ScnR1-Mediated Competitive DNA Binding and Feedback Inhibition Regulate Guvermectin Biosynthesis in Streptomyces caniferus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, Primers, and Growth Conditions
2.2. Gene Overexpression and Disruption
2.3. Detection of Guvermectin
2.4. Isolation of RNA and qRT-PCR Analysis
2.5. Overexpression and Purification of the Three LacI-Family Proteins
2.6. EMSAs
2.7. Prediction of ScnR1 Binding Sites Using AlphaFold 3
2.8. Statistical Analysis
2.9. Bioinformatics Analysis
3. Results
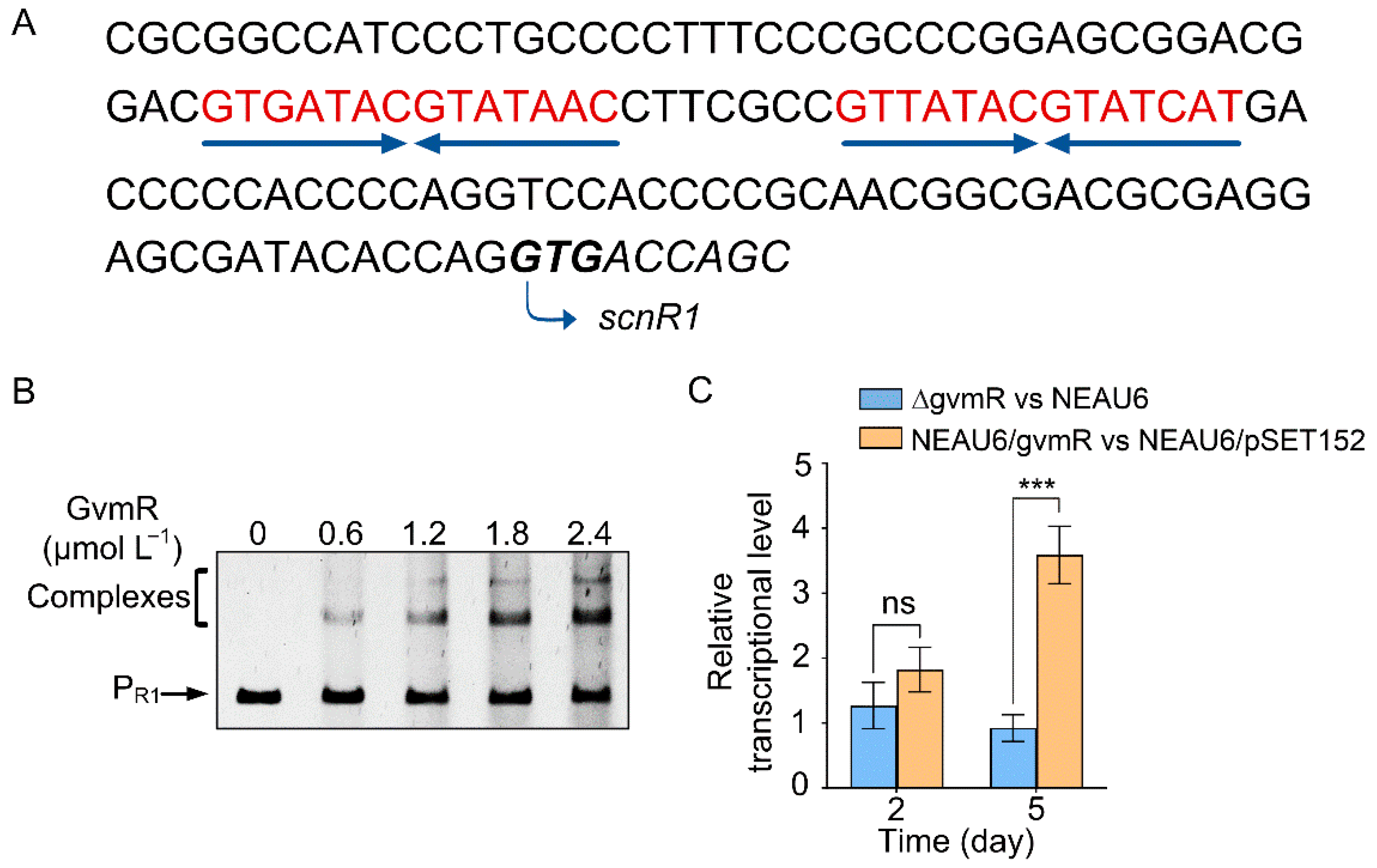
3.1. scnR1, a Highly Conserved LacI-like Regulator, Is a Direct Target of GvmR
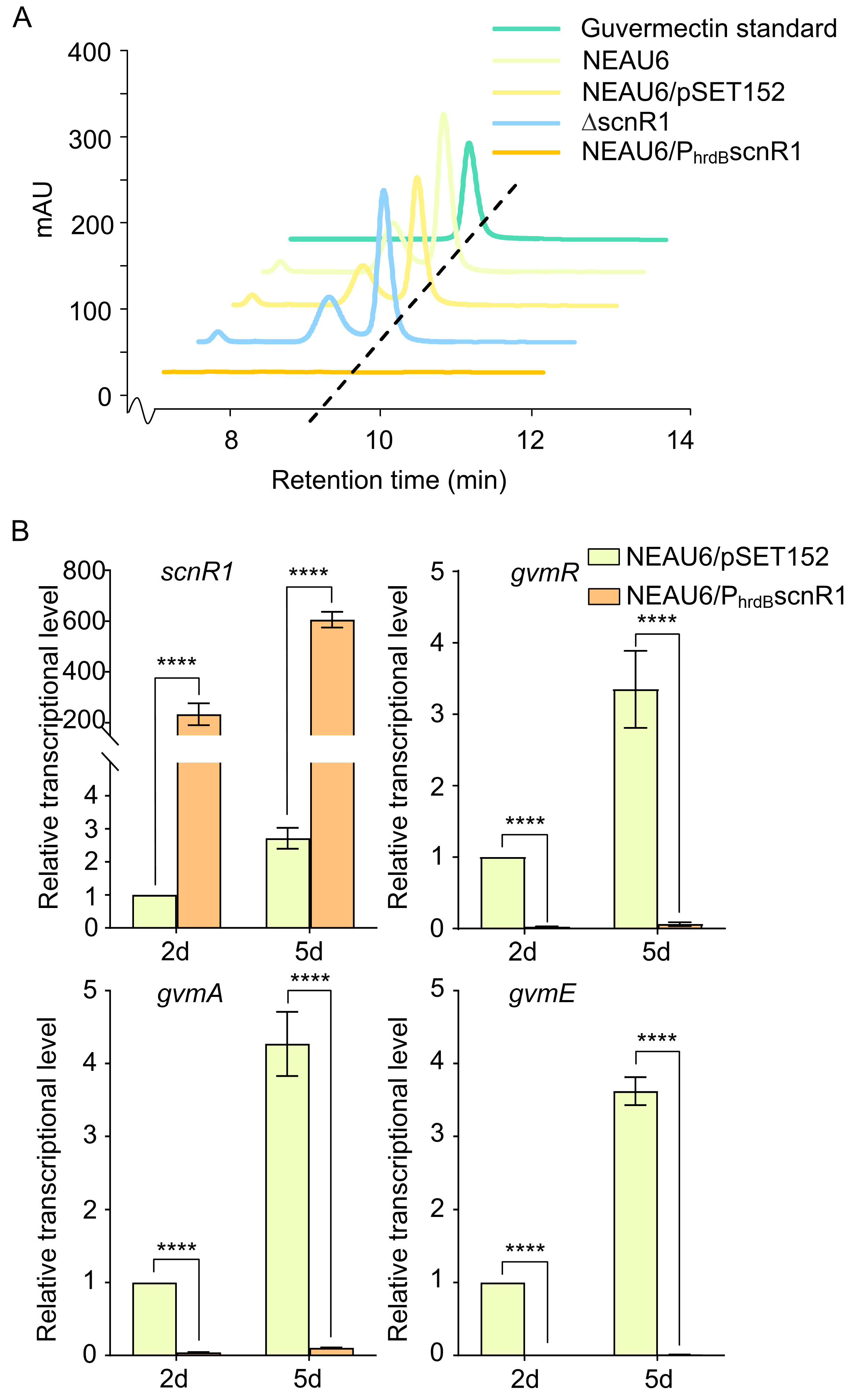
3.2. scnR1 Overexpression Inhibits Guvermectin Production by Suppressing Expression of the Guvermectin BGC
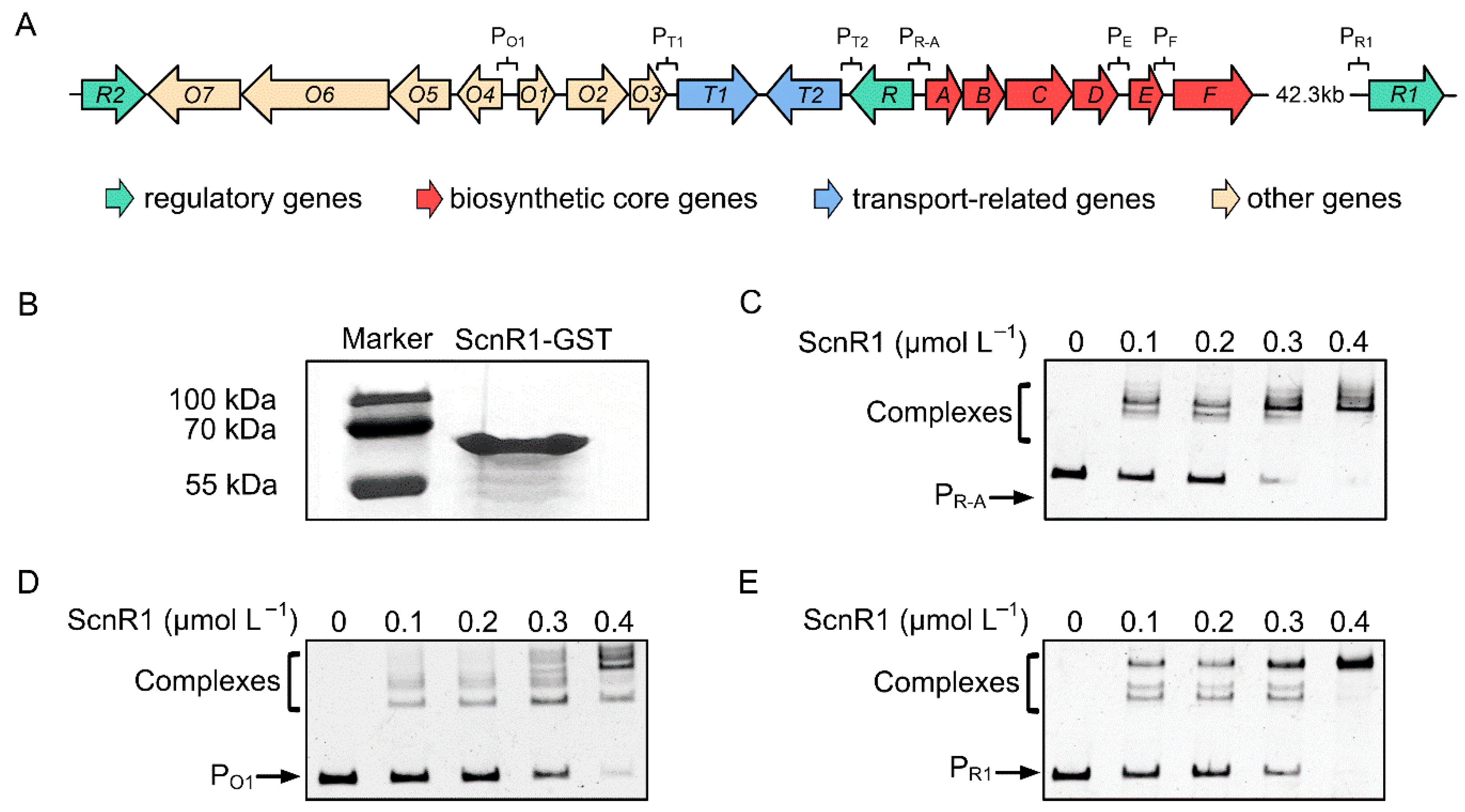
3.3. ScnR1 Binds to the Bidirectional gvmR–gvmA and O1 Promoters
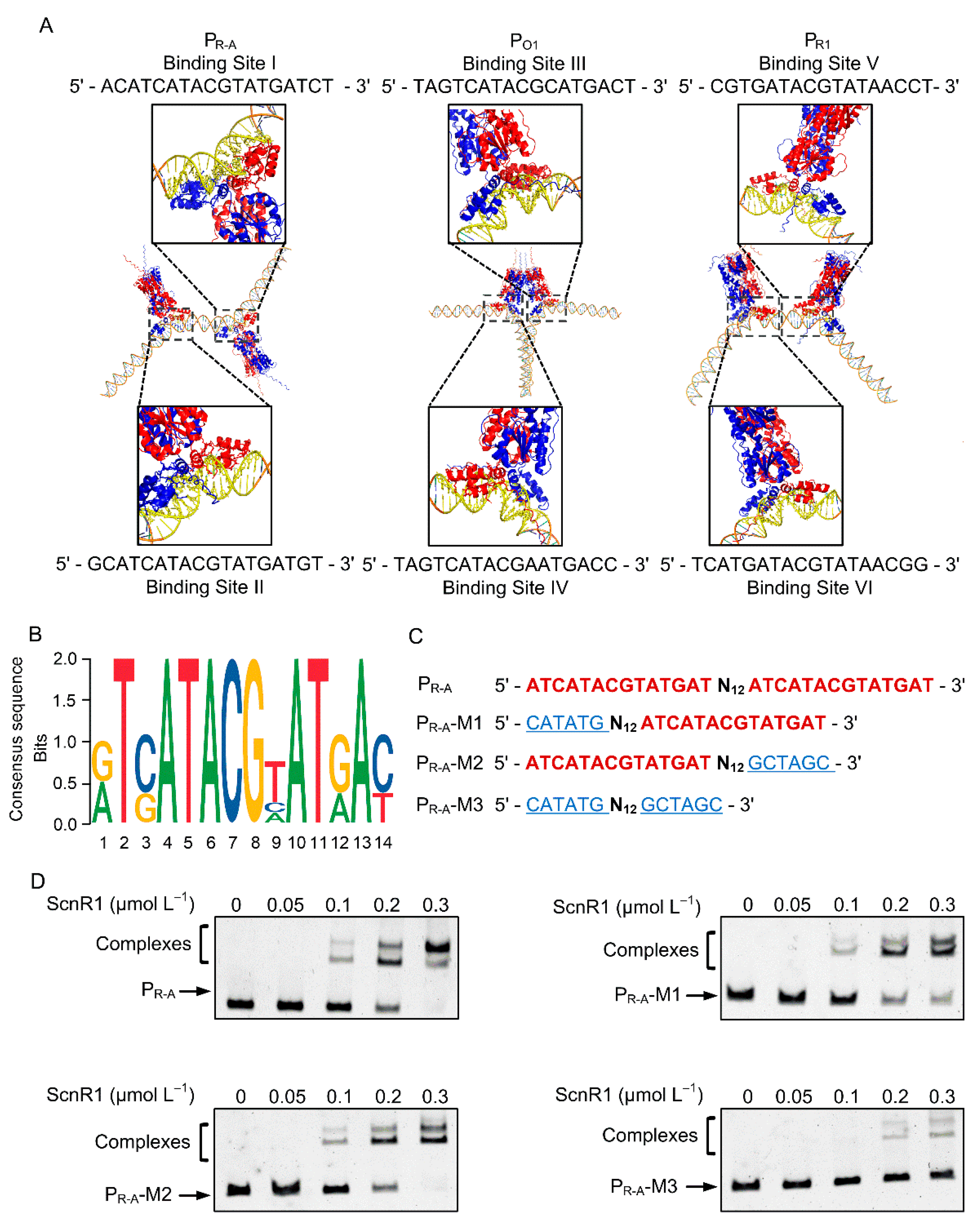
3.4. Identification of ScnR1 Binding Sequences
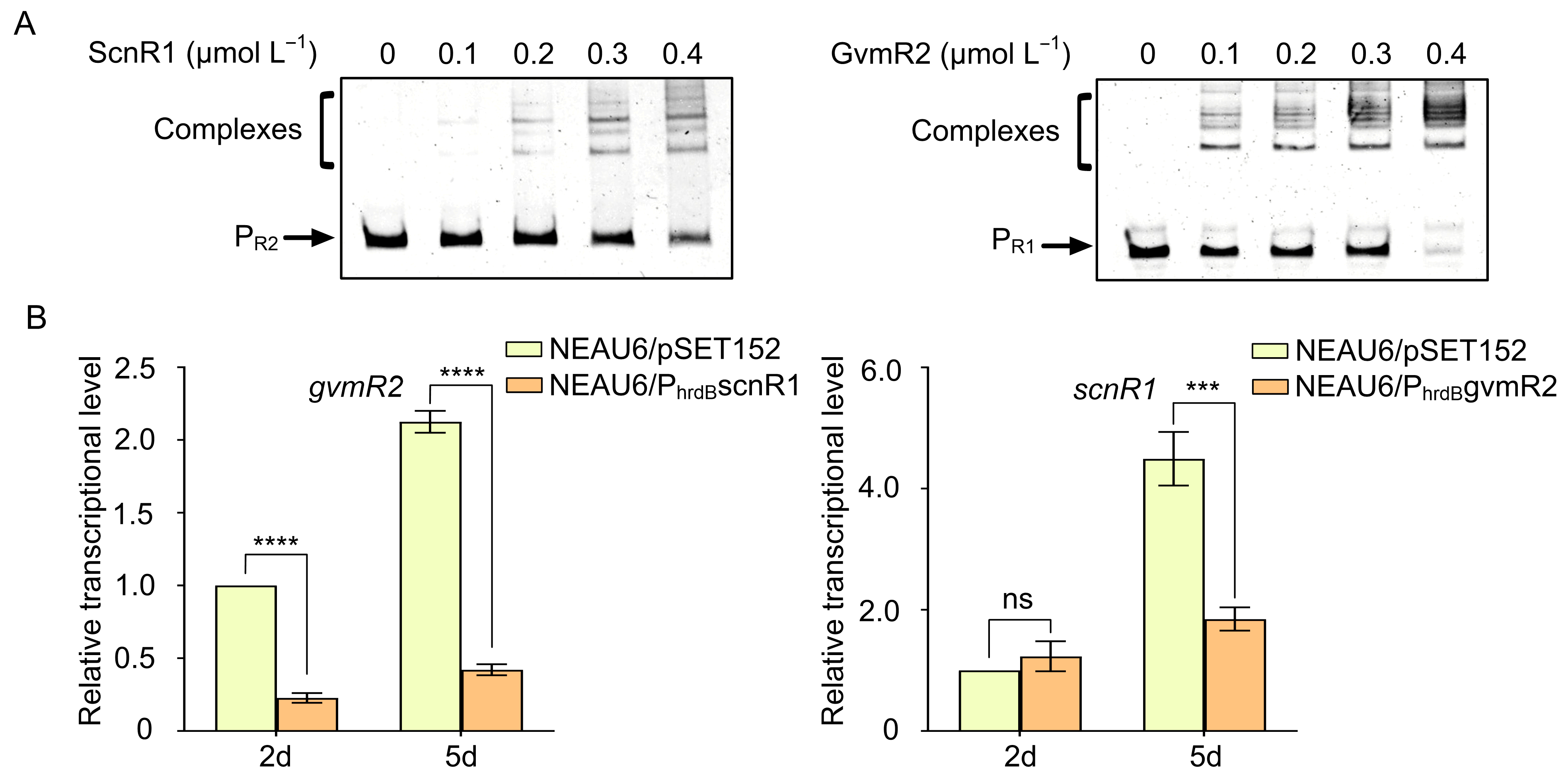
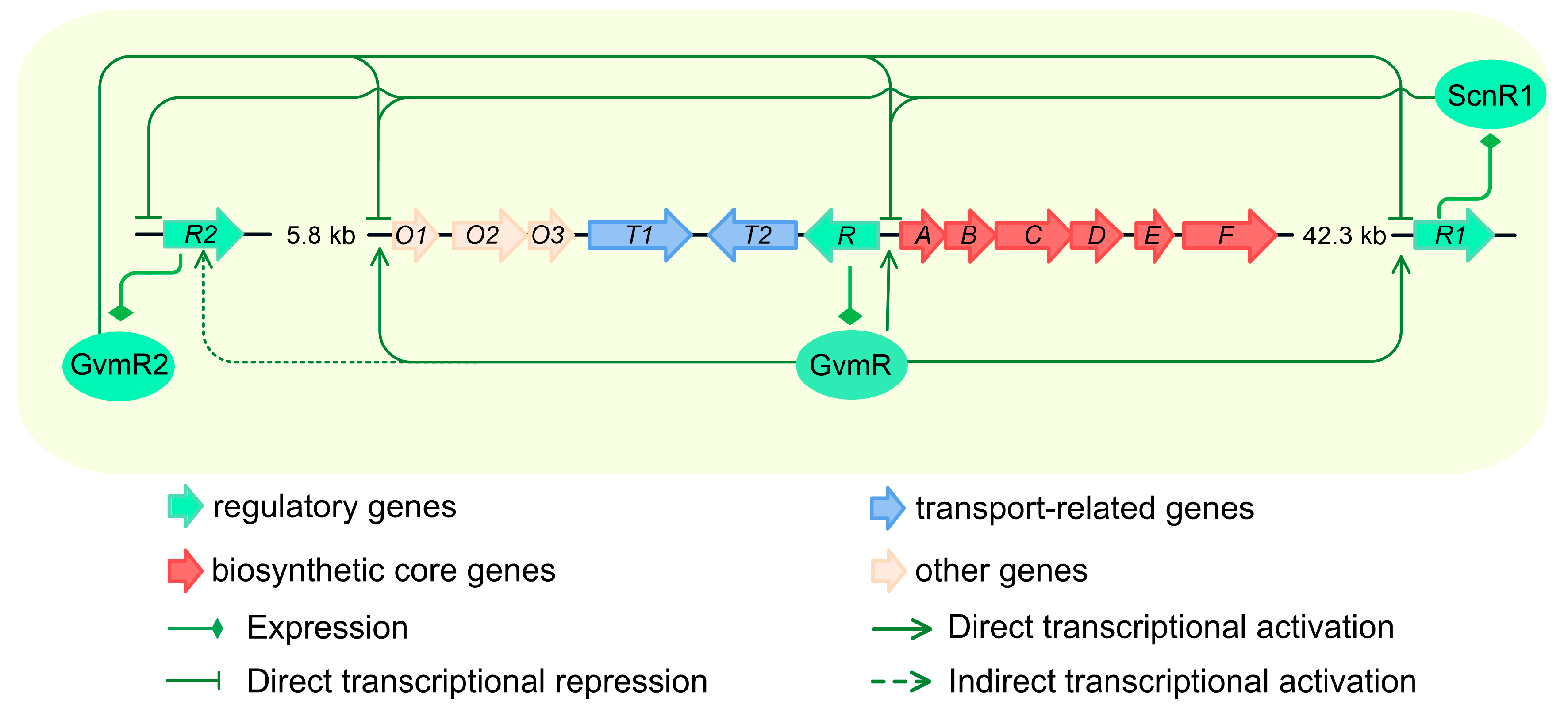
3.5. Reciprocal Transcriptional Repression Between scnR1 and gvmR2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuntsen, H.; Ohkuma, K.; Ishii, Y.; Yonehara, H. Studies on angustmycin. III. J. Antibiot. 1956, 9, 195–201. [Google Scholar]
- Yuntsen, H.; Yonehara, H.; Ui, H. Studies on a new antibiotic, angustmycin. I. J. Antibiot. 1954, 7, 113–115. [Google Scholar]
- Liu, C.; Wang, Z.; Chen, Y.; Yan, Y.; Li, L.; Wang, Y.J.; Bai, L.; Li, S.; Zhang, Y.; Wang, X.; et al. Guvermectin Biosynthesis Revealing the Key Role of a Phosphoribohydrolase and Structural Insight into the Active Glutamate of a Noncanonical Adenine Phosphoribosyltransferase. ACS Chem. Biol. 2023, 18, 102–111. [Google Scholar] [CrossRef]
- Uratani, B.; Lopez, J.M.; Freese, E. Effect of decoyinine on peptidoglycan synthesis and turnover in Bacillus subtilis. J. Bacteriol. 1983, 154, 261–268. [Google Scholar] [CrossRef]
- Liu, C.; Bai, L.; Cao, P.; Li, S.; Huang, S.X.; Wang, J.; Li, L.; Zhang, J.; Zhao, J.; Song, J.; et al. Novel Plant Growth Regulator Guvermectin from Plant Growth-Promoting Rhizobacteria Boosts Biomass and Grain Yield in Rice. J. Agric. Food Chem. 2022, 70, 16229–16240. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Li, C.; Ma, A.; Li, J.; Yang, C.; Chen, X.; Cao, P.; Li, S.; Zhang, Y.; et al. Natural product guvermectin inhibits guanosine 5′-monophosphate synthetase and confers broad-spectrum antibacterial activity. Int. J. Biol. Macromol. 2024, 267, 131510. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, W.; She, Y.; Ma, H.; Cai, Y.S.; Jiang, M.; Deng, Z.; Price, N.P.J.; Chen, W. Efficient biosynthesis of nucleoside cytokinin angustmycin A containing an unusual sugar system. Nat. Commun. 2021, 12, 6633. [Google Scholar] [CrossRef]
- Shi, H.; Wang, J.; Li, S.; Liu, C.; Li, L.; Dong, Z.; Ye, L.; Wang, X.; Zhang, Y.; Xiang, W. Coordinated regulation of two LacI family regulators, GvmR and GvmR2, on guvermectin production in Streptomyces caniferus. Synth. Syst. Biotechnol. 2025, 10, 237–246. [Google Scholar] [CrossRef]
- Zhou, Q.; Ning, S.; Luo, Y. Coordinated regulation for nature products discovery and overproduction in Streptomyces. Synth. Syst. Biotechnol. 2020, 5, 49–58. [Google Scholar] [CrossRef]
- Yan, H.; Lu, X.; Sun, D.; Zhuang, S.; Chen, Q.; Chen, Z.; Li, J.; Wen, Y. BldD, a master developmental repressor, activates antibiotic production in two Streptomyces species. Mol. Microbiol. 2020, 113, 123–142. [Google Scholar] [CrossRef]
- Bibb, M. 1995 Colworth Prize Lecture. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 1996, 142 Pt 6, 1335–1344. [Google Scholar] [CrossRef]
- Wei, J.; He, L.; Niu, G. Regulation of antibiotic biosynthesis in actinomycetes: Perspectives and challenges. Synth. Syst. Biotechnol. 2018, 3, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chater, K.F.; Chandra, G.; Niu, G.; Tan, H. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Y.; Ruan, L.; Yang, S.; Ge, M.; Jiang, W.; Lu, Y. A stepwise increase in pristinamycin II biosynthesis by Streptomyces pristinaespiralis through combinatorial metabolic engineering. Metab. Eng. 2015, 29, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.T.; Li, Y.P.; Xie, H.; Li, J.F.; Lv, Z.Y.; Su, Y.T.; Li, Y.Q. Rational engineering strategies for achieving high-yield, high-quality and high-stability of natural product production in actinomycetes. Metab. Eng. 2021, 67, 198–215. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Found: Norwich, UK, 2000; Volume 291. [Google Scholar]
- Du, D.; Zhu, Y.; Wei, J.; Tian, Y.; Niu, G.; Tan, H. Improvement of gougerotin and nikkomycin production by engineering their biosynthetic gene clusters. Appl. Microbiol. Biotechnol. 2013, 97, 6383–6396. [Google Scholar] [CrossRef]
- Li, C.; He, H.; Wang, J.; Liu, H.; Wang, H.; Zhu, Y.; Wang, X.; Zhang, Y.; Xiang, W. Characterization of a LAL-type regulator NemR in nemadectin biosynthesis and its application for increasing nemadectin production in Streptomyces cyaneogriseus. Sci. China Life Sci. 2019, 62, 394–405. [Google Scholar] [CrossRef]
- Wei, J.; Tian, Y.; Niu, G.; Tan, H. GouR, a TetR family transcriptional regulator, coordinates the biosynthesis and export of gougerotin in Streptomyces graminearus. Appl. Environ. Microbiol. 2014, 80, 714–722. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Delano, W.L. The PyMol Molecular Graphics System. Proteins 2002, 30, 442–454. [Google Scholar]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shi, J.; Molle, V.; Sohlberg, B.; Weaver, D.; Bibb, M.J.; Karoonuthaisiri, N.; Lih, C.J.; Kao, C.M.; Buttner, M.J.; et al. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Mol. Microbiol. 2005, 58, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Kitani, S.; Miyamoto, K.T.; Takamatsu, S.; Herawati, E.; Iguchi, H.; Nishitomi, K.; Uchida, M.; Nagamitsu, T.; Omura, S.; Ikeda, H.; et al. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc. Natl. Acad. Sci. USA 2011, 108, 16410–16415. [Google Scholar] [CrossRef] [PubMed]
- Van Wezel, G.P.; McDowall, K.J. The regulation of the secondary metabolism of Streptomyces: New links and experimental advances. Nat. Prod. Rep. 2011, 28, 1311–1333. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Wang, L.; Tian, X.; Yang, H.; Fan, K.; Yang, K.; Tan, H. “Pseudo” gamma-butyrolactone receptors respond to antibiotic signals to coordinate antibiotic biosynthesis. J. Biol. Chem. 2010, 285, 27440–27448. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, J.; Li, L.; Chen, Z.; Wen, Y.; Li, J. The pathway-specific regulator AveR from Streptomyces avermitilis positively regulates avermectin production while it negatively affects oligomycin biosynthesis. Mol. Genet. Genom. 2010, 283, 123–133. [Google Scholar] [CrossRef]
- Kitani, S.; Ikeda, H.; Sakamoto, T.; Noguchi, S.; Nihira, T. Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis. Appl. Microbiol. Biotechnol. 2009, 82, 1089–1096. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Liu, H.; Wang, H.; Wang, X.; Xiang, W. Characterization of a pathway-specific activator of milbemycin biosynthesis and improved milbemycin production by its overexpression in Streptomyces bingchenggensis. Microb. Cell Fact. 2016, 15, 152. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Robledo-Casados, I.; Sánchez, S. An overview on transcriptional regulators in Streptomyces. Biochim. Biophys. Acta 2015, 1849, 1017–1039. [Google Scholar] [CrossRef]
- Swint-Kruse, L.; Matthews, K.S. Allostery in the LacI/GalR family: Variations on a theme. Curr. Opin. Microbiol. 2009, 12, 129–137. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Bierman, M.; Logan, R.; O’Brien, K.; Seno, E.T.; Rao, R.N.; Schoner, B.E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 1992, 116, 43–49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Wang, J.; Zhang, X.; Zhou, N.; Wang, X.; Xiang, W.; Li, S.; Zhang, Y. ScnR1-Mediated Competitive DNA Binding and Feedback Inhibition Regulate Guvermectin Biosynthesis in Streptomyces caniferus. Biology 2025, 14, 813. https://doi.org/10.3390/biology14070813
Shi H, Wang J, Zhang X, Zhou N, Wang X, Xiang W, Li S, Zhang Y. ScnR1-Mediated Competitive DNA Binding and Feedback Inhibition Regulate Guvermectin Biosynthesis in Streptomyces caniferus. Biology. 2025; 14(7):813. https://doi.org/10.3390/biology14070813
Chicago/Turabian StyleShi, Haoran, Jiabin Wang, Xuedong Zhang, Na Zhou, Xiangjing Wang, Wensheng Xiang, Shanshan Li, and Yanyan Zhang. 2025. "ScnR1-Mediated Competitive DNA Binding and Feedback Inhibition Regulate Guvermectin Biosynthesis in Streptomyces caniferus" Biology 14, no. 7: 813. https://doi.org/10.3390/biology14070813
APA StyleShi, H., Wang, J., Zhang, X., Zhou, N., Wang, X., Xiang, W., Li, S., & Zhang, Y. (2025). ScnR1-Mediated Competitive DNA Binding and Feedback Inhibition Regulate Guvermectin Biosynthesis in Streptomyces caniferus. Biology, 14(7), 813. https://doi.org/10.3390/biology14070813






