3.1. Characterization of the Evolution State of Genome Sequences
Previous work has shown that there are CG- and TA-independent selection modes in genome sequences [
14]. These two kinds of evolution modes can be characterized by the separability values of the CG1 and TA1 8-mer spectra (
Section 2), which are called the CG-independent selection intensity (
δCG1) and the TA-independent selection intensity (
δTA1). There is a mutual inhibition relationship between the two independent selection intensities. With the increase of species evolutionary levels, the CG-independent selection intensity increases, but the TA-independent selection intensity decreases in animals and plants. Therefore, an evolution mechanism of genome sequences was proposed; that is, the CG- and TA-independent selection intensities and the mutual inhibition relationship between them determine the evolution state of genome sequences. It is believed that the mutual inhibition mechanism is the key reason driving the evolution of genome sequences. To evaluate the robustness of the independent selection intensities, we conducted a resampling analysis. Specifically, we performed multiple sampling with replacement on the animal dataset, generating multiple subsamples. For each pair of subsamples, we calculated the CG-independent selection intensities and performed linear regression analysis. The Pearson correlation coefficient (R) ranged from 0.898 to 0.999, and
p-values ranged from 1.94 × 10
−93 to 1.05 × 10
−26. These results indicated that there is a statistically significant correlation between independent selection intensities across different subsamples. This means that the CG-independent selection intensity parameter is highly robust in different data subsets.
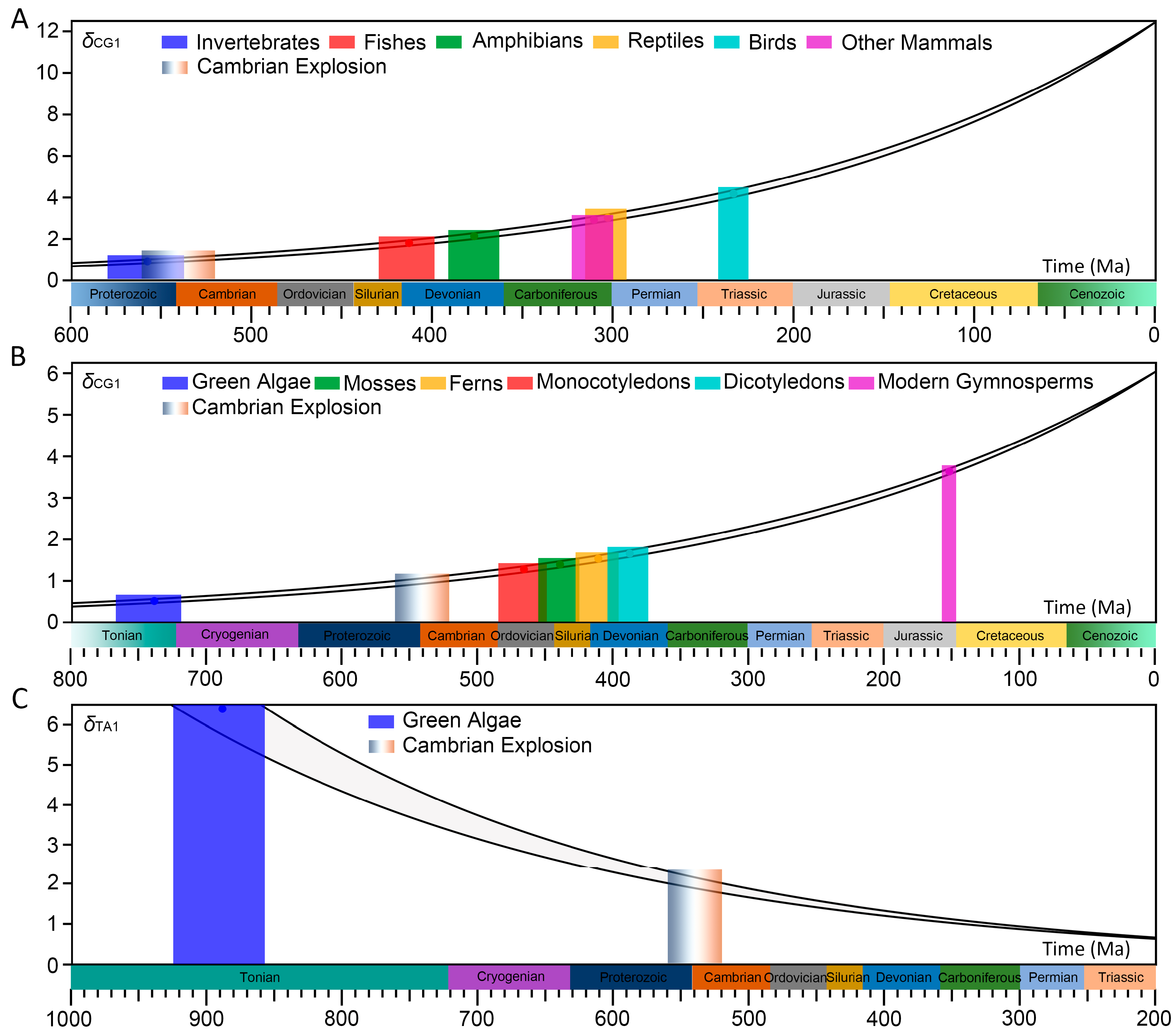
Here, animals are divided into eight branches: invertebrates, fishes, amphibians, reptiles, birds, other mammals, rodents and primates, and plants are divided into six branches: green algae, mosses, ferns, monocotyledons, dicotyledons and modern gymnosperms. The distributions of CG- and TA-independent selection intensities for their genome sequences are given, respectively (
Figure 1). The detailed information is listed in Additional file:
Tables S1 and S2.
In animal genome sequences, it can be seen that the CG-independent selection intensity is positively correlated with the evolutionary levels of animals (
Figure 1B). In invertebrates, fishes, amphibians and reptiles, there is a mutual inhibition relationship between the CG- and TA-independent selection intensities. Starting from other mammals, with the increase in species evolutionary levels, the TA-independent selection mode gradually disappears (
δTA1 ≈ 1). In plant genome sequences, the CG-independent selection intensity is positively correlated with the evolutionary levels of plants (
Figure 1D), and the mutual inhibition relationship exists in all plant branches.
We found that the distribution pattern of a few lower animals is δTA1 > 1 and δCG1 < 1, and that of other animals is δCG1 > 1 and δTA1 < 1. In plants, the distribution pattern of some green algae is δTA1 > 1 and δCG1 < 1, that of some green algae, some mosses, some ferns and some monocotyledons is δCG1 > 1 and δTA1 > 1, and that of other plants is δCG1 > 1 and δTA1 < 1. Overall, the CG- and TA-independent selection intensities are different for different species. Here, we use the CG- or TA-independent selection intensity to characterize the evolution state of species genome sequences.
3.2. Conjecture on the Cause of the Cambrian Explosion
The Cambrian explosion is a revolutionary event in the history of biological evolution, which has the following features in its macroscopic manifestations: (1) the “sudden” prosperity of eukaryotic species; (2) the “sudden” increase in complexity of eukaryotic evolution. From the perspective of genome sequence evolution, we argue that the “sudden” increase in biodiversity and structural complexity of organisms must be reflected in revolutionary changes in the way of genome sequence evolution.
Darwin’s theory stated that higher organisms evolved from lower organisms. According to the distribution patterns of CG- and TA-independent selection intensities along with the evolutionary levels of animals and plants, we found that if we do not distinguish between species, all animals or all plants can be regarded as one kind of organism, which we call the meta-organism. Then, the arrangement of animals or plants according to their evolutionary levels can be regarded as the evolution timeline of the meta-animal or meta-plant (
Figure 1A,C). Then, the change in the CG-independent selection intensity from small to large or the TA-independent selection intensity from large to small corresponds to the change in the evolution time from past to present for the meta-organism. Thus, the evolution time is related to the evolution state for the meta-organism. This approach is inspired by the method used in astronomy to deduce stellar evolution. By observing the “present” image of stars, astronomers analyze the feature differences of stars according to physics theory and deduce their evolutionary history. Similarly, genome sequences of all existing species unfold before our eyes, like the observed “present” image of stars. By analyzing the differences in the evolution state among different species, we can deduce the evolutionary process of the meta-organism based on the evolution mechanism of genome sequences. This meta-organism model was inspired by stellar evolution theories in astronomy, which consider the collective evolution states of modern species as historical evolutionary trajectories. However, unlike stellar systems that strictly follow physical laws, the evolution mechanisms of biological systems involve complex factors such as genetic drift, selection pressure and extinction events. This essential difference makes the direct mapping of astrophysical models in the biological field theoretically limited. We address these limitations by basing our inferences on a model that relies on CG- and TA-independent selection intensities across diverse groups.
When the arrangement of animals or plants by their evolutionary levels is regarded as the evolution timeline of the meta-organism, we found that the distribution curves of the CG-independent selection intensity and the TA-independent selection intensity intersected at a certain time in the past (
Figure 1). The species before and within this intersection region are some lower invertebrates or green algae, while the species after this intersection region are higher eukaryotes. This indicates that in the evolutionary process from lower organisms to higher organisms, there is a transition process from the evolution mode dominated by TA-independent selection to the evolution mode dominated by CG-independent selection. We call this phenomenon the phase transition process of evolution modes. According to the distributions in
Figure 1, we can see that genome sequences adopted the evolution mode dominated by TA-independent selection in the early stage of life evolution, while the CG-independent selection mode was inhibited. As life evolved further, the CG-independent selection intensity gradually increased. Eventually, genome sequences adopted the evolution mode dominated by CG-independent selection, while the TA-independent selection was inhibited or even disappeared.
For the Cambrian explosion, this revolutionary event must be reflected in revolutionary changes in the evolution modes of genome sequences. The phase transition process between the TA- and CG-independent selection modes is a revolutionary event in the evolutionary process of genome sequences. We assumed that the evolution modes of CG- and TA-independent selection have persisted since the Cambrian period. This allows us to infer the evolution states of ancient organisms from modern genome sequences. However, we acknowledge potential limitations, including the possibility that genetic drift, lineage-specific adaptations, or incomplete sampling of modern genomes may obscure ancient evolutionary information. We believe that the evolution rule is immutable from past to present in genome sequences. Although it is impossible to obtain the genome sequences of species in the Cambrian period, we consider that the phase transition phenomenon exhibited by existing species is the main cause of the Cambrian explosion. This phenomenon originated in the Cambrian period and continues to the present. The evolution of organisms from simple to complex eukaryotes is determined by the phase transition phenomenon. Based on our conjecture, we will analyze the relationship between the evolution state and the evolution time of species and deduce the origin time of species branches.
3.3. Relationship Between the Evolution Time and the Evolution State
Considering that the CG-independent selection intensity changes more obviously across species, here we use it to characterize the evolution state of genome sequences. First, we need to determine the two evolution states of genome sequences corresponding to the starting time of the Cambrian explosion and the present time. Second, we need to determine the function that describes the relationship between the evolution time and the evolution state of genome sequences.
We found that regardless of animals or plants, there is such a rule for the transition of evolution mode in the phase transition region: starting from
δTA1 > 1 and
δCG1 < 1, going through the intermediate process of
δTA1 > 1 and
δCG1 > 1, and finally reaching the evolution state of
δCG1 > 1 and
δTA1 < 1 (
Figure 1A,C). According to the definition of independent selection intensity (see “
Section 2”), when
δCG1 ≠ 1 or
δTA1 ≠ 1, the distribution location of the 8-mer spectrum in CG1 or TA1 subsets is apart from the random center. Taking
δCG1 as an example, when
δCG1 > 1, the 8-mer spectrum of the CG1 subset is located at the low-frequency end of the random center. With
δCG1 increases, the distribution conservatism of the 8-mer spectrum of the CG1 subset also increases. When
δCG1 < 1, the 8-mer spectrum of the CG1 subset is located in the high-frequency end of the random center. With
δCG1 decreases, the distribution conservatism of the 8-mer spectrum of the CG1 subset also decreases [
14]. The 8-mer spectral properties of TA1 subsets are the same as those of CG1 subsets. If the 8-mer spectral distribution has high conservatism and is far from the random center, these 8-mers must be functional motifs [
15,
16]. Our results showed that
δCG1 is obviously positively correlated with the evolutionary levels of genome sequences, and the TA-independent selection mode disappears basically (
δTA1 ≈ 1) in mammals (
Figure 1B,D). This indicated that the CG-independent selection mode is a driving force in the evolution of genome sequences, while the TA-independent selection mode is the result of being inhibited. In conclusion, it is reasonable to choose
δCG1 = 1 as the critical point of the phase transition between two evolution modes of animal and plant genome sequences and to choose the time corresponding to
δCG1 = 1 as the starting time of the Cambrian explosion. This choice assumes that the CG- and TA-independent selection intensities observed in modern genomes reflect the evolutionary dynamics of the Cambrian period.
In principle, the selection of the evolution state of the meta-organism genome sequence corresponding to the present time should satisfy two conditions: one is that the evolutionary level of the selected species should be the highest, and the other is that the CG-independent selection intensity of the selected species should be the largest. In animals, it is generally assumed that the maximum value
δCG1,max should appear in primates with the highest evolutionary level. However, as shown in
Figure 1D, we can see high average values of CG-independent selection intensity in primates and rodents, but the highest value is found in other mammals. The maximum values of the CG-independent selection intensity in rodents and primates are obviously lower than in other mammals. This brings confusion to the choice of
δCG1,max. However, in
Figure 1D, we found that the TA-independent selection mode is weak (
δTA1 ≈ 1) in other mammals, and the TA-independent selection mode disappears completely in rodents and primates. That is to say, the mutual inhibition relationship between the two evolution modes is weak or disappears in these genome sequences. The results showed that a new evolution mechanism arose in primates and rodents. Due to the disappearance of the mutual inhibition relation, they no longer obey the general evolution mechanism we proposed. Therefore, the changes in CG-independent selection intensity are no longer dependent on the mutual inhibition relationship.
Therefore, the following analysis does not consider these two branches. It is reasonable for us to select δCG1,max from other mammals. Finally, in animals, we obtained δCG1,max = 12.45, which comes from Sarcophilus harrisii and represents the highest level of the evolution state. We use the evolution state of Sarcophilus harrisii to represent the evolution state of the meta-animal. We record the time corresponding to the evolution state of the meta-animal as the present time. In plants, we obtained δCG1,max = 6.04, which comes from Sequoia sempervirens and represents the highest level of the evolution state. We use the evolution state of Sequoia sempervirens to represent the evolution state of the meta-plant. We record the time corresponding to the evolution state of the meta-plant as the present time.
In animals and plants, the arrangement of species was regarded as the evolution timeline of the meta-animal or meta-plant (
Figure 1A,C). We found that there is a nonlinear positive correlation between the evolution time and the evolution state of the meta-animal or meta-plant. This means that as the CG-independent selection intensity increases, so does the evolution rate (slope of distribution) of species. Therefore, it is inappropriate to directly characterize the evolution time by the CG-independent selection intensity. In
Figure 1B,D, if we pay attention to the overall trend of the distribution of the CG-independent selection intensity for each animal branch or plant branch, we found that there is a linear positive correlation between the evolution state and the evolution time. This is also reflected in the first half of the distribution in
Figure 1A,C. If the evolution rate of the overall trend of the distribution is denoted as the
v0 value, the evolution rate
v0 characterizes the commonality of the evolution of genome sequences across each animal or plant branch. The evolution rates vary widely among species within each animal branch (
Figure 1B). With some exceptions among invertebrates and fishes, the evolution rates of other species are obviously higher than
v0. The evolution rates also differ within each plant branch (
Figure 1D), such as mosses, ferns and modern gymnosperms; their evolution rates are obviously higher than
v0. This difference represents the individuality of the evolution of genome sequences and is closely related to the differences in the living environment of species. In conclusion, the relationship between the evolution state and the evolution time is nonlinear. When exploring the evolution of genome sequences, the commonality and individuality of species evolution must be considered at the same time.
According to the above analysis, we established a functional relationship between the evolution state and the evolution time. As a basic consideration, we assumed that there is a linear positive correlation between evolution rate
v and the evolution state, that is,
v =
kδCG1, where the proportional coefficient
k is a constant. The evolution rate is defined as
v =
dδCG1/
dt. If the starting time of the Cambrian explosion is defined as
t =
t0, the corresponding evolution state is defined as
δCG1 =
δ0 = 1 at the phase transition point. The present time is defined as
t = 0, the corresponding evolution state of animals is defined as
δCG1,max = 12.45, and the corresponding evolution state of plants is defined as
δCG1,max = 6.04. After deduction (
Section 2), we calculated the proportional coefficient
k of animals and plants, respectively. Finally, we obtained the function relationship between the evolution state
δCG1 and the evolution time
t, see Equation (10).
Since the accepted time for the Cambrian explosion event is 560–520 Ma [
1], we present two sets of time
t values to represent the time range corresponding to the evolution state of each species. We calculated the evolution time corresponding to the evolution state of genome sequences in 649 animals and 350 plants. The results are shown in
Figure 2A,B and Additional file:
Tables S1 and S2. In
Figure 2A,B, the upper curve corresponds to the starting time of 560 Ma, and the lower curve corresponds to the starting time of 520 Ma. For example, for
Lithobates catesbeianus, the CG-independent selection intensity is
δCG1 = 2.93, and the corresponding time
t is 321–298 Ma, indicating that the evolution state of this species is equivalent to that of the meta-animal at 321–298 Ma.
3.4. The Origin Time of Each Species Branch
Next, we analyze the origin time of each species branch according to the relationship between the evolution state and the evolution time. Within each species branch, the species with the smallest CG-independent selection intensity should appear earliest. The appearance time of this species can represent the origin time of this species branch, and the evolution state of its genome sequence represents the morphology of the ancestral genome sequence of this species branch. According to this idea, we gave the origin time of six animal branches and six plant branches (
Figure 2). The origin time of each animal branch is shown in
Table 2, and the origin time of each plant branch is shown in
Table 3. The tables also provide representative species of species origin in each branch. It is known that these representative species belong to the ancient species within their corresponding branch. For example, among reptiles,
Sphenodon punctatus is a lizard-like animal of the genus
Sphenodon, which is the only representative of the
Rhyncocephalia remaining from the early Triassic period and is known as a living fossil. Among other mammals, Ornithorhynchus anatinus belongs to monotremes or oviparous mammals, which represents a link in the evolution from reptiles to mammals and is considered to be the oldest mammal.
Among animals, we compared the origin time of the six animal branches with paleontological evidence and found that they are extremely consistent. For invertebrates, paleontological evidence showed that the Ediacaran biota emerged during the Precambrian period (575–542 Ma) [
17,
18]. The origin time we deduced is 579–537 Ma. For fishes, paleontologists have discovered an ancient jawed fish named
Bianchengichthys micros that existed 423 Ma [
19]. More recently, Zhu’s team has discovered a variety of early Silurian jawed fish fossils in the geological time of 439–436 Ma [
20,
21,
22,
23]. The origin time we obtained is 429–398 Ma, which is in the late Silurian and early Devonian. For amphibians, paleontologists believe that
Ichthyostega is the earliest known amphibian [
24,
25]. Its fossils were discovered in strata from the late Devonian period, about 370–360 Ma.
Elpistostege watsoni [
26,
27,
28] and
Tiktaalik roseae [
29], which predate
Ichthyostega, had their fossils found in late Devonian strata around 380 Ma. They have both fish and amphibian characteristics and are considered to be transitional species between fish and tetrapods. So paleontological evidence suggests that amphibians originated around 380–360 Ma. The origin time we gave is 391–363 Ma, which is in the Devonian period. For reptiles, scientists discovered fossil footprints dating back 318 Ma that belong to
Hylonomus lyelli [
30,
31], a small lizard-like animal from the Carboniferous period. However, its bone fossils were discovered in strata of 314 Ma. Paleontological evidence suggests that reptiles originated 318–314 Ma. Our results show that reptiles emerged during the Late Carboniferous and Early Permian periods (315–292 Ma). The origin of birds is a controversial topic with several hypotheses. Some scholars believe that the earliest bird was
Protoavis [
32,
33,
34], whose fossils were discovered in Late Triassic strata, about 225 Ma. However, most scholars do not recognize it as a bird. Some paleontologists also support the hypothesis that birds originated from small dinosaurs. The oldest known small feathered dinosaur is
Anchiornis huxleyi [
35], whose fossils were discovered in Late Jurassic strata, about 161–151 Ma. The origin time we gave is 242–225 Ma in the Triassic period, which is consistent with the geological age of the Protoavis fossils. Our conclusion supports the hypothesis that
Protoavis was the ancestor of birds. The origin time of today’s mammals can be traced back to the Carboniferous period, about 320–315 Ma [
36,
37,
38]. Their ancestors were a group of animals known as non-mammalian synapsids that arose in the amniotes. The origin time of other mammals we calculated is 322–299 Ma. The molecular clock study [
13] has estimated similar divergence times for major animal groups. It supports our findings for invertebrates, fishes, amphibians, reptiles and mammals.
For the Cambrian explosion event, the evidence mainly comes from animals [
1], but there is very little evidence of plants. Although complex land plants did not exist during the Cambrian, we included modern plant data to verify whether the transition of genome evolution mode we proposed, that is, the phase transition from TA-independent selection to CG-independent selection, is universal in animals and plants. This approach allows us to explore whether the genomic dynamics driving the Cambrian explosion apply to a wider range of life forms, thus providing more comprehensive insights into the evolution of complex organisms. Our results showed that the evolution mechanism of animals and plants is the same. We thought that the Cambrian explosion is also reflected in the evolution of plant diversity. Here, the origin time of six plant branches were given. By comparing the origin time with paleontological evidence, we found that our results for the origin time of green algae, mosses and ferns are consistent with paleontological evidence. However, the results for the origin time of angiosperms (monocotyledons and dicotyledons) and gymnosperms differ from paleontological evidence. For green algae, the oldest fossils ever discovered are
Proterocladus antiquus [
39], which appeared 1000 Ma. The molecular clocks calibrated using these fossils suggested that green algae may have originated 1382–797 Ma during the Mesoproterozoic to Neoproterozoic or earlier [
40]. The origin time of green algae we deduced is 767–712 Ma, which is close to paleontological evidence but still leaves some gaps. For mosses, paleontologists discovered the fossils of
Parafunaria sinensis Yang dating back to 520 Ma [
41]. The origin time we obtained is 456–424 Ma, which is only 64 million years later than the paleontological evidence. For ferns, paleontologists believe that the earliest fern,
Baragwanathia, appeared between the Late Silurian and Early Devonian periods (425–395 Ma) [
42]. The origin time we gave is 427–396 Ma, which is consistent with paleontological evidence.
Our results show that the origin time of angiosperms differs obviously from the paleontological evidence. The fossils of an herbaceous plant were discovered in Daohugou of China, which appeared in the Middle Jurassic period about 164 Ma [
43]. However, it did not indicate whether it was monocotyledon or dicotyledon. Paleontologists discovered that
Nanjinganthus dendrostyla gen. et sp. nov. appeared in the early Jurassic period about 174 million years ago and is considered to be the oldest flower fossil in the world [
44]. Additionally, siliconized flora fossils were discovered in eastern Inner Mongolia of China, which appeared 126 Ma [
45]. Furthermore, paleontologists have studied fossils of plants related to the British Jurassic and Antarctic Triassic. Their results showed that the divergence of the close groups of angiosperms (angiophytes) actually began in the late Permian (260–250 Ma), much earlier than when the crown groups of angiosperms appeared in the fossil record [
45]. The fossils of a whole preserved monocotyledons plant,
Sinoherba ningchengensis, were discovered in the Yixian Formation strata of China [
46]. They appeared 125 Ma, which is the earliest reliable fossil record of monocotyledons plants in the world. The fossils of a dicotyledons plant,
Leefructus mirus, were discovered in Lingyuan of China [
47]. They appeared 124 Ma, which is the earliest dicotyledons plant fossils found so far. These paleontological fossil evidences showed that the ancestors of angiosperms may have appeared 260–250 Ma. However, the origin time we gave is 484–449 Ma for monocotyledons and 403–374 Ma for dicotyledons, which is about 200 million years earlier than the existing paleontological evidence.
Although we thought that these findings may reflect an earlier divergence of angiosperms, we acknowledged that they challenge existing fossil records. There are several reasons to support our conclusions about the origin time of angiosperms. First, most of the evidence for the Cambrian explosion appeared in animals. The fossil records of angiosperms are seriously insufficient, and the reference value is unreliable [
1]. Some studies have shown that the earliest angiosperms may have appeared earlier than fossil evidence suggests [
48,
49]. Second, our study showed that animal and plant genome sequences follow the same evolution mechanism. Animals and plants must have a coevolution relationship in similar environments. Comparing
Table 2 and
Table 3, the origin time of plant branches are close to that of animal branches, and the origin time of plant branches is generally earlier than that of animal branches. Our results satisfy the coevolution rule between animals and plants. Third, considering the predator–prey relationship between animals and plants, our conclusions are more convincing. From a macro perspective, there must be a certain percentage of herbivores among various animals. Therefore, the ancestor of angiosperms should appear before the herbivores, not after them. For example, herbivorous fishes appeared about 400 Ma. What did they consume? Definitely not ferns and mosses. Critically, our analysis targets universal macro-level patterns and logical principles governing predator–prey dynamics—not species-specific interactions or micro-level mechanisms. Moreover, it is logically unacceptable that the ancestor of reptiles appeared 318–314 Ma and the ancestor of angiosperms appeared 260–250 Ma [
30,
31,
45]. The origin time of plant branches is generally earlier than that of animal branches, indicating that our conclusions conform to the logical relationship of predator–prey. Fourth, some animals first landed ashore in 380–360 Ma [
24,
25,
26,
27,
28,
29] and needed food to support them. In addition to carnivores, some of the first landed animals were herbivores. Hence, plants should land before animals. After plants landed ashore, they rapidly changed the environment of the Earth’s land and created suitable conditions for the arrival of animals. Therefore, we have reason to believe that plants, probably including the ancestor of angiosperms, landed before animals. However, alternative explanations for the difference include the following: (1) limitations in our model, such as CG- and TA-independent selection intensities, which may not fully capture the evolutionary dynamics of angiosperms; (2) incomplete sampling of plant genomes, particularly for early diverging lineages; (3) potential biases in the fossil record due to preservation challenges for soft-bodied plants. These factors indicate that our deduced origin times should be viewed with caution before obtaining more genomes and paleontological evidence.
For modern gymnosperms, our results are significantly different from the paleontological evidence. Modern gymnosperms originated 158–147 Ma according to our research, while the paleontological evidence shows that gymnosperms originated 395–389 Ma [
50]. We found that only the genome sequences of modern gymnosperms are in current databases, and we do not know why the other gymnosperms are not listed. Existing research results showed that gymnosperms belong to an ancient class of Spermatophyta that originated in the Devonian period of the Paleozoic era (395–389 Ma) [
51,
52]. Gymnosperms have mostly small needle-shaped evergreen leaves with only narrow tracheids in their vascular tissue. They grow slowly and have poor adaptability to harsh environments. Some studies suggested that most gymnosperms became extinct after several major changes in geography and climate during evolution, and only a few species survived. These species are called modern gymnosperms. They have large genome sequences, and their bodies are large, such as conifers; they can thrive in harsh environments. These factors indicate that our deduced origin time for modern gymnosperms is reasonable but may not fully represent the broader gymnosperm lineage. Further studies incorporating additional gymnosperm genomes could help clarify this difference.
The origin time of green algae was derived from the CG-independent selection intensity deduction and was found to be 767–718 Ma, which is still far from the paleontological evidence of 1382–797 Ma (
Table 3). Our results showed that the TA-independent selection intensity is obviously higher in green algae, and
δTA1,max = 6.40. For most green algae, their
δTA1 is larger than
δCG1. About 60% of green algae have
δCG1 < 1. According to the definition of
δCG1 and
δTA1, the results indicated that the evolution of green algae genome sequences is dominated by TA-independent selection mode. Therefore, it is more reasonable to select the TA-independent selection intensity to characterize the evolution state of genome sequences in green algae.
Here, we used the TA-independent selection intensity to characterize the evolution state of genome sequences in green algae. We found that the distribution of the TA-independent selection intensity is also nonlinear (
Figure 3), so we used the same method to deduce the relationship between evolution state and evolution time. We assumed that the evolution rate is
v′ =
kδTA1, and the proportional coefficient
k is the same as that analyzed using the CG-independent selection intensity in plants. The evolution rate is defined as
v′ = −
dδTA1/
dt. The phase transition point is still selected as
δCG1 = 1, which corresponds to
δTA1 = 2 (
Figure 3). Therefore, the starting time of the Cambrian explosion corresponding to
δTA1 =
δ0′ = 2 is denoted as
t =
t0′. Finally, the evolution time
t′ of green algae was obtained; see Equation (17). The results are shown in
Figure 2C and Additional file:
Table S2. The time corresponding to the species with the highest TA-independent selection intensity (
δTA1,max = 6.40) represents the origin time of green algae. Finally, we deduced that green algae originated 922–856 Ma, about 150 million years earlier than the origin time deduced by
δCG1. It showed that the origin time of green algae deduced by
δTA1 is more consistent with paleontological evidence [
39,
40]. It indicates that it is more reasonable to use the dominant evolution mode to characterize the evolution state of species genome sequences. Therefore, we must combine the two characteristic parameters of
δCG1 and
δTA1 to deduce the origin time of each plant branch.
In summary, we deduced the origin time of animal and plant branches based on our conjecture on the cause of the Cambrian explosion; our conclusion is consistent with the paleontological evidence. It verified that the phase transition process of the evolution mode of genome sequences is the cause of the Cambrian explosion.