DIRAS1 Drives Oxaliplatin Resistance in Colorectal Cancer via PHB1-Mediated Mitochondrial Homeostasis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Cell Culture
2.3. Cell Proliferation Assay
2.4. Wound Healing Assay
2.5. Plasmid Constructs and Lentiviral Packaging
2.6. RNA Isolation and Semi-Quantitative Reverse Transcription PCR
2.7. Western Blotting (WB)
2.8. Immunohistochemistry (IHC)
2.9. Annexin V-FITC/PI Apoptosis Assay
2.10. Detection of Mitochondrial Membrane Potential
2.11. Mitochondrial Permeability Transition Pore Assay
2.12. Mitochondrial ROS (mtROS) Measurement
2.13. Immunofluorescence
2.14. Animal Models
2.15. Statistical Analysis
3. Result
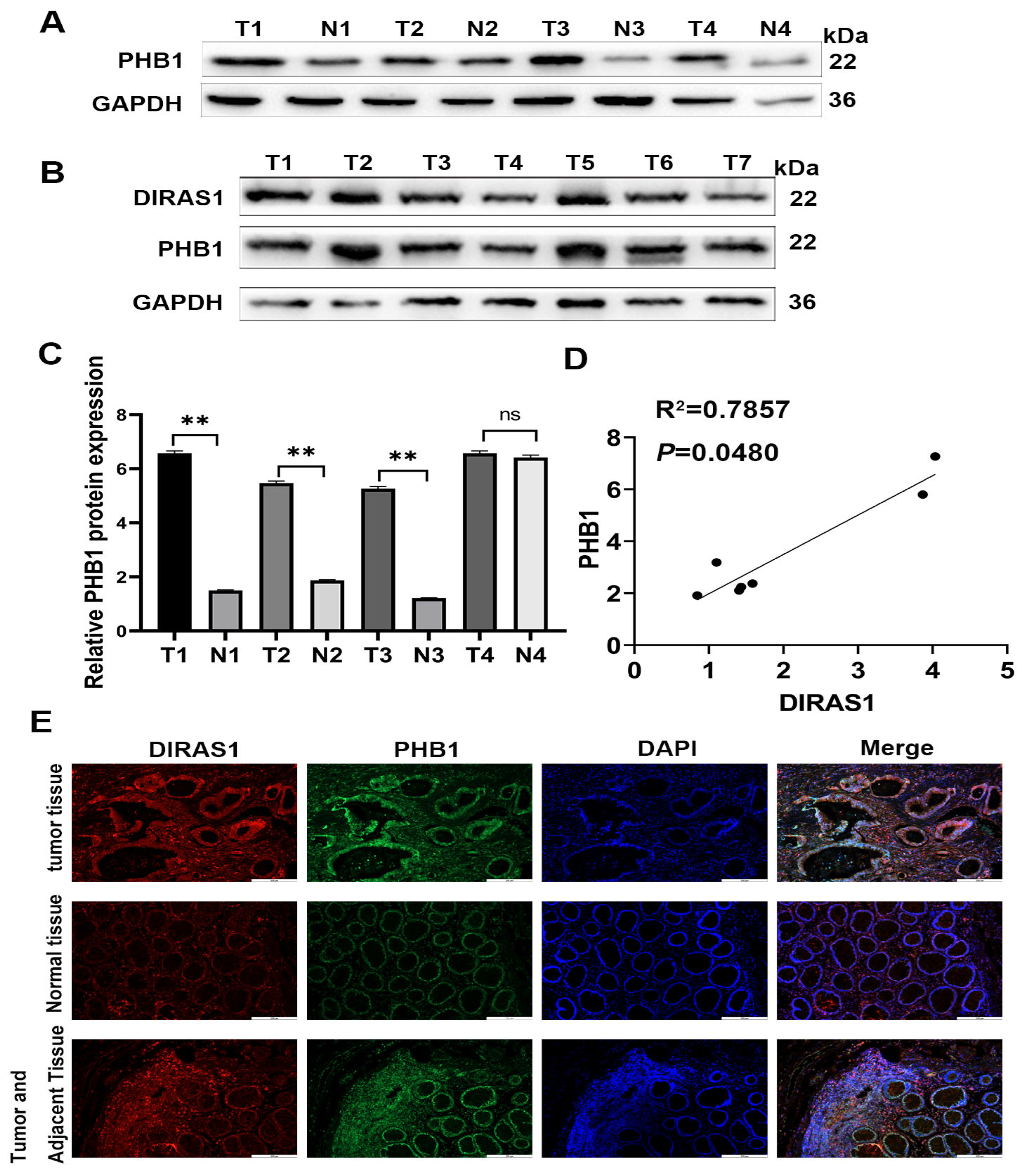
3.1. DIRAS1 Expression Is Significantly Associated with OXA Resistance and Poor Prognosis in CRC Patients
3.2. DIRAS1 Enhances Proliferation and Migration in CRC Cells
3.3. Overexpression of DIRAS1 Promotes OXA Resistance in CRC Cells In Vitro
3.4. DIRAS1 Confers OXA Resistance in a CRC Xenograft Model
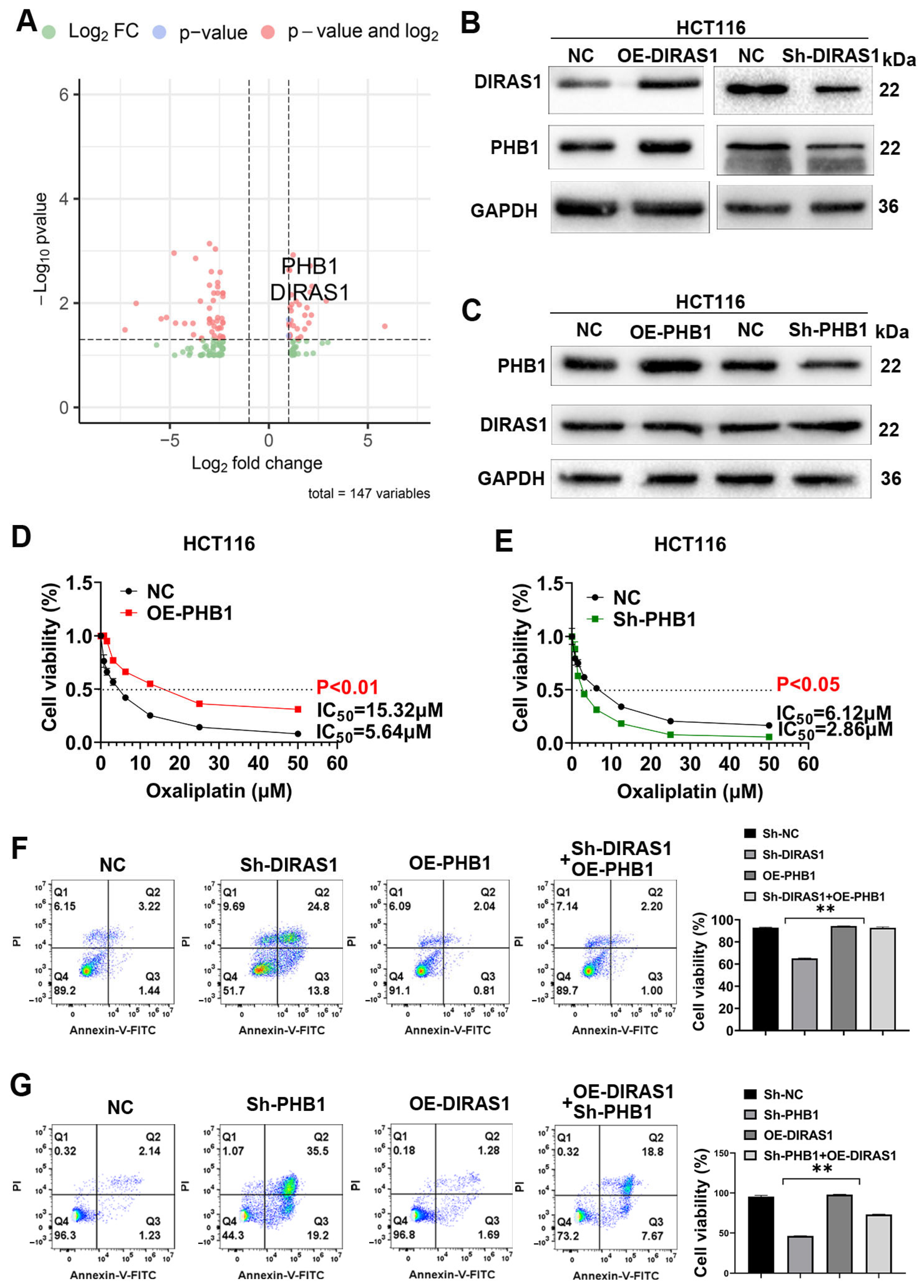
3.5. PHB1 Is a Downstream Target of DIRAS1 That Mediates OXA Resistance
3.6. DIRAS1 Regulates PHB1-Mediated Mitochondrial Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OXA | Oxaliplatin |
| CRC | Colorectal cancer |
| IARC | International Agency for Research on Cancer |
| PHB1 | Prohibitin 1 |
| mtDNA | Mitochondrial DNA |
| WB | Western blotting |
| IHC | Immunohistochemistry |
| mtROS | Mitochondrial ROS |
| mPTP | Mitochondrial permeability transition pore |
| RT | Responder tumor |
| RN | Responder normal |
| NRT | Non-responder tumor |
| NRN | Non-responder normal |
| DEGs | Differentially expressed genes |
| OXPHOS | Oxidative phosphorylation |
| ESCC | Esophageal squamous cell carcinoma |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Abedizadeh, R.; Majidi, F.; Khorasani, H.R.; Abedi, H.; Sabour, D. Colorectal cancer: A comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024, 43, 729–753. [Google Scholar] [CrossRef]
- Yang, L.; Zou, S.; Shu, C.; Song, Y.; Sun, Y.K.; Zhang, W.; Zhou, A.; Yuan, X.; Yang, Y.; Hu, S. CYP2A6 Polymorphisms Associate with Outcomes of S-1 Plus Oxaliplatin Chemotherapy in Chinese Gastric Cancer Patients. Genom. Proteom. Bioinform. 2017, 15, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Yu, M.; Schugar, R.C.; Qian, W.; Tang, F.; Liu, W.; Yang, H.; McDowell, R.E.; Zhao, J.; et al. IL-1 induces mitochondrial translocation of IRAK2 to suppress oxidative metabolism in adipocytes. Nat. Immunol. 2020, 21, 1219–1231. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Wang, J.; Wu, C.; Zhang, L.; Qian, C.; Luo, Y.; Gu, Y.; Wong, W.T.; Xiang, D. A Mitochondria-Targeted Biomimetic Nanomedicine Capable of Reversing Drug Resistance in Colorectal Cancer Through Mitochondrial Dysfunction. Adv. Sci. 2025, 12, e2410630. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, D.; Ge, G.; Cao, N.; Liu, X.; Zhu, N.; Li, F.; Huang, X.; Yu, K.; Zheng, J.; et al. WBP1 regulates mitochondrial function and ferroptosis to modulate chemoresistance in colorectal cancer. Mol. Med. 2025, 31, 93. [Google Scholar] [CrossRef]
- Mahaki, H.; Mansourian, M.; Meshkat, Z.; Avan, A.; Shafiee, M.H.; Mahmoudian, R.A.; Ghorbani, E.; Ferns, G.A.; Manoochehri, H.; Menbari, S.; et al. Nanoparticles Containing Oxaliplatin and the Treatment of Colorectal Cancer. Curr. Pharm. Des. 2023, 29, 3018–3039. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Mathaiyan, J.; Shewade, D.; Dubashi, B.; Sunitha, K. Influence of ABCB-1, ERCC-1 and ERCC-2 gene polymorphisms on response to capecitabine and oxaliplatin (CAPOX) treatment in colorectal cancer (CRC) patients of South India. J. Clin. Pharm. Ther. 2020, 45, 617–627. [Google Scholar] [CrossRef]
- Lin, X.; Xu, L.; Tan, H.; Zhang, X.; Shao, H.; Yao, L.; Huang, X. The potential effects and mechanisms of Gegen Qinlian Decoction in oxaliplatin-resistant colorectal cancer based on network pharmacology. Heliyon 2022, 8, e11305. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Cui, Y.; Zhang, H.; Zhang, S.; Wang, X.; Liu, S.; Gao, Q. Oxaliplatin derived monofunctional triazole-containing platinum(II) complex counteracts oxaliplatin-induced drug resistance in colorectal cancer. Bioorg Chem. 2021, 107, 104636. [Google Scholar] [CrossRef]
- Huang, M.Y.; Huang, Y.J.; Cheng, T.L.; Jhang, W.Y.; Ke, C.C.; Chen, Y.T.; Kuo, S.H.; Lin, I.L.; Huang, Y.H.; Chuang, C.H. XPF-ERCC1 Blocker Improves the Therapeutic Efficacy of 5-FU- and Oxaliplatin-Based Chemoradiotherapy in Colorectal Cancer. Cells 2023, 12, 1475. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Tan, F.; Wang, L.F.; Liu, D.H.; Wang, R.J.; Yin, X.Z. MiR-153-5p promotes sensibility of colorectal cancer cells to oxaliplatin via targeting Bcl-2-mediated autophagy pathway. Biosci. Biotechnol. Biochem. 2020, 84, 1645–1651. [Google Scholar] [CrossRef]
- Morstein, J.; Bowcut, V.; Fernando, M.; Yang, Y.; Zhu, L.; Jenkins, M.L.; Evans, J.T.; Guiley, K.Z.; Peacock, D.M.; Krahnke, S.; et al. Targeting Ras-, Rho-, and Rab-family GTPases via a conserved cryptic pocket. Cell 2024, 187, 6379–6392.e6317. [Google Scholar] [CrossRef] [PubMed]
- Bergom, C.; Hauser, A.D.; Rymaszewski, A.; Gonyo, P.; Prokop, J.W.; Jennings, B.C.; Lawton, A.J.; Frei, A.; Lorimer, E.L.; Aguilera-Barrantes, I.; et al. The Tumor-suppressive Small GTPase DiRas1 Binds the Noncanonical Guanine Nucleotide Exchange Factor SmgGDS and Antagonizes SmgGDS Interactions with Oncogenic Small GTPases. J. Biol. Chem. 2016, 291, 6534–6545, Erratum in J. Biol. Chem. 2016, 291, 10948. https://doi.org/10.1074/jbc.A115.696831. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.C.; Koehn, O.J.; Williams, C.L. SmgGDS: An Emerging Master Regulator of Prenylation and Trafficking by Small GTPases in the Ras and Rho Families. Front. Mol. Biosci. 2021, 8, 685135. [Google Scholar] [CrossRef]
- Sutton, M.N.; Yang, H.; Huang, G.Y.; Fu, C.; Pontikos, M.; Wang, Y.; Mao, W.; Pang, L.; Yang, M.; Liu, J.; et al. RAS-related GTPases DIRAS1 and DIRAS2 induce autophagic cancer cell death and are required for autophagy in murine ovarian cancer cells. Autophagy 2018, 14, 637–653. [Google Scholar] [CrossRef]
- Wielaender, F.; Sarviaho, R.; James, F.; Hytönen, M.K.; Cortez, M.A.; Kluger, G.; Koskinen, L.L.; Arumilli, M.; Kornberg, M.; Bathen-Noethen, A.; et al. Generalized myoclonic epilepsy with photosensitivity in juvenile dogs caused by a defective DIRAS family GTPase 1. Proc. Natl. Acad. Sci. USA 2017, 114, 2669–2674. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ye, L.H.; Zhao, A.Q.; Gao, W.R.; Dai, N.; Yin, Y.; Zhang, X. M6A modification regulates tumor suppressor DIRAS1 expression in cervical cancer cells. Cancer Biol. Ther. 2024, 25, 2306674. [Google Scholar] [CrossRef]
- Zheng, R.; Gao, D.; He, T.; Zhang, M.; Zhang, X.; Linghu, E.; Wei, L.; Guo, M. Methylation of DIRAS1 promotes colorectal cancer progression and may serve as a marker for poor prognosis. Clin. Epigenetics 2017, 9, 50. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Wang, S.; Zheng, X.; Xie, L. RNAa and Vector-Mediated Overexpression of DIRAS1 Suppresses Tumor Growth and Migration in Renal Cell Carcinoma. Mol. Ther. Nucleic Acids 2018, 12, 845–853. [Google Scholar] [CrossRef]
- Verma, S.P.; Agarwal, A.; Das, P. Sodium butyrate induces cell death by autophagy and reactivates a tumor suppressor gene DIRAS1 in renal cell carcinoma cell line UOK146. In Vitro Cell Dev. Biol. Anim. 2018, 54, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Heredia, M.Y.; Chen, X.; Ahmed, M.; Qasim, M.; Callender, T.L.; Hernday, A.D.; Rauceo, J.M. Mitochondrial targeting of Candida albicans SPFH proteins and requirement of stomatins for SDS-induced stress tolerance. Microbiol. Spectr. 2025, 13, e0173324. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Wang, X.; Kim, S.O.; Armstrong, L.C.; Bornstein, P.; Han, J. Metaxin deficiency alters mitochondrial membrane permeability and leads to resistance to TNF-induced cell killing. Protein Cell 2010, 1, 161–173. [Google Scholar] [CrossRef]
- Shimolina, L.; Gulin, A.; Ignatova, N.; Druzhkova, I.; Gubina, M.; Lukina, M.; Snopova, L.; Zagaynova, E.; Kuimova, M.K.; Shirmanova, M. The Role of Plasma Membrane Viscosity in the Response and Resistance of Cancer Cells to Oxaliplatin. Cancers 2021, 13, 6165. [Google Scholar] [CrossRef]
- Xue, X.; You, S.; Zhang, Q.; Wu, Y.; Zou, G.Z.; Wang, P.C.; Zhao, Y.L.; Xu, Y.; Jia, L.; Zhang, X.; et al. Mitaplatin increases sensitivity of tumor cells to cisplatin by inducing mitochondrial dysfunction. Mol. Pharm. 2012, 9, 634–644. [Google Scholar] [CrossRef][Green Version]
- Zampieri, L.X.; Grasso, D.; Bouzin, C.; Brusa, D.; Rossignol, R.; Sonveaux, P. Mitochondria Participate in Chemoresistance to Cisplatin in Human Ovarian Cancer Cells. Mol. Cancer Res. 2020, 18, 1379–1391. [Google Scholar] [CrossRef]
- Ban, T.; Kuroda, K.; Nishigori, M.; Yamashita, K.; Ohta, K.; Koshiba, T. Prohibitin 1 tethers lipid membranes and regulates OPA1-mediated membrane fusion. J. Biol. Chem. 2025, 301, 108076. [Google Scholar] [CrossRef]
- Jung, S.; Park, J.; Ko, K.S. Lipopolysaccharide-induced innate immune responses are exacerbated by Prohibitin 1 deficiency and mitigated by S-adenosylmethionine in murine macrophages. PLoS ONE 2020, 15, e0241224. [Google Scholar] [CrossRef]
- Alula, K.M.; Dowdell, A.S.; LeBere, B.; Lee, J.S.; Levens, C.L.; Kuhn, K.A.; Kaipparettu, B.A.; Thompson, W.E.; Blumberg, R.S.; Colgan, S.P.; et al. Interplay of gut microbiota and host epithelial mitochondrial dysfunction is necessary for the development of spontaneous intestinal inflammation in mice. Microbiome 2023, 11, 256. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; He, P.; Zhuang, H.; Liu, X.; Chen, M.; Zhong, W.; Zhang, Y.; Zhen, C.; Li, Y.; et al. Prohibitin 1 regulates mtDNA release and downstream inflammatory responses. EMBO J. 2022, 41, e111173. [Google Scholar] [CrossRef]
- Kong, B.; Han, C.Y.; Kim, S.I.; Patten, D.A.; Han, Y.; Carmona, E.; Shieh, D.B.; Cheung, A.C.; Mes-Masson, A.M.; Harper, M.E.; et al. Prohibitin 1 interacts with p53 in the regulation of mitochondrial dynamics and chemoresistance in gynecologic cancers. J. Ovarian Res. 2022, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, W.; Jeong, J.H.; Rokavec, M.; Wei, R.; Feng, S.; Schroth, W.; Brauch, H.; Zhong, S.; Luo, J.L. Cytokines-activated nuclear IKKα-FAT10 pathway induces breast cancer tamoxifen-resistance. Sci. China Life Sci. 2024, 67, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Zou, Z.; Cai, K.; Xu, Y.I.; Chen, X.; Yi, W.; Luo, J.; Luo, Z. ARG1 functions as a tumor suppressor in breast cancer. Acta Biochim. Biophys. Sin. 2020, 52, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Y.; Long, M.; Chen, X.; Zhong, S.; Huang, C.; Wei, R.; Luo, J.L. DDX5 Functions as a Tumor Suppressor in Tongue Cancer. Cancers 2023, 15, 5882. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Zhong, S.; Wei, R.; Luo, J.L. Triple-Negative Breast Cancer Intrinsic FTSJ1 Favors Tumor Progression and Attenuates CD8+ T Cell Infiltration. Cancers 2024, 16, 597. [Google Scholar] [CrossRef]
- Qu, M.; Miao, L.; Chen, H.; Zhang, X.; Wang, Y. SKN-1/Nrf2-dependent regulation of mitochondrial homeostasis modulates transgenerational toxicity induced by nanoplastics with different surface charges in Caenorhabditis elegans. J. Hazard. Mater. 2023, 457, 131840. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Freire-Brito, L.; Oliveira, P.F.; Alves, M.G. Evaluation of Human Spermatozoa Mitochondrial Membrane Potential Using the JC-1 Dye. Curr. Protoc. 2022, 2, e531. [Google Scholar] [CrossRef]
- Yadav, P.; Beura, S.K.; Panigrahi, A.R.; Kulkarni, P.P.; Yadav, M.K.; Munshi, A.; Singh, S.K. Lysophosphatidylcholine induces oxidative stress and calcium-mediated cell death in human blood platelets. Cell Biol. Int. 2024, 48, 1266–1284. [Google Scholar] [CrossRef]
- Luo, W.; Zou, Z.; Nie, Y.; Luo, J.; Ming, Z.; Hu, X.; Luo, T.; Ouyang, M.; Liu, M.; Tang, H.; et al. ASS1 inhibits triple-negative breast cancer by regulating PHGDH stability and de novo serine synthesis. Cell Death Dis. 2024, 15, 319. [Google Scholar] [CrossRef]
- Liu, H.; Shu, W.; Liu, T.; Li, Q.; Gong, M. Analysis of the function and mechanism of DIRAS1 in osteosarcoma. Tissue Cell 2022, 76, 101794. [Google Scholar] [CrossRef]
- Jackson, D.N.; Panopoulos, M.; Neumann, W.L.; Turner, K.; Cantarel, B.L.; Thompson-Snipes, L.; Dassopoulos, T.; Feagins, L.A.; Souza, R.F.; Mills, J.C.; et al. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut 2020, 69, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, C.; Huth, T.; Sugiyanto, R.N.; Pusch, S.; Goeppert, B.; Singer, S.; Tabti, R.; Hausser, I.; Schirmacher, P.; Désaubry, L.; et al. Prohibitin, STAT3 and SH2D4A physically and functionally interact in tumor cell mitochondria. Cell Death Dis. 2020, 11, 1023. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Fu, L.; Chen, L.; Qin, Y.R.; Liu, H.; Xie, F.; Zeng, T.; Dong, S.S.; Li, J.; Li, Y.; et al. Downregulation of the novel tumor suppressor DIRAS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Res. 2013, 73, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- García-Torres, D.; Fierke, C.A. The chaperone SmgGDS-607 has a dual role, both activating and inhibiting farnesylation of small GTPases. J. Biol. Chem. 2019, 294, 11793–11804. [Google Scholar] [CrossRef]
- Chen, G.; Gong, T.; Wang, Z.; Wang, Z.; Lin, X.; Chen, S.; Sun, C.; Zhao, W.; Kong, Y.; Ai, H.; et al. Colorectal cancer organoid models uncover oxaliplatin-resistant mechanisms at single cell resolution. Cell Oncol. 2022, 45, 1155–1167. [Google Scholar] [CrossRef]
- Li, Y.; Yan, W.; Qin, Y.; Zhang, L.; Xiao, S. The Anthraquinone Derivative C2 Enhances Oxaliplatin-Induced Cell Death and Triggers Autophagy via the PI3K/AKT/mTOR Pathway. Int. J. Mol. Sci. 2024, 25, 6468. [Google Scholar] [CrossRef] [PubMed]
- García-Chávez, D.; Domínguez-Martín, E.; Kawasaki, L.; Ongay-Larios, L.; Ruelas-Ramírez, H.; Mendoza-Martinez, A.E.; Pardo, J.P.; Funes, S.; Coria, R. Prohibitins, Phb1 and Phb2, function as Atg8 receptors to support yeast mitophagy and also play a negative regulatory role in Atg32 processing. Autophagy 2024, 20, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Wang, Y.K.; Wang, M.Q.; Deng, J.; Gao, N.; Li, M.; Li, Y.P.; Zhang, X.; Jia, X.L.; Liu, X.T.; et al. Prohibitin 1 inhibits cell proliferation and induces apoptosis via the p53-mediated mitochondrial pathway in vitro. World J. Gastrointest. Oncol. 2024, 16, 398–413. [Google Scholar] [CrossRef]
- Qi, A.; Lamont, L.; Liu, E.; Murray, S.D.; Meng, X.; Yang, S. Essential Protein PHB2 and Its Regulatory Mechanisms in Cancer. Cells 2023, 12, 1211. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Wang, S.; Weishaeupl, C.; Wernitznig, A.; Chetta, P.; Pinto, C.; Ho, J.; Dutcher, D.; Gorman, P.N.; Kroe-Barrett, R.; et al. Selective Tumor Cell Apoptosis and Tumor Regression in CDH17-Positive Colorectal Cancer Models using BI 905711, a Novel Liver-Sparing TRAILR2 Agonist. Mol. Cancer Ther. 2021, 20, 96–108. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Z.; Wang, Y.; Chen, Y.; Zhang, H.; Lu, Y.; Xu, L.; Shen, H.; Huang, C.; Zhao, M.; et al. The E3 Ubiquitin Ligase ARIH1 Facilitates Colorectal Cancer Progression by Promoting Oxidative Phosphorylation via the Mitochondrial Translocation of K63-Linked Ubiquitinated PHB1. Adv. Sci. 2025, e2501017. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, J.; Yin, P.; Liang, F. The landscape of mitophagy in sepsis reveals PHB1 as an NLRP3 inflammasome inhibitor. Front. Immunol. 2023, 14, 1188482. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Jiang, L.; Zhang, L.; Xiang, M.; Ren, H. FoxM1 Induced Paclitaxel Resistance via Activation of the FoxM1/PHB1/RAF-MEK-ERK Pathway and Enhancement of the ABCA2 Transporter. Mol. Ther. Oncolytics 2019, 14, 196–212. [Google Scholar] [CrossRef] [PubMed]





| Gene | Sequences |
|---|---|
| DIRAS1-EGFP-F | CGAGCTCAAGCTTCGAATTCTATGCCGGAACAGAGTAACGA |
| DIRAS1-EGFP-R | GGGCGGGATCCGCGGCCGCTTAAGCGTAGTCTGGGACGTCGTATGGGTACATGAGGGTGCATTTGCC |
| DIRAS1-Sh-F | CCGGCCACAAATGTAGCAACCAGAACTCGAGTTCTGGTTGCTACATTTGTGGTTTTTG |
| DIRAS1-Sh-R | AATTCAAAAACCACAAATGTAGCAACCAGAACTCGAGTTCTGGTTGCTACATTTGTGG |
| PHB1-OE-F | CTAGCTAGCGCCACCATGGCTGCCAAAGTGTTTGA |
| PHB1-OE-R | CCGCTCGAGTTAAGCGTAGTCTGGGACGTCGTATGGGTACTGGGGCAGCTGGAGGAGCA |
| PHB1-Sh-F | CCGGTGTCATCTTTGACCGATTCCGCTCGAGCGGAATCGGTCAAAGATGACATTTTTG |
| PHB1-Sh-R | AATTCAAAAATGTCATCTTTGACCGATTCCGCTCGAGCGGAATCGGTCAAAGATGACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; Ouyang, Q.; Wen, J.; Zeng, X.; Xu, Z.; Zhong, S.; Huang, C.; Luo, J.-L. DIRAS1 Drives Oxaliplatin Resistance in Colorectal Cancer via PHB1-Mediated Mitochondrial Homeostasis. Biology 2025, 14, 819. https://doi.org/10.3390/biology14070819
Long M, Ouyang Q, Wen J, Zeng X, Xu Z, Zhong S, Huang C, Luo J-L. DIRAS1 Drives Oxaliplatin Resistance in Colorectal Cancer via PHB1-Mediated Mitochondrial Homeostasis. Biology. 2025; 14(7):819. https://doi.org/10.3390/biology14070819
Chicago/Turabian StyleLong, Min, Qian Ouyang, Jingyi Wen, Xuan Zeng, Zihui Xu, Shangwei Zhong, Changhao Huang, and Jun-Li Luo. 2025. "DIRAS1 Drives Oxaliplatin Resistance in Colorectal Cancer via PHB1-Mediated Mitochondrial Homeostasis" Biology 14, no. 7: 819. https://doi.org/10.3390/biology14070819
APA StyleLong, M., Ouyang, Q., Wen, J., Zeng, X., Xu, Z., Zhong, S., Huang, C., & Luo, J.-L. (2025). DIRAS1 Drives Oxaliplatin Resistance in Colorectal Cancer via PHB1-Mediated Mitochondrial Homeostasis. Biology, 14(7), 819. https://doi.org/10.3390/biology14070819







