Metabolic Responses, Uptake, and Export of Copper in Cyanobacteria
Simple Summary
Abstract
1. Introduction
2. Cu Essentiality, Deficiency, and Toxicity
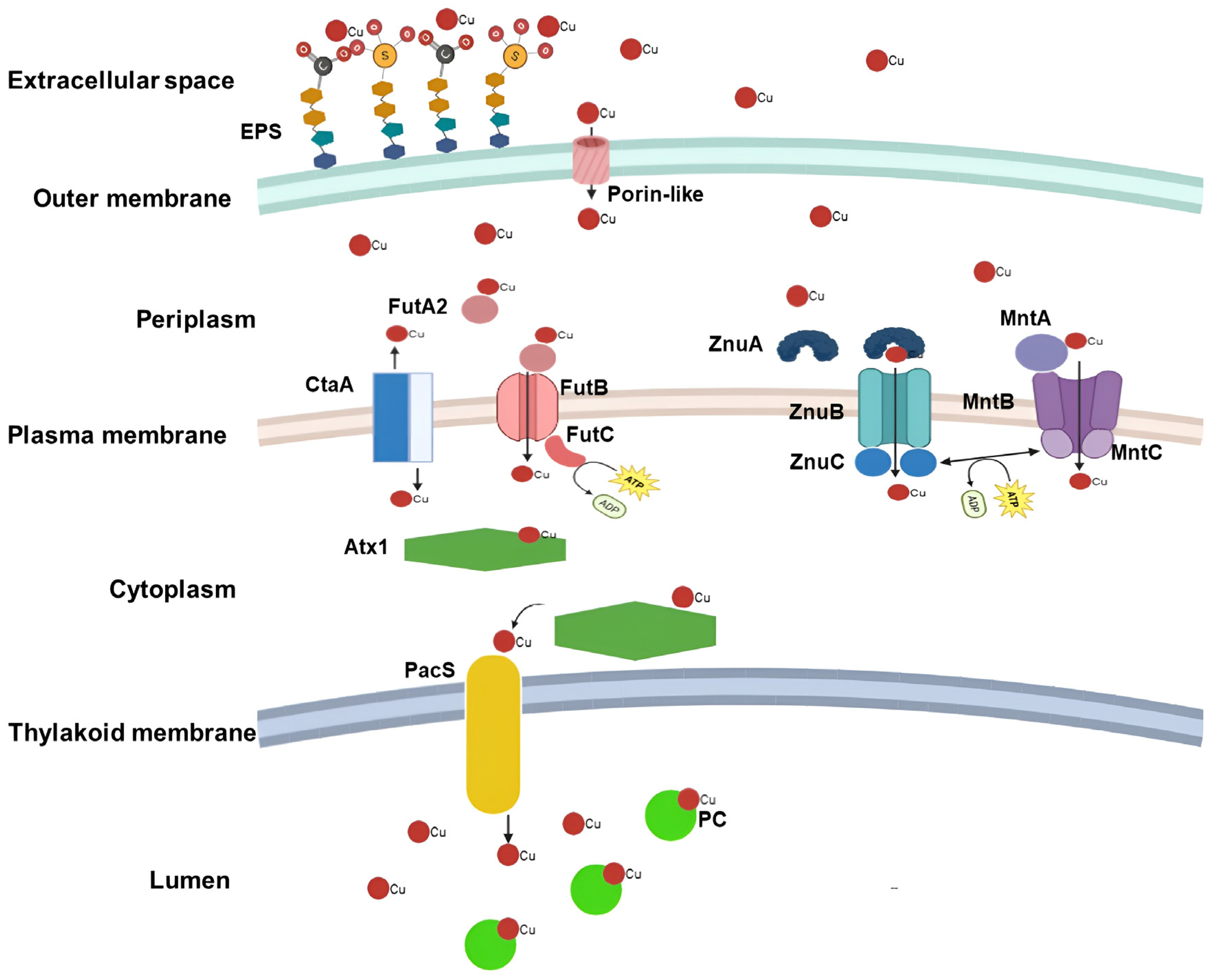
3. Cu Transport: Extracellular to Intracellular Space
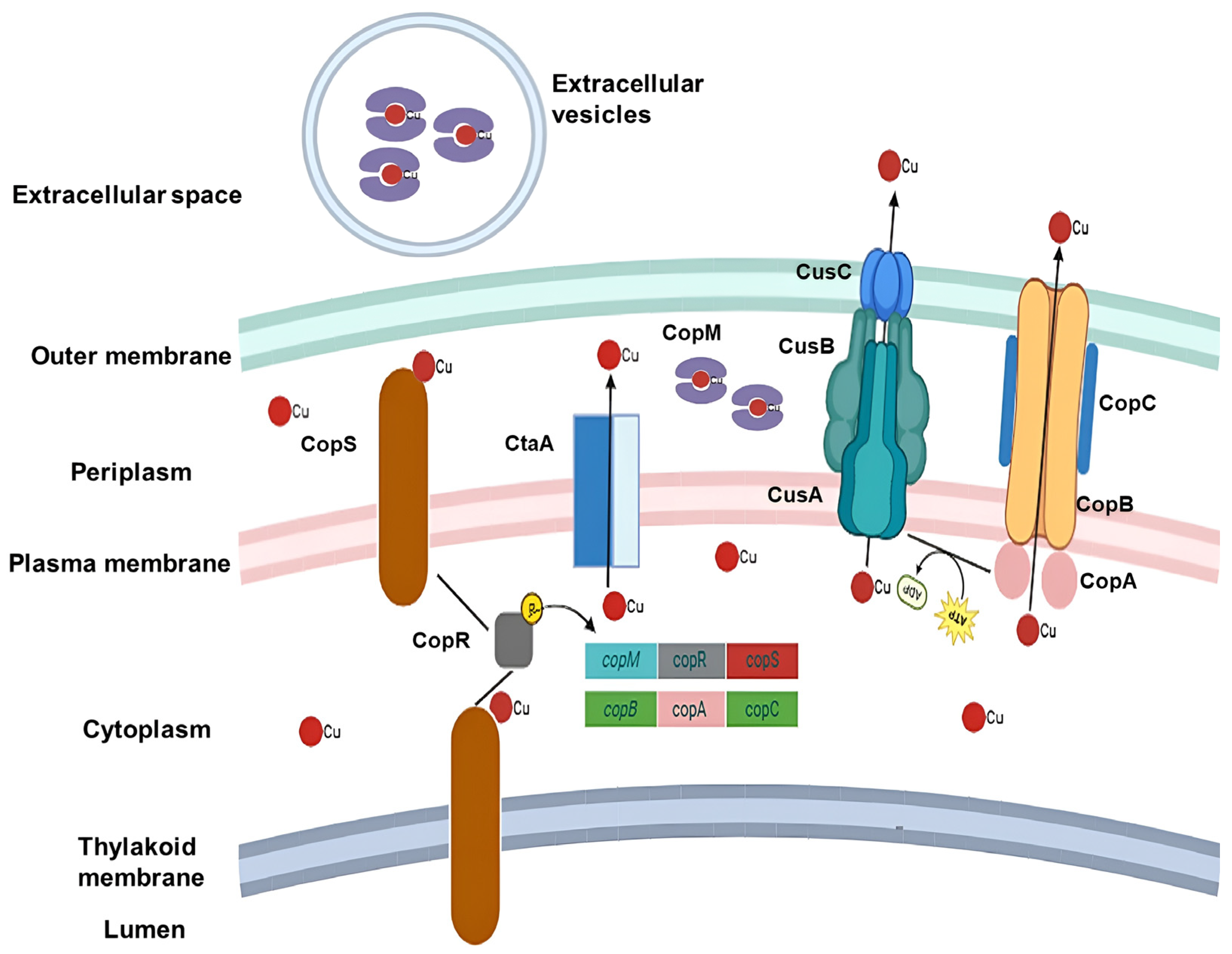
4. Response to Excess Cu
5. Exopolysaccharides (EPS) Interactions
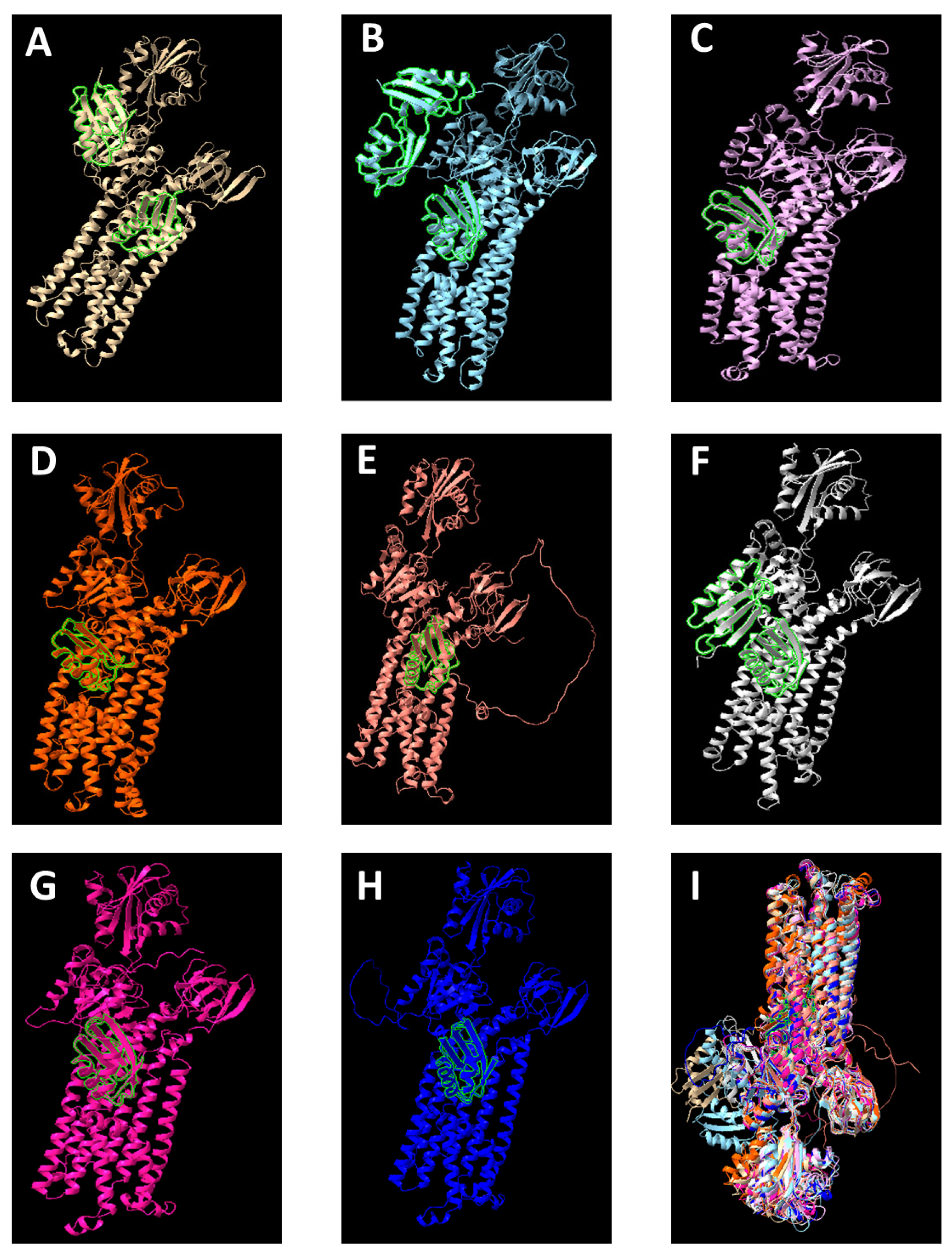
6. Phylogeny and Structure of the CopA Protein
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cd | cadmium |
| CPS | capsular exopolysaccharides |
| Cr | chromium |
| Cu | copper |
| Cyt c6 | cytochrome c6 |
| EPS | exopolysaccharides |
| Fe-S | iron-sulfur |
| Hg | mercury |
| HK | histidine kinase |
| LC50 | lethal concentration 50 |
| Mn | manganese |
| Ni | nickel |
| NCBI | National Center for Biotechnology Information |
| PC | plastocyanin |
| Pb | lead |
| PSI | photosystem I |
| PSII | photosystem II |
| RND | Nodulation-Cell Division |
| ROS | reactive oxygen species |
| RPS | released exopolysaccharides |
| SLH | S-layer Homologous |
| SOD | superoxide dismutase |
| Zn | zinc |
References
- Sinha, R.P.; Häder, D.-P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289. [Google Scholar] [CrossRef]
- De Los Ríos, A.; Grube, M.; Sancho, L.G.; Ascaso, C. Ultrastructural and genetic characteristics of endolithic cyanobacterial biofilms colonizing Antarctic granite rocks. FEMS Microbiol. Ecol. 2007, 59, 386–395. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Samson, S.O.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Versatile applications of cyanobacteria in biotechnology. Microorganisms 2022, 10, 2318. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, C.; Jia, X.; Xu, Y.; Wu, C.; Chen, L.; Wang, F. Characteristics of γ-hexachlorocyclohexane biodegradation by a nitrogen-fixing cyanobacterium, Anabaena azotica. J. Appl. Phycol. 2012, 24, 221–225. [Google Scholar] [CrossRef]
- Cepoi, L.; Donţu, N.; Şalaru, V.; Şalaru, V. Removal of organic pollutants from wastewater by cyanobacteria. In Cyanobacteria for Bioremediation of Wastewaters; Springer: Cham, Switzerland, 2016; pp. 27–43. [Google Scholar]
- Parikh, A.; Madamwar, D. Textile dye decolorization using cyanobacteria. Biotechnol. Lett. 2005, 27, 323–326. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Tiwari, B.S. Cyanotherapeutics: An emerging field for future drug discovery. Appl. Phycol. 2020, 1, 44–57. [Google Scholar] [CrossRef]
- Kalita, N.; Baruah, P.P. Cyanobacteria as a potent platform for heavy metals biosorption: Uptake, responses and removal mechanisms. J. Hazard. Mater. Adv. 2023, 11, 100349. [Google Scholar] [CrossRef]
- Huertas, M.J.; López-Maury, L.; Giner-Lamia, J.; Sánchez-Riego, A.M.; Florencio, F.J. Metals in cyanobacteria: Analysis of the copper, nickel, cobalt and arsenic homeostasis mechanisms. Life 2014, 4, 865–886. [Google Scholar] [CrossRef]
- Chevalier, P.; Proulx, D.; Lessard, P.; Vincent, W.F.; De la Noüe, J. Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J. Appl. Phycol. 2000, 12, 105–112. [Google Scholar] [CrossRef]
- Lynch, F.; Santana-Sánchez, A.; Jämsä, M.; Sivonen, K.; Aro, E.M.; Allahverdiyeva, Y. Screening native isolates of cyanobacteria and a green alga for integrated wastewater treatment, biomass accumulation and neutral lipid production. Algal Res. 2015, 11, 411–420. [Google Scholar] [CrossRef]
- Kabariya, J.H.; Ramani, V.M. Dairy wastewater treatment by cyanobacteria for removal of nutrients with extraction of high value compounds from biomass. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1527–1538. [Google Scholar] [CrossRef]
- Mukherjee, C.; Chowdhury, R.; Sutradhar, T.; Begam, M.; Ghosh, S.M.; Basak, S.K.; Ray, K. Parboiled rice effluent: A wastewater niche for microalgae and cyanobacteria with growth coupled to comprehensive remediation and phosphorus biofertilization. Algal Res. 2016, 19, 225–236. [Google Scholar] [CrossRef]
- Del Valle-Real, M.; Franco-Morgado, M.; García-García, R.; Guardado-Félix, D.; Gutiérrez-Uribe, J.A. Wastewater from maize lime-cooking as growth media for alkaliphilic microalgae–cyanobacteria consortium to reduce chemical oxygen demand and produce biomass with high protein content. Int. J. Food Sci. Technol. 2023, 58, 6775–6783. [Google Scholar] [CrossRef]
- Nagasathya, A.; Thajuddin, N. Decolourization of paper mill effluent using hypersaline cyanobacterium. Sci. Alert 2008, 2, 408–414. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E. Cyanobacteria provide a new paradigm in the regulation of cofactor dependence. Proc. Natl. Acad. Sci. USA 2021, 118, e2100281118. [Google Scholar] [CrossRef]
- Kong, L. Copper requirement and acquisition by marine microalgae. Microorganisms 2022, 10, 1853. [Google Scholar] [CrossRef]
- Boden, J.S.; Konhauser, K.O.; Robbins, L.J.; Sánchez-Baracaldo, P. Timing the evolution of antioxidant enzymes in cyanobacteria. Nat. Commun. 2021, 12, 4742. [Google Scholar] [CrossRef]
- Kalita, N.; Baruah, P.P. Copper removal efficacy and stress tolerance potential of Leptolyngbya sp. GUEco1015. Heliyon 2024, 10, 8. [Google Scholar] [CrossRef]
- National Research Council, Commission on Life Sciences, Board on Environmental Studies; Committee on Copper in Drinking Water. Copper in Drinking Water. 2000. Available online: https://nap.nationalacademies.org/catalog/9782/copper-in-drinking-water (accessed on 17 January 2025).
- Castielli, O.; De la Cerda, B.; Navarro, J.A.; Hervás, M.; De la Rosa, M.A. Proteomic analyses of the response of cyanobacteria to different stress conditions. FEBS Lett. 2009, 583, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- García-Cañas, R.; Giner-Lamia, J.; Florencio, F.J.; López-Maury, L. A protease-mediated mechanism regulates the cytochrome c 6/plastocyanin switch in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2021, 118, e2017898118. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, D.R.; Sanschagrin, M.; Beaudoin, J.; Labbé, S. A novel copper-regulated promoter system for expression of heterologous proteins in Schizosaccharomyces pombe. Gene 2001, 273, 191–198. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishihama, A. Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 2006, 70, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Mager, M.; Pineda Hernandez, H.; Brandenburg, F.; López-Maury, L.; McCormick, A.J.; Nürnberg, D.J.; Orthwein, T.; Russo, D.A.; Victoria, J.A.; Wang, X.; et al. Interlaboratory reproducibility in growth and reporter expression in the cyanobacterium Synechocystis sp. PCC 6803. ACS Synth. Biol. 2023, 12, 1823–1835. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Waterbury, J.B.; Stanier, R.Y. Isolation and growth of cyanobacteria from marine and hypersaline environments. In The prokaryotes: A Handbook on Habitats, Isolation, and Identification of Bacteria; Springer: Berlin/Heidelberg, Germany, 1981; pp. 221–223. [Google Scholar]
- Kotai, J. Instruction of preparations of modified nutrient medium Z8 for algae. Norw. Inst. Water Res. Blind. Oslo. 1972, 15, 32–39. [Google Scholar]
- Zarrouk, C. Contribution a L’etude D’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina mixima. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Ahad, R.I.A.; Syiem, M.B. Copper and cadmium-induced toxicity on the cyanobacterium Nostoc muscorum Meg 1: A comparative study. EurAsian J. Biosci. 2018, 12, 333–345. [Google Scholar]
- Mohy El Din, S. Effect of copper and lead on growth and some metabolic activities of cyanobacterium Spirulina platensis (Nordstedt). Egypt. J. Bot. 2017, 57, 445–456. [Google Scholar] [CrossRef]
- Giner-Lamia, J.; López-Maury, L.; Florencio, F.J. Global transcriptional profiles of the copper responses in the cyanobacterium Synechocystis sp. PCC 6803. PLoS ONE 2014, 9, e108912. [Google Scholar] [CrossRef]
- Wu, Z.X.; Gan, N.Q.; Huang, Q.; Song, L.R. Response of Microcystis to copper stress–do phenotypes of Microcystis make a difference in stress tolerance? Environ. Pollut. 2007, 147, 324–330. [Google Scholar] [CrossRef]
- Wang, H.; Ebenezer, V.; Ki, J. Photosynthetic and biochemical responses of the freshwater green algae Closterium ehrenbergii Meneghini (Conjugatophyceae) exposed to the metal coppers and its implication for toxicity testing. J. Microbiol. 2018, 56, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, D.I.; Duquette, A.; Goodson, A.; Keppler, C.J.; Williams, S.H.; Brock, L.M.; Stackley, K.D.; White, D.; Wilde, S.B. The effects of three chemical algaecides on cell numbers and toxin content of the cyanobacteria Microcystis aeruginosa and Anabaenopsis sp. Environ. Manag. 2014, 54, 1110–1120. [Google Scholar] [CrossRef]
- Coyne, K.J.; Wang, Y.; Johnson, G. Algicidal bacteria: A review of current knowledge and applications to control harmful algal blooms. Front. Microbiol. 2022, 13, 871177. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Kajjumba, G.W.; Ejjada, M.; Masrura, S.U.; Marti, E.J.; Khan, E.; Jones-Lepp, T.L. Recent advancements in the removal of cyanotoxins from water using conventional and modified adsorbents—A contemporary review. Water 2020, 12, 2756. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, W.J.; Ji, B.H. Degradation of cyanotoxin cylindrospermopsin by TiO2-assisted ozonation in water. J. Environ. Sci. Health 2015, 50, 1116–1126. [Google Scholar] [CrossRef]
- Fan, J.; Hobson, P.; Ho, L.; Daly, R.; Brookes, J. The effects of various control and water treatment processes on the membrane integrity and toxin fate of cyanobacteria. J. Hazard. Mater. 2014, 264, 313–322. [Google Scholar] [CrossRef]
- Gijsbertsen-Abrahamse, A.J.; Schmidt, W.; Chorus, I.; Heijman, S.G.J. Removal of cyanotoxins by ultrafiltration and nanofiltration. J. Membr. Sci. 2006, 276, 252–259. [Google Scholar] [CrossRef]
- Verma, S.K.; Singh, S.P. Factors regulating copper uptake in a cyanobacterium. Curr. Microbiol. 1990, 21, 33–37. [Google Scholar] [CrossRef]
- Andrei, A.; Öztürk, Y.; Khalfaoui-Hassani, B.; Rauch, J.; Marckmann, D.; Trasnea, P.I.; Daldal, F.; Koch, H.G. Cu homeostasis in bacteria: The ins and outs. Membranes 2020, 10, 242. [Google Scholar] [CrossRef]
- Kowata, H.; Tochigi, S.; Takahashi, H.; Kojima, S. Outer membrane permeability of cyanobacterium Synechocystis sp. strain PCC 6803: Studies of passive diffusion of small organic nutrients reveal the absence of classical porins and intrinsically low permeability. J. Bacteriol. 2017, 199, 10.1128. [Google Scholar] [CrossRef]
- Kojima, S.; Muramoto, K.; Kusano, T. Outer membrane proteins derived from non-cyanobacterial lineage cover the peptidoglycan of Cyanophora paradoxa cyanelles and serve as a cyanelle diffusion channel. J. Biol. Chem. 2016, 291, 20198–20209. [Google Scholar] [CrossRef] [PubMed]
- Wylie, J.L.; Worobec, E.A. The OprB porin plays a central role in carbohydrate uptake in Pseudomonas aeruginosa. J. Bacteriol. 1995, 177, 3021–3026. [Google Scholar] [CrossRef]
- Schätzle, H.; Brouwer, E.M.; Liebhart, E.; Stevanovic, M.; Schleiff, E. Comparative phenotypic analysis of Anabaena sp. PCC 7120 mutants of porinlike genes. J. Microbiol. Biotechnol. 2021, 31, 645. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Lima, S.; Matinha-Cardoso, J.; Tamagnini, P.; Oliveira, P. The role of outer membrane protein (s) harboring SLH/OprB-domains in extracellular vesicles’ production in Synechocystis sp. PCC 6803. Plants 2021, 10, 2757. [Google Scholar] [CrossRef] [PubMed]
- Coines, J.; Acosta-Gutierrez, S.; Bodrenko, I.; Rovira, C.; Ceccarelli, M. Glucose transport via the pseudomonad porin OprB: Implications for the design of Trojan Horse anti-infectives. Phys. Chem. Chem. Phys. 2019, 21, 8457–8463. [Google Scholar] [CrossRef]
- Pederick, V.G.; Eijkelkamp, B.A.; Begg, S.L.; Ween, M.P.; McAllister, L.J.; Paton, J.C.; McDevitt, C.A. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 13139. [Google Scholar] [CrossRef]
- Cavet, J.S.; Borrelly, G.P.M.; Robinson, N.J. Zn, Cu and Co in cyanobacteria: Selective control of metal availability. FEMS Microbiol. Rev. 2003, 27, 165–181. [Google Scholar] [CrossRef]
- Banerjee, S.; Wei, B.; Bhattacharyya-Pakrasi, M.; Pakrasi, H.B.; Smith, T.J. Structural determinants of metal specificity in the zinc transport protein ZnuA from Synechocystis 6803. J. Mol. Biol. 2003, 333, 1061–1069. [Google Scholar] [CrossRef]
- Yatsunyk, L.A.; Easton, J.A.; Kim, L.R.; Sugarbaker, S.A.; Bennett, B.; Breece, R.M.; Vorontsov, I.I.; Tierney, D.L.; Crowder, M.W.; Rosenzweig, A.C. Structure and metal binding properties of ZnuA, a periplasmic zinc transporter from Escherichia coli. JBIC J. Biol. Inorg. Chem. 2008, 13, 271–288. [Google Scholar] [CrossRef]
- Patzer, S.I.; Hantke, K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 1998, 28, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Hudek, L.; Pearson, L.A.; Michalczyk, A.; Neilan, B.A.; Ackland, M.L. Molecular and cellular characterisation of the zinc uptake (Znu) system of Nostoc punctiforme. FEMS Microbiol. Ecol. 2013, 86, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Bartsevich, V.V.; Pakrasi, H.B. Manganese transport in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 1996, 271, 26057–26061. [Google Scholar] [CrossRef] [PubMed]
- Al-Tameemi, H.; Beavers, W.N.; Norambuena, J.; Skaar, E.P.; Boyd, J.M. Staphylococcus aureus lacking a functional MntABC manganese import system has increased resistance to copper. Mol. Microbiol. 2021, 115, 554–573. [Google Scholar] [CrossRef]
- Lim, K.H.; Jones, C.E.; Vanden Hoven, R.N.; Edwards, J.L.; Falsetta, M.L.; Apicella, M.A.; Jennings, M.P.; McEwan, A.G. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect. Immun. 2008, 76, 3569–3576. [Google Scholar] [CrossRef]
- Giner-Lamia, J.; López-Maury, L.; Reyes, J.C.; Florencio, F.J. The CopRS two-component system is responsible for resistance to copper in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2012, 159, 1806–1818. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, A.C.; Argüello, J.M. Toward a molecular understanding of metal transport by P1B-Type ATPases. In Current topics in Membranes; Academic Press: Cambridge, MA, USA, 2012; pp. 113–136. [Google Scholar]
- Williams, L.E.; Mills, R.F. P1B-ATPases–an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005, 10, 491–502. [Google Scholar] [CrossRef]
- Salustros, N.; Grønberg, C.; Abeyrathna, N.S.; Lyu, P.; Orädd, F.; Wang, K.; Andersson, M.; Meloni, G.; Gourdon, P. Structural basis of ion uptake in copper-transporting P1B-type ATPases. Nat. Commun. 2022, 13, 5121. [Google Scholar] [CrossRef]
- Hederstedt, L.; Lewin, A.; Throne-Holst, M. Heme A synthase enzyme functions dissected by mutagenesis of Bacillus subtilis CtaA. J. Bacteriol. 2005, 187, 8361–8369. [Google Scholar] [CrossRef]
- Raimunda, D.; González-Guerrero, M.; Leeber, B., III; Argüello, J. The transport mechanism of bacterial Cu+-ATPases: Distinct efflux rates adapted to different function. BioMetals 2011, 24, 467–475. [Google Scholar] [CrossRef]
- Phung, L.T.; Ajlani, G.; Haselkorn, R. P-type ATPase from the cyanobacterium Synechococcus 7942 related to the human Menkes and Wilson disease gene products. Proc. Natl. Acad. Sci. USA 1994, 91, 9651–9654. [Google Scholar] [CrossRef]
- Yuan, D.S.; Dancis, A.; Klausner, R.D. Restriction of copper export in Saccharomyces cerevisiae to a late Golgi or post-Golgi compartment in the secretory pathway. J. Biol. Chem. 1997, 272, 25787–25793. [Google Scholar] [CrossRef]
- Zhen, Z.H.; Qin, S.; Ren, Q.M.; Wang, Y.; Ma, Y.Y.; Wang, Y.C. Reciprocal effect of copper and iron regulation on the proteome of Synechocystis sp. PCC 6803. Front. Bioeng. Biotechnol. 2021, 9, 673402. [Google Scholar] [CrossRef]
- Katoh, H.; Hagino, N.; Ogawa, T. Iron-binding activity of FutA1 subunit of an ABC-type iron transporter in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 2001, 42, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Waldron, K.J.; Tottey, S.; Yanagisawa, S.; Dennison, C.; Robinson, N.J. A periplasmic iron-binding protein contributes toward inward copper supply. J. Biol. Chem. 2007, 282, 3837–3846. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Hahn, A.; Nicolaisen, K.; Mirus, O.; Schleiff, E. The components of the putative iron transport system in the cyanobacterium Anabaena sp. PCC 7120. Environ. Microbiol. 2012, 14, 1655–1670. [Google Scholar] [CrossRef]
- Aliaga, M.E.; López-Alarcón, C.; Bridi, R.; Speisky, H. Redox-implications associated with the formation of complexes between copper ions and reduced or oxidized glutathione. J. Inorg. Biochem. 2016, 154, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, A.; Khatua, K.; Shanbhag, V.; Comba, P.; Datta, A. Cu2+ selective chelators relieve copper-induced oxidative stress in vivo. Chem. Sci. 2018, 9, 7916–7930. [Google Scholar] [CrossRef]
- Markossian, K.A.; Kurganov, B.I. Copper chaperones, intracellular copper trafficking proteins. Function, structure, and mechanism of action. Biochemistry 2003, 68, 827–837. [Google Scholar] [CrossRef]
- Arnesano, F.; Banci, L.; Bertini, I.; Huffman, D.L.; O’Halloran, T.V. Solution structure of the Cu(I) and apo forms of the yeast metallochaperone, Atx1. Biochemistry 2001, 40, 1528–1539. [Google Scholar] [CrossRef]
- Hu, Q.; Sitsel, O.; Bågenholm, V.; Grønberg, C.; Lyu, P.; Pii Svane, A.S.; Andersen, K.R.; Laursen, N.S.; Meloni, G.; Nissen, P.; et al. Transition metal transporting P-type ATPases: Terminal metal-binding domains serve as sensors for autoinhibitory tails. FEBS J. 2024, 292, 1654–1674. [Google Scholar] [CrossRef] [PubMed]
- Gounder, P.E.; Davamani, V.; Kalaiselvi, P.; Sebastian, S.P.; Ilakiya, T. Metallothioneins: Diverse Protein Family to Bind Metallic Ions. In Heavy Metals—Their Environmental Impacts and Mitigation; IntechOpen: London, UK, 2021. [Google Scholar]
- Bourdineaud, J.P.; Baudrimont, M.; Gonzalez, P.; Moreau, J.L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. [Google Scholar] [CrossRef]
- Dziegiel, P.; Pula, B.; Kobierzycki, C.; Stasiolek, M.; Podhorska-Okolow, M.; Podhorska-Okolow, M. Metallothioneins: Structure and functions. Met. Norm. Cancer Cells 2016, 3, 20. [Google Scholar]
- Robinson, N.J.; Wilson, J.R.; Turner, J.S. Expression of the type 2 metallothionein-like gene MT2 from Arabidopsis thaliana in Zn2+-metallothionein-deficient Synechococcus PCC 7942: Putative role for MT2 in Zn2+ metabolism. Plant Mol. Biol. 1996, 30, 1169–1179. [Google Scholar] [CrossRef]
- Munson, G.P.; Lam, D.L.; Outten, F.W.; O’Halloran, T.V. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J. Bacteriol. 2000, 182, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Rouch, D.A.; Brown, N.L. Copper-inducible transcriptional regulation at two promoters in the Escherichia coli copper resistance determinant pco. Microbiology 1997, 143, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Giner-Lamia, J.; Pereira, S.B.; Bovea-Marco, M.; Futschik, M.E.; Tamagnini, P.; Oliveira, P. Extracellular proteins: Novel key components of metal resistance in cyanobacteria? Front. Microbiol. 2016, 7, 878. [Google Scholar] [CrossRef]
- Gittins, J.R. Cloning of a copper resistance gene cluster from the cyanobacterium Synechocystis sp. PCC 6803 by recombineering recovery. FEBS Lett. 2015, 589, 1872–1878. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, X.; Niu, G.; Dong, W.; Wang, J.; Fang, Y.; Lin, Y.; Liu, L. Structural basis for copper/silver binding by the Synechocystis metallochaperone CopM. Biol. Crystallogr. 2016, 72, 997–1005. [Google Scholar]
- Yang, J.; Gao, M.; Wang, J.; He, C.; Wang, X.; Liu, L. Structural basis of copper binding by a dimeric periplasmic protein forming a six-helical bundle. J. Inorg. Biochem. 2022, 229, 111728. [Google Scholar] [CrossRef]
- Lima, S.; Matinha-Cardoso, J.; Giner-Lamia, J.; Couto, N.; Pacheco, C.C.; Florencio, F.J.; Wright, P.C.; Tamagnini, P.; Oliveira, P. Extracellular vesicles as an alternative copper-secretion mechanism in bacteria. J. Hazard. Mater. 2022, 431, 128594. [Google Scholar] [CrossRef]
- Lawton, T.J.; Kenney, G.E.; Hurley, J.D.; Rosenzweig, A.C. The CopC family: Structural and bioinformatic insights into a diverse group of periplasmic copper binding proteins. Biochemistry 2016, 55, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Erill, I.; Cusick, K.D. Linking Copper-Associated Signal Transduction Systems with Their Environment in Marine Bacteria. Microorganisms 2023, 11, 1012. [Google Scholar] [CrossRef] [PubMed]
- Rensing, C.; Fan, B.; Sharma, R.; Mitra, B.; Rosen, B.P. CopA: An Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 2000, 97, 652–656. [Google Scholar] [CrossRef]
- Gómez-Santos, N.; Pérez, J.; Sánchez-Sutil, M.C.; Moraleda-Muñoz, A.; Muñoz-Dorado, J. CorE from Myxococcus xanthus is a copper-dependent RNA polymerase sigma factor. PLoS Genet. 2011, 7, e1002106. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Cooksey, D.A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919. [Google Scholar] [CrossRef]
- Cha, J.S.; Cooksey, D.A. Copper hypersensitivity and uptake in Pseudomonas syringae containing cloned components of the copper resistance operon. Appl. Environ. Microbiol. 1993, 59, 1671–1674. [Google Scholar] [CrossRef]
- Olivan-Muro, I.; Sarasa-Buisan, C.; Guio, J.; Arenas, J.; Sevilla, E.; Fillat, M.F. Unbalancing Zur (FurB)-mediated homeostasis in Anabaena sp. PCC7120: Consequences on metal trafficking, heterocyst development and biofilm formation. Environ. Microbiol. 2023, 25, 2142–2162. [Google Scholar] [CrossRef]
- Behlau, F.; Canteros, B.I.; Minsavage, G.V.; Jones, J.B.; Graham, J.H. Molecular characterization of copper resistance genes from Xanthomonas citri subsp. citri and Xanthomonas alfalfae subsp. citrumelonis. Appl. Environ. Microbiol. 2011, 77, 4089–4096. [Google Scholar]
- Mills, S.D.; Jasalavich, C.A.; Cooksey, D.A. A two-component regulatory system required for copper-inducible expression of the copper resistance operon of Pseudomonas syringae. J. Bacteriol. 1993, 175, 1656–1664. [Google Scholar] [CrossRef]
- Franke, S.; Grass, G.; Rensing, C.; Nies, D.H. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 2003, 185, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Long, F.; Zimmermann, M.T.; Rajashankar, K.R.; Jernigan, R.L.; Yu, E.W. Crystal structure of the CusBA heavy-metal efflux complex of Escherichia coli. Nature 2011, 470, 558–562. [Google Scholar] [CrossRef]
- Hahn, A.; Stevanovic, M.; Mirus, O.; Lytvynenko, I.; Pos, K.M.; Schleiff, E. The outer membrane TolC-like channel HgdD is part of tripartite resistance-nodulation-cell division (RND) efflux systems conferring multiple-drug resistance in the cyanobacterium Anabaena sp. PCC7120. J. Biol. Chem. 2013, 288, 31192–31205. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, K.; Hahn, A.; Valdebenito, M.; Moslavac, S.; Samborski, A.; Maldener, I.; Wilken, C.; Valladares, A.; Flores, E.; Hantke, K.; et al. The interplay between siderophore secretion and coupled iron and copper transport in the heterocyst-forming cyanobacterium Anabaena sp. PCC 7120. Biochim. Et. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 2131–2140. [Google Scholar] [CrossRef]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from cyanobacteria: Strategies for bioprocess development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Muthuraj, M.; Bhunia, B.; Bandyopadhyay, T.K.; Annapurna, K.; Sahu, M.; Indrama, T. Biosynthesis, purification and structure-property relationships of new cyanobacterial exopolysaccharides. Polym. Test. 2020, 89, 106592. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Nogueira, P.F.M.; Melão, M.G.G.; Lombardi, A.T.; Nogueira, M.M.; Vieira, A.A.H. The effects of Anabaena spiroides exopolysaccharides on copper accumulation in an aquatic food chain. Aquat. Toxicol. 2009, 93, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Peng, L.; Chen, A.; Zeng, Q.; Luo, S.; Shao, J. Enhancement mechanisms of copper (II) adsorption onto kaolinite by extracellular polymeric substances of Microcystis aeruginosa (cyanobacterium). Int. Biodeterior. Biodegrad. 2019, 138, 8–14. [Google Scholar] [CrossRef]
- Paperi, R.; Micheletti, E.; De Philippis, R. Optimization of copper sorbing–desorbing cycles with confined cultures of the exopolysaccharide-producing cyanobacterium Cyanospira capsulata. J. Appl. Microbiol. 2006, 101, 1351–1356. [Google Scholar] [CrossRef]
- Ciani, M.; Decorosi, F.; Ratti, C.; De Philippis, R.; Adessi, A. Semi-continuous cultivation of EPS-producing marine cyanobacteria: A green biotechnology to remove dissolved metals obtaining metal-organic materials. New Biotechnol. 2024, 82, 33–42. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Pagli, C.; Chamizo, S.; Migliore, G.; Rugnini, L.; De Giudici, G.; Braglia, R.; Canini, A.; Cantón, Y. Isolation of biocrust cyanobacteria and evaluation of Cu, Pb, and Zn immobilisation potential for soil restoration and sustainable agriculture. Sci. Total Environ. 2024, 946, 174020. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Diwan, B. Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Asthana, R.K.; Kayastha, A.M.; Pandey, S.; Chaudhary, A.K.; Singh, S.P. Thiol and exopolysaccharide production in a cyanobacterium under heavy metal stress. Process Biochem. 1999, 35, 63–68. [Google Scholar] [CrossRef]
- De Philippis, R.; Paperi, R.; Sili, C. Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation 2007, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.P.; Hurd, C.L.; Sander, S.G.; Armstrong, E.; Fernández, P.A.; Suhrhoff, T.J.; Roleda, M.Y. Copper pollution exacerbates the effects of ocean acidification and warming on kelp microscopic early life stages. Sci. Rep. 2018, 8, 14763. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard. Mater. 2008, 151, 185–193. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oder, J.C.; Sateles, T.E.; de Souza, L.B.; Nunes-Nesi, A.; Araújo, W.L.; Alvarenga-Lucius, L. Metabolic Responses, Uptake, and Export of Copper in Cyanobacteria. Biology 2025, 14, 798. https://doi.org/10.3390/biology14070798
Oder JC, Sateles TE, de Souza LB, Nunes-Nesi A, Araújo WL, Alvarenga-Lucius L. Metabolic Responses, Uptake, and Export of Copper in Cyanobacteria. Biology. 2025; 14(7):798. https://doi.org/10.3390/biology14070798
Chicago/Turabian StyleOder, Jean Coutinho, Thamires Emidio Sateles, Laila Barros de Souza, Adriano Nunes-Nesi, Wagner L. Araújo, and Luna Alvarenga-Lucius. 2025. "Metabolic Responses, Uptake, and Export of Copper in Cyanobacteria" Biology 14, no. 7: 798. https://doi.org/10.3390/biology14070798
APA StyleOder, J. C., Sateles, T. E., de Souza, L. B., Nunes-Nesi, A., Araújo, W. L., & Alvarenga-Lucius, L. (2025). Metabolic Responses, Uptake, and Export of Copper in Cyanobacteria. Biology, 14(7), 798. https://doi.org/10.3390/biology14070798





