Circulating Antimicrobial Peptides as Biomarkers of Inflammation and Airway Dysfunction After Marathon Running
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Setting
2.1.1. Laboratory Procedures
2.1.2. Detection of Proteins in Serum
2.1.3. Statistical Methods
3. Results
3.1. Demographic and Spirometry Data
3.2. Serum Measurement of Circulating Antimicrobial Peptides
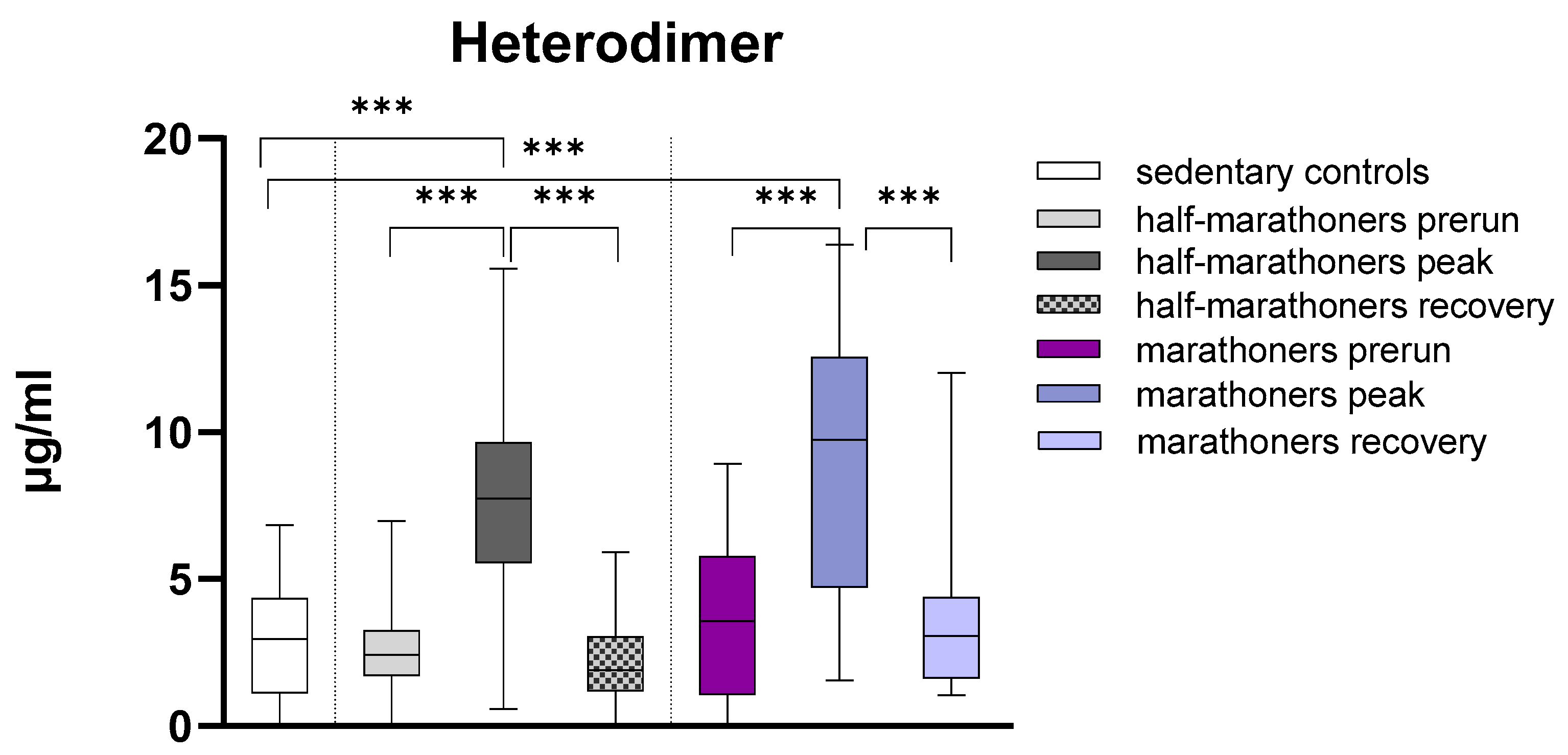
3.2.1. Calprotectin
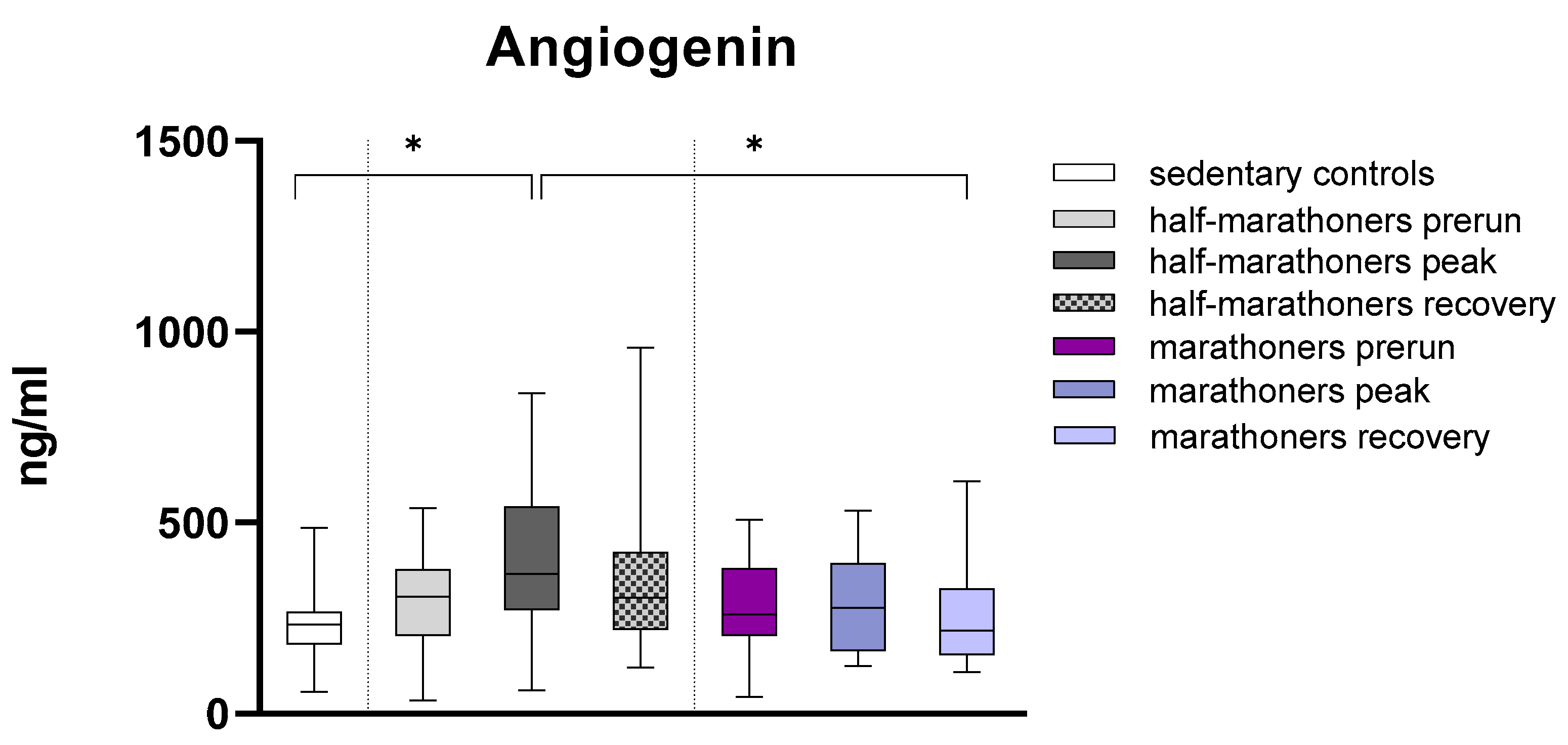
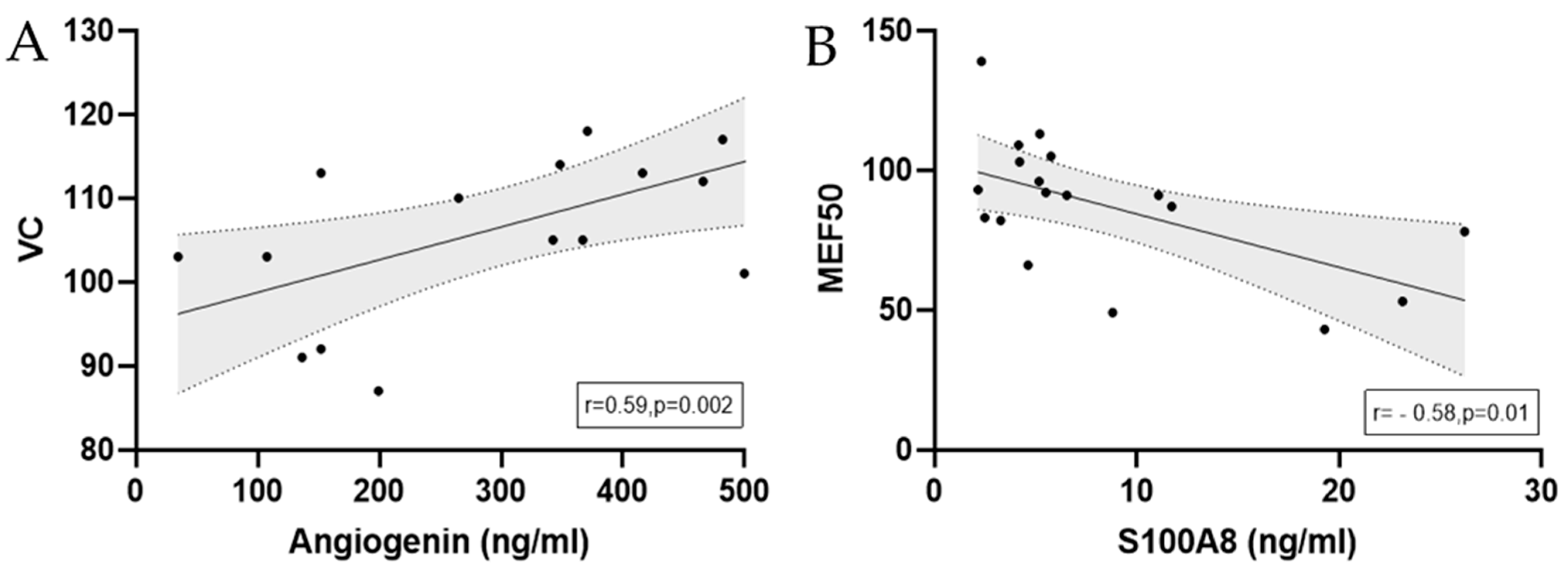
3.2.2. Angiogenin
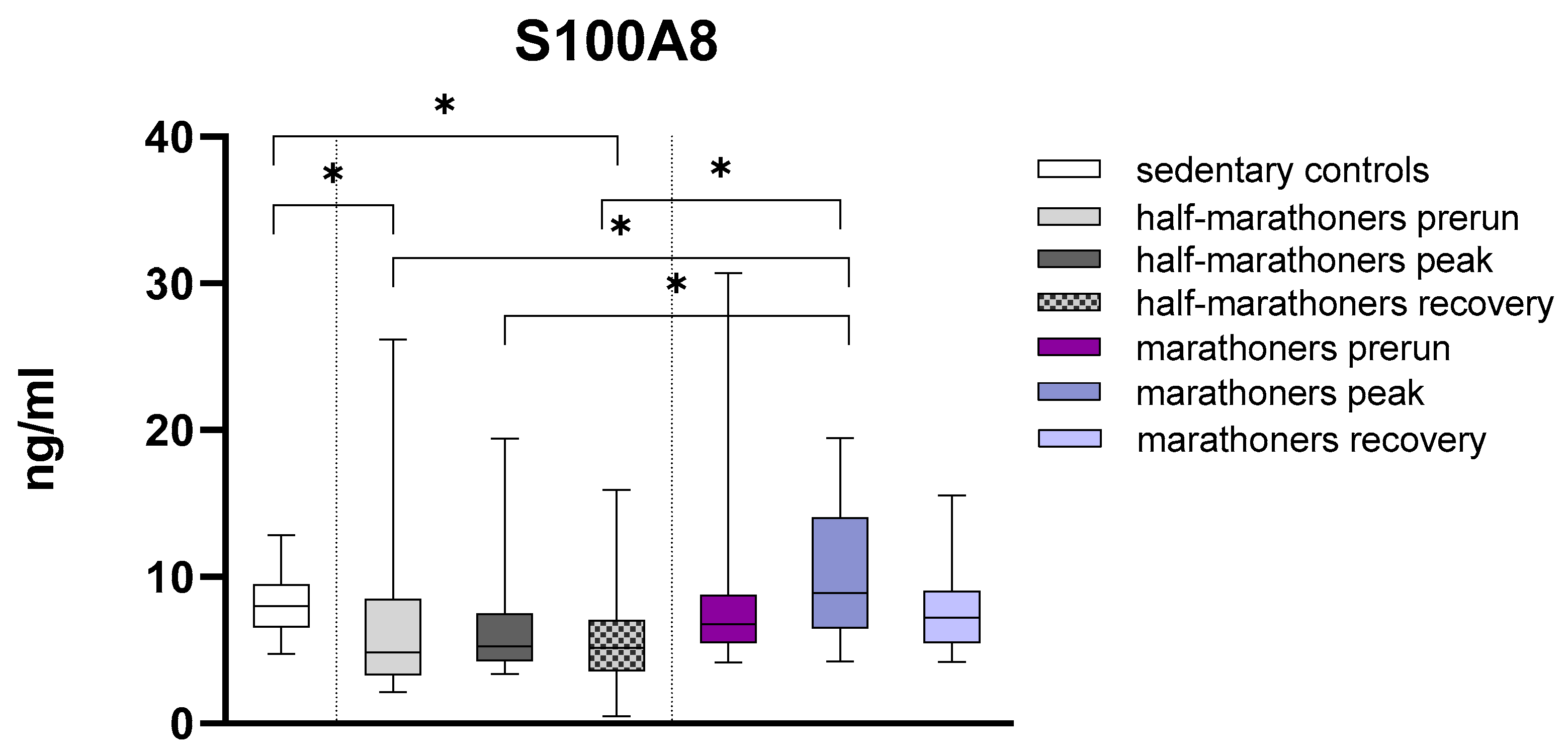
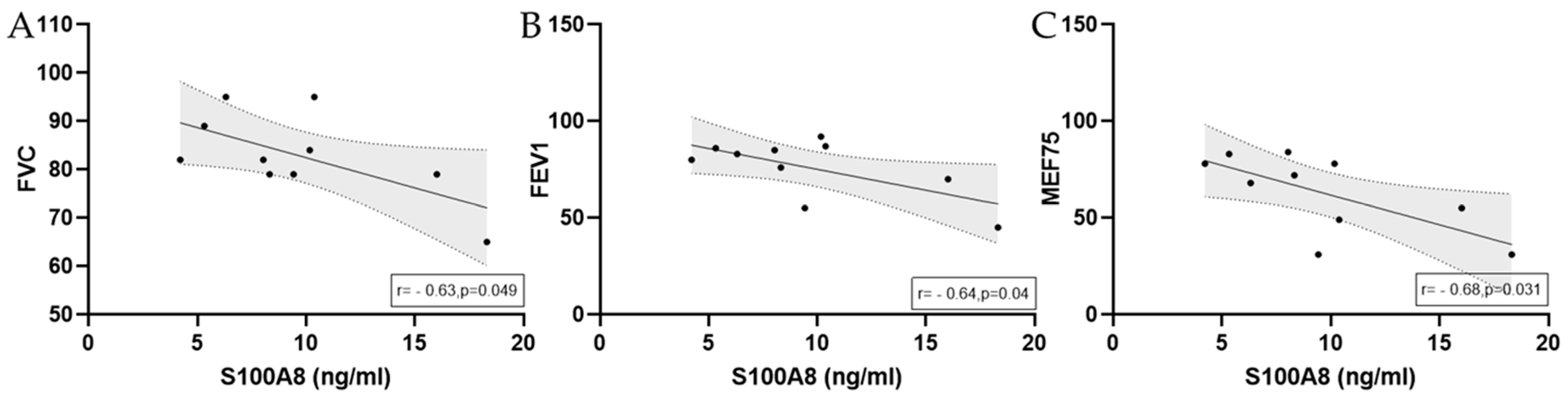
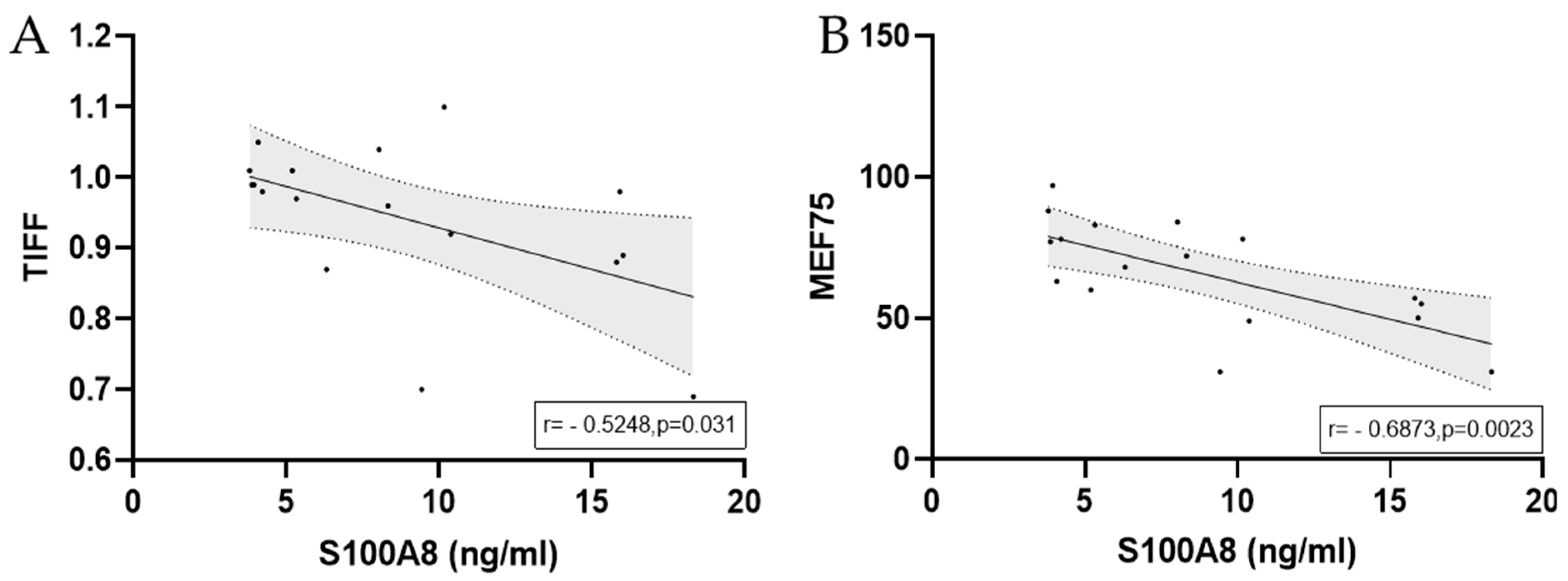
3.2.3. S100A8
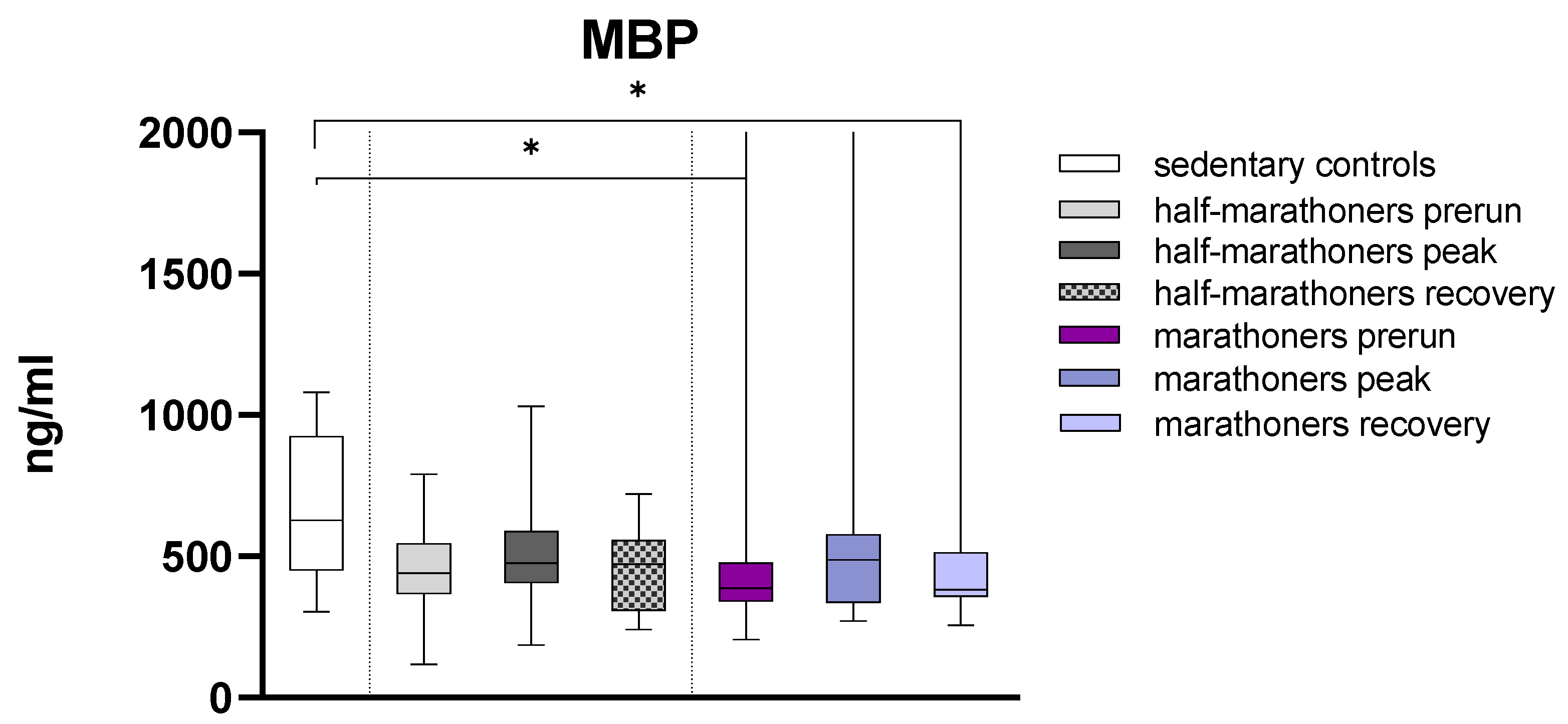
3.2.4. Major Basic Protein
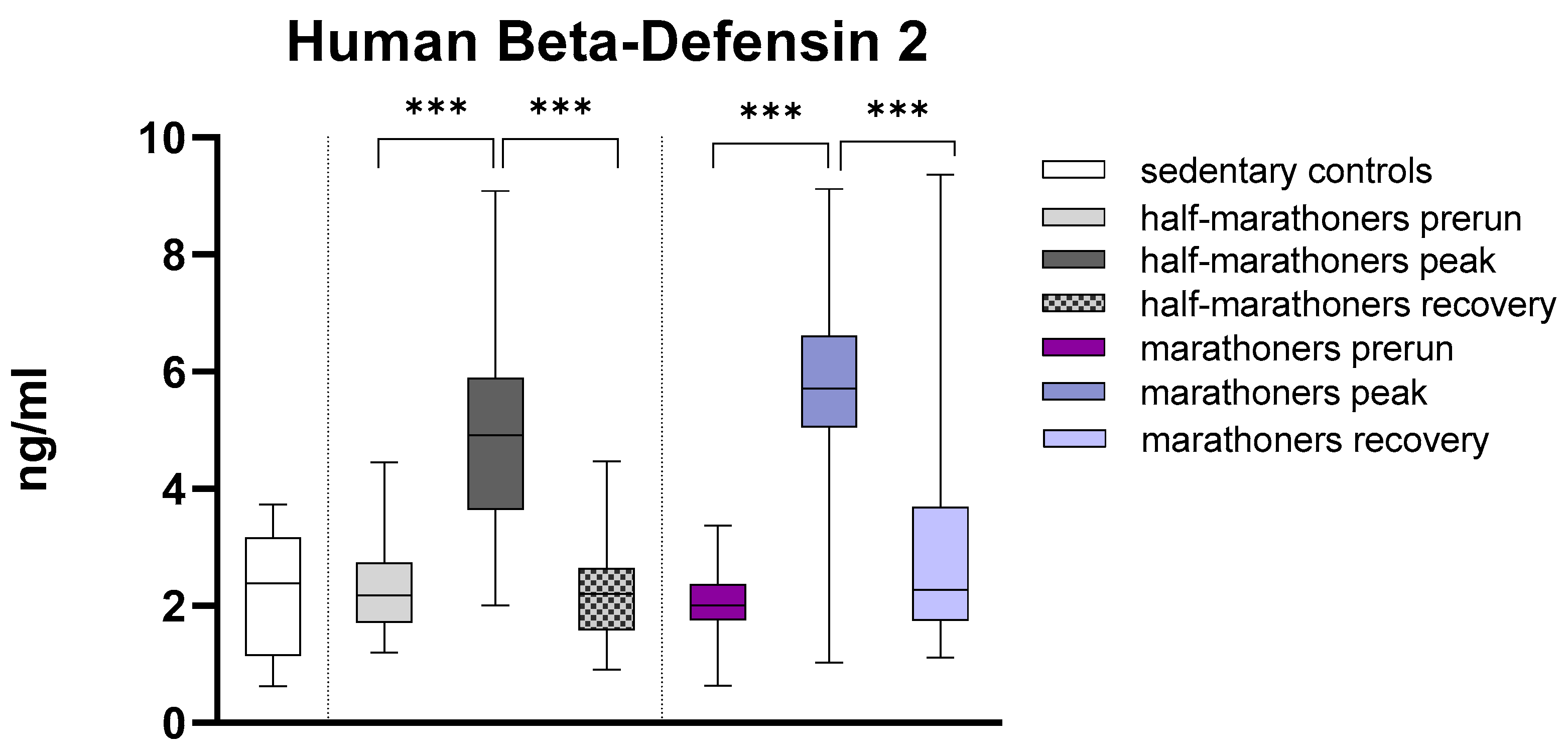
3.2.5. Human Beta-Defensin 2
3.3. EIB
Correlations of Cytokines and Calculated Antimicrobial Peptides in Half-Marathoners and Marathoners with EIB
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGECML | Advanced glycation end products–carboxymethyllysine |
| AMP | Antimicrobial peptide |
| ARDS | Acute respiratory distress syndrome |
| CRP | C-reactive protein |
| DAMP | Damage-associated molecular pattern |
| EIB | Exercise-induced bronchoconstriction |
| ELISA | Enzyme-linked immunosorbent assay |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| HBDF2/hBD-2 | Human beta-defensin 2 |
| HCT | Hematocrit |
| HM | Half-marathoner(s) |
| HMGB1 | High mobility group box 1 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| ICU | Intensive care unit |
| IL-1RA | Interleukin 1 receptor antagonist |
| IL-6 | Interleukin 6 |
| IL-33 | Interleukin 33 |
| IQR | Interquartile range |
| MAPK | Mitogen-activated protein kinase |
| MBP | Major basic protein |
| MEF25/50/75 | Maximal expiratory flow at 25%/50%/75% of FVC |
| MPV | Mean platelet volume |
| MPO | Myeloperoxidase |
| MXD | Mixed cell percentage |
| NE | Neutrophil elastase |
| NF-κB | Nuclear factor kappa B |
| PDW | Platelet distribution width |
| PLT | Platelet count |
| RAGE | Receptor for advanced glycation end products |
| ROS | Reactive oxygen species |
| r | Correlation coefficient |
| SD | Standard deviation |
| sRAGE | Soluble receptor for advanced glycation end products |
| ST2 | Suppression of tumorigenicity 2 |
| Th2 | T-helper cell type 2 |
| TIFF | Tiffeneau–Pinelli index (FEV1/FVC ratio) |
| TLR4 | Toll-like receptor 4 |
| VEGF | Vascular endothelial growth factor |
| WBC | White blood cell count |
References
- Poirier, P.; Després, J.P. Exercise in weight management of obesity. Cardiol. Clin. 2001, 19, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Buresh, R. Exercise and glucose control. J. Sports Med. Phys. Fitness 2014, 54, 373–382. [Google Scholar]
- Marwaha, R.K.; Puri, S.; Tandon, N.; Dhir, S.; Agarwal, N.; Bhadra, K.; Saini, N. Effects of sports training & nutrition on bone mineral density in young Indian healthy females. Indian J. Med. Res. 2011, 134, 307–313. [Google Scholar]
- Thune, I.; Furberg, A.S. Physical activity and cancer risk: Dose-response and cancer, all sites and site-specific. Med. Sci. Sports Exerc. 2001, 33, S530–S550; discussion S609. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Giardini, G.; Tonacci, A.; Mastorci, F.; Mercuri, A.; Mrakic-Sposta, S.; Moretti, S.; Andreassi, M.G.; Pratali, L. Chronic and acute effects of endurance training on telomere length. Mutagenesis 2015, 30, 711–716. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Smith, L.L.; Utter, A.C.; Vinci, D.M.; Davis, J.M.; Kaminsky, D.E.; Shute, M. Cytokine changes after a marathon race. J. Appl. Physiol. 2001, 91, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.C.; Wegberger, C.; Tscharre, M.; Haller, P.M.; Piackova, E.; Vujasin, I.; Kassem, M.; Tentzeris, I.; Freynhofer, M.K.; Jäger, B.; et al. Effect of marathon and ultra-marathon on inflammation and iron homeostasis. Scand. J. Med. Sci. Sports 2021, 31, 542–552. [Google Scholar] [CrossRef]
- Braschler, L.; Nikolaidis, P.T.; Thuany, M.; Chlíbková, D.; Rosemann, T.; Weiss, K.; Wilhelm, M.; Knechtle, B. Physiology and Pathophysiology of Marathon Running: A narrative Review. Sports Med. Open 2025, 11, 10. [Google Scholar] [CrossRef]
- Santos, V.C.; Sierra, A.P.R.; Oliveira, R.; Caçula, K.G.; Momesso, C.M.; Sato, F.T.; Silva, M.B.B.; Oliveira, H.H.; Passos, M.E.P.; de Souza, D.R.; et al. Marathon race affects neutrophil surface molecules: Role of inflammatory mediators. PLoS ONE 2016, 11, e0166687. [Google Scholar] [CrossRef]
- Predel, H.-G. Marathon run: Cardiovascular adaptation and cardiovascular risk. Eur. Heart J. 2014, 35, 3091–3098. [Google Scholar] [CrossRef]
- Cantó, E.; Roca, E.; Perea, L.; Rodrigo-Troyano, A.; Suarez-Cuartin, G.; Giner, J.; Feliu, A.; Soria, J.M.; Nescolarde, L.; Vidal, S.; et al. Salivary immunity and lower respiratory tract infections in non-elite marathon runners. PLoS ONE 2018, 13, e0206059. [Google Scholar] [CrossRef]
- Santos, J.d.M.B.D.; Bachi, A.L.L.; Luna Junior, L.A.; Foster, R.; Sierra, A.P.R.; Benetti, M.; Araújo, J.R.; Ghorayeb, N.; Kiss, M.A.P.D.; Vieira, R.P.; et al. The Relationship of IL-8 and IL-10 Myokines and Performance in Male Marathon Runners Presenting Exercise-Induced Bronchoconstriction. Int. J. Environ. Res. Public Health 2020, 17, 2622. [Google Scholar] [CrossRef] [PubMed]
- Górecka, M.; Krzemiński, K.; Buraczewska, M.; Kozacz, A.; Dąbrowski, J.; Ziemba, A.W. Effect of mountain ultra-marathon running on plasma angiopoietin-like protein 4 and lipid profile in healthy trained men. Eur. J. Appl. Physiol. 2020, 120, 117–125. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Wang, G. The antimicrobial peptide database is 20 years old: Recent developments and future directions. Protein Sci. 2023, 32, e4778. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Renshaw, G.; Cripps, A.W. Antimicrobial peptides and proteins, exercise and innate mucosal immunity. FEMS Immunol. Med. Microbiol. 2006, 48, 293–304. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Human β-Defensin 2 and Its Postulated Role in Modulation of the Immune Response. Cells 2021, 10, 2991. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.J.P.; Aanerud, M.; Hardie, J.A.; Miodini Nilsen, R.; Bakke, P.S.; Eagan, T.M.; Hiemstra, P.S. Antimicrobial peptide levels are linked to airway inflammation, bacterial colonisation and exacerbations in chronic obstructive pulmonary disease. Eur. Respir. J. 2017, 49, 1601328. [Google Scholar] [CrossRef]
- Sonna, L.A.; Angel, K.C.; Sharp, M.A.; Knapik, J.J.; Patton, J.F.; Lilly, C.M. The prevalence of exercise-induced bronchospasm among US Army recruits and its effects on physical performance. Chest 2001, 119, 1676–1684. [Google Scholar] [CrossRef]
- Ng’ang’a, L.W.; Odhiambo, J.A.; Mungai, M.W.; Gicheha, C.M.; Nderitu, P.; Maingi, B.; Macklem, P.T.; Becklake, M.R. Prevalence of exercise induced bronchospasm in Kenyan school children: An urban-rural comparison. Thorax 1998, 53, 919–926. [Google Scholar] [CrossRef]
- Kukafka, D.S.; Lang, D.M.; Porter, S.; Rogers, J.; Ciccolella, D.; Polansky, M.; D’Alonzo, G.E. Exercise-induced bronchospasm in high school athletes via a free running test: Incidence and epidemiology. Chest 1998, 114, 1613–1622. [Google Scholar] [CrossRef]
- Anderson, S.D.; Kippelen, P. Exercise-induced bronchoconstriction: Pathogenesis. Curr. Allergy Asthma Rep. 2005, 5, 116–122. [Google Scholar] [CrossRef]
- Anderson, S.D.; Kippelen, P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J. Allergy Clin. Immunol. 2008, 122, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.M.; Harder, J. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 1999, 31, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.S.; Blaum, B.S.; Vargues, T.; De Cecco, M.; Deakin, J.A.; Lyon, M.; Barran, P.E.; Campopiano, D.J.; Uhrín, D. Interaction of human β-defensin 2 (HBD2) with glycosaminoglycans. Biochemistry 2010, 49, 10486–10495. [Google Scholar] [CrossRef]
- Yanagi, S.; Ashitani, J.; Imai, K.; Kyoraku, Y.; Sano, A.; Matsumoto, N.; Nakazato, M. Significance of human beta-defensins in the epithelial lining fluid of patients with chronic lower respiratory tract infections. Clin. Microbiol. Infect. 2007, 13, 63–69. [Google Scholar] [CrossRef]
- Chen, L.; Sun, B.B.; Wang, T.; Wang, X.; Li, J.Q.; Wang, H.X.; Zhang, S.F.; Liu, D.S.; Liu, L.; Xu, D.; et al. Cigarette smoke enhances β-defensin 2 expression in rat airways via nuclear factor- κB activation. Eur. Respir. J. 2010, 36, 638–645. [Google Scholar] [CrossRef]
- Patricia Rosete Olvera, D.; Cabello Gutiérrez, C. Multifunctional Activity of the β-Defensin-2 during Respiratory Infections. In Immune Response Activation and Immunomodulation; Tyagi, R.K., Bisen, P.S., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-151-9. [Google Scholar]
- Borchers, N.S.; Santos-Valente, E.; Toncheva, A.A.; Wehkamp, J.; Franke, A.; Gaertner, V.D.; Nordkild, P.; Genuneit, J.; Jensen, B.A.H.; Kabesch, M. Human β-Defensin 2 Mutations Are Associated with Asthma and Atopy in Children and Its Application Prevents Atopic Asthma in a Mouse Model. Front. Immunol. 2021, 12, 636061. [Google Scholar] [CrossRef]
- Liu, S.; He, L.-R.; Wang, W.; Wang, G.-H.; He, Z.-Y. Prognostic value of plasma human β-defensin 2 level on short-term clinical outcomes in patients with community-acquired pneumonia: A preliminary study. Respir. Care 2013, 58, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ouyang, Y.; Li, Z.; Wang, X.; Ma, J. S100A8 and S100A9 in cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188891. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Roth, J.; Goebeler, M.; Sorg, C. S100A8 and S100A9 in inflammatory diseases. Lancet 2001, 357, 1041. [Google Scholar] [CrossRef]
- Trøstrup, H.; Lerche, C.J.; Christophersen, L.; Jensen, P.Ø.; Høiby, N.; Moser, C. Immune Modulating Topical S100A8/A9 Inhibits Growth of Pseudomonas aeruginosa and Mitigates Biofilm Infection in Chronic Wounds. Int. J. Mol. Sci. 2017, 18, 1359. [Google Scholar] [CrossRef]
- Lin, C.-R.; Bahmed, K.; Criner, G.J.; Marchetti, N.; Tuder, R.M.; Kelsen, S.; Bolla, S.; Mandapati, C.; Kosmider, B. S100A8 Protects Human Primary Alveolar Type II Cells against Injury and Emphysema. Am. J. Respir. Cell Mol. Biol. 2019, 60, 299–307. [Google Scholar] [CrossRef]
- Coveney, A.P.; Wang, W.; Kelly, J.; Liu, J.H.; Blankson, S.; Wu, Q.D.; Redmond, H.P.; Wang, J.H. Myeloid-related protein 8 induces self-tolerance and cross-tolerance to bacterial infection via TLR4- and TLR2-mediated signal pathways. Sci. Rep. 2015, 5, 13694. [Google Scholar] [CrossRef]
- Vogl, T.; Ludwig, S.; Goebeler, M.; Strey, A.; Thorey, I.S.; Reichelt, R.; Foell, D.; Gerke, V.; Manitz, M.P.; Nacken, W.; et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 2004, 104, 4260–4268. [Google Scholar] [CrossRef]
- Zhao, J.; Endoh, I.; Hsu, K.; Tedla, N.; Endoh, Y.; Geczy, C.L. S100A8 modulates mast cell function and suppresses eosinophil migration in acute asthma. Antioxid. Redox Signal. 2011, 14, 1589–1600. [Google Scholar] [CrossRef]
- Xu, Y.-D.; Cui, J.-M.; Wang, Y.; Yin, L.-M.; Gao, C.-K.; Liu, Y.-Y.; Yang, Y.-Q. The early asthmatic response is associated with glycolysis, calcium binding and mitochondria activity as revealed by proteomic analysis in rats. Respir. Res. 2010, 11, 107. [Google Scholar] [CrossRef]
- Chakraborty, D.; Zenker, S.; Rossaint, J.; Hölscher, A.; Pohlen, M.; Zarbock, A.; Roth, J.; Vogl, T. Alarmin S100A8 Activates Alveolar Epithelial Cells in the Context of Acute Lung Injury in a TLR4-Dependent Manner. Front. Immunol. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Decaesteker, T.; Bos, S.; Lorent, N.; Everaerts, S.; Vanoirbeek, J.; Bullens, D.; Dupont, L.J. Elevated serum calprotectin (S100A8/A9) in patients with severe asthma. J. Asthma 2022, 59, 1110–1115. [Google Scholar] [CrossRef]
- Burkhardt, K.; Schwarz, S.; Pan, C.; Stelter, F.; Kotliar, K.; Von Eynatten, M.; Sollinger, D.; Lanzl, I.; Heemann, U.; Baumann, M. Myeloid-related protein 8/14 complex describes microcirculatory alterations in patients with type 2 diabetes and nephropathy. Cardiovasc. Diabetol. 2009, 8, 10. [Google Scholar] [CrossRef]
- Kane, D.; Roth, J.; Frosch, M.; Vogl, T.; Bresnihan, B.; FitzGerald, O. Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum. 2003, 48, 1676–1685. [Google Scholar] [CrossRef]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 2015, 110, 444–454. [Google Scholar] [CrossRef]
- Ji, X.; Nie, C.; Yao, Y.; Ma, Y.; Huang, H.; Hao, C. S100A8/9 modulates perturbation and glycolysis of macrophages in allergic asthma mice. PeerJ 2024, 12, e17106. [Google Scholar] [CrossRef]
- Berry, A.; Busse, W.W. Biomarkers in asthmatic patients: Has their time come to direct treatment? J. Allergy Clin. Immunol. 2016, 137, 1317–1324. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Gu, Q.; Lin, A.-H.; Khosravi, M.; Gleich, G. Airway hypersensitivity induced by eosinophil granule-derived cationic proteins. Pulm. Pharmacol. Ther. 2019, 57, 101804. [Google Scholar] [CrossRef]
- Plager, D.A.; Loegering, D.A.; Weiler, D.A.; Checkel, J.L.; Wagner, J.M.; Clarke, N.J.; Naylor, S.; Page, S.M.; Thomas, L.L.; Akerblom, I.; et al. A novel and highly divergent homolog of human eosinophil granule major basic protein. J. Biol. Chem. 1999, 274, 14464–14473. [Google Scholar] [CrossRef]
- Tai, P.C.; Ackerman, S.J.; Spry, C.J.; Dunnette, S.; Olsen, E.G.; Gleich, G.J. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet 1987, 1, 643–647. [Google Scholar] [CrossRef]
- Gleich, G.J.; Flavahan, N.A.; Fujisawa, T.; Vanhoutte, P.M. The eosinophil as a mediator of damage to respiratory epithelium: A model for bronchial hyperreactivity. J. Allergy Clin. Immunol. 1988, 81, 776–781. [Google Scholar] [CrossRef]
- Stelts, D.; Egan, R.W.; Falcone, A.; Garlisi, C.G.; Gleich, G.J.; Kreutner, W.; Kung, T.T.; Nahrebne, D.K.; Chapman, R.W.; Minnicozzi, M. Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 1998, 18, 463–470. [Google Scholar] [CrossRef]
- Evans, C.M.; Fryer, A.D.; Jacoby, D.B.; Gleich, G.J.; Costello, R.W. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J. Clin. Investig. 1997, 100, 2254–2262. [Google Scholar] [CrossRef]
- Gundel, R.H.; Letts, L.G.; Gleich, G.J. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J. Clin. Investig. 1991, 87, 1470–1473. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Morici, G.; Riccobono, L.; Insalaco, G.; Bonanno, A.; Profita, M.; Paternò, A.; Vassalle, C.; Mirabella, A.; Vignola, A.M. Airway inflammation in nonasthmatic amateur runners. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L668–L676. [Google Scholar] [CrossRef]
- Hu, H.; Gao, X.; Sun, Y.; Zhou, J.; Yang, M.; Xu, Z. Alpha-actinin-2, a cytoskeletal protein, binds to angiogenin. Biochem. Biophys. Res. Commun. 2005, 329, 661–667. [Google Scholar] [CrossRef]
- Tello-Montoliu, A.; Patel, J.V.; Lip, G.Y.H. Angiogenin: A review of the pathophysiology and potential clinical applications. J. Thromb. Haemost. 2006, 4, 1864–1874. [Google Scholar] [CrossRef]
- Fett, J.W.; Strydom, D.J.; Lobb, R.R.; Alderman, E.M.; Bethune, J.L.; Riordan, J.F.; Vallee, B.L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 1985, 24, 5480–5486. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.-P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Strydom, D.J. The angiogenins. Cell. Mol. Life Sci. 1998, 54, 811–824. [Google Scholar] [CrossRef]
- Olson, K.A.; Verselis, S.J.; Fett, J.W. Angiogenin is regulated in vivo as an acute phase protein. Biochem. Biophys. Res. Commun. 1998, 242, 480–483. [Google Scholar] [CrossRef]
- Bekos, C.; Zimmermann, M.; Unger, L.; Janik, S.; Hacker, P.; Mitterbauer, A.; Koller, M.; Fritz, R.; Gäbler, C.; Kessler, M.; et al. Non-professional marathon running: RAGE axis and ST2 family changes in relation to open-window effect, inflammation and renal function. Sci. Rep. 2016, 6, 32315. [Google Scholar] [CrossRef] [PubMed]
- Fallon, K.E. The acute phase response and exercise: The ultramarathon as prototype exercise. Clin. J. Sport Med. 2001, 11, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bekos, C.; Zimmermann, M.; Unger, L.; Janik, S.; Mitterbauer, A.; Koller, M.; Fritz, R.; Gäbler, C.; Didcock, J.; Kliman, J.; et al. Exercise-induced bronchoconstriction, temperature regulation and the role of heat shock proteins in non-asthmatic recreational marathon and half-marathon runners. Sci. Rep. 2019, 9, 4168. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Mooren, F.C.; Lechtermann, A.; Fobker, M.; Brandt, B.; Sorg, C.; Völker, K.; Nacken, W. The response of the novel pro-inflammatory molecules S100A8/A9 to exercise. Int. J. Sports Med. 2006, 27, 751–758. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.D.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef]
- Halayko, A.J.; Ghavami, S. S100A8/A9: A mediator of severe asthma pathogenesis and morbidity? Can. J. Physiol. Pharmacol. 2009, 87, 743–755. [Google Scholar] [CrossRef]
- Guillot, L.; Medjane, S.; Le-Barillec, K.; Balloy, V.; Danel, C.; Chignard, M.; Si-Tahar, M. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: Evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 2004, 279, 2712–2718. [Google Scholar] [CrossRef]
- MacRedmond, R.; Greene, C.; Taggart, C.C.; McElvaney, N.; O’Neill, S. Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir. Res. 2005, 6, 116. [Google Scholar] [CrossRef]
- Conti, P.; DiGioacchino, M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001, 22, 133–137. [Google Scholar] [CrossRef]
- Scott, H.A.; Wood, L.G.; Williams, E.J.; Weaver, N.; Upham, J.W. Comparing the Effect of Acute Moderate and Vigorous Exercise on Inflammation in Adults with Asthma: A Randomized Controlled Trial. Ann. Am. Thorac. Soc. 2022, 19, 1848–1855. [Google Scholar] [CrossRef]
- Li, S.; Hu, G.-F. Emerging role of angiogenin in stress response and cell survival under adverse conditions. J. Cell. Physiol. 2012, 227, 2822–2826. [Google Scholar] [CrossRef]
- Eleuteri, E.; Mezzani, A.; Di Stefano, A.; Vallese, D.; Gnemmi, I.; Delle Donne, L.; Taddeo, A.; Della Bella, S.; Giannuzzi, P. Aerobic training and angiogenesis activation in patients with stable chronic heart failure: A preliminary report. Biomarkers 2013, 18, 418–424. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamabe, H.; Osawa, H.; Nakamura, N.; Shimada, M.; Kumasaka, R.; Murakami, R.; Fujita, T.; Osanai, T.; Okumura, K. Hypoxic conditions stimulate the production of angiogenin and vascular endothelial growth factor by human renal proximal tubular epithelial cells in culture. Nephrol. Dial. Transplant. 2006, 21, 1489–1495. [Google Scholar] [CrossRef]
- Hartmann, A.; Kunz, M.; Köstlin, S.; Gillitzer, R.; Toksoy, A.; Bröcker, E.B.; Klein, C.E. Hypoxia-induced up-regulation of angiogenin in human malignant melanoma. Cancer Res. 1999, 59, 1578–1583. [Google Scholar]
- Rajashekhar, G.; Loganath, A.; Roy, A.C.; Chong, S.S.; Wong, Y.C. Hypoxia up-regulated angiogenin and down-regulated vascular cell adhesion molecule-1 expression and secretion in human placental trophoblasts. J. Soc. Gynecol. Investig. 2005, 12, 310–319. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Sierra, A.P.R.; Benetti, M.; Ghorayeb, N.; Sierra, C.A.; Kiss, M.A.P.D.M.; Cury-Boaventura, M.F. Impact of hot environment on fluid and electrolyte imbalance, renal damage, hemolysis, and immune activation postmarathon. Oxid. Med. Cell. Longev. 2017, 2017, 9824192. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2019, 10, 1550. [Google Scholar] [CrossRef]
- Legaz-Arrese, A.; George, K.; Carranza-García, L.E.; Munguía-Izquierdo, D.; Moros-García, T.; Serrano-Ostáriz, E. The impact of exercise intensity on the release of cardiac biomarkers in marathon runners. Eur. J. Appl. Physiol. 2011, 111, 2961–2967. [Google Scholar] [CrossRef]
- Kim, B.J.; Choi, J.-Y.; Oh, S.-J.; Heo, J.-H. Endurance Exercise Training Prevents Elevation of Soluble ST2 in Mice with Doxorubicin-Induced Myocardial Injury. Int. J. Heart Fail. 2021, 3, 59–68. [Google Scholar] [CrossRef]
- Roy, I.; Jover, E.; Matilla, L.; Alvarez, V.; Fernández-Celis, A.; Beunza, M.; Escribano, E.; Gainza, A.; Sádaba, R.; López-Andrés, N. Soluble ST2 as a new oxidative stress and inflammation marker in metabolic syndrome. Int. J. Environ. Res. Public Health 2023, 20, 2579. [Google Scholar] [CrossRef]
- Drosatos, I.-A.; Tsoporis, J.N.; Izhar, S.; Gupta, S.; Tsirebolos, G.; Sakadakis, E.; Triantafyllis, A.S.; Rigopoulos, A.; Rigopoulos, D.; Rallidis, L.S.; et al. Differential regulation of circulating soluble receptor for advanced glycation end products (srages) and its ligands S100A8/A9 four weeks post an exercise intervention in a cohort of young army recruits. Biomolecules 2021, 11, 1354. [Google Scholar] [CrossRef]
- Wood, L.G.; Baines, K.J.; Fu, J.; Scott, H.A.; Gibson, P.G. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest 2012, 142, 86–93. [Google Scholar] [CrossRef]
- Bertolini, F.; Carriero, V.M.A.; Arrigo, E.; Guida, G.; Levra, S.; Pizzimenti, S.; Profita, M.; Gnemmi, I.; Di Stefano, A.; Ricciardolo, F.L.M. Vascular remodeling and TSLP/angiogenin overexpression in severe mixed asthma. Respir. Res. 2025, 26, 78. [Google Scholar] [CrossRef]
- Van Nevel, S.; van Ovost, J.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Braun, H.; Maes, T.; Bachert, C.; Krysko, O. Neutrophils Affect IL-33 Processing in Response to the Respiratory Allergen Alternaria alternata. Front. Immunol. 2021, 12, 677848. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yan, W.; Geczy, C.L.; Brown, M.A.; Thomas, R. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, R39. [Google Scholar] [CrossRef]
- Gustafsson, T.; Kraus, W.E. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front. Biosci. 2001, 6, D75–D89. [Google Scholar]
- Guerrero, C.; Collado-Boira, E.; Martinez-Navarro, I.; Hernando, B.; Hernando, C.; Balino, P.; Muriach, M. Impact of Plasma Oxidative Stress Markers on Post-race Recovery in Ultramarathon Runners: A Sex and Age Perspective Overview. Antioxidants 2021, 10, 355. [Google Scholar] [CrossRef]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxid. Med. Cell. Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, C.; Che, N.; Jing, L.; Ge, R. Hydrogen-rich saline attenuates eosinophil activation in a guinea pig model of allergic rhinitis via reducing oxidative stress. J. Inflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Calhoun, W.J.; Reed, H.E.; Moest, D.R.; Stevens, C.A. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am. Rev. Respir. Dis. 1992, 145, 317–325. [Google Scholar] [CrossRef]
- Gillum, T.L.; Kuennen, M.; Gourley, C.; Schneider, S.; Dokladny, K.; Moseley, P. Salivary antimicrobial protein response to prolonged running. Biol. Sport 2013, 30, 3–8. [Google Scholar] [CrossRef]
- Usui, T.; Yoshikawa, T.; Orita, K.; Ueda, S.-Y.; Katsura, Y.; Fujimoto, S.; Yoshimura, M. Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur. J. Appl. Physiol. 2011, 111, 2005–2014. [Google Scholar] [CrossRef]
- Shimizu, K.; Hanaoka, Y.; Akama, T.; Kono, I. Ageing and free-living daily physical activity effects on salivary beta-defensin 2 secretion. J. Sports Sci. 2017, 35, 617–623. [Google Scholar] [CrossRef]
- Ito, R.; Uchino, T.; Uchida, M.; Fujie, S.; Iemitsu, K.; Kojima, C.; Nakamura, M.; Shimizu, K.; Tanimura, Y.; Shinohara, Y.; et al. Acute salivary antimicrobial peptide secretion response to different exercise intensities and durations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 327, R616–R622. [Google Scholar] [CrossRef]

| MARATHON (N = 34) | HALF-MARATHON (N = 36) | CONTROLS (N = 30) | |
|---|---|---|---|
| AGE (YEARS) | 36.5 (32–44) | 37.0 (30.00–42.00) | 29.00 (25.00–41.00) |
| F:M (QUANTITY) | 10:24 | 17:19 | 13:17 |
| BMI (KG/M2) | 22.51 (20.93–24,13) | 23.47 (20.93–25.23) | 22.99 (20.86–27.08) |
| WEIGHT (KG) | 71.00 (63.20–76.40) | 71.85 (58.60–86.35) | 73.00 (64.45–83.75) |
| BODY FAT PERCENTAGE (%) | 17.40 (12.80–23.00) | 24.45 (20.85–30.33) | 21.45 (16.03–27.40) |
| TARGET TIME (MIN) | 230.00 (201.75–251.75) | 112.00 (106.00–128.50) | 0 |
| KM PER WEEK | 60.00 (40.00–80.00) | 35.00 (30.00–40.00) | 0 |
| TRAINING DAYS/WEEK | 4.00 (4.00–5.00) | 3.00 (3.00–4.00) | 0 |
| Sedentary Controls | HM Baseline | HM Peak | HM Recovery | M Baseline | M Peak | M Recovery | |
|---|---|---|---|---|---|---|---|
| FVC | 94.5 (85.00–105.25) | 100.00 (90.00–105.00) | 93.00 (80.00–101.50) | 94.00 (85.00–103.00) | 99.50 (94.00–105.00) | 87.00 (79.00–95.50) | 102.00 (90.00–108.75) |
| FEV1 | 90.50 (79.00–101.00) | 96.00 (88.00–101.00) | 88.00 (81.50–96.50) | 91.00 (83.00–101.00) | 95.50 (90.00–102.00) | 87.50 (81.00–95.25) | 96.50 (87.00–101.00) |
| VC | 91.50 (82.00–100.50) | 101.00 (87.00–110) | 93.00 (83.50–102.00) | 99.00 (91.00–105.00) | 97.50 (87.25–107.50) | 87.00 (78.00–93.00) | 99.00 (89.00–114.00) |
| TIFF | 0.97 (0.90–1.02) | 0.97 (0.94–0.99) | 0.97 (0.93–1.01) | 0.96 (0.92–0.99) | 0.97 (0.92–1.00) | 0.98 (0.92–1.07) | 0.96 (0.90–0.99) |
| MEF75% | 85.00 (68.75–96.00) | 84.00 (71.00–99.5) | 80.00 (67.50–88.00) | 86.00 (76.50–102.00) | 80.00 (68.00–89.75) | ||
| MEF50% | 89.00 (64.75–103.00) | 86.50 (74.00–103.75) | 83.00 (67.00–90.50) | 91.00 (76.00–105.00) | 80.50 (66.25–94.50) | ||
| MEF25% | 81.00 (55.25–106.50) | 86.00 (69.00–109.00) | 82.00 (65.00–105.00) | 82.00 (68.00–107.00) | 82.50 (67.50–105.00) | ||
| CRP (mg/dL) | 0.11 (0.00–0.28) | 0.05 (0.00–0.16) | 0.05 (0.00–0.14) | 0.10 (0.05–0.23) | 0.00 (0.00–0.07) | 0.00 (0.00–0.053) | 0.13 (0.08–0.25) |
| IL33 (pg/mL) | 44.81 (16.25–292.85) | 64.02 (0.00–507.88) | 77.37 (0.00–483.78) | 71.52 (0.00–335.42) | 58.28 (0.00–562.75) | 86.18 (15.93–638.16) | 66.30 (0.00–439.65) |
| AGE-CML (ng/mL) | 33.37 (24.71–42.52) | 52.73 (39.86–61.31) | 61.77 (53.31–70.96) | 51.83 (44.79–64.31) | 34.46 (26.88–48.56) | 41.91 (36.45–53.50) | 34.70 (28.76–44.08) |
| esRAGE (ng/mL) | 0.32 (0.23–0.43) | 0.34 (0.24–0.44) | 0.48 (0.36–0.60) | 0.38 (0.27–0.51) | 0.31 (0.25–0.37) | 0.36 (0.28–0.51) | 0.29 (0.21–0.38) |
| Sedentary Controls | HM Baseline | HM Peak | HM Recovery | M Baseline | M Peak | M Recovery | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Angiogenin (ng/mL) | 233 (182–267) | 306 (204–379) | 365 (272–542) | 303 (221–424) | 260 (204–381) | 277 (164–393) | 218 (155–328) | 0.015 |
| S100A8/A9 (µg/mL) | 2.96 (1.12–4.36) | 2.43 (1.71–3.26) | 7.75 (5.56–9.66) | 3.04 (1.20–3.04) | 3.58 (1.05–5.78) | 9.75 (4.72–12.56) | 9.75 (4.72–12.56) | <0.001 |
| S100A8 (ng/mL) | 8.02 (6.56–9.49) | 4.85 (3.31–8478) | 5.25 (4.24–7.49) | 5.16 (3.59–7.04) | 6.80 (5.50–8.75) | 8.89 (6.48–14.04) | 7.23 (5.49–9.03) | <0.001 |
| Major Basic Protein (ng/mL) | 627 (450–926) | 440 (368–547) | 476 (405–589) | 472 (307–558) | 388 (341–477) | 488 (335–578) | 383 (357–514) | 0.022 |
| Human Beta-Defensin 2 (ng/mL) | 2.38 (1.15–3.16) | 2.18 (1.72–2.74) | 4.91(3.64–5.89) | 2.21 (1.60–2.64) | 2.02 (1.77–2.37) | 5.72 (5.10–6.61) | 2.27 (1.75–3.69) | <0.001 |
| Group | Subgroup | Variables Correlated | Correlation Coefficient (r) | p-Value |
|---|---|---|---|---|
| Baseline | HM | ST2 ↔ Angiogenin | 0.70 | 0.002 |
| Baseline | M | ST2 ↔ Angiogenin | 0.84 | 0.004 |
| Baseline | HM | S100A8 ↔ esRAGE | 0.89 | 0.007 |
| Baseline | M | MBP ↔ esRAGE | 0.95 | <0.001 |
| Baseline | M | S100A8 ↔ CRP | 0.68 | 0.02 |
| Peak | HM | S100A8/A9 ↔ MBP | 0.90 | 0.006 |
| Peak | M | S100A8/A9 ↔ IL33 | 0.79 | 0.01 |
| Peak | M | S100A8 ↔ AGECML | −0.64 | 0.05 |
| Recovery | HM | Angiogenin ↔ HBDF2 | 0.84 | 0.02 |
| Recovery | M | MBP ↔ esRAGE | 0.84 | 0.003 |
| Recovery | HM | Angiogenin ↔ HBDF2 | 0.85 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lingitz, M.-T.; Kühtreiber, H.; Auer, L.; Mildner, M.; Krenn, C.G.; Aigner, C.; Moser, B.; Bekos, C.; Ankersmit, H.J. Circulating Antimicrobial Peptides as Biomarkers of Inflammation and Airway Dysfunction After Marathon Running. Biology 2025, 14, 825. https://doi.org/10.3390/biology14070825
Lingitz M-T, Kühtreiber H, Auer L, Mildner M, Krenn CG, Aigner C, Moser B, Bekos C, Ankersmit HJ. Circulating Antimicrobial Peptides as Biomarkers of Inflammation and Airway Dysfunction After Marathon Running. Biology. 2025; 14(7):825. https://doi.org/10.3390/biology14070825
Chicago/Turabian StyleLingitz, Marie-Therese, Hannes Kühtreiber, Lisa Auer, Michael Mildner, Claus G. Krenn, Clemens Aigner, Bernhard Moser, Christine Bekos, and Hendrik Jan Ankersmit. 2025. "Circulating Antimicrobial Peptides as Biomarkers of Inflammation and Airway Dysfunction After Marathon Running" Biology 14, no. 7: 825. https://doi.org/10.3390/biology14070825
APA StyleLingitz, M.-T., Kühtreiber, H., Auer, L., Mildner, M., Krenn, C. G., Aigner, C., Moser, B., Bekos, C., & Ankersmit, H. J. (2025). Circulating Antimicrobial Peptides as Biomarkers of Inflammation and Airway Dysfunction After Marathon Running. Biology, 14(7), 825. https://doi.org/10.3390/biology14070825









