Unraveling the Role of Spicules in Shaping Sponge Body Structure: Evidence from the Early Cambrian Shuijingtuo Formation
Simple Summary
Abstract
1. Introduction
2. Geological Background
3. Materials and Methods
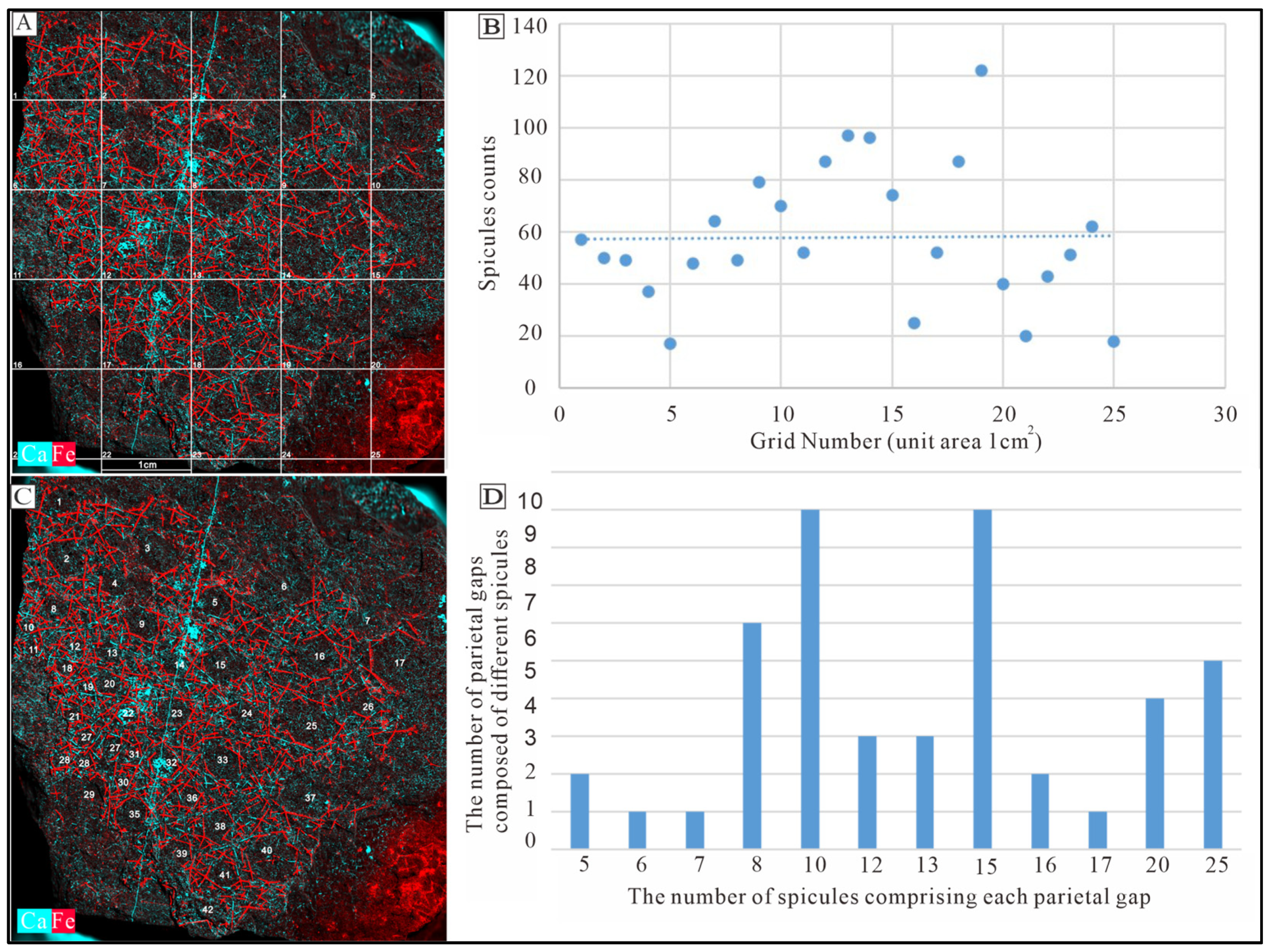
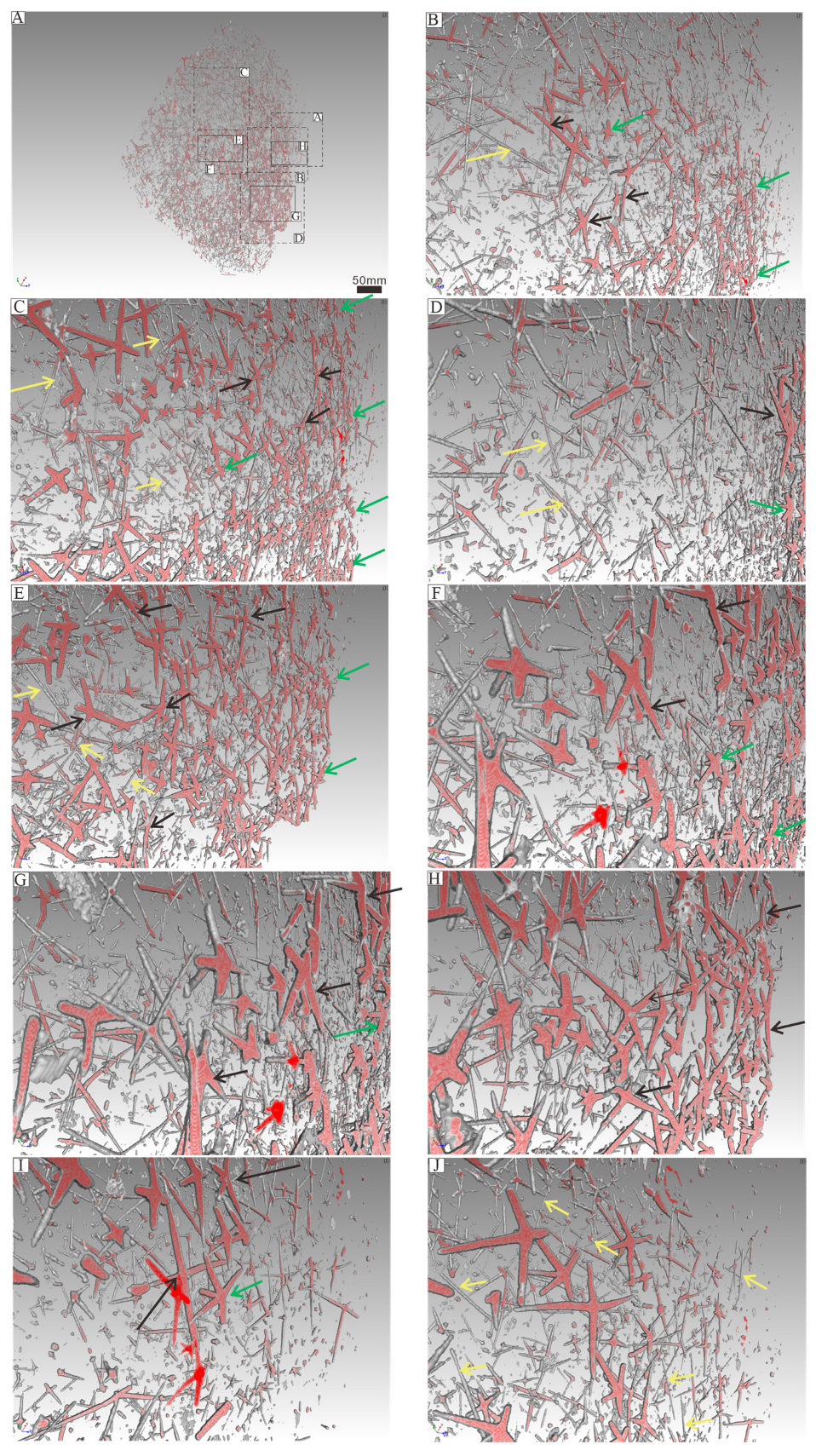
4. Results
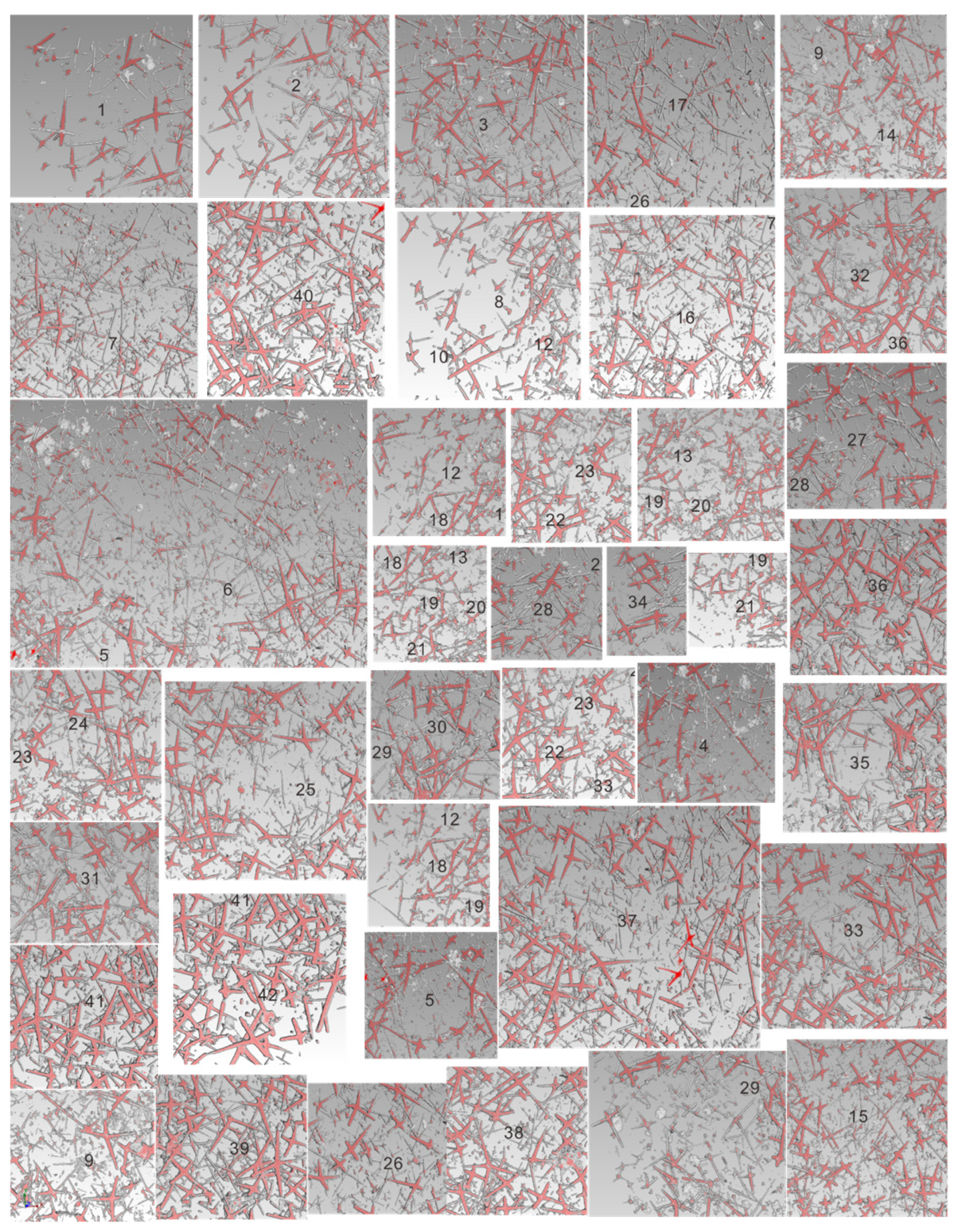
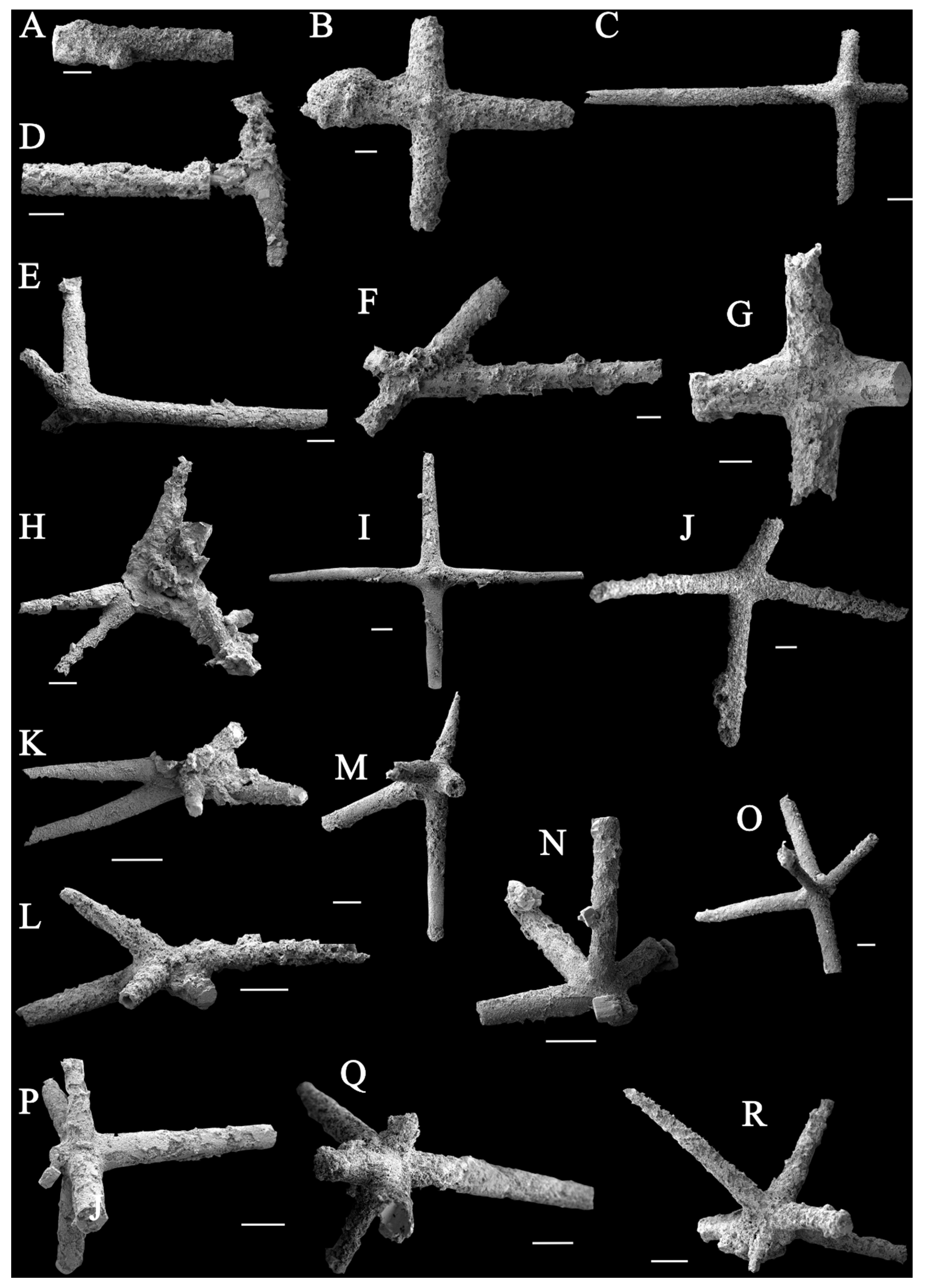
4.1. Sponge Spicules Extracted from Limestones
4.2. Articulated Sponge Fossils Preserved in Black Shales
4.3. Spicule Morphological Affinities
5. Discussion
5.1. Taxonomic Identification
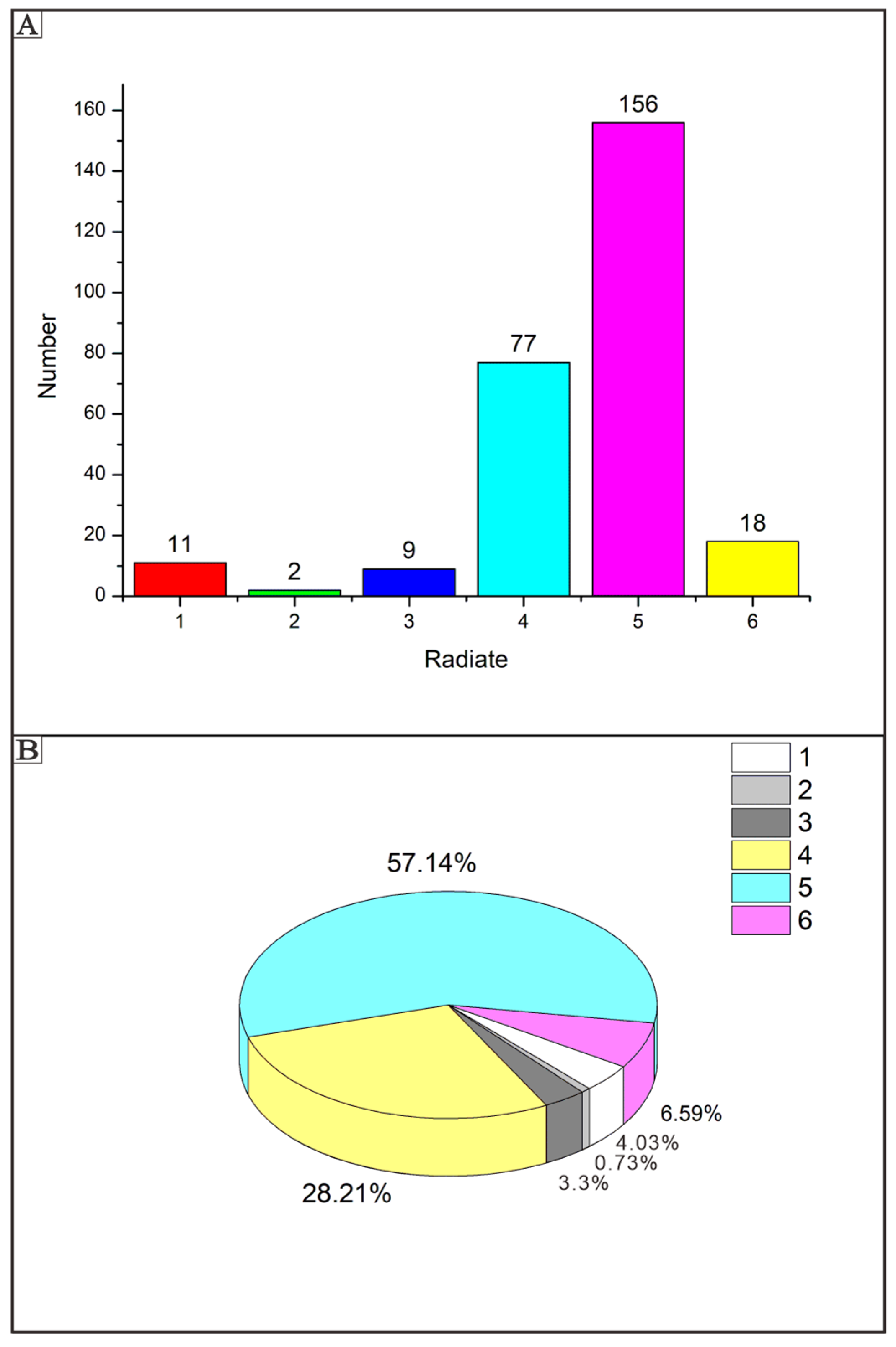
5.2. Spicule Orientation and the Function of Spicules in Sponge Framework Construction
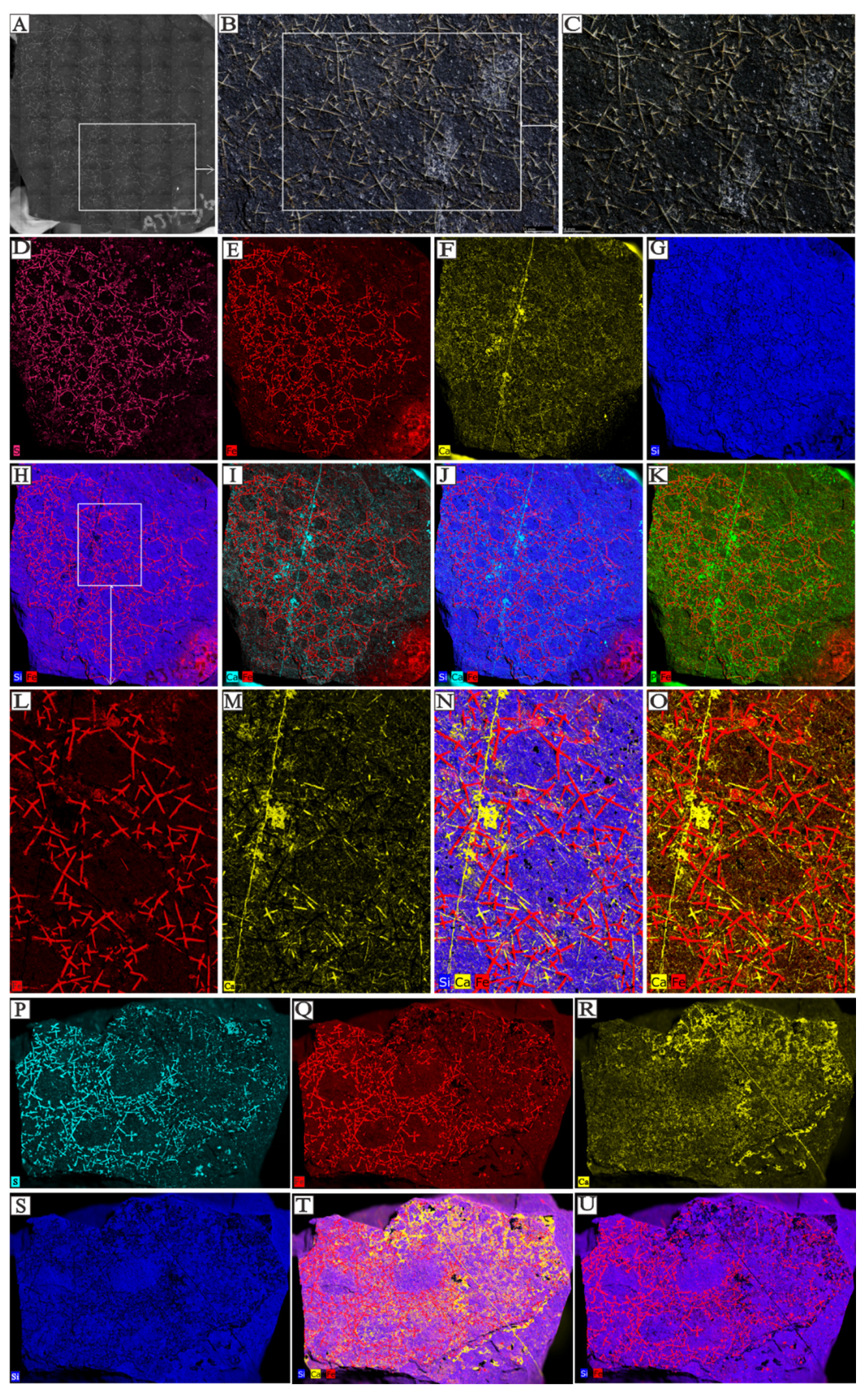
5.3. Integrating Acid Residues with Macroscale Sponge Specimens
5.4. The Significance of Combining Non-Destructive X-Ray Microscopy Techniques with Specimens from Acid Residues
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | scanning electron microscope |
| XRF | X-ray fluorescence spectrometer |
| PCA | principal component analysis |
Appendix A
Appendix B
| Number | X × Y | Name |
|---|---|---|
| 1 | 1 × 1 | monaxons diactine |
| 2 | 2 × 2 | bixon triactines |
| 3 | 2 × 3 | bixon triactines |
| 4 | 2 × 4 | bixon tetractines |
| 5 | 3 × 3 | triaxon triactines |
| 6 | 3 × 4 | triaxon tetractines |
| 7 | 3 × 5 | triaxon pentactines |
| 8 | 3 × 6 | triaxon hexactines/hexactinestrixons |
| 9 | 4 × 4 | tetraxon tetractines |
| 10 | 4 × 5 | tetraxon pentactines |
| 11 | 5 × 5 | pentaxon pentactines |
| 12 | 6 × 6 | hexactines |
| 13 | three forks at the top with one long rod | triaenes |
| 14 | Uniform 2 × 4 | orthotetractines |
| 15 | T-shaped, like 2 × y | T-form bixones |
| 16 | Multi-rayed | polyactinals |
Appendix C
| No. | Sponge Spicule Number (Micro-XRF) | Bigger Spicules (Parietal Gaps Framework) (Micro-XRF) | Smaller Spicules (Linkage of Parietal Gaps Framework) (Micro-XRF) | Sponge Spicule Number (Micro-CT) |
|---|---|---|---|---|
| 17 | 5 | 1 | 4 | 5 |
| 6 | 13 | 2 | 11 | 13 |
| 11 | 6 | 2 | 5 | 7 |
| 1 | 12 | 3 | 9 | 12 |
| 7 | 7 | 3 | 4 | 7 |
| 9 | 13 | 3 | 10 | 13 |
| 12 | 17 | 3 | 14 | 17 |
| 13 | 10 | 3 | 16 | 19 |
| 22 | 15 | 3 | 12 | 15 |
| 26 | 8 | 3 | 6 | 9 |
| 30 | 10 | 3 | 9 | 12 |
| 31 | 8 | 3 | 8 | 11 |
| 34 | 15 | 3 | 12 | 15 |
| 38 | 10 | 3 | 9 | 12 |
| 2 | 15 | 4 | 18 | 22 |
| 4 | 10 | 4 | 6 | 10 |
| 15 | 25 | 4 | 24 | 28 |
| 18 | 10 | 4 | 6 | 10 |
| 19 | 10 | 4 | 6 | 10 |
| 21 | 10 | 4 | 6 | 10 |
| 24 | 16 | 4 | 12 | 16 |
| 27 | 15 | 4 | 11 | 15 |
| 28 | 12 | 4 | 8 | 12 |
| 33 | 20 | 4 | 16 | 20 |
| 36 | 25 | 4 | 9 | 13 |
| 37 | 20 | 4 | 16 | 20 |
| 41 | 20 | 4 | 16 | 20 |
| 42 | 10 | 4 | 18 | 22 |
| 8 | 16 | 5 | 11 | 16 |
| 16 | 15 | 5 | 16 | 21 |
| 10 | 8 | 5 | 3 | 8 |
| 20 | 8 | 5 | 9 | 14 |
| 23 | 25 | 5 | 24 | 29 |
| 35 | 15 | 5 | 10 | 15 |
| 3 | 25 | 6 | 18 | 24 |
| 25 | 15 | 6 | 12 | 18 |
| 29 | 15 | 6 | 18 | 24 |
| 32 | 12 | 6 | 10 | 16 |
| 39 | 10 | 6 | 10 | 16 |
| 14 | 15 | 7 | 10 | 17 |
| 40 | 25 | 7 | 18 | 25 |
| 5 | 20 | 8 | 13 | 21 |
Appendix D
Appendix E
| No. | Spicules’ Axis Number | Spicules’ Radiate Number | Spicules Developed from the Same Center (Yes = 1, No = 0) | Spicules Developed from Different Centers (Yes = 1, No = 0) |
|---|---|---|---|---|
| 16 | 0 | 0 | 0 | 0 |
| 1 | 1 | 1 | 1 | 0 |
| 4 | 1 | 1 | 1 | 0 |
| 5 | 1 | 1 | 1 | 0 |
| 7 | 1 | 1 | 1 | 0 |
| 9 | 1 | 1 | 1 | 0 |
| 11 | 1 | 1 | 1 | 0 |
| 12 | 1 | 1 | 1 | 0 |
| 19 | 1 | 1 | 1 | 0 |
| 20 | 1 | 1 | 1 | 0 |
| 21 | 1 | 1 | 1 | 0 |
| 22 | 1 | 1 | 1 | 0 |
| 23 | 1 | 1 | 1 | 0 |
| 24 | 1 | 1 | 1 | 0 |
| 6 | 2 | 2 | 1 | 0 |
| 1 | 3 | 3 | 1 | 0 |
| 2 | 3 | 3 | 1 | 0 |
| 3 | 3 | 3 | 1 | 0 |
| 4 | 3 | 3 | 1 | 0 |
| 5 | 3 | 3 | 1 | 0 |
| 6 | 3 | 3 | 1 | 0 |
| 7 | 3 | 3 | 1 | 0 |
| 8 | 3 | 3 | 1 | 0 |
| 9 | 3 | 3 | 1 | 0 |
| 10 | 3 | 3 | 1 | 0 |
| 11 | 3 | 3 | 1 | 0 |
| 12 | 3 | 3 | 1 | 0 |
| 13 | 3 | 3 | 1 | 0 |
| 14 | 3 | 3 | 1 | 0 |
| 15 | 3 | 3 | 1 | 0 |
| 17 | 3 | 3 | 1 | 0 |
| 19 | 3 | 3 | 1 | 0 |
| 20 | 3 | 3 | 1 | 0 |
| 21 | 3 | 3 | 1 | 0 |
| 22 | 3 | 3 | 1 | 0 |
| 23 | 3 | 3 | 1 | 0 |
| 24 | 3 | 3 | 1 | 0 |
| 1 | 4 | 4 | 1 | 0 |
| 2 | 4 | 4 | 1 | 0 |
| 4 | 4 | 4 | 1 | 0 |
| 6 | 4 | 4 | 1 | 0 |
| 12 | 4 | 4 | 1 | 0 |
| 15 | 4 | 4 | 1 | 0 |
| 19 | 4 | 4 | 1 | 0 |
| 20 | 4 | 4 | 1 | 0 |
| 21 | 4 | 4 | 1 | 0 |
| 22 | 4 | 4 | 1 | 0 |
| 23 | 4 | 4 | 1 | 0 |
| 24 | 4 | 4 | 1 | 0 |
| 4 | 7 | 7 | 1 | 0 |
| 4 | 8 | 8 | 1 | 0 |
| 4 | 22 | 22 | 1 | 0 |
| 4 | 30 | 30 | 1 | 0 |
Appendix F
| No. | Spicules’ Axis Number | Spicules’ Radiate Number | Spicules Developed from the Same Center (Yes = 1, No = 0) | Spicules Developed from Different Centers (Yes = 1, No = 0) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 0 |
| 2 | 1 | 1 | 1 | 0 |
| 3 | 1 | 1 | 1 | 0 |
| 4 | 1 | 1 | 1 | 0 |
| 6 | 1 | 1 | 1 | 0 |
| 7 | 1 | 1 | 1 | 0 |
| 8 | 1 | 1 | 1 | 0 |
| 9 | 1 | 1 | 1 | 0 |
| 10 | 1 | 1 | 1 | 0 |
| 11 | 1 | 1 | 1 | 0 |
| 12 | 1 | 1 | 1 | 0 |
| 13 | 1 | 1 | 1 | 0 |
| 14 | 1 | 1 | 1 | 0 |
| 2 | 2 | 2 | 1 | 0 |
| 6 | 2 | 2 | 0 | 1 |
| 7 | 2 | 2 | 0 | 1 |
| 8 | 2 | 2 | 0 | 1 |
| 12 | 3 | 3 | 1 | 0 |
| 13 | 3 | 3 | 1 | 0 |
| 1 | 4 | 4 | 1 | 0 |
| 4 | 4 | 4 | 1 | 0 |
| 8 | 4 | 4 | 0 | 1 |
| 10 | 4 | 4 | 0 | 1 |
| 10 | 4 | 4 | 1 | 0 |
| 11 | 4 | 4 | 0 | 1 |
| 12 | 4 | 4 | 1 | 0 |
| 14 | 4 | 4 | 0 | 1 |
| 3 | 5 | 5 | 0 | 1 |
| 8 | 5 | 5 | 1 | 0 |
| 12 | 5 | 5 | 1 | 0 |
| 13 | 5 | 5 | 0 | 1 |
| 13 | 5 | 5 | 1 | 0 |
| 16 | 5 | 5 | 1 | 0 |
| 1 | 6 | 6 | 1 | 0 |
| 2 | 6 | 6 | 0 | 1 |
| 10 | 6 | 6 | 1 | 0 |
| 10 | 6 | 6 | 1 | 0 |
| 11 | 6 | 6 | 0 | 1 |
| 12 | 6 | 6 | 0 | 1 |
| 16 | 6 | 6 | 1 | 0 |
| 8 | 7 | 7 | 1 | 0 |
| 8 | 7 | 7 | 0 | 0 |
| 10 | 7 | 7 | 1 | 0 |
| 12 | 7 | 7 | 0 | 1 |
| 13 | 7 | 7 | 0 | 1 |
| 15 | 7 | 7 | 0 | 1 |
| 1 | 8 | 8 | 1 | 0 |
| 8 | 8 | 8 | 0 | 1 |
| 10 | 8 | 8 | 1 | 0 |
| 12 | 8 | 8 | 0 | 1 |
| 14 | 8 | 8 | 1 | 0 |
| 14 | 8 | 8 | 0 | 1 |
| 12 | 9 | 9 | 1 | 0 |
| 15 | 9 | 9 | 1 | 0 |
| 13 | 10 | 10 | 0 | 1 |
| 12 | 11 | 11 | 0 | 1 |
| 13 | 11 | 11 | 0 | 1 |
| 13 | 11 | 11 | 0 | 1 |
| 14 | 12 | 12 | 0 | 1 |
| 9 | 15 | 15 | 0 | 1 |
| 14 | 15 | 15 | 0 | 1 |
| 15 | 15 | 15 | 0 | 1 |
| 8 | 17 | 17 | 0 | 1 |
| 5 | 21 | 21 | 0 | 1 |
| 10 | 22 | 22 | 0 | 1 |
| 9 | 24 | 24 | 0 | 1 |
| 6 | 26 | 26 | 0 | 1 |
| 8 | 26 | 26 | 0 | 1 |
| 10 | 44 | 44 | 0 | 1 |
| 10 | 70 | 70 | 0 | 1 |
| 7 | 96 | 96 | 0 | 1 |
| 10 | 200 | 200 | 1 | 0 |
Appendix G
| No. | Spicules’ Axis Number | Spicules’ Radiate Number | Spicules Developed from the Same Center (Yes = 1, No = 0) | Spicules Developed from Different Centers (Yes = 1, No = 0) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 0 |
| 2 | 1 | 1 | 1 | 0 |
| 3 | 1 | 1 | 1 | 0 |
| 5 | 1 | 1 | 1 | 0 |
| 6 | 1 | 1 | 1 | 0 |
| 7 | 1 | 1 | 1 | 0 |
| 9 | 1 | 1 | 1 | 0 |
| 11 | 1 | 1 | 1 | 0 |
| 13 | 1 | 1 | 1 | 0 |
| 23 | 1 | 1 | 1 | 0 |
| 6 | 2 | 2 | 1 | 0 |
| 9 | 2 | 2 | 0 | 1 |
| 2 | 2 | 3 | 1 | 0 |
| 3 | 3 | 3 | 1 | 0 |
| 7 | 3 | 3 | 1 | 0 |
| 10 | 3 | 3 | 1 | 0 |
| 11 | 3 | 3 | 1 | 0 |
| 12 | 2 | 3 | 1 | 0 |
| 14 | 2 | 3 | 1 | 0 |
| 20 | 3 | 3 | 1 | 0 |
| 22 | 2 | 3 | 1 | 0 |
| 23 | 3 | 3 | 1 | 0 |
| 25 | 3 | 3 | 1 | 0 |
| 30 | 3 | 3 | 1 | 0 |
| 1 | 4 | 4 | 1 | 0 |
| 2 | 2 | 4 | 1 | 0 |
| 3 | 4 | 4 | 1 | 0 |
| 5 | 2 | 4 | 1 | 0 |
| 5 | 3 | 4 | 1 | 0 |
| 6 | 4 | 4 | 1 | 0 |
| 6 | 2 | 4 | 1 | 0 |
| 6 | 2 | 4 | 1 | 0 |
| 7 | 4 | 4 | 1 | 0 |
| 7 | 4 | 4 | 0 | 1 |
| 7 | 4 | 4 | 1 | 0 |
| 8 | 4 | 4 | 1 | 0 |
| 9 | 4 | 4 | 0 | 1 |
| 10 | 2 | 4 | 1 | 0 |
| 14 | 2 | 4 | 1 | 0 |
| 15 | 2 | 4 | 1 | 0 |
| 19 | 2 | 4 | 1 | 0 |
| 21 | 2 | 4 | 1 | 0 |
| 22 | 2 | 4 | 1 | 0 |
| 23 | 4 | 4 | 1 | 0 |
| 23 | 2 | 4 | 1 | 0 |
| 26 | 3 | 4 | 1 | 0 |
| 30 | 2 | 4 | 1 | 0 |
| 30 | 4 | 4 | 1 | 0 |
| 31 | 2 | 4 | 1 | 0 |
| 32 | 3 | 4 | 1 | 0 |
| 36 | 2 | 4 | 1 | 0 |
| 1 | 3 | 5 | 1 | 0 |
| 4 | 5 | 5 | 1 | 0 |
| 5 | 4 | 5 | 1 | 0 |
| 6 | 3 | 5 | 1 | 0 |
| 7 | 3 | 5 | 1 | 0 |
| 7 | 5 | 5 | 1 | 0 |
| 7 | 5 | 5 | 1 | 0 |
| 7 | 5 | 5 | 1 | 0 |
| 7 | 5 | 5 | 1 | 0 |
| 10 | 5 | 5 | 1 | 0 |
| 11 | 3 | 5 | 1 | 0 |
| 11 | 5 | 5 | 1 | 0 |
| 13 | 3 | 5 | 1 | 0 |
| 15 | 3 | 5 | 1 | 0 |
| 17 | 3 | 5 | 1 | 0 |
| 20 | 3 | 5 | 1 | 0 |
| 21 | 3 | 5 | 1 | 0 |
| 22 | 3 | 5 | 1 | 0 |
| 23 | 3 | 5 | 1 | 0 |
| 23 | 5 | 5 | 1 | 0 |
| 24 | 3 | 5 | 1 | 0 |
| 25 | 3 | 5 | 1 | 0 |
| 27 | 3 | 5 | 1 | 0 |
| 28 | 3 | 5 | 1 | 0 |
| 29 | 3 | 5 | 1 | 0 |
| 30 | 3 | 5 | 1 | 0 |
| 30 | 4 | 5 | 1 | 0 |
| 33 | 3 | 5 | 1 | 0 |
| 35 | 3 | 5 | 1 | 0 |
| 1 | 3 | 6 | 1 | 0 |
| 2 | 3 | 6 | 1 | 0 |
| 3 | 5 | 6 | 1 | 0 |
| 3 | 6 | 6 | 1 | 0 |
| 4 | 6 | 6 | 1 | 0 |
| 4 | 3 | 6 | 1 | 0 |
| 5 | 6 | 6 | 1 | 0 |
| 5 | 3 | 6 | 1 | 0 |
| 5 | 6 | 6 | 1 | 0 |
| 6 | 3 | 6 | 1 | 0 |
| 6 | 46 | 6 | 0 | 1 |
| 6 | 6 | 6 | 1 | 0 |
| 7 | 3 | 6 | 1 | 0 |
| 7 | 6 | 6 | 1 | 0 |
| 7 | 6 | 6 | 1 | 0 |
| 7 | 6 | 6 | 1 | 0 |
| 7 | 6 | 6 | 0 | 1 |
| 8 | 3 | 6 | 1 | 0 |
| 10 | 6 | 6 | 1 | 0 |
| 11 | 3 | 6 | 1 | 0 |
| 11 | 6 | 6 | 0 | 1 |
| 13 | 3 | 6 | 1 | 0 |
| 13 | 6 | 6 | 0 | 1 |
| 15 | 3 | 6 | 1 | 0 |
| 16 | 3 | 6 | 1 | 0 |
| 17 | 3 | 6 | 1 | 0 |
| 18 | 3 | 6 | 1 | 0 |
| 19 | 3 | 6 | 1 | 0 |
| 20 | 3 | 6 | 1 | 0 |
| 21 | 3 | 6 | 1 | 0 |
| 23 | 3 | 6 | 1 | 0 |
| 27 | 3 | 6 | 1 | 0 |
| 28 | 3 | 6 | 0 | 1 |
| 28 | 6 | 6 | 0 | 1 |
| 29 | 3 | 6 | 1 | 0 |
| 29 | 3 | 6 | 1 | 0 |
| 33 | 3 | 6 | 1 | 0 |
| 34 | 3 | 6 | 1 | 0 |
| 35 | 3 | 6 | 1 | 0 |
| 36 | 3 | 6 | 1 | 0 |
| 5 | 7 | 7 | 0 | 1 |
| 7 | 7 | 7 | 1 | 0 |
| 7 | 7 | 7 | 1 | 0 |
| 7 | 7 | 7 | 0 | 1 |
| 7 | 7 | 7 | 1 | 0 |
| 7 | 7 | 7 | 0 | 1 |
| 8 | 7 | 7 | 0 | 1 |
| 8 | 7 | 7 | 1 | 0 |
| 10 | 6 | 7 | 0 | 1 |
| 11 | 7 | 7 | 1 | 0 |
| 11 | 7 | 7 | 1 | 0 |
| 12 | 7 | 7 | 1 | 0 |
| 23 | 4 | 7 | 0 | 1 |
| 28 | 7 | 7 | 0 | 1 |
| 5 | 8 | 8 | 0 | 1 |
| 5 | 8 | 8 | 0 | 1 |
| 6 | 7 | 8 | 0 | 1 |
| 7 | 8 | 8 | 0 | 1 |
| 7 | 8 | 8 | 1 | 0 |
| 7 | 8 | 8 | 1 | 0 |
| 7 | 8 | 8 | 0 | 1 |
| 8 | 8 | 8 | 0 | 1 |
| 11 | 8 | 8 | 1 | 0 |
| 17 | 8 | 8 | 1 | 0 |
| 17 | 8 | 8 | 1 | 0 |
| 20 | 8 | 8 | 1 | 0 |
| 23 | 8 | 8 | 1 | 0 |
| 30 | 8 | 8 | 0 | 1 |
| 32 | 8 | 8 | 0 | 1 |
| 3 | 9 | 9 | 1 | 0 |
| 3 | 9 | 9 | 0 | 1 |
| 4 | 9 | 9 | 1 | 0 |
| 4 | 9 | 9 | 0 | 1 |
| 5 | 9 | 9 | 0 | 1 |
| 6 | 8 | 9 | 0 | 1 |
| 6 | 9 | 9 | 0 | 1 |
| 7 | 9 | 9 | 1 | 0 |
| 7 | 9 | 9 | 1 | 0 |
| 7 | 9 | 9 | 1 | 0 |
| 7 | 9 | 9 | 1 | 0 |
| 8 | 9 | 9 | 0 | 1 |
| 8 | 9 | 9 | 1 | 0 |
| 11 | 9 | 9 | 1 | 0 |
| 12 | 9 | 9 | 1 | 0 |
| 26 | 9 | 9 | 0 | 1 |
| 33 | 9 | 9 | 0 | 1 |
| 3 | 10 | 10 | 1 | 0 |
| 4 | 10 | 10 | 1 | 0 |
| 5 | 10 | 10 | 0 | 1 |
| 5 | 10 | 10 | 0 | 1 |
| 6 | 7 | 10 | 0 | 1 |
| 7 | 10 | 10 | 0 | 1 |
| 7 | 10 | 10 | 1 | 0 |
| 8 | 10 | 10 | 0 | 1 |
| 11 | 10 | 10 | 0 | 1 |
| 32 | 10 | 10 | 0 | 1 |
| 5 | 11 | 11 | 0 | 1 |
| 6 | 11 | 11 | 0 | 1 |
| 6 | 10 | 11 | 0 | 1 |
| 7 | 11 | 11 | 1 | 0 |
| 7 | 11 | 11 | 1 | 0 |
| 7 | 11 | 11 | 0 | 1 |
| 11 | 11 | 11 | 1 | 0 |
| 12 | 11 | 11 | 1 | 0 |
| 13 | 11 | 11 | 0 | 1 |
| 24 | 11 | 11 | 0 | 1 |
| 2 | 12 | 12 | 0 | 1 |
| 4 | 12 | 12 | 0 | 1 |
| 5 | 12 | 12 | 0 | 1 |
| 6 | 12 | 12 | 0 | 1 |
| 7 | 12 | 12 | 1 | 0 |
| 7 | 12 | 12 | 0 | 1 |
| 7 | 12 | 12 | 1 | 0 |
| 8 | 12 | 12 | 0 | 1 |
| 10 | 12 | 12 | 0 | 1 |
| 11 | 12 | 12 | 0 | 1 |
| 14 | 12 | 12 | 0 | 1 |
| 15 | 12 | 12 | 0 | 1 |
| 20 | 12 | 12 | 0 | 1 |
| 21 | 12 | 12 | 1 | 0 |
| 22 | 12 | 12 | 0 | 1 |
| 25 | 12 | 12 | 0 | 1 |
| 4 | 13 | 13 | 0 | 1 |
| 5 | 13 | 13 | 0 | 1 |
| 7 | 13 | 13 | 1 | 0 |
| 7 | 13 | 13 | 1 | 0 |
| 7 | 13 | 13 | 1 | 0 |
| 23 | 13 | 13 | 0 | 1 |
| 29 | 13 | 13 | 0 | 1 |
| 32 | 13 | 13 | 0 | 1 |
| 4 | 14 | 14 | 0 | 1 |
| 6 | 14 | 14 | 0 | 1 |
| 7 | 14 | 14 | 1 | 0 |
| 7 | 14 | 14 | 1 | 0 |
| 10 | 13 | 14 | 0 | 1 |
| 17 | 14 | 14 | 0 | 1 |
| 20 | 14 | 14 | 0 | 1 |
| 26 | 14 | 14 | 0 | 1 |
| 35 | 14 | 14 | 0 | 1 |
| 36 | 14 | 14 | 0 | 1 |
| 3 | 15 | 15 | 0 | 1 |
| 4 | 15 | 15 | 0 | 1 |
| 7 | 15 | 15 | 0 | 1 |
| 8 | 15 | 15 | 1 | 0 |
| 10 | 13 | 15 | 0 | 1 |
| 25 | 15 | 15 | 0 | 1 |
| 5 | 16 | 16 | 0 | 1 |
| 6 | 16 | 16 | 0 | 1 |
| 7 | 16 | 16 | 1 | 0 |
| 8 | 16 | 16 | 0 | 1 |
| 14 | 16 | 16 | 0 | 1 |
| 15 | 16 | 16 | 0 | 1 |
| 19 | 16 | 16 | 0 | 1 |
| 20 | 16 | 16 | 0 | 1 |
| 25 | 16 | 16 | 0 | 1 |
| 30 | 16 | 16 | 0 | 1 |
| 4 | 17 | 17 | 1 | 0 |
| 4 | 17 | 17 | 0 | 1 |
| 6 | 17 | 17 | 0 | 1 |
| 4 | 18 | 18 | 0 | 1 |
| 6 | 18 | 18 | 0 | 1 |
| 7 | 18 | 18 | 1 | 0 |
| 8 | 18 | 18 | 0 | 1 |
| 11 | 18 | 18 | 0 | 1 |
| 4 | 19 | 19 | 0 | 1 |
| 19 | 19 | 19 | 0 | 1 |
| 30 | 19 | 19 | 0 | 1 |
| 35 | 19 | 19 | 0 | 1 |
| 6 | 20 | 20 | 0 | 1 |
| 7 | 20 | 20 | 0 | 1 |
| 10 | 20 | 20 | 0 | 1 |
| 11 | 20 | 20 | 0 | 1 |
| 12 | 20 | 20 | 0 | 1 |
| 16 | 20 | 20 | 0 | 1 |
| 22 | 20 | 20 | 0 | 1 |
| 5 | 21 | 21 | 0 | 1 |
| 11 | 21 | 21 | 0 | 1 |
| 24 | 21 | 21 | 0 | 1 |
| 7 | 22 | 22 | 0 | 1 |
| 14 | 22 | 22 | 0 | 1 |
| 19 | 22 | 22 | 0 | 1 |
| 32 | 22 | 22 | 0 | 1 |
| 7 | 23 | 23 | 1 | 0 |
| 11 | 23 | 23 | 0 | 1 |
| 23 | 23 | 23 | 0 | 1 |
| 1 | 24 | 24 | 1 | 0 |
| 2 | 24 | 24 | 0 | 1 |
| 4 | 24 | 24 | 0 | 1 |
| 20 | 24 | 24 | 0 | 1 |
| 25 | 24 | 24 | 0 | 1 |
| 26 | 24 | 24 | 0 | 1 |
| 29 | 24 | 24 | 0 | 1 |
| 6 | 25 | 25 | 0 | 1 |
| 36 | 25 | 25 | 0 | 1 |
| 11 | 26 | 26 | 0 | 1 |
| 24 | 26 | 26 | 0 | 1 |
| 10 | 27 | 27 | 0 | 1 |
| 7 | 28 | 28 | 0 | 1 |
| 7 | 28 | 28 | 0 | 1 |
| 6 | 30 | 30 | 0 | 1 |
| 27 | 30 | 30 | 0 | 1 |
| 30 | 30 | 30 | 0 | 1 |
| 11 | 31 | 31 | 0 | 1 |
| 12 | 32 | 32 | 0 | 1 |
| 16 | 32 | 32 | 1 | 0 |
| 19 | 33 | 33 | 0 | 1 |
| 4 | 34 | 34 | 0 | 1 |
| 23 | 35 | 35 | 0 | 1 |
| 2 | 36 | 36 | 0 | 1 |
| 17 | 36 | 36 | 0 | 1 |
| 26 | 36 | 36 | 0 | 1 |
| 19 | 38 | 38 | 0 | 1 |
| 36 | 38 | 38 | 0 | 1 |
| 27 | 40 | 40 | 0 | 1 |
| 19 | 44 | 44 | 1 | 0 |
| 10 | 48 | 48 | 0 | 1 |
| 12 | 48 | 48 | 0 | 1 |
| 16 | 48 | 48 | 0 | 1 |
| 12 | 49 | 49 | 1 | 0 |
| 13 | 56 | 56 | 0 | 1 |
| 19 | 56 | 56 | 0 | 1 |
| 6 | 60 | 60 | 0 | 1 |
| 7 | 60 | 60 | 0 | 1 |
| 30 | 35 | 65 | 0 | 1 |
| 24 | 68 | 68 | 0 | 1 |
| 19 | 75 | 75 | 0 | 1 |
| 13 | 80 | 80 | 0 | 1 |
| 29 | 80 | 80 | 0 | 1 |
| 17 | 84 | 84 | 0 | 1 |
| 23 | 85 | 85 | 0 | 1 |
| 13 | 90 | 90 | 0 | 1 |
| 19 | 100 | 100 | 1 | 0 |
| 19 | 108 | 108 | 0 | 1 |
| 13 | 138 | 138 | 0 | 1 |
| 13 | 150 | 150 | 0 | 1 |
| 12 | 162 | 162 | 0 | 1 |
| 19 | 210 | 210 | 0 | 1 |
| 12 | 216 | 216 | 0 | 1 |
| 19 | 224 | 224 | 0 | 1 |
| 19 | 240 | 240 | 0 | 1 |
| 12 | 350 | 350 | 0 | 1 |
| 19 | 432 | 432 | 0 | 1 |
| 17 | 640 | 640 | 0 | 1 |
Appendix H
Appendix I
References
- Walter, M.R. The Biology of Reefs and Reef Organisms; Chicago University Press: Chicago, IL, USA, 2013; 410p. [Google Scholar] [CrossRef]
- Bernard, M.D.; Sally, L.; Claire, L. Sponge development and antiquity of animal pattern formation. Integr. Comp. Biol. 2005, 45, 335–341. [Google Scholar] [CrossRef]
- Yang, J.Y.; Ma, H.D.; Chen, A.L.; Hou, X.G. A New Leptomitid Sponge from the Early Cambrian Chengjiang Biota. Geol. J. China Univ. 2019, 25, 480, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Li, L.X.; Yan, G.Z.; Wei, X.; Gong, F.Y.; Oliver, L.; Wu, R.C. Late Ordovician sponge spicules from the Yangtze Platform, South China: Biostratigraphical and palaeobiogeographical significance. J. Asian Earth Sci. 2025, 277, 106380. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.X. Early Cambrian Sponge Spicule Fossils from Zhenba County, Southern Shaanxi Province. Acta Micropalaeontologica Sin. 2006, 23, 281–294, (In Chinese with English Abstract). [Google Scholar]
- Zhang, Z.F.; Liang, Y.; Liu, F.; Hu, Y.Z.; Yao, J.L.; Song, B.P.; Luo, M.; Zhang, C.B.; Wang, J.Y.; Chen, Y.L.; et al. New perspectives on Cambrian Explosion: Construction of the First Animal Consumer-Driven Marine Ecosystem on Earth. Acta Palaeontol. Sin. 2023, 62, 463–515. (In Chinese) [Google Scholar] [CrossRef]
- Kolesnikov, K.A.; Botting, J.P.; Ivantsov, A.Y.; Zhuravlev, A.Y. New early Cambrian sponges of the Siberian platform and the origins of spiculate crown-group demosponges. Pap. Palaeontol. 2024, 10, e1582. [Google Scholar] [CrossRef]
- Bengston, S. Siliceous microfossils from the Upper Cambrian of Queensland. Alcheringa 1986, 10, 195–216. [Google Scholar] [CrossRef]
- Peel, J.S. Sponge spicule assemblages from the Cambrian (Series 2–3) of North Greenland (Laurentia): Systematics and biogeography. GFF 2019, 141, 133–161. [Google Scholar] [CrossRef]
- Ma, Q.F. Geobiological Study on the Biota from the Chiungchussuan Shuijingtuo Formation (Cambrian Series 2), Western Hubei Province, South China. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2019. [Google Scholar]
- Yang, X.L.; Zhao, Y.L.; Zhu, M.Y.; Cui, T.; Yang, K.D. Sponges from the Early Cambrian Niutitang Formation at Danzhai, Guizhou and their environmental background. Acta Palaeontol. Sin. 2010, 49, 348–359, (In Chinese with English Abstract). [Google Scholar]
- Fu, D.; Tong, G.; Dai, T.; Liu, W.; Yang, Y.; Zhang, Y.; Cui, L.; Li, L.; Yun, H.; Wu, Y.; et al. The Qingjiang biota-A Burgess Shale-type fossil Lagerstätte from the early Cambrian of South China. Science 2019, 363, 1338–1342. [Google Scholar] [CrossRef]
- Botting, J.P.; Muir, L.A.; Ma, J.Y. Teganium (Porifera, Hexactinellida) from the Middle Ordovician Castle Bank fauna of Avalonia (Wales, UK). Palaeontol. Electron. 2023, 26, a21. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, L.; Chang, S.; Feng, Q.L. A sponge fossil fauna from the Cambrian Shuijingtuo Formation, Qiaojiaping Village, Yichang, Hubei Province. Acta Palaeontol. Sin. 2021, 60, 69–86, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Chang, S.H.; Zhang, L.; Clausen, S.; Bottjer, D.J.; Feng, Q.L. The Ediacaran-Cambrian rise of siliceous sponges and development of modern oceanic ecosystems. Precambrian Res. 2019, 333, 105–438. [Google Scholar] [CrossRef]
- Han, J.; Zhang, X.L. Precambrian sponge records and the study of Chengjiang sponge group in Yunnan province. Northwestern Geol. 1999, 32, 5. (In Chinese) [Google Scholar]
- Gehling, J.; Rigby, J. Long expected sponges from the Neoproterozoic Ediacara fauna of South Australia. J. Paleontol. 1996, 70, 185–195. [Google Scholar] [CrossRef]
- Wang, X.P.; Alexander, G.L.; Chen, Z.H.; Wu, C.X.; Liu, Y.R.; Wan, B.; Pang, K.; Zhou, C.M.; Yuan, X.L.; Xiao, S.H. A late-Ediacaran crown-group sponge animal. Nature 2024, 630, 905–911. [Google Scholar] [CrossRef]
- Ding, W.M.; Qian, Y. Small shelly fossils from Late Sinian to Early Cambrian in Yangjiaping, Shimen, Hunan. Acta Micropalaeontologica Sin. 1988, 1, 39–55. (In Chinese) [Google Scholar]
- Antcliffe, J.B.; Callow, R.H.T.; Brasier, M.D. Giving the early fossil record of sponges a squeeze. Biol. Rev. 2014, 89, 972–1004. [Google Scholar] [CrossRef]
- Chang, S.; Feng, Q.L.; Clausen, S.; Zhang, L. Sponge spicules from the lower Cambrian in the Yanjiahe Formation, South China: The earliest biomineralizing sponge record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 474, 36–44. [Google Scholar] [CrossRef]
- Mehl, D.; Erdtmann, B.D. Sanshapentella dapingi n. gen. n. sp.—A new hexactinellid sponge from the Early Cambrian (Tommotian) of China. Berl. Geowiss. Abh. Reihe E 1994, 13, 315–319. [Google Scholar]
- Yun, H.; Luo, C.; Chang, C.; Li, L.; Reitner, J.; Zhang, X.L. Adaptive specialization of a unique sponge body from the Cambrian Qingjiang biota. Proc. R. Soc. B. 2022, 289, 20220804. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Luo, C.; Janussen, D.; Zhang, X.L.; Reitner, J. Adaptive evolution of hexactinellid sponges from the Cambrian black shale Lagerstätten of China. In Proceedings of the Fossillagerstätten and Taphonomy: New Ideas and Approaches, Göttingen, Germany, 23–25 November 2023. [Google Scholar] [CrossRef]
- Steiner, M.; Mehl, D.; Reitner, J.; Erdtmann, B.D. Oldest entirely preserved sponges and other fossils from the Lowermost Cambrian and a new facies reconstruction of the Yangtze platform (China). Berl. Geowiss. Abh. 1993, 9, 293–329. [Google Scholar]
- Yuan, X.L.; Xiao, S.H.; Parsley, R.L.; Zhou, C.M.; Chen, Z.; Hu, J. Towering sponges in an Early Cambrian Lagerstätte: Disparity between nonbilaterian and bilaterian epifaunal tierers at the Neoproterozoic-Cambrian transition. Geology 2002, 30, 363–366. [Google Scholar] [CrossRef]
- Wu, W.; Yang, A.H.; Janussen, D.; Steiner, M.; Zhu, M.Y. Hexactinellid sponges from the Early Cambrian black shale of South Anhui, China. J. Paleontol. 2005, 79, 1043–1051. [Google Scholar] [CrossRef]
- Rigby, J.K. Treatise on invertebrate paleontology. Paleontol. Soc. 2003, 2, 1–226. Available online: https://journals.ku.edu/InvertebratePaleo (accessed on 14 August 2024).
- Rigby, J.K. Sponges of the Burgess Shale (Middle Cambrian), British Columbia, Palaeontographica Canadiana. Can. Soc. Pet. Geol. 1986, 2, 1–105. [Google Scholar]
- Yuan, X.L.; Wang, Q.F.; Zhang, Y. Late Precambrian Wengán Biotafrom Guizhou, Southwest China. Acta Micropalaeontologica Sin. 1993, 10, 409–423. [Google Scholar]
- Zhou, C.M.; Yuan, X.L.; Xue, Y.S. Spongespicule tike pseudofossils from the Neoproterozoie Doushantuo Formationin Wengan, Guizhou, China. Acta Micropalaeontologica Sin. 1998, 15, 380–384. [Google Scholar]
- Yin, L.; Xiao, S.H.; Yuan, X.L. New observations on spieule like structures from Doushantuo phosphorites at Wengan, Guizhou Province. Chin. Sci. Bull. 2001, 46, 1828–1832. [Google Scholar] [CrossRef]
- Guo, J.F.; Li, Y.; Li, G.X. Small shelly fossils from the early Cambrian Yanjiahe Formation, Yichang, Hubei, China. Gondwana Res. 2014, 25, 999–1007. [Google Scholar] [CrossRef]
- Wang, X.F. Biostratigraphy of the Yangtze Gorge Area Early Palaeozoic Era; Geological Publishing House: Beijing, China, 1987; p. 489, (In Chinese with English summary). [Google Scholar]
- Zhang, Z.F.; Zhang, Z.L.; Li, G.X.; Holmer, L.E. The Cambrian brachiopod fauna from the first-trilobite age Shuijingtuo Formation in the Three Gorges area of China. Palaeoworld 2015, 25, 333–355. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, J.H.; Santosh, M. Tectonic evolution of the Qinling orogenic belt, Central China: New evidence from geochemical zircon U-Pb geochronology and Hf isotopes. Precambrian Res. 2013, 231, 19–60. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, Z.F.; Holmer, L. Studies on the shell ultrastructure and ontogeny of the oldest Acrotretid Brachiopods from south China. Acta Palaeontol. Sin. 2017, 56, 483–503. [Google Scholar] [CrossRef]
- Zhu, M.Y. The origin and Cambrian explosion of animals: Fossil evidences from China. Acta Palaeontol. Sin. 2010, 49, 269–287. (In Chinese) [Google Scholar]
- Zhu, M.Y.; Yang, A.H.; Yuan, J.L.; Li, G.X.; Zhang, J.M.; Zhao, F.C.; Ahn, S.Y.; Miao, L.Y. Cambrian integrative stratigraphy and timescale of China. Sci. China Earth Sci. 2019, 62, 25–60. [Google Scholar] [CrossRef]
- Wang, J.; Song, B.; Liang, Y.; Liang, K.; Zhang, Z. The Internal Anatomy and Water Current System of Cambrian Archaeocyaths of South China. Life 2024, 14, 167. [Google Scholar] [CrossRef]
- Ingo, S.; Gert, W. Structure and composition of calcareous sponge spicules: A review and comparison to structurally related biominerals. Micron 2008, 39, 209–228. [Google Scholar] [CrossRef]
- Li, C.W.; Chen, J.Y.; Hua, T. Precambrian sponges with cellular structures. Science 1998, 279, 879–882. [Google Scholar] [CrossRef]
- Ma, J.Y.; Yang, Q. Early divergence dates of demosponges based on mitogenomics and evaluated fossil calibrations. Palaeoworld 2016, 25, 292–302. [Google Scholar] [CrossRef]
- Love, G.D.; Grosjean, E.; Stalvies, C.; Fike, D.A.; Grotzinger, J.P.; Bradley, A.S.; Kelly, A.E.; Bhatia, M.; Meredith, W.; Snape, C.E.; et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 2009, 457, 718–721. [Google Scholar] [CrossRef]
- Uriz, M.J. Mineral skeletogenesis in sponges. Can. J. Zool. 2006, 84, 322–356. [Google Scholar] [CrossRef]
- Joseph, P.B. ‘Cambrian’ demosponges in the Ordovician of Morocco: Insights into the early evolutionary history of sponges. Geobios 2007, 40, 737–748. [Google Scholar]
- Cárdenas, P.; Perez, T.; Boury-Esnault, N. Sponge Systematics Facing New Challenges. Adv. Mar. Biol. 2012, 61, 79–209. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, M.Y.; Steiner, M. Composition and tiering of the Cambrian sponge communities. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 86–96. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhao, Y.L.; Zhu, M.Y. New sponges from the lower Cambrian of Guizhou. Acta Palaeontol. Sin. 2005, 44, 454–463. (In Chinese) [Google Scholar] [CrossRef]
- Li, L.; Reitner, J.; Gong, F.; Yan, G.; Wu, R. A new stiodermatid (Hexactinellida, Porifera) from the latest Ordovician of Anhui, South China and its significance for searching the missing link between the Cambrian and late Palaeozoic stiodermatid lineage. Hist. Biol. 2022, 35, 116–126. [Google Scholar] [CrossRef]
- Xiao, S.H.; Hu, J.; Yuan, X.L.; Parsley, R.L.; Cao, R.J. Articulated sponges from the Lower Cambrian Hetang Formation in southern Anhui, South China: Their age and implications for the early evolution of sponges. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 220, 89–117. [Google Scholar] [CrossRef]
- Forchielli, A.; Steiner, M.; Kasbohm, J.; Hu, S.X.; Keupp, H. Taphonomic traits of clay-hosted early Cambrian Burgess Shale-type fossil Lagersttten in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 59–85. [Google Scholar] [CrossRef]
- Skinner, E.S. Taphonomy and depositional circumstances of exceptionally preserved fossils from the Kinzers Formation (Cambrian), southeastern Pennsylvania. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 220, 167–192. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, M.; Steiner, M.; Luo, H.; Zhao, F.; Liu, Q. Biodiversity and taphonomy of the Early Cambrian Guanshan biota, eastern Yunnan. Sci. China Earth Sci. 2010, 9, 24–33. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Lin, Y.; Zhuang, X.G.; Hoang, V.L.; Wu, P.; Luo, X.; Zhang, H.; Zhang, X.Y. Mineralogy and geochemistry of the Cambrain Shuijingtuo Formation black shales from Western Hubei, China: Implications on enrichment of critical metals and paleoenvironment. Front. Mar. Sci. 2024, 11, 1457964. [Google Scholar] [CrossRef]
- O’Flynn, R.J.; Liu, Y.; Hou, X.G.; Mai, H.J.; Yu, M.X.; Zhuang, S.L.; Williams, M.; Guo, J.; Edgecombe, G.D. The early Cambrian Kylinxia zhangi and evolution of the arthropod head. Curr. Biol. 2023, 33, 4006–4013. [Google Scholar] [CrossRef]
- Ma, H.D.; Yang, J.Y.; Chen, A.L.; Hou, X.G.; Tang, F. New species of prospongiid sponges from the early cambrain Chengjiang biota in Yunnan. Acta Geol. Sin. 2019, 93, 2715–2728. (In Chinese) [Google Scholar] [CrossRef]
- Botting, J.P.; Yuan, X.L.; Lin, R.B. Tetraradial symmetry in early poriferans. Chin. Sci. Bull. 2014, 59, 796–802. [Google Scholar] [CrossRef]
- Wang, P.L.; Zhao, Y.L.; Yang, X.L.; Yang, R.J. Crumillospongia Biporosa (sponge) from the Early Cambrian Niutitang Biota in Guizhou Province. Acta Micropalaeontologica Sin. 2005, 22, 196–201. (In Chinese) [Google Scholar]
- Yang, X.L.; Zhu, M.Y.; Zhao, Y.L.; Wang, Y.B. Cambrian sponge assemblages from Guizhou. Acta Micropalaeontologica Sin. 2005, 22, 295–303. (In Chinese) [Google Scholar] [CrossRef]
- Uriz, M.; Turon, X.; Becerro, M.; Agell, G. Siliceous spicules and skeleton frameworks in sponges origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003, 62, 279–299. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Zhou, C.M.; Xiao, S.H.; Yuan, X.L. The sponge fauna of Hetang Formation from the early Cambrian in southern Anhui. Chin. Sci. Bull. 2004, 49, 1399–1402. (In Chinese) [Google Scholar] [CrossRef]
- Joseph, P.B.; Lucy, A.M. Early sponge evolution: A review and phylogenetic framework. Palaeoworld 2018, 27, 1–29. [Google Scholar] [CrossRef]
- Pruss, S.B.; Dwyer, C.H.; Smith, E.F.; Macdonald, F.A.; Tosca, N.J. Phosphatized early Cambrian archaeocyaths and small shelly fossils (SSFs) of southwestern Mongolia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 513, 166–177. [Google Scholar] [CrossRef]
- Chu, Y.L. The Taxonomy and Zoogeography of the Calcare (Porifera) from China Seas. Master’s Thesis, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China, 2020. (In Chinese). [Google Scholar]
- Chen, C. The Ediacaran-Cambrian Boundary and Evolution of the Fossil Assemblage in the Southeastern Margin of the Yangtze Platform. Ph.D. Thesis, China University of Geosciences: Beijing, China, 2022. (In Chinese) [Google Scholar]
- Yang, J.Y.; Ma, H.D.; Chen, A.L.; Hou, X.G. An extraordinary new protomonaxonid sponge—Ovulispongia multa gen. et sp. nov. from the Chengjiang Biota. Acta Geol. Sin. 2022, 96, 3750–3759. (In Chinese) [Google Scholar] [CrossRef]
- Han, Y.J.; You, X.L.; Xing, W.H.; Zhang, Z.; Zou, W.G. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. The investigation of energy metabolism in osteoblasts and osteoclasts. West China J. Stomatol. 2021, 39, 501–509, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Lin, M.X.; Wang, J.T.; Li, L.T.; Wang, C.D. KIAA0753 promotes glucose and energy metabolism in osteoblasts inhibited by diabetes. J. Army Med. Univ. 2024, 46, 2291–2300. [Google Scholar] [CrossRef]
- Mu, J. Study on the influence of coarse aggregate properties on concrete properties. Int. Archit. 2021, 3, 10–13, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Ehrlich, H.; Miksik, I.; Tsurkan, M.V.; Simon, P.; Porzucek, F.; Rybka, J.D.; Mankowska, M.; Galli, R.; Viehweger, C.; Brendler, E.; et al. Discovery of mammalian collagens I and III within ancient poriferan biopolymer spongin. Nat. Commun. 2025, 16, 2515. [Google Scholar] [CrossRef]
- Deng, Z.Q.; Kong, L. Middle Triassic sponges from Qingyan, Guizhou. Acta Micropalaeontologica Sin. 2005, 44, 283–295. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, S.; Khan, M.Z.; Feng, Q.L.; Danelian, T.; Clausen, S.; Tribovillard, N.; Steiner, M. The link between metazoan diversity and paleo-oxygenation in the early Cambrian: An integrated palaeontological and geochemical record from the eastern Three Gorges Region of South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 495, 24–41. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Hu, Y.; Strotz, L.C.; Luo, M.; Zhang, C.; Zhang, Z. Unraveling the Role of Spicules in Shaping Sponge Body Structure: Evidence from the Early Cambrian Shuijingtuo Formation. Biology 2025, 14, 826. https://doi.org/10.3390/biology14070826
Ren X, Hu Y, Strotz LC, Luo M, Zhang C, Zhang Z. Unraveling the Role of Spicules in Shaping Sponge Body Structure: Evidence from the Early Cambrian Shuijingtuo Formation. Biology. 2025; 14(7):826. https://doi.org/10.3390/biology14070826
Chicago/Turabian StyleRen, Xinyi, Yazhou Hu, Luke C. Strotz, Mei Luo, Caibin Zhang, and Zhifei Zhang. 2025. "Unraveling the Role of Spicules in Shaping Sponge Body Structure: Evidence from the Early Cambrian Shuijingtuo Formation" Biology 14, no. 7: 826. https://doi.org/10.3390/biology14070826
APA StyleRen, X., Hu, Y., Strotz, L. C., Luo, M., Zhang, C., & Zhang, Z. (2025). Unraveling the Role of Spicules in Shaping Sponge Body Structure: Evidence from the Early Cambrian Shuijingtuo Formation. Biology, 14(7), 826. https://doi.org/10.3390/biology14070826









