Cyclin Pairs. J. Mol. Biol. 2025, 437, 168952. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Su, Y.; Zho, U.D. Cloning of CyclinB gene from Sipunculus nudus and its expression analysis in Oocytes. South. J. Agric. 2021, 52, 1980–1990. [Google Scholar] [CrossRef]
- Zariwala, M.; Liu, J.; Xiong, Y. Oncogene; Springer Nature: Berlin/Heidelberg, Germany, 1997; pp. 2787–2798. [Google Scholar]
- Xu, P.; McIntyre, L.M.; Scardina, J.; Wheeler, P.A.; Thorgaard, G.H.; Nichols, K.M. Transcriptome profiling of embryonic development rate in rainbow trout advanced backcross introgression lines. Mar. Biotechnol. 2011, 13, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Tanaka, M. Gonadal development in fish. Sex Dev. 2014, 8, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Odajima, J.; Saini, S.; Jung, P.; Ndassa-Colday, Y.; Ficaro, S.; Geng, Y.; Marco, E.; Michowski, W.; Wang, Y.E.; DeCaprio, J.A.; et al. Proteomic landscape of tissue-specific cyclin E functions in vivo. PLoS Genet. 2016, 12, e1006429. [Google Scholar] [CrossRef] [PubMed]
- Parisi, T.; Beck, A.R.; Rougier, N.; McNeil, T.; Lucian, L.; Werb, Z.; Amati, B. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003, 22, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Encinas, P.; Carbajosa, S.; Cuesta, A.; Chaves-Pozo, E.; Tafalla, C.; Estepa, A.; Coll, J.M. Transfection improvements of fish cell lines by using deacylated polyethylenimine of selected molecular weights. Fish Shellfish. Immunol. 2009, 26, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Xu, G.; Shi, W.; Gu, W.; Wang, G.; Ge, K.; Fan, P.; Sun, Y.; Li, D.; Huang, T. The effect of interfering with CyclinE1 gene expression on gonadal development in Rainbow Trout. Chin. J. Fish. 2024. Available online: https://link.cnki.net/urlid/23.1363.S.20241119.1444.002 (accessed on 22 March 2025).
- Xie, X.H.; An, H.J.; Kang, S.; Hong, S.; Choi, Y.P.; Kim, Y.T.; Choi, Y.D.; Cho, N.H. Loss of Cyclin B1 followed by downregulation of Cyclin A/Cdk2, apoptosis and antiproliferation in Hela cell line. Int. J. Cancer 2005, 116, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Yoshii, A.; Yokota, T.; Sakai, C.; Hori, H.; Kanamori, A.; Yamashita, M. Structural components of the synaptonemal complex, SYCP1 and SYCP3, in the medaka fish Oryzias latipes. Exp. Cell Res. 2006, 312, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- De Vries, F.A.; De Boer, E.; Van den Bosch, M.; Baarends, W.M.; Ooms, M.; Yuan, L.; Liu, J.-G.; van Zeeland, A.A.; Heyting, C.; Pastink, A. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 2005, 19, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.-F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, F.; Ren, S.; Cai, Z.; Huang, K.; Wu, X.; Lin, Y.; Tong, W.; Li, Q.; Zheng, S. Preparation and immunological study of ichthyophthirius multifiliis β-tubulin DNA vaccine in grass carp (Ctenopharyngodon idella). Aquac. Rep. 2024, 39, 102415. [Google Scholar] [CrossRef]
- Tao, M.; Liu, S.; Long, Y.; Zeng, C.; Liu, J.; Liu, L.; Zhang, C.; Duan, W.; Liu, Y. The cloning of Dmc1 cDNAs and a comparative study of its expression in different ploidy cyprinid fishes. Sci. China Ser. C Life Sci. 2008, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Feitsma, H.; Leal, M.C.; Moens, P.B.; Cuppen, E.; Schulz, R.W. Mlh1 deficiency in zebrafish results in male sterility and aneuploid as well as triploid progeny in females. Genetics 2007, 175, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Meng, L.; Xu, W.; Cui, Z.; Zhang, N.; Guo, H.; Wang, N.; Shao, C.; Chen, S. The autosomal gsdf gene plays a role in male gonad development in chinese tongue sole (Cynoglossus semilaevis). Sci. Rep. 2018, 8, 17716. [Google Scholar] [CrossRef] [PubMed]
- Baloch, A.R.; Franěk, R.; Saito, T.; Pšenička, M. Dead-end (dnd) protein in fish—A review. Fish Physiol. Biochem. 2021, 47, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Silva de Assis, H.C.; Navarro-Martín, L.; Fernandes, L.S.P.; Cardoso, C.C.; Pavoni, D.P.; Trudeau, V.L. Cloning, partial sequencing and expression analysis of the neural form of P450 aromatase (cyp19a1b) in the South America catfish Rhamdia quelen. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 221–222, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, S.; Ji, X.; Shao, C. Molecular cloning, characterization and expression analysis of Sox9a and Foxl2 genes in half-smooth tongue sole (Cynoglossus semilaevis). Acta Oceanol. Sin. 2011, 30, 68–77. [Google Scholar] [CrossRef]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish—Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, L.; Sun, B.; Wei, Y.; Liang, M. Transcriptomic analysis of potential “lncRNA–mRNA” interactions in liver of the marine teleost Cynoglossus semilaevis fed diets with different DHA/EPA ratios. Front. Physiol. 2019, 10, 331. [Google Scholar] [CrossRef] [PubMed]




| Groups | Sequence | Length/bp |
|---|---|---|
| siRNA-Cyclin E1 | GGAACAACCUGUUGAAGAA | 19 |
| siRNA-Cyclin E2 | GGAACAACCUGUUGAAGAA | 19 |
| NC | UUCUCCGAACGUGUCACGUTT | 21 |
| Primer Name | Primer Sequence (5′-3′) | Usage |
|---|---|---|
| CCNE1-F | GCGGGAGAATATTTTTAGGGTCTAT | Partial mRNA cloning |
| CCNE1-R | TGCAATATCTTGGTCCTGTCTTGAG | |
| CCNE2-F | AGTGGATCATTTTCGGTGGAACTCT | |
| CCNE2-R | CAGAAGATCAACACGAGGAGACCCT | |
| CCNE1-qF | GTCTTCCCCTCTTCCTGTGCTA | Real-time PCR |
| CCNE1-qR | AGCCAGTCCAGAAGAATAGCCC | |
| CCNE2-qF | GTGGTCGCATCACATTGAAAGC | |
| CCNE2-qR | GACTTGCCCCTTCTTCTGACCA | |
| β-actin-F | CTCACCGACTACCTGATGAAGATC | |
| β-actin-R | GTAGCACAGCTTCTCCTTGATGTC | |
| Amh-F | GGGAATAACCATGCTATCCTGCTT | |
| Amh-R | CTCCACCACCTTGAGGTCCTCATAGT | |
| Foxl2a-F | TGTGCTGGATTTGTTTTTTGTT | |
| Foxl2a-R | GTGTCGTGGACCATCAGGGCCA | |
| Cyp19a1b-F | TGAGGAAGGCACTGGAAGATGAC | |
| Cyp19a1b-R | GGCTGGAAGAAACGACTGGGC | |
| Dnd-F | GCTAGGGAGAGAAAATAACTTGCAA | |
| Dnd-R | CTGTTTCTACATGCATCATTCCCAC | |
| IncRNA-F | AACGCCCAAACAAGGACT | |
| IncRNA-R | GCCACGAGGACATTGACA | |
| Sycp1-F | ACCGAAGCTCTCAGAACTCC | |
| Sycp1-R | TGTTCCGAGCTGTCAGACTT | |
| Sycp3-F | AGCCATGCAAGCCAAGAGAA | |
| Sycp3-R | GACAGTGGCCATCTCTTGCT | |
| Mlh1-F | TAAAGACCCAACCCAAACCC | |
| Mlh1-R | CTGCTCACCACCTCCACAAT | |
| Dmc1-F | CATCAGCAATTCCCCCTGGA | |
| Dmc1-R | GTTGGATTTTGTGGCAGCCA | |
| β-tubulin-F | TTGGATGTGGTGAGGAAAGA | |
| β-tubulin-R | ATAGGTGGGCGTGGTAAGTT | |
| Sox9a-F | CACATCTCTTCCGGTGACATC | |
| Sox9a-R | AAGTACTGGTCGAACTCATGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, E.; Song, H.; Gu, W.; Wang, G.; Fan, P.; Ge, K.; Sun, Y.; Li, D.; Xu, G.; Huang, T. Functional Analysis of the Cyclin E Gene in the Reproductive Development of Rainbow Trout (Oncorhynchus mykiss). Biology 2025, 14, 862. https://doi.org/10.3390/biology14070862
Liu E, Song H, Gu W, Wang G, Fan P, Ge K, Sun Y, Li D, Xu G, Huang T. Functional Analysis of the Cyclin E Gene in the Reproductive Development of Rainbow Trout (Oncorhynchus mykiss). Biology. 2025; 14(7):862. https://doi.org/10.3390/biology14070862
Chicago/Turabian StyleLiu, Enhui, Haixia Song, Wei Gu, Gaochao Wang, Peng Fan, Kaibo Ge, Yunchao Sun, Datian Li, Gefeng Xu, and Tianqing Huang. 2025. "Functional Analysis of the Cyclin E Gene in the Reproductive Development of Rainbow Trout (Oncorhynchus mykiss)" Biology 14, no. 7: 862. https://doi.org/10.3390/biology14070862
APA StyleLiu, E., Song, H., Gu, W., Wang, G., Fan, P., Ge, K., Sun, Y., Li, D., Xu, G., & Huang, T. (2025). Functional Analysis of the Cyclin E Gene in the Reproductive Development of Rainbow Trout (Oncorhynchus mykiss). Biology, 14(7), 862. https://doi.org/10.3390/biology14070862






