The Effects of Extender Energetic Substrate Type on Goat Sperm Stored at 17 °C
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Media
2.2. Semen Collection and Processing
2.3. In Vitro Sperm Quality Evaluation
2.3.1. Sperm Motility Analysis
2.3.2. Flow Cytometry Analysis
2.4. Experimental Design
2.4.1. Experiment 1: Effects of Glucose, Fructose, Pyruvate, or Lactate as Energetic Extender Substrates on Sperm Quality Following Storage at 17 °C for 48 h
2.4.2. Experiment 2: Effects of Glucose, Pyruvate, NaCl Supplementation, and Osmolarity on Sperm Quality Following Storage at 17 °C for 48 h
2.5. Statistical Analysis
3. Results
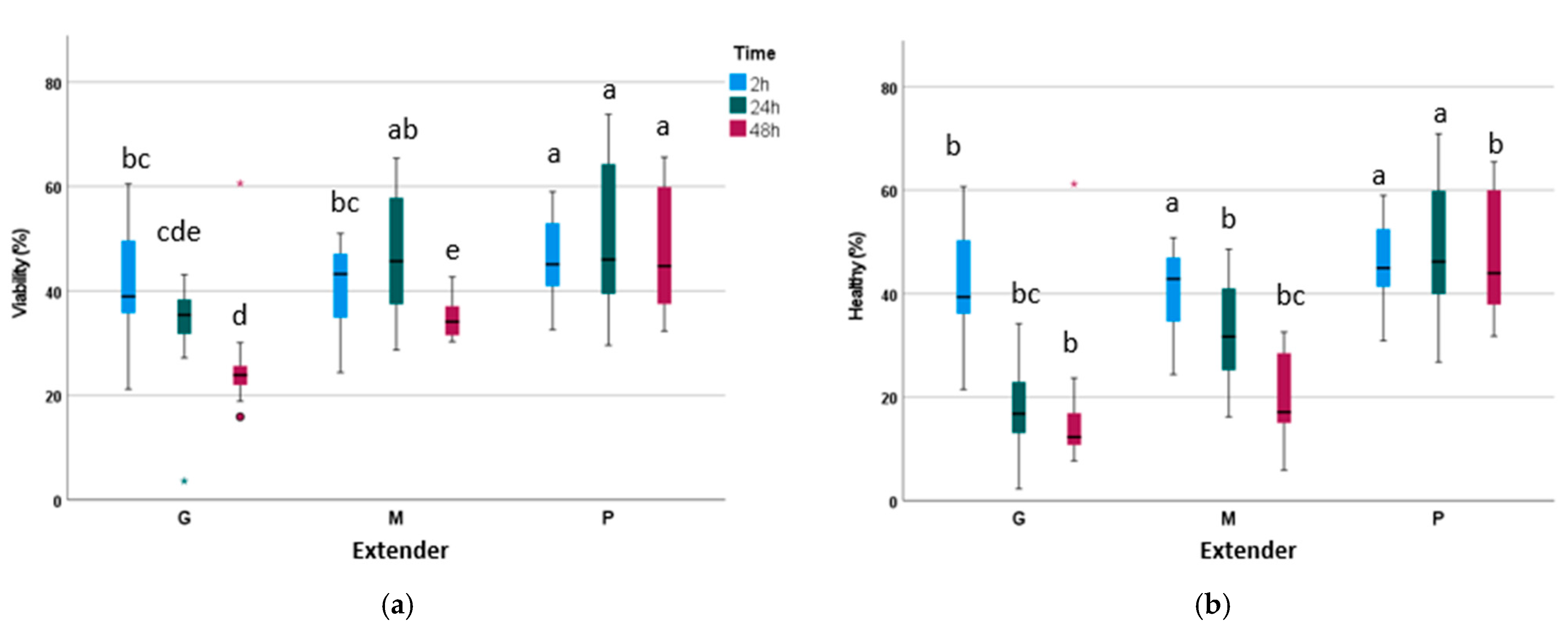
3.1. Experiment 1: Effects of Glucose, Fructose, Pyruvate, or Lactate as Energetic Extender Substrates on Sperm Quality Following Storage at 17 °C for 48 h
3.2. Experiment 2: Effects of Glucose, Pyruvate, NaCl, Osmolarity, and Energetic Substrate Concentration on Sperm Quality Following Storage at 17 °C for 48 h
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leboeuf, B.; Guillouet, P.; Batellier, F.; Bernelas, D.; Bonné, J.L.; Forgerit, Y.; Renaud, G.; Magistrini, M. Effect of native phosphocaseinate on the in vitro preservation of fresh semen. Theriogenology 2003, 60, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Mocé, E.; Mocé, M.L.; Lozano-Palazón, S.A.; Bernácer, J.; Martínez-Granell, M.M.; Esteve, I.C.; Bernat, F.; Contreras, S.J.; Villalba, I.; Gómez, E.A. Fertility prediction in dairy goats from Murciano-Granadina breed: The role of sperm evaluation and female traits. Animal 2022, 16, 100525. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, B.; Restall, B.; Salamon, S. Production and storage of goat semen for artificial insemination. Anim. Reprod. Sci. 2000, 62, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Ortiz-Rodríguez, J.M.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega-Ferrusola, C.; Martín-Cano, F.E. An integrated overview on the regulation of sperm metabolism (glycolysis-Krebs cycle-oxidative phosphorylation). Anim. Reprod. Sci. 2022, 246, 106805. [Google Scholar] [CrossRef]
- Peña, F.J.; Gibb, Z. Oxidative stress and reproductive function: Oxidative stress and the long-term storage of horse spermatozoa. Reproduction 2022, 164, F135–F144. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; Van Der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Hernández-Avilés, C.; Ramírez-Agámez, L.; Love, C.C.; Friedrich, M.; Pearson, M.; Kelley, D.E.; Beckham, A.M.N.; Teague, S.R.; LaCaze, K.A.; Brinsko, S.P.; et al. The effects of metabolic substrates glucose, pyruvate, and lactate added to a skim milk-based semen extender for cooled storage of stallion sperm. Theriogenology 2021, 161, 83–97. [Google Scholar] [CrossRef]
- Setiawan, R.; Christi, R.F.; Alhuur, K.R.G.; Widyastuti, R.; Solihati, N.; Rasad, S.D.; Hidajat, K.; Do, D.N. Impact of glucose and pyruvate on adenosine triphosphate production and sperm motility in goats. Anim. Biosci. 2024, 37, 631–639. [Google Scholar] [CrossRef]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar]
- Qiu, J.; Li, Y.; Xie, H.; Li, Q.; Dong, H.; Sun, M. Effects of glucose metabolism pathways on sperm motility and oxidative status during long-term liquid storage of goat semen. Theriogenology 2016, 86, 839–849. [Google Scholar] [CrossRef]
- Chunrong, L.; Larbi, A.; Wu, G.; Hong, Q.; Quan, G. Improving the quality of cryopreserved goat semen with a commercial bull extender supplemented with resveratrol. Anim. Reprod. Sci. 2019, 208, 106127. [Google Scholar]
- Mocé, E.; Lozano-Palazón, S.A.; Martínez-Granell, M.D.M.; Mocé, M.L.; Gómez, E.A. Effect of the Refrigeration System on In Vitro Quality and In Vivo Fertility of Goat Buck Sperm. Animals 2020, 10, 2399. [Google Scholar] [CrossRef] [PubMed]
- Gororo, E.; Zulu, P.T.; Chatiza, F.P.; Mhuka, C. Effects of different extenders and storage temperatures on longevity of small East African goat (Capra hircus) semen. Small Rumin. Res. 2019, 175, 83–89. [Google Scholar] [CrossRef]
- Roca, J.; Carrizosa, J.A.; Campos, I.; Lafuente, A.; Vazquez, J.M.; Martinez, E. Viability and fertility of unwashed Murciano-Granadina goat spermatozoa diluted in Tris-egg yolk extender and stored at 5 °C. Small Rumin. Res. 1997, 25, 147–153. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, J.; Zhao, B.; Lan, G.; Luo, M.; Chang, Z.; Sui, H.; Tan, J. Liquid Storage of Goat Semen in Chemically Defined Extenders. Reprod. Domest. Anim. 2009, 44, 771–778. [Google Scholar] [CrossRef]
- Silvestre, M.A.; Sánchez, J.P.; Gómez, E.A. Vitrification of goat, sheep, and cattle skin samples from whole ear extirpated after death and maintained at different storage times and temperatures. Cryobiology 2004, 49, 221–229. [Google Scholar] [CrossRef]
- Konyali, C.; Tomás, C.; Blanch, E.; Gómez, E.A.; Graham, J.K.; Mocé, E. Optimizing conditions for treating goat semen with cholesterol-loaded cyclodextrins prior to freezing to improve cryosurvival. Cryobiology 2013, 67, 124–131. [Google Scholar] [CrossRef]
- Gacem, S.; Castello-Ruiz, M.; Hidalgo, C.O.; Tamargo, C.; Santolaria, P.; Soler, C.; Yániz, J.L.; Silvestre, M.A. Bull Sperm SWATH-MS-Based Proteomics Reveals Link between High Fertility and Energy Production, Motility Structures, and Sperm-Oocyte Interaction. J. Proteome Res. 2023, 22, 3607–3624. [Google Scholar] [CrossRef]
- Varner, D.D.; Blanchard, T.L.; Love, C.L.; Garcia, M.C.; Kenney, R.M. Effects of semen fractionation and dilution ratio on equine spermatozoal motility parameters. Theriogenology 1987, 28, 709–723. [Google Scholar] [CrossRef]
- Batista, M.; Niño, T.; Alamo, D.; Castro, N.; Santana, M.; González, F.; Cabrera, F.; Gracia, A. Successful artificial insemination using semen frozen and stored by an ultrafreezer in the Majorera goat breed. Theriogenology 2009, 71, 1307–1315. [Google Scholar] [CrossRef]
- Gangwar, C.; Kharche, S.D.; Ranjan, R.; Kumar, S.; Goel, A.K.; Jindal, S.K.; Agrawal, S.K. Effect of vitamin C supplementation on freezability of Barbari buck semen. Small Rumin. Res. 2015, 129, 104–107. [Google Scholar] [CrossRef]
- Naing, S.W.; Wahid, H.; Mohd Azam, K.; Rosnina, Y.; Zuki, A.B.; Kazhal, S.; Bukar, M.M.; Thein, M.; Kyaw, T.; San, M.M. Effect of sugars on characteristics of Boer goat semen after cryopreservation. Anim. Reprod. Sci. 2010, 122, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ritar, A.J.; Salam, S. Fertility of fresh and frozen -thawed semen of the angora goat. Aust. J. Biol. Sci. 1983, 36, 49–60. [Google Scholar] [CrossRef]
- Salvador, I.; Viudes-De-Castro, M.P.; Bernacer, J.; Gómez, E.A.; Silvestre, M.A. Factors affecting pregnancy rate in artificial insemination with frozen semen during non-breeding season in Murciano-Granadina goats: A field assay. Reprod. Domest. Anim. 2005, 40, 526–529. [Google Scholar] [CrossRef]
- Salvador, I.; Yániz, J.; Viudes-de-Castro, M.P.; Gómez, E.A.; Silvestre, M.A. Effect of solid storage on caprine semen conservation at 5 °C. Theriogenology 2006, 66, 974–981. [Google Scholar] [CrossRef]
- Nunes, J.F.; Corteel, J.-M.; Combarnous, Y.; Baril, G.; Leboeuf, B. Rôle du plasma séminal dans la survie in vitro des spermatozoïdes de bouc. Reprod. Nutr. Dév. 1982, 22, 611–620. [Google Scholar] [CrossRef]
- Galián, S.; Peinado, B.; Almela, L.; Poto, Á.; Ruiz, S. Post-Thaw Quality of Spermatozoa Frozen with Three Different Extenders in the Murciano Granadina Goat Breed. Animals 2023, 13, 309. [Google Scholar] [CrossRef]
- Sadeghi, S.; Del Gallego, R.; García-Colomer, B.; Gómez, E.A.; Yániz, J.L.; Gosálvez, J.; López-Fernández, C.; Silvestre, M.A. Effect of sperm concentration and storage temperature on goat spermatozoa during liquid storage. Biology 2020, 9, 300. [Google Scholar] [CrossRef]
- Amaral, A. Energy metabolism in mammalian sperm motility. WIREs Mech. Dis. 2022, 14, e1569. [Google Scholar] [CrossRef]
- Van de Hoek, M.; Rickard, J.P.; de Graaf, S.P. Manipulation of metabolism to improve liquid preservation of mammalian spermatozoa. Anim. Reprod. Sci. 2024, 271, 107631. [Google Scholar] [CrossRef]
- Tsujii, H.; Ohta, E.; Miah, A.G.; Hossain, S.; Salma, U. Effect of fructose on motility, acrosome reaction and in vitro fertilization capability of boar spermatozoa. Reprod. Med. Biol. 2006, 5, 255. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, S.; Su, J.; Zhang, J.; Bao, X.; Ding, R.; Shi, P.; Li, S.; Wu, C.; Zhao, G.; et al. The effects of cryopreservation on the acrosome structure, enzyme activity, motility, and fertility of bovine, ovine, and goat sperm. Anim. Reprod. 2021, 17, e20200219. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R.S. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Ismail, N.F.B.; Calvert, S.J.; Pacey, A.A.; Paley, M.N.J. Evidence for Rapid Oxidative Phosphorylation and Lactate Fermentation in Motile Human Sperm by Hyperpolarized 13 C Magnetic Resonance Spectroscopy. Sci. Rep. 2017, 7, 4322. [Google Scholar] [CrossRef]
- Hereng, T.H.; Elgstøen, K.B.P.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Sklhegg, B.S.; Rosendal, K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef]
- Ramírez-Agámez, L.; Hernández-Avilés, C.; Ortíz, I.; Love, C.C.; Varner, D.D.; Hinrichs, K. Lactate as the sole energy substrate induces spontaneous acrosome reaction in viable stallion spermatozoa. Andrology 2023, 12, 459–471. [Google Scholar] [CrossRef]
- Becerro-Rey, L.; Martín-Cano, F.E.; Ferrusola, C.O.; Rodríguez-Martínez, H.; Gaitskell-Phillips, G.; da Silva-Álvarez, E.; Silva-Rodríguez, A.; Gil, M.C.; Peña, F.J. Aging of stallion spermatozoa stored in vitro is delayed at 22 °C using a 67 mm glucose-10 mm pyruvate-based media. Andrology 2024, 12, 1170–1185. [Google Scholar] [CrossRef]
- Atashfaraz, E.; Farokhi, F.; Najafi, G. Protective Effect of Ethyl Pyruvate on Epididymal Sperm Characteristics, Oxidative Stress and Testosterone Level in Methotrexate Treated Mice. J. Reprod. Infertil. 2013, 14, 190. [Google Scholar]
- Thompson, J.A.; Love, C.C.; Stich, K.L.; Brinsko, S.P.; Blanchard, T.L.; Varner, D.D. A Bayesian approach to prediction of stallion daily sperm output. Theriogenology 2004, 62, 1607–1617. [Google Scholar] [CrossRef]
- Varma, S.D.; Devamanoharan, P.S.; Morris, S.M. Photoinduction of cataracts in rat lens in vitro. Preventive effect of pyruvate. Exp. Eye Res. 1990, 50, 805–812. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1993, 10 (Suppl. S1), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Andrae, U.; Singh, J.; Ziegler-Skylakakis, K. Pyruvate and related α-ketoacids protect mammalian cells in culture against hydrogen peroxide-induced cytotoxicity. Toxicol. Lett. 1985, 28, 93–98. [Google Scholar] [CrossRef]
- Brand, K.A.; Hermfisse, U. Aerobic glycolysis by proliferating cells: A protective strategy against reactive oxygen species. FASEB J. 1997, 11, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Foutouhi, A.; Meyers, S. Comparative oxidative metabolism in mammalian sperm. Anim. Reprod. Sci. 2022, 247, 107095. [Google Scholar] [CrossRef] [PubMed]
- Darr, C.R.; Varner, D.D.; Teague, S.; Cortopassi, G.A.; Datta, S.; Meyers, S.A. Lactate and pyruvate are major sources of energy for stallion sperm with dose effects on mitochondrial function, motility, and ROS production. Biol. Reprod. 2016, 95, 1–11. [Google Scholar] [CrossRef]
- Upreti, G.C.; Jensen, K.; Munday, R.; Duganzich, D.M.; Vishwanath, R.; Smith, J.F. Studies on aromatic amino acid oxidase activity in ram spermatozoa: Role of pyruvate as an antioxidant. Anim. Reprod. Sci. 1998, 51, 275–287. [Google Scholar] [CrossRef]
- Sattar, A.; Farooq, M.; Khan, M.; Rehman, A.; Javed, K. 43 Effect of addition of sodium pyruvate in extender on post-thaw quality of Beetal buck semen. J. Anim. Sci. 2018, 96, 456. [Google Scholar] [CrossRef]
- Korkmaz, F.; Malama, E.; Siuda, M.; Leiding, C.; Bollwein, H. Effects of sodium pyruvate on viability, synthesis of reactive oxygen species, lipid peroxidation and DNA integrity of cryopreserved bovine sperm. Anim. Reprod. Sci. 2017, 185, 18–27. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Quadrelli, J.; Smith, N.D.; Aitken, R.J. L-carnitine and pyruvate are prosurvival factors during the storage of stallion spermatozoa at room temperature. Biol. Reprod. 2015, 93, 1–9. [Google Scholar] [CrossRef]
- Breininger, E.; Beconi, M.T. Ascorbic acid or pyruvate counteracts peroxidative damage in boar sperm cryopreserved with or without alpha-tocopherol. Anim. Sci. Pap. Rep. 2014, 32, 15–23. [Google Scholar]
- Bruemmert, J.E.; Coy, R.C.; Squires, E.L.; Graham, J.K. Effect of pyruvate on the function of stallion spermatozoa stored for up to 48 hours. J. Anim. Sci. 2002, 80, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Morel, I.; Chevanne, M.; Monnier, M.; Cillard, J.; Delamarche, A. Free radical scavenging and antioxidant effects of lactate ion: An in vitro study. J. Appl. Physiol. 2000, 89, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hugentobler, S.A.; Humpherson, P.G.; Leese, H.J.; Sreenan, J.M.; Morris, D.G. Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle. Mol. Reprod. Dev. 2008, 75, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, J.M.; Martín-Cano, F.E.; Gaitskell-Phillips, G.L.; Silva, A.; Ortega-Ferrusola, C.; Gil, M.C.; Peña, F.J. Low glucose and high pyruvate reduce the production of 2-oxoaldehydes, improving mitochondrial efficiency, redox regulation, and stallion sperm function. Biol. Reprod. 2021, 105, 519–532. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Wen, F.; Xian, M.; Guo, S.; Zhang, X.; Feng, X.; Hu, Z.; Hu, J. Glucose Starvation Inhibits Ferroptosis by Activating the LKB1/AMPK Signaling Pathway and Promotes the High Speed Linear Motility of Dairy Goat Sperm. Animals 2023, 13, 1442. [Google Scholar] [CrossRef]
- Martín-Cano, F.E.; Gaitskell-Phillips, G.; Becerro-Rey, L.; da Silva, E.; Masot, J.; Redondo, E.; Silva-Rodríguez, A.; Ortega- Ferrusola, C.; Gil, M.C.; Peña, F.J. Pyruvate enhances stallion sperm function in high glucose media improving overall metabolic efficiency. Theriogenology 2024, 215, 113–124. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef]
- Mortimer, S.T. A critical review of the physiological importance and analysis of sperm movement in mammals. Hum. Reprod. Update 1997, 3, 403–439. [Google Scholar] [CrossRef]
- Dorado, J.; Molina, I.; Muñoz-Serrano, A.; Hidalgo, M. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Florida goats. Theriogenology 2010, 74, 795–804. [Google Scholar] [CrossRef]
- Purdy, P.H. A review on goat sperm cryopreservation. Small Rumin. Res. 2006, 63, 215–225. [Google Scholar] [CrossRef]
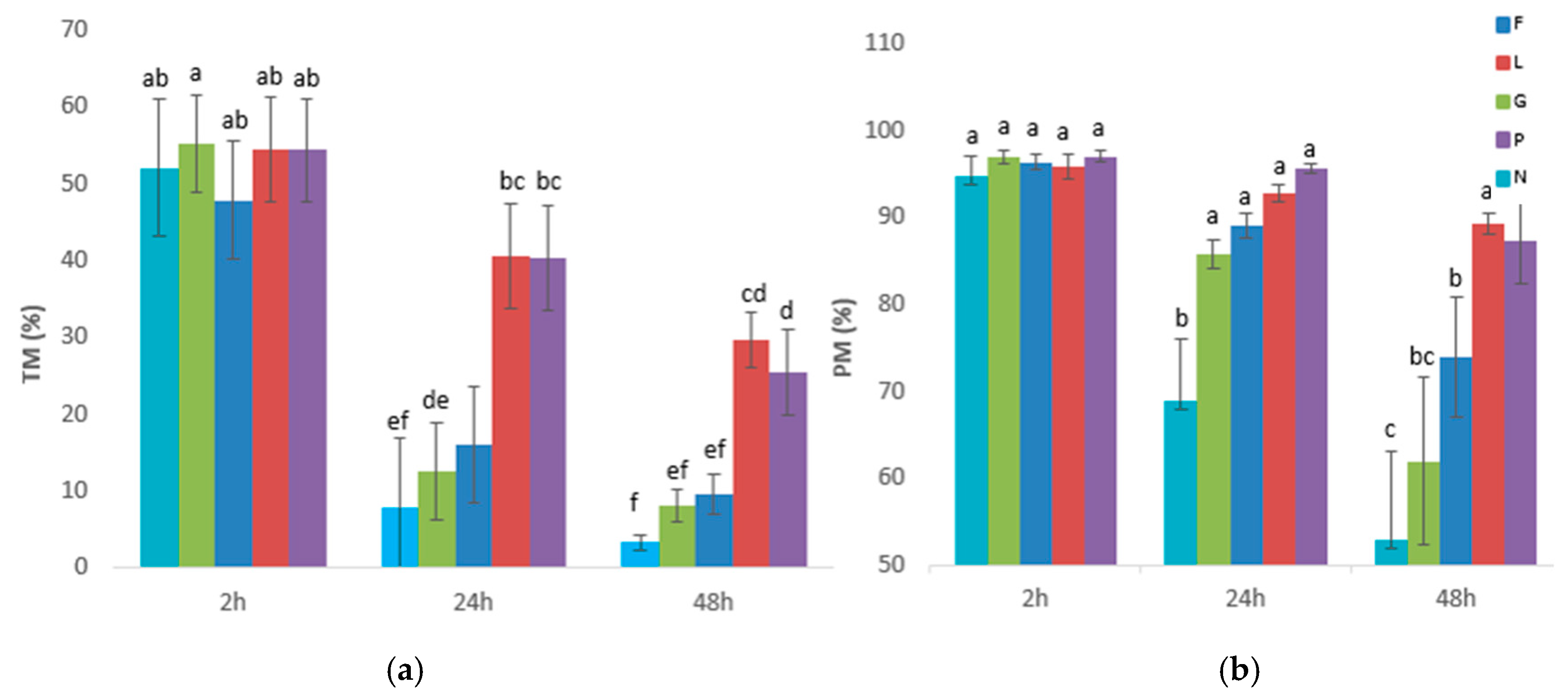
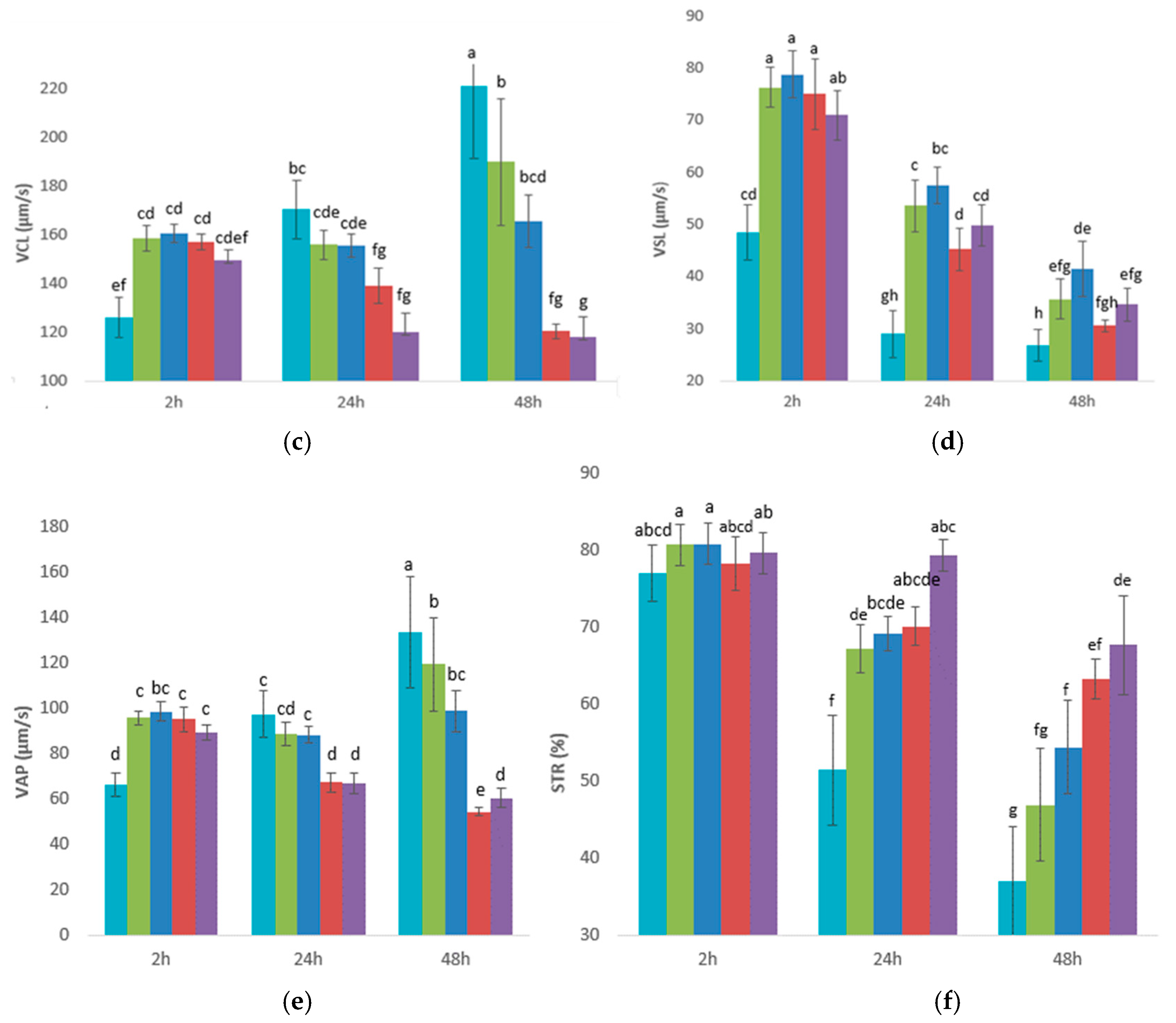
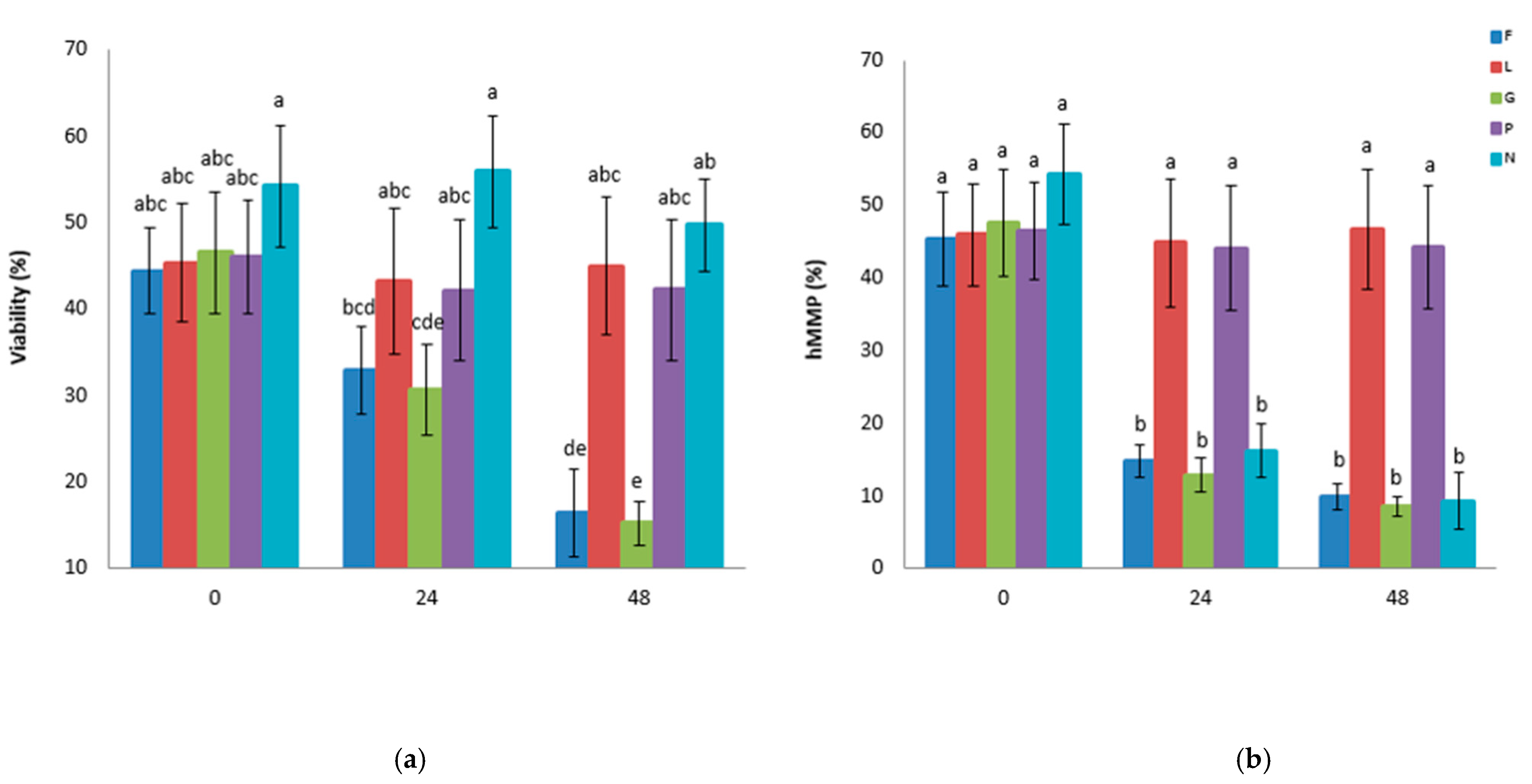
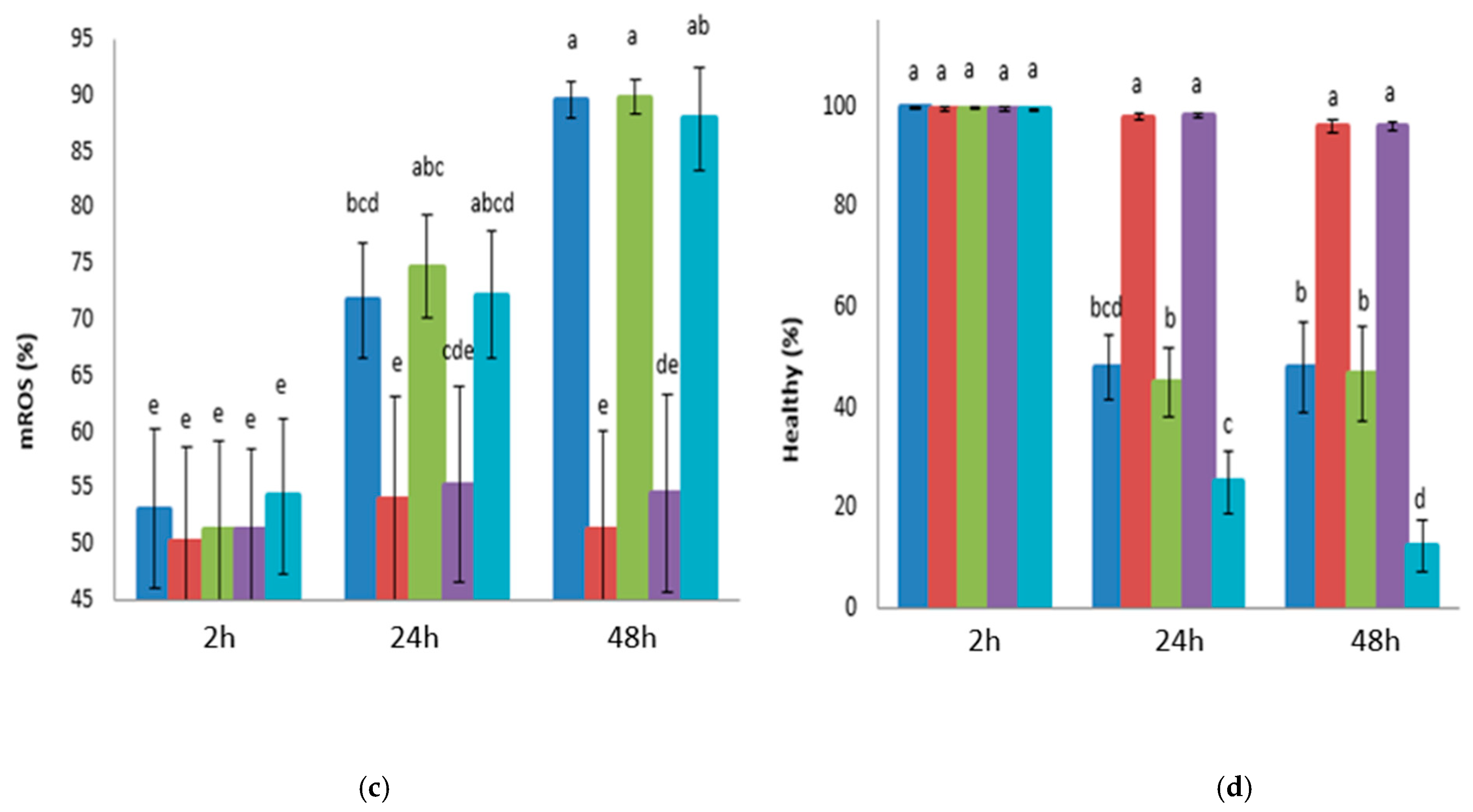
| PBS-Based Extender | Osmolarity Group | D(+) Glucose Anhydrous (mM) | D(-) Fructose (mM) | Sodium Pyruvate (mM) | Sodium DL-Lactate 60% (w/w) (mM) | NaCl (mM) | pH | Osmolarity (mOsm) |
|---|---|---|---|---|---|---|---|---|
| Experiment 1 | ||||||||
| N | - | - | - | - | 204.5 | 6.8 | 339 | |
| G | 35 | - | - | - | 102.3 | 7.0 | 345 | |
| F | - | 35 | - | - | 102.3 | 7.0 | 340 | |
| P | - | - | 35 | - | - | 6.9 | 343 | |
| L | - | - | - | 35 | - | 6.9 | 342 | |
| Experiment 2 | ||||||||
| G70 | H | 70 | - | - | - | - | 6.8 | 323 |
| G35/NaCl 18 | H | 35 | - | - | - | 17.5 | 7.0 | 342 |
| G18/NaCl 9 | H | 17.5 | - | - | - | 8.8 | 6.9 | 321 |
| G35 | L | 35 | - | - | - | - | 6.8 | 298 |
| P35 | L | - | - | 35 | - | - | 6.9 | 303 |
| P18 | L | - | - | 17.5 | - | - | 6.9 | 312 |
| P18/NaCl 9 | H | - | - | 17.5 | - | 17.5 | 6.9 | 337 |
| G35/P18 | H | 35 | - | 17.5 | - | - | 7.0 | 348 |
| G18/P9 | L | 17.5 | - | 8.75 | - | - | 7.0 | 309 |
| TM (%) | PM (%) | VCL (µm/s) | VAP (µm/s) | VSL (µm/s) | STR (%) | LIN (%) | ||
|---|---|---|---|---|---|---|---|---|
| NaCl supplementation | ||||||||
| N | 37.43 ± 1.64 | 95.21 ± 0.34 | 134.56 ± 1.86 | 92.54 ± 1.67 | 74.06 ± 1.83 | 81.42 ± 0.58 | 57.54 ± 0.91 | |
| S | 38.76 ± 2.23 | 95 ± 0.65 | 136.19 ± 2.6 | 92.14 ± 2.21 | 73.22 ± 2.57 | 80.65 ± 0.97 | 56.04 ± 1.39 | |
| Osmolarity | ||||||||
| H | 37.22 ± 1.79 | 95.02 ± 0.45 | 134.09 ± 2.07 | 91.65 ± 1.8 | 72.86 ± 2.01 * | 81.15 ± 0.68 | 56.98 ± 1.01 | |
| L | 38.69 ± 1.97 | 95.3 ± 0.41 | 136.38 ± 2.21 | 93.35 ± 1.98 | 74.94 ± 2.21 | 81.19 ± 0.76 | 57.12 ± 1.17 | |
| Extender | ||||||||
| G | 36.7 ± 1.9 | 95.32 ± 0.4 | 134.92 ± 2.11 | 93.39 ± 1.77 | 75.33 ± 2 | 82.04 ± 0.64 | 58.56 ± 1.05 | |
| M | 36.49 ± 2.84 | 94.75 ± 0.66 | 133.27 ± 3.26 | 92.91 ± 2.71 | 74.11 ± 3.02 | 81.42 ± 1.08 | 58.58 ± 1.55 | |
| P | 40.36 ± 2.28 | 95.16 ± 0.63 | 136.58 ± 2.85 | 90.74 ± 2.69 | 71.5 ± 2.95 | 79.83 ± 1.01 | 53.99 ± 1.43 |
| Viability (%) | hMMP (%) | mROS (%) | Healthy (%) | ||
|---|---|---|---|---|---|
| NaCl supplementation | |||||
| N | 40.37 ± 1.16 | 41.55 ± 2.07 | 61.45 ± 1.44 | 34.29 ± 1.51 | |
| S | 38.73 ± 1.83 | 40.34 ± 2.85 | 63.11 ± 2.30 | 32.88 ± 2.46 | |
| Osmolarity | |||||
| H | 40.11 ± 1.31 | 42.07 ± 2.24 | 61.51 ± 1.62 | 34.79 ± 1.7 | |
| L | 39.45 ± 1.5 | 39.97 ± 2.53 | 62.64 ± 1.88 | 32.59 ± 2 | |
| Extender | |||||
| G | 33.18 ± 1.28 c | 35.73 ± 2.57 b | 69.93 ± 1.74 a | 24.98 ± 1.78 c | |
| M | 40.85 ± 1.64 b | 40.24 ± 3.36 b | 62.55 ± 2.09 b | 31.13 ± 2.07 b | |
| P | 47.87 ± 1.54 a | 48.87 ± 2.62 a | 51.23 ± 1.61 c | 47.23 ± 1.51 a |
| Viability | hMMP | mROS | Healthy | TM | PM | VCL | VAP | VSL | STR | LIN | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time × NaCl suppl | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Time × Osmolarity | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS |
| Time × Extender | ** | * | ** | ** | NS | NS | NS | NS | NS | NS | NS |
| Time × NaCl suppl × Osmolarity | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Time × NaCl suppl × Extender | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Time × Osmolarity × Extender | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Glucose | Pyruvate | ||||
|---|---|---|---|---|---|
| 18 | 35 | 70 | 18 | 35 | |
| Sperm quality parameters | |||||
| Viability (%) | 40.07 ± 1.16 a | 33.98 ± 1.32 ab | 31.93 ± 2.05 b | 52.12 ± 1.95 a | 40.29 ± 4.37 b |
| hMMP (%) | 40.89 ± 1.19 b | 30.91 ± 1.86 c | 54.43 ± 6.24 a | 36.86 ± 3.82 b | 42.47 ± 5.76 a |
| mROS (%) | 59.83 ± 1.14 c | 62.98 ± 1.31 b | 74.75 ± 3.08 a | 48.13 ± 2.45 b | 60.58 ± 5.14 a |
| Healthy (%) | 99.36 ± 0.2 a | 90.38 ± 2.65 b | 64.93 ± 5.93 c | 87.47 ± 3.39 a | 73.84 ± 5.74 b |
| Sperm motility parameters | |||||
| TM (%) | 31.78 ± 3.03 b | 48.81 ± 2.47 a | 20.79 ± 2.71 c | 34.98 ± 2.31 b | 56.53 ± 3.81 a |
| PM (%) | 93.52 ± 1.37 b | 96.57 ± 0.38 a | 94.22 ± 0.65 b | 93.91 ± 0.96 b | 97.04 ± 0.42 a |
| VCL (µm/s) | 141.57 ± 4.43 b | 149.78 ± 3.13 a | 112.76 ± 2.56 c | 133.06 ± 3.28 b | 140.74 ± 3.25 a |
| VAP (µm/s) | 93.83 ± 4.75 b | 103.58 ± 2.75 a | 79.96 ± 2.8 c | 87.66 ± 2.92 b | 101.15 ± 1.79 a |
| VSL (µm/s) | 73.06 ± 5.95 b | 84.94 ± 2.75 a | 63.22 ± 2.15 c | 67.22 ± 3.75 b | 83 ± 1.77 a |
| STR (%) | 80.42 ± 2.06 b | 80.6 ± 0.73 b | 84.85 ± 0.59 a | 79.32 ± 1.43 a | 80.89 ± 0.75 a |
| LIN (%) | 53.78 ± 2.87 c | 57.54 ± 1.17 b | 61.61 ± 1.37 a | 53.2 ± 1.97 b | 60.64 ± 1.37 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacem, S.; Mocé, E.; Gozalbo, C.; Albuixech-Benetó, M.; Esteve, I.C.; Martínez-Talaván, A.; Silvestre, M.A. The Effects of Extender Energetic Substrate Type on Goat Sperm Stored at 17 °C. Biology 2025, 14, 782. https://doi.org/10.3390/biology14070782
Gacem S, Mocé E, Gozalbo C, Albuixech-Benetó M, Esteve IC, Martínez-Talaván A, Silvestre MA. The Effects of Extender Energetic Substrate Type on Goat Sperm Stored at 17 °C. Biology. 2025; 14(7):782. https://doi.org/10.3390/biology14070782
Chicago/Turabian StyleGacem, Sabrina, Eva Mocé, Carmen Gozalbo, Marta Albuixech-Benetó, Inés C. Esteve, Amparo Martínez-Talaván, and Miguel A. Silvestre. 2025. "The Effects of Extender Energetic Substrate Type on Goat Sperm Stored at 17 °C" Biology 14, no. 7: 782. https://doi.org/10.3390/biology14070782
APA StyleGacem, S., Mocé, E., Gozalbo, C., Albuixech-Benetó, M., Esteve, I. C., Martínez-Talaván, A., & Silvestre, M. A. (2025). The Effects of Extender Energetic Substrate Type on Goat Sperm Stored at 17 °C. Biology, 14(7), 782. https://doi.org/10.3390/biology14070782






