An Exploratory Investigation of Heart Rate Variability in Response to Exercise Training and Detraining in Young and Middle-Aged Men
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
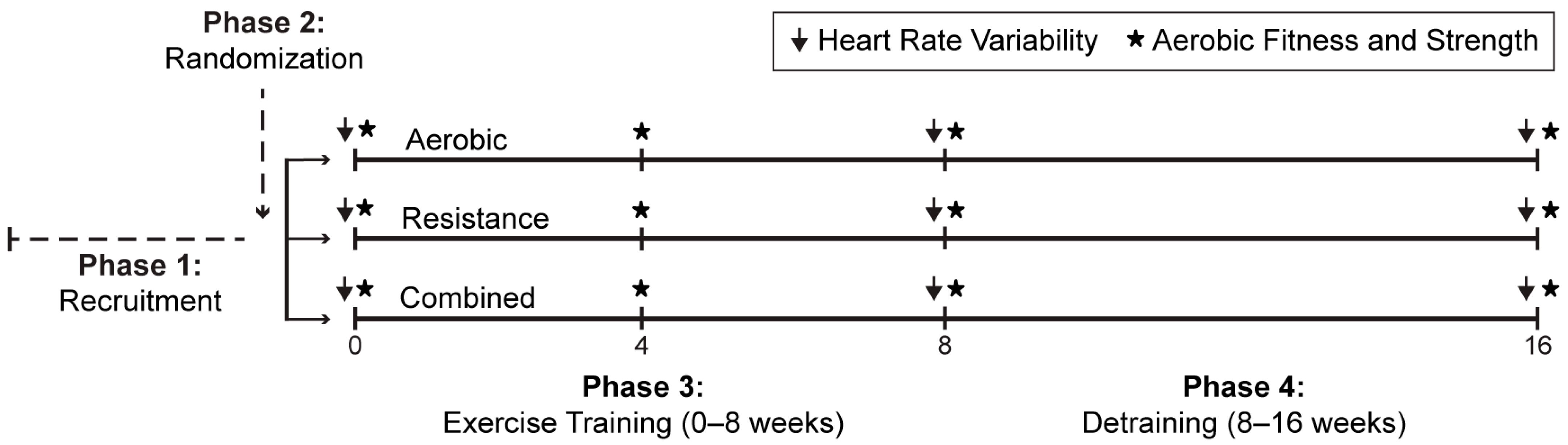
2.2. Study Design
2.3. Outcome Measurements
2.4. Exercise Training and Detraining Protocol
2.5. Statistical Analysis
3. Results
3.1. Age-Related Differences in Participant Characteristics, Peak Volume of Oxygen Consumption, and Muscular Strength
3.2. Comparison of Heart Rate and Heart Rate Variability Between Exercise Modalities
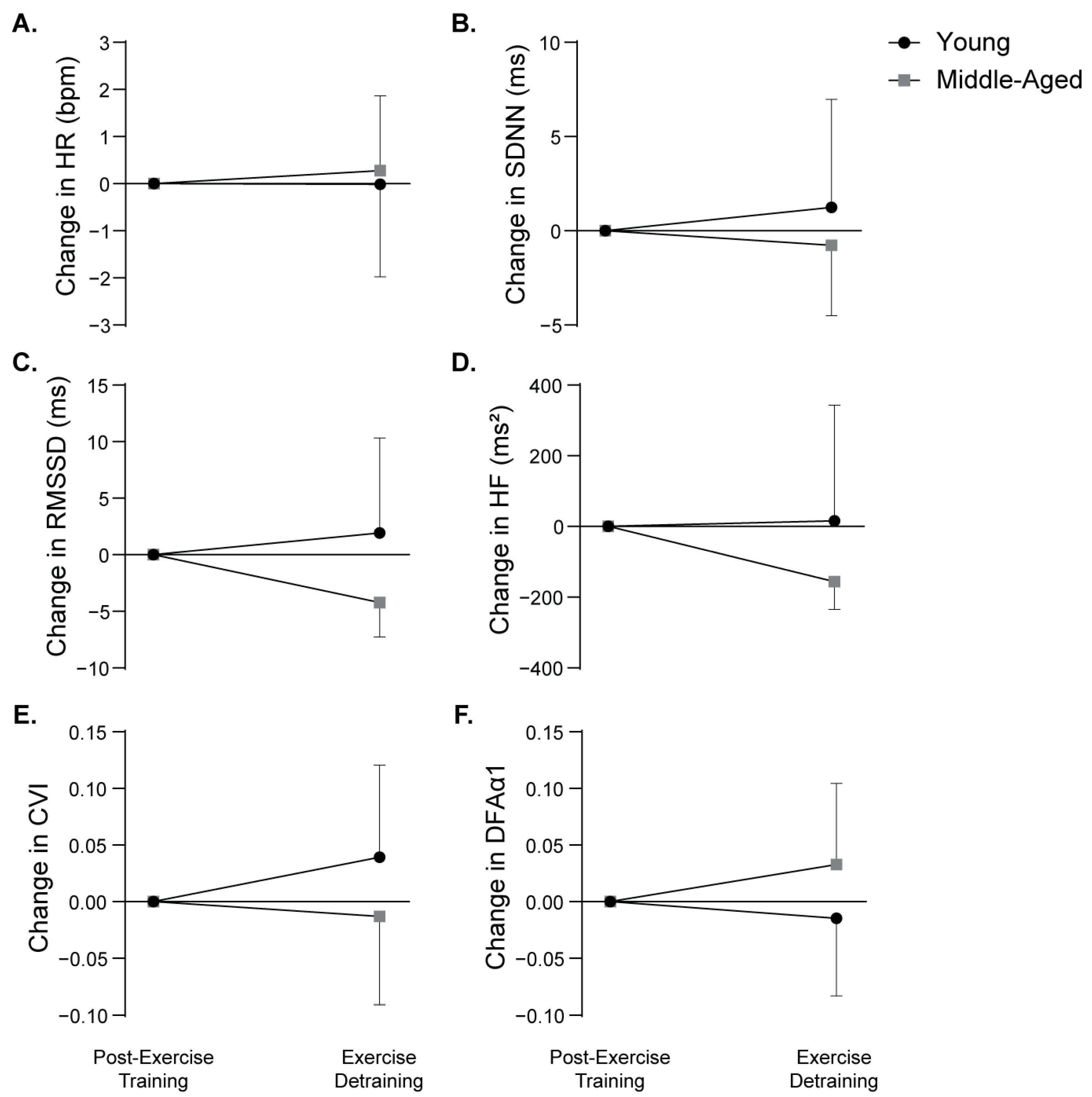
3.3. Age-Related Differences in Heart Rate and Heart Rate Variability
3.4. Associations Between Percent Change in Heart Rate Variability Indices and Arterial Blood Pressure in Response to Exercise Detraining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1RM | One-repetition maximum |
| BMI | Body mass index |
| COVID-19 | Coronavirus disease 2019 |
| CVI | Cardiac vagal index |
| DFAα1 | Detrended fluctuation analysis alpha 1 |
| HF | High frequency |
| HR | Heart rate |
| HRV | Heart rate variability |
| RMSSD | Square root of the mean of squared differences between successive RR intervals |
| RR | Time interval between heart beats |
| SDNN | Standard deviation of normal RR intervals |
| VO2peak | Peak volume of oxygen consumption |
References
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Dinas, P.C.; Koutedakis, Y.; Flouris, A.D. Effects of Active and Passive Tobacco Cigarette Smoking on Heart Rate Variability. Int. J. Cardiol. 2013, 163, 109–115. [Google Scholar] [CrossRef]
- Almeida-Santos, M.A.; Barreto-Filho, J.A.; Oliveira, J.L.M.; Reis, F.P.; Da Cunha Oliveira, C.C.; Sousa, A.C.S. Aging, Heart Rate Variability and Patterns of Autonomic Regulation of the Heart. Arch. Gerontol. Geriatr. 2016, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arantes, F.S.; Rosa Oliveira, V.; Leão, A.K.M.; Afonso, J.P.R.; Fonseca, A.L.; Fonseca, D.R.P.; Mello, D.A.C.P.G.; Costa, I.P.; Oliveira, L.V.F.; Da Palma, R.K. Heart Rate Variability: A Biomarker of Frailty in Older Adults? Front. Med. 2022, 9, 1008970. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Santos, I.S.; Dantas, E.M.; Andreão, R.V.; Mill, J.G.; Lotufo, P.A.; Bensenor, I. Reduced Heart-Rate Variability and Increased Risk of Hypertension—A Prospective Study of the ELSA-Brasil. J. Hum. Hypertens. 2021, 35, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Larson, M.G.; Tsuji, H.; Evans, J.C.; O’Donnell, C.J.; Levy, D. Reduced Heart Rate Variability and New-Onset Hypertension: Insights Into Pathogenesis of Hypertension: The Framingham Heart Study. Hypertension 1998, 32, 293–297. [Google Scholar] [CrossRef]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of Different Training Interventions on Heart Rate Variability and Cardiovascular Health and Risk Factors in Young and Middle-Aged Adults: A Systematic Review. Front. Physiol. 2021, 12, 657274. [Google Scholar] [CrossRef]
- Raffin, J.; Barthélémy, J.-C.; Dupré, C.; Pichot, V.; Berger, M.; Féasson, L.; Busso, T.; Da Costa, A.; Colvez, A.; Montuy-Coquard, C.; et al. Exercise Frequency Determines Heart Rate Variability Gains in Older People: A Meta-Analysis and Meta-Regression. Sports Med. 2019, 49, 719–729. [Google Scholar] [CrossRef]
- Chen, P.; Mao, L.; Nassis, G.P.; Harmer, P.; Ainsworth, B.E.; Li, F. Coronavirus Disease (COVID-19): The Need to Maintain Regular Physical Activity While Taking Precautions. J. Sport Health Sci. 2020, 9, 103–104. [Google Scholar] [CrossRef]
- Daanen, H.; Bose-O’Reilly, S.; Brearley, M.; Flouris, D.A.; Gerrett, N.M.; Huynen, M.; Jones, H.M.; Lee, J.K.W.; Morris, N.; Norton, I.; et al. COVID-19 and Thermoregulation-Related Problems: Practical Recommendations. Temperature 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Zwolski, C.M.; Paterno, M.V.; Rethorn, T.J.; Thomas, S.M.; Quatman-Yates, C.C.; Schmitt, L.C. Physical, Psychological, and Environmental Shifts Experienced during the Young Athlete Journey after ACL Reconstruction. Phys. Ther. Sport 2024, 70, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Polero, P.; Rebollo-Seco, C.; Adsuar, J.C.; Pérez-Gómez, J.; Rojo-Ramos, J.; Manzano-Redondo, F.; Garcia-Gordillo, M.Á.; Carlos-Vivas, J. Physical Activity Recommendations during COVID-19: Narrative Review. Int. J. Environ. Res. Public Health 2020, 18, 65. [Google Scholar] [CrossRef]
- Park, A.H.; Zhong, S.; Yang, H.; Jeong, J.; Lee, C. Impact of COVID-19 on Physical Activity: A Rapid Review. J. Glob. Health 2022, 12, 05003. [Google Scholar] [CrossRef] [PubMed]
- Pla, R.; Bosquet, L.; McGibbon, K.; Mujika, I.; Aubry, A. Heart Rate Variability in Elite Swimmers before, during and after COVID-19 Lockdown: A Brief Report on Time Domain Analysis. Appl. Sci. 2021, 11, 8106. [Google Scholar] [CrossRef]
- Gamelin, F.; Berthoin, S.; Sayah, H.; Libersa, C.; Bosquet, L. Effect of Training and Detraining on Heart Rate Variability in Healthy Young Men. Int. J. Sports Med. 2007, 28, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Dinas, P.C.; Krase, A.; Nintou, E.; Georgakopoulos, A.; Granzotto, M.; Metaxas, M.; Karachaliou, E.; Rossato, M.; Vettor, R.; Georgoulias, P.; et al. Human White-Fat Thermogenesis: Experimental and Meta-Analytic Findings. Temperature 2021, 8, 39–52. [Google Scholar] [CrossRef]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A Study of Concurrent and Construct Validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability Measures of the Short International Physical Activity Questionnaire (IPAQ) in Greek Young Adults. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2009, 50, 283–294. [Google Scholar]
- Tsitoglou, K.I.; Koutedakis, Y.; Dinas, P.C. Validation of the Polar RS800CX for Assessing Heart Rate Variability during Rest, Moderate Cycling and Post-Exercise Recovery. F1000Research 2018, 7, 1501. [Google Scholar] [CrossRef]
- Michael, S.; Graham, K.S.; Davis, G.M. Cardiac Autonomic Responses during Exercise and Post-Exercise Recovery Using Heart Rate Variability and Systolic Time Intervals—A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Macartney, M.J.; Meade, R.D.; Notley, S.R.; Herry, C.L.; Seely, A.J.E.; Kenny, G.P. Fluid Loss during Exercise-Heat Stress Reduces Cardiac Vagal Autonomic Modulation. Med. Sci. Sports Exerc. 2020, 52, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of Scaling Exponents and Crossover Phenomena in Nonstationary Heartbeat Time Series. Chaos Interdiscip. J. Nonlinear Sci. 1995, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Bonnemeier, H.; Wiegand, U.K.H.; Brandes, A.; Kluge, N.; Katus, H.A.; Richardt, G.; Potratz, J. Circadian Profile of Cardiac Autonomic Nervous Modulation in Healthy Subjects: Differing Effects of Aging and Gender on Heart Rate Variability. J. Cardiovasc. Electrophysiol. 2003, 14, 791–799. [Google Scholar] [CrossRef]
- Olga, N.D.; Rogozhkina, E.A.; Shvartz, V.A.; Shvartz, E.N.; Kiselev, A.R.; Drapkina, O.M. Indices of Heart Rate Variability Are Not Associated With Obesity In Patients 30–60 Years Of Age Without Chronic Noncommunicable Diseases. Russ. Open Med. J. 2023, 12, e0408. [Google Scholar] [CrossRef]
- Plaza-Florido, A.; Ruiz, J.R.; Alcantara, J.M.A. Resting Heart Rate but Not Heart Rate Variability Is Associated with the Normal-weight Obesity Phenotype. Am. J. Hum. Biol. 2024, 36, e24043. [Google Scholar] [CrossRef]
- De Sousa, T.L.W.; Ostoli, T.L.V.D.P.; Sperandio, E.F.; Arantes, R.L.; Gagliardi, A.R.D.T.; Romiti, M.; Da Silva, R.P.; Dourado, V.Z. Dose-Response Relationship between Very Vigorous Physical Activity and Cardiovascular Health Assessed by Heart Rate Variability in Adults: Cross-Sectional Results from the EPIMOV Study. PLoS ONE 2019, 14, e0210216. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Silveira, E.A.; Rosa, L.; Santos, A.; Rodrigues, A.P.; Mendonça, C.; Silva, L.; Gentil, P.; Rebelo, A.C. Risk Factors Associated with Cardiac Autonomic Modulation in Obese Individuals. J. Obes. 2020, 2020, 7185249. [Google Scholar] [CrossRef]
- Chen, L.Y.; Zmora, R.; Duval, S.; Chow, L.S.; Lloyd-Jones, D.M.; Schreiner, P.J. Cardiorespiratory Fitness, Adiposity, and Heart Rate Variability: The Coronary Artery Risk Development in Young Adults Study. Med. Sci. Sports Exerc. 2019, 51, 509–514. [Google Scholar] [CrossRef]
- De Sousa Fortes, L.; Pinheiro Paes, P.; Tavares Paes, S.; Oliveira Carvalho, F.; Serpeloni Cyrino, E. Clustering vs Multi-Sets Method in Resistance Training: Effect on Heart Rate Variability. Asian J. Sports Med. 2017, 9, e14576. [Google Scholar] [CrossRef]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F. Effect of Exercise Training on Heart Rate Variability in Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0251863. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.S.; Fahs, C.A.; Shinsako, K.K.; Jae, S.Y.; Fernhall, B. Heart Rate Recovery and Heart Rate Complexity Following Resistance Exercise Training and Detraining in Young Men. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H3180–H3186. [Google Scholar] [CrossRef]
- Bhati, P.; Moiz, J.A.; Menon, G.R.; Hussain, M.E. Does Resistance Training Modulate Cardiac Autonomic Control? A Systematic Review and Meta-Analysis. Clin. Auton. Res. 2019, 29, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, J.D.; Figueroa, A. Acute and Training Effects of Resistance Exercise on Heart Rate Variability. Clin. Physiol. Funct. Imaging 2016, 36, 179–187. [Google Scholar] [CrossRef]
- Ammar, A.; Boukhris, O.; Halfpaap, N.; Labott, B.K.; Langhans, C.; Herold, F.; Grässler, B.; Müller, P.; Trabelsi, K.; Chtourou, H.; et al. Four Weeks of Detraining Induced by COVID-19 Reverse Cardiac Improvements from Eight Weeks of Fitness-Dance Training in Older Adults with Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2021, 18, 5930. [Google Scholar] [CrossRef]
- Kaftan, A.H.; Kaftan, O. QT Intervals and Heart Rate Variability in Hypertensive Patients. Jpn. Heart J. 2000, 41, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Honkalampi, K.; Järvelin-Pasanen, S.; Tarvainen, M.P.; Saaranen, T.; Vauhkonen, A.; Kupari, S.; Perkiö-Mäkelä, M.; Räsänen, K.; Oksanen, T. Heart Rate Variability and Chronotype—A Systematic Review. Chronobiol. Int. 2021, 38, 1786–1796. [Google Scholar] [CrossRef]
- Shurkevich, N.P.; Vetoshkin, A.S.; Kareva, M.A.; Gubin, D.G. Arterial Hypertension and COVID-19 in Arctic Rotating Shift Work: The Impact of Chronostructure Disruptions on Circadian Blood Pressure Rhythm in Relation to Echocardiographic Parameters. Russ. Open Med. J. 2024, 13, e0408. [Google Scholar] [CrossRef]
- Al Abbad, M.; Nuhmani, S.; Ahsan, M.; Muaidi, Q. Chronotype and Athletes’ Performance in Sports: A Narrative Review. Electron. J. Gen. Med. 2023, 20, em484. [Google Scholar] [CrossRef]
| Exercise Modality | Week | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4–8 | |
| Aerobic | 30 min of cycling at 65% VO2peak 3 days/week | 45 min of cycling at 65% VO2peak 3 days/week | 45 min of cycling at 65% VO2peak 5 days/week | 60 min of cycling at 65% VO2peak 5 days/week |
| Resistance | * Resistance training familiarization session 4 days/week | 2 sets of 8–10 reps at 65% of 1RM 4 days/week | 3 sets of 8–10 reps at 65% of 1RM 4 days/week | 4 sets of 8–10 reps at 65% of 1RM 4 days/week |
| Combined | 15 min of cycling at 65% VO2peak and * resistance training familiarization session 3 days/week | 23 min of cycling at 65% VO2peak and 1 set of 8–10 reps at 65% of 1RM 4 days/week | 23 min of cycling at 65% VO2peak and 2 sets of 8–10 reps at 65% of 1RM 4 days/week | 30 min of cycling at 65% VO2peak and 2 sets of 8–10 reps at 65% of 1RM 5 days/week |
| Variable | Young (n = 8) | Middle-Aged (n = 10) | ||||
|---|---|---|---|---|---|---|
| Baseline | Exercise Training | Detraining | Baseline | Exercise Training | Detraining | |
| Age (years) | 27.8 (3.8) | -- | -- | 41.9 (3.8) * | -- | -- |
| BMI (kg/m2) | 26.0 (4.9) | 26.3 (5.0) | 26.1 (4.9) | 28.9 (4.7) | 29.2 (4.7) | 29.2 (5.1) |
| Percent BF (%) | 24.7 (8.8) | 24.9 (8.1) | 25.4 (8.6) | 32.8 (7.9) | 32.3 (8.6) | 32.3 (7.7) |
| Systolic BP (mmHg) | 120.8 (9.8) | 112.8 (10.0) | 116.9 (11.1) | 126.2 (10.9) | 124.2 (13.0) | 123.1 (12.7) |
| Diastolic BP (mmHg) | 81.6 (7.6) | 79.1 (4.2) | 82.3 (8.7) | 87.5 (9.8) | 85.2 (12.2) | 85.8 (10.6) |
| VO2peak (mL/kg/min) | 31.2 (8.5) | 34.1 (7.8) | 32.4 (7.3) | 27.0 (9.0) | 30.8 (8.3) | 29.2 (7.4) |
| 1RM Leg Press (kg) | 67.0 (17.2) | 69.8 (14.1) | 67.1 (13.4) | 85.1 (54.3) | 112.2 (87.2) | 95.9 (69.5) |
| 1RM Chest Press (kg) | 56.0 (19.7) | 58.9 (22.4) | 56.1 (19.9) | 58.0 (20.8) | 62.2 (19.1) | 57.6 (21.6) |
| Variable | Mean (SD) | Unadjusted Difference | Adjusted Difference | |||
|---|---|---|---|---|---|---|
| Young (n = 8) | Middle-Aged (n = 10) | Mean [95% CI] | p-Value | Mean [95% CI] | p-Value | |
| Heart Rate (bpm) | ||||||
| Baseline | 62.2 (5.4) | 62.2 (7.2) | 0.0 [−6.5, 6.6] | 0.996 | – | – |
| Exercise Training | 60.6 (4.3) | 61.5 (8.6) | 0.8 [−6.3, 8.0] | 0.805 | 0.8 [−5.1, 6.80] | 0.771 |
| Detraining | 60.6 (4.9) | 61.7 (7.0) | 1.1 [−5.1, 7.3] | 0.709 | 1.1 [−3.0, 5.2] | 0.577 |
| SDNN (ms) | ||||||
| Baseline | 50.0 (11.7) | 34.3 (12.0) | −15.7 [−27.7, −3.9] | 0.013 * | – | – |
| Exercise Training | 54.3 (15.8) | 39.6 (19.8) | −14.6 [−32.9, 3.6] | 0.109 | −6.2 [−27.8, 15.5] | 0.554 |
| Detraining | 55.6 (13.9) | 38.9 (17.7) | −16.7 [−32.9, −0.5] | 0.045 * | −7.2 [−25.7, 11.3] | 0.417 |
| RMSSD (ms) | ||||||
| Baseline | 51.7 (19.3) | 32.6 (11.6) | −19.1 [−34.6, −3.5] | 0.019 * | – | – |
| Exercise Training | 56.6 (30.6) | 39.5 (20.8) | −17.1 [−37.9, 3.7] | 0.100 | −3.1 [−24.7, 18.5] | 0.763 |
| Detraining | 58.5 (15.1) | 35.3 (15.2) | −23.2 [−38.5, −8.0] | 0.005 * | −18.6 [−37.0, −0.3] | 0.047 * |
| Log HF | ||||||
| Baseline | 2.9 (0.3) | 2.5 (0.3) | −0.5 [−0.8, −0.2] | 0.005 * | – | – |
| Exercise Training | 2.9 (0.3) | 2.6 (0.5) | −0.4 [−0.8, 0.0] | 0.057 | 0.0 [−0.5, 0.4] | 0.841 |
| Detraining | 3.0 (0.2) | 2.5 (0.3) | −0.5 [−0.8, −0.2] | 0.002 * | −0.3 [−0.6, 0.0] | 0.084 |
| CVI | ||||||
| Baseline | 4.5 (0.3) | 4.1 (0.3) | −0.4 [−0.6, −0.1] | 0.007 * | – | – |
| Exercise Training | 4.6 (0.2) | 4.2 (0.5) | −0.4 [−0.8, 0.0] | 0.062 | −0.1 [−0.6, 0.4] | 0.657 |
| Detraining | 4.6 (0.2) | 4.2 (0.4) | −0.4 [−0.7, −0.1] | 0.012 * | −0.2 [−0.5, 0.2] | 0.319 |
| DFAα1 | ||||||
| Baseline | 1.0 (0.2) | 1.1 (0.3) | 0.1 [−0.2, 0.3] | 0.581 | – | – |
| Exercise Training | 1.0 (0.3) | 1.1 (0.2) | 0.0 [−0.2, 0.3] | 0.748 | 0.0 [−0.2, 0.2] | 0.897 |
| Detraining | 1.0 (0.1) | 1.1 (0.3) | 0.1 [−0.2, 0.3] | 0.477 | 0.0 [−0.2, 0.2] | 0.648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo, A.E.; Dinas, P.C.; Gkiata, P.; Ferri, A.R.; Kenny, G.P.; Koutedakis, Y.; Jamurtas, A.Z.; Metsios, G.S.; Flouris, A.D. An Exploratory Investigation of Heart Rate Variability in Response to Exercise Training and Detraining in Young and Middle-Aged Men. Biology 2025, 14, 794. https://doi.org/10.3390/biology14070794
Carrillo AE, Dinas PC, Gkiata P, Ferri AR, Kenny GP, Koutedakis Y, Jamurtas AZ, Metsios GS, Flouris AD. An Exploratory Investigation of Heart Rate Variability in Response to Exercise Training and Detraining in Young and Middle-Aged Men. Biology. 2025; 14(7):794. https://doi.org/10.3390/biology14070794
Chicago/Turabian StyleCarrillo, Andres E., Petros C. Dinas, Paraskevi Gkiata, Alexa R. Ferri, Glen P. Kenny, Yiannis Koutedakis, Athanasios Z. Jamurtas, George S. Metsios, and Andreas D. Flouris. 2025. "An Exploratory Investigation of Heart Rate Variability in Response to Exercise Training and Detraining in Young and Middle-Aged Men" Biology 14, no. 7: 794. https://doi.org/10.3390/biology14070794
APA StyleCarrillo, A. E., Dinas, P. C., Gkiata, P., Ferri, A. R., Kenny, G. P., Koutedakis, Y., Jamurtas, A. Z., Metsios, G. S., & Flouris, A. D. (2025). An Exploratory Investigation of Heart Rate Variability in Response to Exercise Training and Detraining in Young and Middle-Aged Men. Biology, 14(7), 794. https://doi.org/10.3390/biology14070794










