Simple Summary
Rain and other extreme weather can lower salt levels in coastal waters, making it harder to farm Japanese scallops. This study tested how scallops respond to low-salt water over time. When salt levels dropped too much, scallops grew poorly, used more energy, and many died. Some were able to adjust at moderate salt levels, but at very low levels, their defenses stopped working. The study also looked at changes in gene activity to understand how scallops respond to stress. These results can help improve scallop farming by showing which salt levels are safe and which should be avoided.
Abstract
Extreme weather events such as heavy rainfall significantly reduce surface salinity in coastal waters, presenting considerable challenges to the aquaculture of Japanese scallops (Mizuhopecten yessoensis) in shallow cage systems. This study investigated the effects of chronic low-salinity stress on the growth performance, antioxidant capacity, and gene expression profile of M. yessoensis using a 60-day salinity gradient experiment. S33 represents the control treatment with normal seawater salinity (33‰), while S30, S28, and S26 represent experimental groups with progressively lower salinities of 30‰, 28‰, and 26‰, respectively. A decline in salinity was accompanied by an increase in oxygen consumption. The S26 group exhibited a higher ammonia excretion rate (2.73 μg/g·h) than other groups, indicating intensified nitrogen metabolism. Growth was inhibited under low-salinity conditions. The S33 group exhibited greater weight gain (16.7%) and shell growth (8.4%) compared to the S26 group (11.6% and 6%), which also showed a substantially higher mortality rate (46%) compared to the control (13%). At 28‰, antioxidant enzyme activities (T-AOC, SOD, CAT, POD) were elevated, indicating a moderate level of stress. However, at the lowest salinity (26‰), these indicators decreased, reflecting the exhaustion of the antioxidant systems and indicating that the mollusks’ adaptive capacity had been exceeded, leading to a state of stress fatigue. NAD-MDH activity was elevated in the S26 group, reflecting enhanced aerobic metabolism under stress. Transcriptome analysis revealed 564 differentially expressed genes (DEGs) between the S33 and S26 groups. Functional enrichment analysis indicated that these DEGs were mainly associated with immune and stress response pathways, including NF-κB, TNF, apoptosis, and Toll/Imd signaling. These genes are involved in key metabolic processes, such as alanine, aspartate, and glutamate metabolism. Genes such as GADD45, ATF4, TRAF3, and XBP1 were upregulated, contributing to stress repair and antioxidant responses. Conversely, the expressions of CASP3, IKBKA, BIRC2/3, and LBP were downregulated, potentially mitigating apoptosis and inflammatory responses. These findings suggest that M. yessoensis adapts to chronic low-salinity stress through the activation of antioxidant systems, modulation of immune responses, and suppression of excessive apoptosis. This study provides new insights into the molecular mechanisms underlying salinity adaptation in bivalves and offers valuable references for scallop aquaculture and selective breeding programs.
1. Introduction
Mizuhopecten yessoensis is a large cold-water bivalve widely distributed along the coastal waters of Northeast Asia. It is considered a major species in regional aquaculture. In northern coastal China, it is regarded as one of the most important cultivated bivalves [1]. This species also plays a significant ecological role. As a filter-feeding mollusk, it helps purify water by consuming phytoplankton. Its carbon-rich shell and tissues contribute to long-term CO2 sequestration, thus supporting marine carbon sink functions [2,3]. Previous studies have shown that filter-feeding bivalves have high carbon content. Consequently, the expansion of coastal bivalve aquaculture may contribute to marine carbon fixation [4]. Therefore, M. yessoensis holds not only economic value for fisheries but also ecological significance as a key species in carbon sequestration and ecosystem functioning [5].
In recent years, increasing fluctuations in surface salinity in nearshore waters have become a prominent feature of coastal aquaculture environments [6]. These fluctuations are primarily attributed to the increasing frequency of extreme weather events—such as heavy rainfall and prolonged drought—driven by global climate change. Such events may cause abrupt and localized changes in salinity, imposing physiological stress on marine organisms and, under severe conditions, resulting in mass mortality [7,8]. For M. yessoensis, which inhabits surface waters in suspended raft cages, sudden drops in salinity following rainstorms may trigger acute low-salinity stress [9,10]. This species is highly sensitive to salinity variation. When salinity levels temporarily fall below its tolerance range (25‰), it may experience stress responses and physiological disorders that seriously threaten its survival [11]. Consequently, the growing prevalence of low-salinity conditions in coastal waters presents a critical challenge for the sustainable cultivation of M. yessoensis. This highlights the importance of understanding its physiological and molecular responses to salinity stress.
Salinity fluctuations exert profound effects on the physiological metabolism and immune–oxidative functions of bivalves. When salinity deviates from optimal levels, bivalves often adjust their metabolic activity to maintain osmotic balance. These adjustments are typically reflected in changes in energy metabolism indicators, such as oxygen consumption and ammonia excretion rates. Disruptions in these processes may lead to energy imbalance, growth impairment, or even mortality [12]. Moreover, low-salinity stress is often accompanied by oxidative damage and immune activation [13]. Sudden changes in salinity are known to disrupt the physiological balance of bivalves, particularly through the induction of oxidative stress and immune dysfunction. Several studies have shown that acute low-salinity exposure significantly alters key antioxidant and immune indicators. For instance, under salinity shock, oysters exhibit changes in the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) alongside fluctuations in immune-related enzymes like alkaline phosphatase (AKP) and lysozyme (LSZ) [14]. Additionally, levels of malondialdehyde (MDA), a marker of lipid peroxidation and oxidative damage, can increase dramatically—up to 100-fold in extreme cases [15]. While these physiological changes clearly reflect stress responses, interpreting them solely based on linear cause–effect models may oversimplify the underlying mechanisms. In fact, increasing evidence in redox biology suggests that the relationship between oxidative stress and cellular response is often non-linear and biphasic. This phenomenon, commonly referred to as hormesis or a bell-shaped response, posits that low to moderate levels of reactive oxygen species (ROS) can activate beneficial adaptive mechanisms—such as the upregulation of antioxidant defenses and stress-responsive genes—whereas excessive ROS production overwhelms the system, leading to cellular damage, inflammation, and apoptosis [16,17,18].
To better understand how bivalves adapt to salinity stress, recent studies have explored their molecular responses to low-salinity conditions. Evidence suggests that bivalves or mollusks activate various stress-related signaling pathways and gene expression responses under low-salinity exposure [19,20]. Transcriptomic analyses indicate that low-salinity stress leads to the reprogramming of key biological pathways. These include NF-κB and TNF signaling pathways, which are involved in innate immunity and inflammation, as well as apoptosis and energy metabolism pathways, such as the tricarboxylic acid (TCA) cycle and amino acid metabolism [21]. For example, under low-salinity conditions, bivalves may improve survival by regulating apoptosis-related gene expression. Anti-apoptotic genes, such as those encoding BIR domain-containing proteins (BIRC2/3), are often upregulated, whereas pro-apoptotic genes like caspase-3 (CASP3) may be downregulated. This expression profile suggests that marine organisms may adapt to salinity stress by limiting excessive apoptosis [21].
However, most existing studies have concentrated on the physiological, biochemical, or molecular responses to acute or short-term salinity stress, while much less is known about how bivalves respond to chronic low-salinity conditions. In particular, comprehensive transcriptomic analyses examining long-term gene expression changes under extended low-salinity exposure remain scarce. This knowledge gap limits our understanding of the mechanisms underlying salinity tolerance in bivalves. It also constrains the development of effective aquaculture management strategies and targeted selective breeding programs.
In light of these challenges, the present study investigated the responses of M. yessoensis to chronic low-salinity stress using a 60-day exposure experiment. High-throughput RNA sequencing (RNA-seq) was employed to systematically profile transcriptomic changes in scallops subjected to prolonged low-salinity exposure. The analysis focused on the regulation of antioxidant enzyme activity, modulation of apoptosis-related signaling pathways, and reprogramming of energy metabolism. By examining adaptive responses in oxidative defense, apoptotic regulation, and metabolic adjustment, this study aims to elucidate the physiological and molecular mechanisms by which M. yessoensis adapts to chronic low-salinity stress. The findings will provide a theoretical foundation for improving environmental management practices and advancing the development of salinity-tolerant scallop strains.
2. Materials and Methods
2.1. Ethics Statement
M. yessoensis individuals used in this study were all artificially bred from the same cohort in Xiaoyaowan, Jinzhou Development Zone, Dalian, China. All experimental procedures were conducted in strict accordance with the relevant national guidelines of China and the institutional regulations of Dalian Ocean University.
2.2. Experimental Animals and Stress Design
The salinity stress experiment was conducted at the Key Laboratory of Mariculture, Ministry of Agriculture, Dalian Ocean University. All experimental M. yessoensis individuals were 1.5 years old. Prior to the experiment, surface debris was removed from the M. yessoensis, and sand-filtered seawater was prepared for the stress treatments. The scallops were maintained in a recirculating aquaculture system and acclimated by feeding Chlorella for one week. A total of 180 healthy individuals were randomly assigned to 12 square tanks (100 L each), with 15 scallops per tank. Prior to the experiment, the shell length, shell width, shell height, and wet weight were measured for each individual. Chlorella was administered twice daily at 7:00 a.m. and 4:30 p.m. Seawater was renewed every three days via siphoning, and feces were removed on a daily basis. The rearing conditions were maintained under low-light intensity, with the natural seawater temperature gradually declining from 18 °C to 13 °C. The pH was maintained between 7.6 and 8.3; dissolved oxygen levels were kept above 6 mg/L; and ammonia nitrogen and nitrite concentrations were maintained below 0.05 mg/L.
Chronic low-salinity stress was simulated through the proportional addition of distilled water, reducing salinity by 2‰ every three days to avoid abrupt drops at the beginning of the experiment. Four salinity levels were established: 33‰, 30‰, 28‰, and 26‰, designated as S33, S30, S28, and S26, respectively. Since the salinity of normal seawater is around 33‰, salinity of 33‰ was set as the control group. Each salinity treatment group included three replicates. The experiment lasted for 60 days, during which salinity was adjusted using a mixture of seawater and distilled water and monitored with a salinometer.
2.3. Sample Collection
At the beginning and end of the experiment, 100 mL of seawater was collected from each tank to measure the dissolved oxygen consumption and ammonia excretion rates. Upon completion of the experiment, all Mizuhopecten yessoensis individuals in each tank were measured for shell length, shell width, shell height, wet weight, soft tissue weight, and shell weight. Subsequently, ten individuals were randomly selected from each tank for tissue sampling. The soft tissue, adductor muscle, and visceral mass were dissected, immediately flash-frozen in liquid nitrogen, and transferred into 1.5 mL RNase-free microcentrifuge tubes (Axygen, Union City, CA, USA). Samples were stored at −80 °C for subsequent enzyme activity assays, gene expression analysis, and transcriptomic sequencing.
2.4. Growth and Survival Assessment
During the experiment, we considered the scallops dead if their shells remained open and could not close again. These individuals were included in the mortality count. At the end of the experiment, we measured the shell length, shell width, and shell height of each scallop using a vernier caliper. The wet weight, soft body weight, and shell weight of each scallop were measured using an electronic balance.
2.5. Measurement of Oxygen Consumption and Ammonia Excretion Rates
Dissolved oxygen (DO) levels at the start and end of the experiment were recorded using a HACH water quality analyzer. Ammonium nitrogen (NH4+-N) concentrations were determined using the Nessler’s reagent method, appropriate for seawater.
The oxygen consumption rate (RO) and ammonia excretion rate (RN) per unit dry mass were calculated using the following formulas:
where DO and NH4+-N concentrations (mg/L or μmol/L) were measured at the start and end of the experiment, volume represents the volume of water (L), dry mass is the soft tissue mass (g), and time refers to the total experimental duration (h).
RO = [(Initial DO − Final DO) × Volume]/(Dry Mass × Time)
RN = [(Final NH4+-N − Initial NH4+-N) × Volume]/(Dry Mass × Time)
2.6. Enzyme Activity Assays
Visceral mass tissues collected at the end of the experiment were homogenized for antioxidant and immune enzyme assays. The parameters included catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), malondialdehyde (MDA) content, NAD-dependent malate dehydrogenase (NAD-MDH) activity, and total protein content. All assays were conducted using commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s protocols.
2.7. Sample Collection and RNA Extraction
RNA Isolation and cDNA Synthesis: Total RNA was extracted from the body wall of M. yessoensis by using TianGen’s RNA Easy Fast kit (DP451) and FastKing gDNA Dispelling RT SuperMix (KR118) (TianGen, Beijing, China) [22]. The RNA integrity was confirmed via agarose gel electrophoresis by using 50X TAE Buffer (B548101) and Agarose, Regular (A620014) (Sangon Biotech, Shanghai, China) [23]. cDNA synthesis was performed under the following conditions: incubation at 37 °C for 15 min, denaturation at 85 °C for 5 s, and storage of the cDNA at −20 °C.
Primer Design: Primers for RT-PCR were devised using NCBI’s Primer BLAST (2.15.0), targeting specific sequences within the coding regions identified in the NCBI database. This rigorous approach yielded eight pairs of primers, including the reference gene primer Cytochrome b (Cytb-R), cited directly from the relevant literature [24] (Table 1).

Table 1.
Real-time PCR primers used in the present study.
Quantitative PCR Setup and Analysis: The qPCR was performed by using the FastKing One Step RT-PCR Kit (KR123) (TianGen, Beijing, China) [25]. The qPCR employed SYBR Green I chemistry in a 20 μL reaction mixture containing 10 μL 2 × TransStart® Tip Green qPCR Super Mix, 0.8 μL each of forward and reverse primers (20μM), 2 μL of cDNA, and 6.4 μL of RNase-free water. PCR cycling consisted of an initial denaturation at 95 °C for 120 s, followed by 45 cycles of 95 °C for 5 s, and 95 °C for 10 s for annealing/extension, concluding with a melt curve analysis.
Standard Curve and Dilution: Standard curves for the reference and target genes were established using high-expression samples at an initial concentration of 50 ng/μL, followed by a tenfold dilution series across five points, each replicated three times. The optimal reaction cDNA concentration was determined to be 5 ng/μL, achieved by diluting 50 ng/μL cDNA tenfold with DEPC-treated water.
Relative expression levels were calculated using the 2−ΔΔCT method. This involved computing ΔCT as the difference between the CT values of the target and reference genes, normalizing ΔCT to a control group to get ΔΔCT, and determining the fold change as 2−ΔΔCT. Results were expressed as the means and standard deviations.
2.8. Library Construction and High-Throughput Sequencing
Qualified RNA samples were submitted to Annoroad Gene Technology (Beijing, China) for library preparation and sequencing on an Illumina HiSeq™ 2500 platform (Illumina, San Diego, CA, USA). Poly(A) tails were isolated to enrich mRNA, which was then fragmented and reverse-transcribed to synthesize cDNA. Double-stranded cDNA underwent end repair, A-tailing, adapter ligation, and PCR amplification. Library fragments were approximately 300 bp in length. Sequencing was conducted using a 150 bp paired-end (PE150) strategy, generating over 20 million clean reads per sample (ASM211388v2).
2.9. Quality Control and Sequence Assembly
Raw sequence data were assessed using FastQC (v0.11.9). Low-quality reads, adapter sequences, and reads with high N content were removed using Trimmomatic to obtain clean reads. Trinity (v2.15.1) software was used for de novo transcriptome assembly with default parameters. Unigenes were annotated by aligning against the NR, COG/KOG, GO, Swiss-Prot, eggNOG, and KEGG databases. All transcriptome data were deposited in the NCBI database (Accession: PRJNA1263710).
2.10. Differential Gene Expression Analysis
Read counts were normalized using DESeq2 (v1.40.2) software. Differentially expressed genes (DEGs) were identified using a false discovery rate (FDR) < 0.05 and |log2FoldChange| > 1. Volcano plots were used for visualization. KEGG pathway enrichment of DEGs was performed using the clusterProfiler (v4.8.2) package, applying Fisher’s exact test and FDR correction.
2.11. Data Analysis
Data were analyzed using SPSS 22.0 statistical software. Initially, the normality of data distribution was assessed with the Shapiro–Wilk test, and the homogeneity of variances was evaluated with Levene’s test. Subsequently, one-way analysis of variance (ANOVA) was employed to determine the significance among dietary groups. If significance (p < 0.05) was detected, Tukey’s multiple comparisons were used to identify the significant differences between dietary groups. All data were presented as means ± standard errors (SEs).
3. Results
3.1. Effects of Different Low-Salinity Levels on Shell Length Growth Rate, Wet Weight Gain, and Mortality of M. yessoensis
The effects of different salinity levels on the growth and survival of M. yessoensis are shown in Figure 1. No significant differences in shell length were observed among the groups (p > 0.05), although a decreasing trend was noted with reduced salinity. The highest shell length growth rate was observed in the S33 group (8.4%) and the lowest in the S26 group (6%). Some groups showed differences in wet weight gain rate (p < 0.05); S33 (16.7%) and S30 (16.6%) were higher than S26 (11.6%), and an overall decreasing trend was associated with lower salinity. Mortality increased with declining salinity, with S26 reaching 46%, while the minimum observed value was 13% in the S33 group.

Figure 1.
Shell length growth rate, wet weight growth rate, and mortality rate of Mizuhopecten yessoensis under low-salt stress. Different letters represent significance (p < 0.05), while the same letters do not represent significance (p > 0.05).
3.2. Effects of Different Low-Salinity Levels on Oxygen Consumption and Ammonia Excretion
Oxygen consumption and ammonia excretion under different salinity levels are shown in Figure 2. Oxygen consumption showed no significant differences among groups (p > 0.05) but tended to increase as salinity decreased. Ammonia excretion showed differences among some groups (p < 0.05); in particular, the S26 group had the highest value, reaching 2.73 μg/g·h.

Figure 2.
Oxygen consumption rate and ammonia excretion rate of Mizuhopecten yessoensis under low-salt stress. Different letters represent significance (p < 0.05), while the same letters do not represent significance (p > 0.05).
3.3. Effects of Different Low-Salinity Levels on Enzyme Activities in M. yessoensis
Under different salinity conditions, significant variations were observed in the activity or content of CAT, MDA, POD, SOD, T-AOC, and NAD-MDH in the visceral mass (Figure 3). CAT activity (Figure 3b) fluctuated with salinity following a “rise–fall–rise–fall” pattern and peaked at 28‰ (238.02 U/mg prot). MDA content (Figure 3c) showed a “rise–fall–rise” trend. No differences were observed between the 28‰ and 30‰ groups (p ≥ 0.05). POD activity (Figure 3e) followed a “rise–fall–rise” pattern, with a maximum at 28‰ (203.22 U/mg prot) and a minimum at 30‰. SOD activity (Figure 3f) showed a “fall–rise–fall” pattern, with a peak at 28‰ (27.68 U/mg prot), and the lowest value in the 30‰ group. T-AOC (Figure 3a) exhibited a similar “rise–fall” trend and reached a maximum at 28‰ (0.44 mmol/g), indicating enhanced total antioxidant capacity at this salinity level. NAD-MDH activity (Figure 3d) also followed a fluctuating trend, peaking at 26‰ (22,580.31 U/g), and was higher than in the other groups.

Figure 3.
Changes in enzyme activities of Mizuhopecten yessoensis under low-salt stress. Different letters indicate statistically significant differences (p < 0.05), while the same letters indicate no significant difference (p > 0.05). Panel (a) shows the activity of T-AOC (Total Antioxidant Capacity), (b) shows the activity of CAT (Catalase), (c) shows the level of MDA (Malondialdehyde), (d) shows the activity of NAD-MDH (NAD-dependent Malate Dehydrogenase), (e) shows the activity of POD (Peroxidase), and (f) shows the activity of SOD (Superoxide Dismutase).
3.4. Differential Gene Expression
We observed that the S26 group exhibited more severe physiological damage compared to the other experimental groups. Therefore, transcriptomic analysis was conducted between the S26 and S33 groups to investigate the molecular responses under extreme salinity stress. Differentially expressed genes (DEGs) were screened using |log2FoldChange| > 1 and p < 0.05. As shown in Figure 4, a total of 564 DEGs were identified, including 264 upregulated and 300 downregulated genes.

Figure 4.
Volcano map of differential genes.
Representative DEGs and their functions are summarized in Table 2. Key genes included CASP3, IKBKA, and BIRC2/3, involved in NF-κB signaling and apoptosis inhibition; ABCG1 and LBP, associated with lipid transport and LPS recognition; ATF4 and GADD45, linked to stress response and DNA repair; TRAF3, participating in TNF receptor signaling; and several metabolism-related genes such as CAPN1, ASNS, glnA, and gadB, involved in maintaining osmotic balance and energy metabolism.

Table 2.
Key genes in the key KEGG pathways.
3.5. KEGG Pathway Enrichment
According to the KEGG enrichment results (Figure 5), DEGs were mapped to several signaling and metabolic pathways. Immune and stress-related pathways included the Toll and Imd signaling pathways, TNF signaling pathway, PI3K-Akt pathway, MAPK pathway, NF-κB signaling, and FoxO pathway. These enrichments suggest that low-salinity stress may trigger immune and inflammatory responses in M. yessoensis.
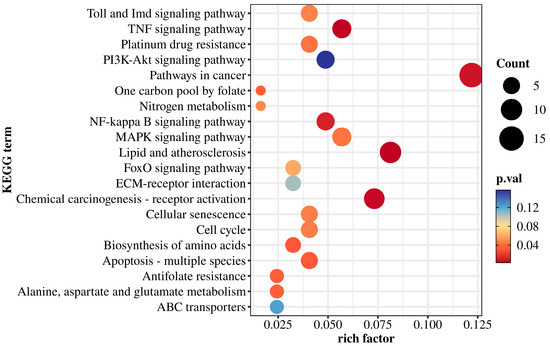
Figure 5.
KEGG enrichment bubble plot.
Pathways associated with cell fate regulation were also enriched, including cellular senescence, cell cycle, apoptosis (multiple species), and cancer-related pathways. These results imply possible activation of cell cycle arrest and apoptotic mechanisms under low-salinity stress.
Additionally, several metabolic pathways were enriched, such as nitrogen metabolism, one-carbon pool by folate, amino acid biosynthesis, alanine, aspartate and glutamate metabolism, and ABC transporters.
Five pathways were closely related to low-salinity stress response, including Pathways in cancer (map05200), Lipid and atherosclerosis (map05417), Apoptosis (map04210), NF-kappa B signaling (map04064), and TNF signaling (map04668). These pathways contained multiple DEGs with potential biological relevance in oxidative stress, apoptosis, inflammation, and metabolic regulation.
In the lipid oxidation pathway (Figure 6a), key upregulated genes included LDLR, APOB, ABCA1, TLR2, MYD88, TRAF3, ATF4, and XBP1; downregulated genes included LBP, ABCG1, IKBKA, and CASP3. In the TNF signaling pathway (Figure 6b), upregulated genes included TRAF3 and CREB1; downregulated genes included BIRC2/3, MAP3K8, IKBKA, CASP3, RPS6KA5, and IKBKG. In the NF-κB pathway (Figure 6c), MYD88, TRAF3, and GADD45 were upregulated, whereas BIRC2/3, IKBKA, and LBP were downregulated. In the apoptosis pathway (Figure 7a), ATF4, CAPN1, TUBA, ACTB-G, and GADD45 were upregulated, while IKBKA, BIRC2/3, and CASP3 were downregulated. In the cancer pathway (Figure 7c), five genes (WNT1, DLL, TRAF1, GST, and GADD45) were upregulated, while CASP3, IKBKA, STAT5A, RPS6KA5, BIRC5, BIRC2/3, and SKP2 were downregulated.
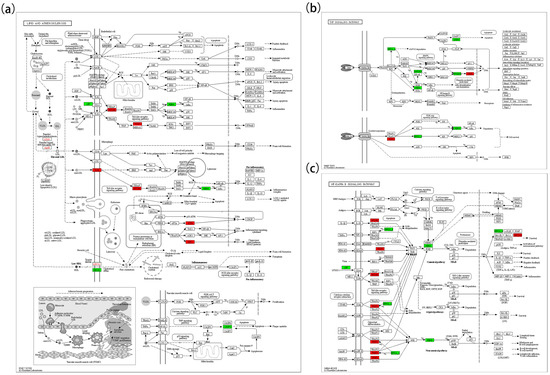
Figure 6.
KEGG map 1. (a) Represents the Lipid and Atherosclerosis pathway (map05417); (b) represents the TNF signaling pathway (map04668); (c) represents the NF-κB signaling pathway (map04064).
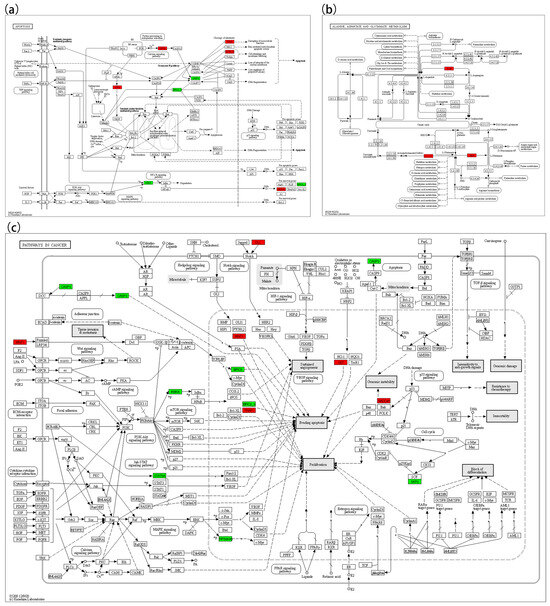
Figure 7.
KEGG map 2. (a) Represents Apoptosis (map4010); (b) represents the Alanine, Aspartate and Glutamate Metabolism pathway (map00250); (c) represents Pathways in cancer (05200).
3.6. qRT-PCR and RNA-Seq
The differential expression of genes was validated by qRT-PCR. The gene validation results indicated that the relative expression levels of 11 tested genes measured by qRT-PCR were generally consistent with the trends observed in the RNA-Seq data (Figure 8).

Figure 8.
qRT-PCR validation of differentially expressed genes.
4. Discussion
4.1. Antioxidant Response
In a sustained low-salinity environment, Mizuhopecten yessoensis is challenged not only by osmotic stress but also by complex, multilayered physiological responses. Due to being cultured in suspended cages, this species is particularly susceptible to salinity fluctuations, which can affect growth, energy metabolism, immune function, and gene expression. Salinity is a well-established ecological factor influencing bivalve adaptability; it regulates cellular osmosis and modulates multiple physiological pathways through signaling mechanisms, thereby influencing development, reproduction, and stress resistance.
After 60 days of low-salinity exposure, the antioxidant capacity of the scallops in the S26 group changed markedly compared to the S33 group. T-AOC and key antioxidant enzyme activities, such as those of superoxide dismutase (SOD) and peroxidase (POD), increased, while malondialdehyde (MDA) levels also rose, suggesting oxidative stress induced by low salinity [5]. Osmotic imbalance under low-salinity conditions can lead to excessive reactive oxygen species (ROS) production [26,27], which in turn activates antioxidant enzymes that scavenge superoxide radicals and hydrogen peroxide [28]. Transcriptomic data further showed upregulation of glutathione S-transferase (GST), an enzyme involved in ROS detoxification [29,30]. In addition, stress-related transcription factors ATF4 and XBP1 were upregulated, potentially indicating endoplasmic reticulum (ER) stress activation [30]. The unfolded protein response (UPR) initiated by ER stress can enhance molecular chaperone expression and elevate antioxidant defenses, acting synergistically with classical antioxidant enzymes [30].
Nevertheless, the elevated MDA levels indicate that ROS generation may have outpaced elimination, leading to oxidative damage [27]. When ROS production exceeds the antioxidant capacity, lipid peroxidation ensues, resulting in MDA accumulation and potentially initiating apoptosis [26,30]. These findings suggest that while the scallops activated antioxidant defenses in response to chronic low-salinity stress, a persistent redox imbalance still occurred. This pattern can be interpreted through the lens of the hormetic model, which offers a meaningful framework for understanding oxidative stress responses in bivalves under salinity fluctuations. According to this model, moderate increases in ROS can stimulate protective adaptations, including the upregulation of antioxidant enzymes—a trend reflected in the enhanced SOD, CAT, and POD activities observed under moderate salinity reduction. However, when salinity drops below a critical threshold, the balance shifts. The sharp elevation in MDA levels, together with signs of impaired enzyme function, suggests that ROS accumulation exceeded cellular tolerance, pushing the system from adaptation into damage. As Hong noted, ROS at physiological levels play crucial roles in signaling and homeostasis, but excessive ROS accumulation transforms their role from functional to cytotoxic [31].
Therefore, the redox state of scallops under chronic low-salinity stress appears to follow a biphasic response curve: initial activation of defenses under tolerable stress, followed by oxidative injury as stress intensity surpasses adaptive limits. This insight underscores the importance of identifying salinity thresholds beyond which protective mechanisms begin to fail.
4.2. Regulation of Apoptosis Signaling
Chronic low-salinity stress also affected apoptotic pathways. Transcriptomic data revealed significant changes in apoptosis-related gene expression in the S26 group. CASP3, a key executor of apoptosis, was downregulated, indicating suppression of the terminal apoptotic cascade, possibly to prevent excessive cell death [32]. Simultaneously, genes from the inhibitor of apoptosis protein (IAP) family, including BIRC2, BIRC3, and BIRC5, were also downregulated. Although IAPs normally inhibit caspase activity to prevent premature apoptosis [33], their downregulation may allow for the selective clearance of irreparably damaged cells, while the concurrent downregulation of CASP3 raises the apoptotic threshold [34,35]. This dual-regulation strategy may help maintain tissue homeostasis under stress.
Additionally, GADD45, a stress-responsive gene, was upregulated. GADD45 family members respond to osmotic and oxidative stress by inducing cell cycle arrest and DNA repair [36], and they modulate cell fate through interactions with kinases such as p38 and JNK [37]. GADD45 may thus promote cell survival via repair or facilitate programmed cell death depending on the severity of damage.
Alterations were also observed in the NF-κB signaling pathway. The expressions of IKK complex components IKBKA and IKBKG, which are essential for NF-κB activation, were downregulated in the S26 group. NF-κB signaling typically promotes cell survival and inflammatory responses by inducing anti-apoptotic genes [38]. Its suppression, along with decreased CASP3 and IAP expressions, suggests a finely tuned apoptotic control mechanism [39].
CAPN1 upregulation, together with increased expressions of cytoskeletal proteins such as alpha-tubulin and ACTB-G, suggests cytoskeletal remodeling and involvement of calcium signaling. Calpain, a calcium-dependent protease, participates in protein turnover and stress signaling, potentially aiding in both apoptosis and structural maintenance [40]. The upregulation of cytoskeletal components may help stabilize cell structure under osmotic stress [30]. Collectively, these findings suggest that under chronic low-salinity conditions, M. yessoensis reprogrammed apoptotic signaling to balance cell survival and the removal of damaged cells [41].
4.3. Adaptation of Energy Metabolism
M. yessoensis also exhibited metabolic adaptations in response to prolonged low-salinity stress. Increased NAD-MDH activity in the S26 group indicates enhanced aerobic metabolism via the TCA cycle [42,43]. Energy is required to maintain osmotic homeostasis, including Na+/K+-ATPase activity and osmolyte synthesis. The upregulation of TCA cycle activity likely supported this elevated ATP demand [44,45,46]. Similar responses have been reported in other euryhaline bivalves, where low salinity stimulates fatty acid β-oxidation [44].
Transcriptome data also showed differential expression of lipid metabolism genes, particularly in the “lipid and atherosclerosis” pathway. Upregulation of LDLR and APOB suggests enhanced lipid uptake and redistribution [47], while increased ABCA1 expression promotes cholesterol efflux. Interestingly, ABCG1 was downregulated, suggesting a preferential reliance on ABCA1-mediated transport under stress. Such lipid transport adjustments may facilitate the removal of oxidized lipids, stabilize membranes, and deliver energy substrates to peripheral tissues.
Membrane remodeling likely contributed to osmoregulation. Exposure to low salinity can cause osmotic swelling or shrinkage, and shifts in lipid composition help preserve membrane fluidity [44]. Upregulation of genes involved in glycerophospholipid metabolism is consistent with previous reports in bivalves under salinity stress [47].
Energy allocation strategies also shifted. Downregulation of growth- and proliferation-related genes such as STAT5A and SKP2 suggests reduced energy investment in growth and a reallocation toward survival and repair processes [48,49]. Meanwhile, CREB1 was upregulated in the S26 group, potentially compensating for reduced NF-κB signaling and helping to maintain energy and survival-related gene expression [50,51].
Stress-responsive transcription factors ATF4 and XBP1, upregulated under ER stress, may support not only antioxidant defense but also metabolic adjustments through the regulation of protein synthesis and lipid metabolism [52,53]. These changes collectively indicate a shift from a “growth mode” to a “maintenance mode” under chronic low-salinity stress [54].
Finally, enrichment of the “Pathways in cancer” category merits attention. Genes such as WNT1 and DLL, typically associated with cell proliferation and differentiation, were upregulated. Wnt and Notch signaling may contribute to tissue repair or immune regulation under stress [55]. Although these pathways are also implicated in tumorigenesis, their expressions in adult scallops likely reflect controlled activation aimed at maintaining homeostasis. Similarly, TRAF1 upregulation may serve as a compensatory mechanism for reduced IAP expression, ensuring survival signaling remains functional [56,57].
5. Conclusions
This study investigated the physiological and transcriptomic responses of M. yessoensis during 60 days of chronic low-salinity stress. The findings revealed phase-dependent adaptive mechanisms involving antioxidative regulation, apoptosis control, and metabolic remodeling. At moderate salinity reduction (28‰), enhanced activities of SOD, POD, and T-AOC, together with the upregulation of genes such as GST, ATF4, and XBP1, indicated the activation of antioxidant defenses and effective stress adaptation. In contrast, the 26‰ group showed reduced antioxidant enzyme activities and a high mortality rate (46%), suggesting that the adaptive mechanisms had reached their limits, leading to oxidative imbalance and physiological dysfunction.
Selective apoptosis regulation was observed through upregulation of GADD45 and CAPN1 and downregulation of CASP3 and BIRC2/3, facilitating damaged cell clearance while minimizing excessive cell loss. Modulation of NF-κB signaling, with upregulation of TRAF3 and downregulation of IKBKA and IKBKG, may contribute to the control of chronic inflammation. Metabolically, elevated NAD-MDH activity and upregulated expression of lipid metabolism genes (ABCA1, LDLR) and amino acid metabolism genes (asnB, GlsA, GABA-T) suggested enhanced aerobic respiration and nitrogen regulation under moderate stress.
In summary, M. yessoensis exhibited conditional physiological plasticity under chronic low-salinity stress, but this adaptability diminished as salinity dropped below a critical threshold, resulting in oxidative damage and increased mortality. These findings help define salinity conditions that pose physiological risks, providing practical guidance for improving scallop health and survival in variable aquaculture environments.
Author Contributions
Conceptualization, J.D.; H.X. was responsible for conceptualization, methodology, formal analysis, investigation, and writing—original draft; X.J. handled formal analysis, investigation, visualization, and writing—original draft; Z.W. contributed to formal analysis, visualization, and investigation; Q.Y. was responsible for writing—original draft; W.L. was responsible for formal analysis, investigation; L.H. was responsible for formal analysis, investigation, and visualization; J.D. was responsible for conceptualization, methodology, writing—review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Dalian Jinshitanwan Laboratory Demonstration Project (Dljswsf202401), the Major Agricultural Project of the Liaoning Provincial Science and Technology Department (Project No.: 2023JH1/10200007), and the Liaoning Province “Xingliao Talent Plan” Leading Talents Project (Project No. XLYC2202001, for Jun Ding).
Institutional Review Board Statement
Not applicable. Ethical review and approval were waived for this study due to the categorization of scallops as invertebrates.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nan, X.; Wei, H.; Zhang, H.; Nie, H. Factors influencing the interannual variation in biomass of bottom-cultured Yesso scallop (Patinopecten yessoensis) in the Changhai Sea area, China. Front. Mar. Sci. 2022, 8, 798359. [Google Scholar] [CrossRef]
- Newell, R.I. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. J. Shellfish Res. 2004, 23, 51–62. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Biological aspects, fisheries, and aquaculture of yesso scallops in Russian waters of the Sea of Japan. Diversity 2022, 14, 399. [Google Scholar] [CrossRef]
- Namikawa, Y.; Suzuki, M. Atmospheric CO2 Sequestration in Seawater Enhanced by Molluscan Shell Powders. Environ. Sci. Technol. 2024, 58, 2404–2412. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, Y.; Chang, Y.; Hao, Z. Behavioral characteristics and related physiological and ecological indexes of cultured scallops (Mizuhopecten yessoensis) in response to predation by the crab Charybdis japonica. Fishes 2024, 9, 389. [Google Scholar] [CrossRef]
- Gibson, R.; Barnes, M.; Atkinson, R. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanogr. Mar. Biol. Annu. Rev. 2002, 40, 233. [Google Scholar] [CrossRef]
- Mackay, A. Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. J. Environ. Qual. 2022, 37, 3056. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Cote, I.M.; Dodson, J.J.; Fleming, I.A.; Jennings, S.; Mantua, N.J.; Peterman, R.M.; Riddell, B.E.; Weaver, A.J. Climate change, fisheries, and aquaculture: Trends and consequences for Canadian marine biodiversity. Environ. Rev. 2012, 20, 220–311. [Google Scholar] [CrossRef]
- Hao, Z.; Tang, X.; Zhan, Y.; Tian, Y.; Yang, L.; Ding, J.; Chang, Y. A Comparative Study of Survival, Metabolism, Immune Indicators, and Proteomics, in Five Batches of Japanese Scallop Mizuhopecten yessoensis under Short-Term High Temperature Stress. Isr. J. Aquac. 2016, 68, 20828. [Google Scholar] [CrossRef]
- Peng, M.; Liu, X.; Niu, D.; Ye, B.; Lan, T.; Dong, Z.; Li, J. Survival, growth and physiology of marine bivalve (Sinonovacula constricta) in long-term low-salt culture. Sci. Rep. 2019, 9, 2819. [Google Scholar] [CrossRef]
- Liu, J.; Changg, Y.; Yang, Y.; Liu, Y.; Zhang, J.; Zhang, W.; Wang, Y. Effects of Gradual Salinity Changes on Immune Parameters of Scallop (Patinopecten yessoensis). J. Agric. Sci. Technol. 2011, 13, 7. [Google Scholar]
- LüFu; Pan, L.Q.; Wang, A.M.; Hu, Y. Effects of salinity on oxygen consumption rate and ammonia excretion rate of allogynogemetic crucian carp. Acta Hydrobiol. Sin. 2010, 34, 1000–3207. Available online: http://ssswxb.ihb.ac.cn/en/article/id/23af8a68-c7ef-4ade-9274-a28dd2aceb5f (accessed on 30 January 2025).
- Falfushynska, H.; Wu, F.; Sokolov, E.P.; Sokolova, I.M. Salinity variation modulates cellular stress response to ZnO nanoparticles in a sentinel marine bivalve, the blue mussel Mytilus sp. Mar. Environ. Res. 2023, 183, 105834. [Google Scholar] [CrossRef]
- Knowles, G.; Handlinger, J.; Jones, B.; Moltschaniwskyj, N. Hemolymph chemistry and histopathological changes in Pacific oysters (Crassostrea gigas) in response to low salinity stress. J. Invertebr. Pathol. 2014, 121, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Peteiro, L.G.; Woodin, S.A.; Wethey, D.S.; Costas-Costas, D.; Martínez-Casal, A.; Olabarria, C.; Vázquez, E. Responses to salinity stress in bivalves: Evidence of ontogenetic changes in energetic physiology on Cerastoderma edule. Sci. Rep. 2018, 8, 8329. [Google Scholar] [CrossRef] [PubMed]
- Berezina, N. Biomarkers of stress in common coastal amphipods and bivalves under salinity gradient and pollution influence (the White Sea). Žurnal Obŝej Biol. 2024, 85, 445–459. [Google Scholar] [CrossRef]
- Gostyukhina, O.L.; Kladchenko, E.S.; Chelebieva, E.S.; Tkachuk, A.A.; Lavrichenko, D.S.; Andreyeva, A.Y. Short-time salinity fluctuations are strong activators of oxidative stress in Mediterranean mussel (Mytilus galloprovincialis). Ecol. Montenegrina 2023, 63, 46–58. [Google Scholar] [CrossRef]
- Braga, A.C.; Pereira, V.; Marçal, R.; Marques, A.; Guilherme, S.; Costa, P.R.; Pacheco, M. DNA damage and oxidative stress responses of mussels Mytilus galloprovincialis to paralytic shellfish toxins under warming and acidification conditions—Elucidation on the organ-specificity. Aquat. Toxicol. 2020, 228, 105619. [Google Scholar] [CrossRef]
- Yang, H.; Wang, P.; Zhang, T.; Wang, J.; He, Y.; Zhang, F. Effects of reduced salinity on oxygen consumption and ammonia-N excretion of Chlamys farreri. Chin. J. Oceanol. Limnol. 1999, 17, 207–211. [Google Scholar] [CrossRef]
- Wei, S.; Xie, Z.; Liu, C.; Sokolova, I.; Sun, B.; Mao, Y.; Xiong, K.; Peng, J.; Fang, J.K.-H.; Hu, M. Antioxidant response of the oyster Crassostrea hongkongensis exposed to diel-cycling hypoxia under different salinities. Mar. Environ. Res. 2022, 179, 105705. [Google Scholar] [CrossRef]
- Yan, L.; Su, J.; Wang, Z.; Yan, X.; Yu, R.; Ma, P.; Li, Y.; Du, J. Transcriptomic analysis of Crassostrea sikamea × Crassostrea angulata hybrids in response to low salinity stress. PLoS ONE 2017, 12, e0171483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-m.; Yao, X.-m.; Cheng, Y.; Xing, Y.-j.; Sun, Y.; Hua, Q.; Wan, S.-j.; Meng, X.-j. Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide. Heliyon 2024, 10, e24432. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and its derivatives as supports for enzyme immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, J.L.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Yao, H.; Hu, Y.; Tong, H.; Shi, S. Dimethylglycine Alleviates Metabolic Dysfunction-Associated Fatty Liver Disease by Improving the Circulating Estrogen Level via Gut Staphylococcus. J. Agric. Food Chem. 2023, 72, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Choi, C.Y. Temporal changes in physiological responses of Bay Scallop: Performance of antioxidant mechanism in Argopecten irradians in response to sudden changes in habitat salinity. Antioxidants 2021, 10, 1673. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Dong, H.; Wang, Y.; Liu, Q.; Li, H. Effect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2016, 49, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, A.-G.; Yan, J.-K.; Liu, Z.-H.; Sun, X.-J.; Zhou, L.-Q.; Zhang, G.-M. Effects of acute low-salinity stress on the activities of catalase (CAT), superoxide dismutase (SOD) and glutathiones-transferase (GST) in Scapharca broughtonii. J. Mol. Biol. Res. 2019, 9, 172. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Cheng, D.; Tan, K.; Ye, T.; Ma, H.; Li, S.; Zheng, H. Differential responses of a pi-class glutathione S-transferase (CnGSTp) expression and antioxidant status between golden and brown noble scallops under pathogenic stress. Fish Shellfish Immunol. 2020, 105, 144–151. [Google Scholar] [CrossRef]
- Tang, D.; Wu, Y.; Huang, S.; Wu, L.; Luo, Y.; Wang, Z. Transcriptome reveals the mechanism of immunity in the low salinity stress of the Chinese shrimp (Fenneropenaeus chinensis). Thalass. Int. J. Mar. Sci. 2022, 38, 977–987. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, Y.; Xu, Z.; Zheng, Z.; Wan, S. Involvement of caspase-3 activity and survivin downregulation in cinobufocini-induced apoptosis in A 549 cells. Exp. Biol. Med. 2009, 234, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Tamm, I.; Wang, Y.; Sausville, E.; Scudiero, D.A.; Vigna, N.; Oltersdorf, T.; Reed, J.C. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998, 58, 5315–5320. [Google Scholar] [PubMed]
- Xu, J.-H.; Wang, A.-x.; Huang, H.-Z.; Wang, J.-G.; Pan, C.-B.; Zhang, B. Survivin shRNA induces caspase-3-dependent apoptosis and enhances cisplatin sensitivity in squamous cell carcinoma of the tongue. Oncol. Res. 2010, 18, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Li, G.; Meng, K.; Guan, X.; Wang, C. Expressions of survivin and caspase-3 in human non-small cell lung cancer and their relationship with cell apoptosis. Zhongguo Fei Ai Za Zhi 2005, 8, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.; Hecla, J.; Wu, D.; Lai, X.; Boers, T.; Yang, K.; Moulton, T.; Kenyon, S.; Arzoumanian, Z.; Krull, W. Stationary computed tomography for space and other resource-constrained environments. Sci. Rep. 2018, 8, 14195. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, B.; Talikoti, A.T.; Nelamangala, K.; Krishnamurthy, D. Effect of Oral Pregabalin as Preemptive Analgesic in Patients Undergoing Lower Limb Orthopedic Surgeries under Spinal Anaesthesia. J. Clin. Diagn. Res. JCDR 2016, 10, Uc01–Uc04. [Google Scholar] [CrossRef]
- Ding, L.; Li, W.; Li, N.; Liang, L.; Zhang, X.; Jin, H.; Shi, H.; Storey, K.B.; Hong, M. Antioxidant responses to salinity stress in an invasive species, the red-eared slider (Trachemys scripta elegans) and involvement of a TOR-Nrf2 signaling pathway. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 219, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Lopez-Chavez, A.; Citrin, D.; Janik, J.E.; Morris, J.C. Impacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivin. Mol. Cancer 2011, 10, 35. [Google Scholar] [CrossRef]
- Feng, K.; Lu, J.; Chen, Y.; Luo, Y.; Hu, Y.; Li, X.; Zhong, S.; Cheng, L. The coordinated alterations in antioxidative enzymes, PeCu/ZnSOD and PeAPX2 expression facilitated in vitro Populus euphratica resistance to salinity stress. Plant Cell Tissue Organ Cult. 2022, 150, 399–416. [Google Scholar] [CrossRef]
- Santhanam, M.; Pandey, K.; Shteinfer-Kuzmine, A.; Paul, A.; Abusiam, N.; Zalk, R.; Shoshan-Barmatz, V. Interaction of SMAC/Diablo with a survivin/BIRC5-derived peptide alters essential cancer hallmarks: Tumor growth, inflammation, and immunosuppression. Mol. Ther. 2024, 32, 1934–1955. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Zhang, X.; Gao, G.; Niu, M.; Wang, H.; Chen, L.; Wang, C.; Mu, C.; Wang, F. Metabolic Response in the Gill of Portunus trituberculatus Under Short-Term Low Salinity Stress Based on GC-MS Technique. Front. Mar. Sci. 2022, 9, 881016. [Google Scholar] [CrossRef]
- Arense, P.; Bernal, V.; Iborra, J.; Cánovas, M. Metabolic adaptation of Escherichia coli to long-term exposure to salt stress. Process Biochem. 2010, 45, 1459–1467. [Google Scholar] [CrossRef]
- Liu, T.; Nie, H.; Ding, J.; Huo, Z.; Yan, X.-W. Physiological and transcriptomic analysis provides new insights into osmoregulation mechanism of Ruditapes philippinarum under low and high salinity stress. Sci. Total Environ. 2024, 935, 173215. [Google Scholar] [CrossRef]
- Giffard-Mena, I.; Ponce-Rivas, E.; Sigala-Andrade, H.; Uganda-Solís, C.; Re, A.D.; Díaz, F.; Camacho-Jiménez, L. Evaluation of the osmoregulatory capacity and three stress biomarkers in white shrimp Penaeus vannamei exposed to different temperature and salinity conditions: Na+/K+ ATPase, Heat Shock Proteins (HSP), and Crustacean Hyperglycemic Hormones (CHHs). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 271, 110942. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, X. Expression of Na+/K+-ATPase Was Affected by Salinity Change in Pacific abalone Haliotis discus hannai. Front. Physiol. 2018, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Shen, Y.; Li, X.; Wu, Z.; Jiao, L.; Li, J.; Zhou, Q.-C.; Jin, M. A New Insight Into the Underlying Adaptive Strategies of Euryhaline Marine Fish to Low Salinity Environment: Through Cholesterol Nutrition to Regulate Physiological Responses. Front. Nutr. 2022, 9, 855369. [Google Scholar] [CrossRef] [PubMed]
- La Fortezza, M.; Schenk, M.; Cosolo, A.; Kolybaba, A.; Grass, I.; Classen, A.-K. JAK/STAT signalling mediates cell survival in response to tissue stress. Development 2016, 143, 2907–2919. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, X.; Wang, B.; Yu, B.; Ge, J. Deubiquitinase inhibitor b-AP15 activates endoplasmic reticulum (ER) stress and inhibits Wnt/Notch1 signaling pathway leading to the reduction of cell survival in hepatocellular carcinoma cells. Eur. J. Pharmacol. 2018, 825, 10. [Google Scholar] [CrossRef]
- Guan, Y.; Yao, W.; Yu, H.; Feng, Y.; Zhao, Y.; Zhan, X.; Wang, Y. Chronic stress promotes colorectal cancer progression by enhancing glycolysis through β2-AR/CREB1 signal pathway. Int. J. Biol. Sci. 2023, 19, 2006–2019. [Google Scholar] [CrossRef]
- Mambetsariev, N.; Lin, W.; Stunz, L.; Hanson, B.; Arkee, T.; Bishop, G. Nuclear TRAF3 Inhibits CREB-mediated survival and metabolic reprogramming in B lymphocytes (TUM10P.1045). J. Immunol. 2015, 194, 211-26. [Google Scholar] [CrossRef]
- Barcelos, E.S.; Rompietti, C.; Adamo, F.; Dorillo, E.; De Falco, F.; Del Papa, B.; Baldoni, S.; Nogarotto, M.; Esposito, A.; Capoccia, S.; et al. NOTCH1-mutated chronic lymphocytic leukemia displays high endoplasmic reticulum stress response with druggable potential. Front. Oncol. 2023, 13, 1218989. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, C.; Yang, H.; Chen, Y.; Feng, X.; Li, B.; Fan, H. P-STAT3 Inhibition Activates Endoplasmic Reticulum Stress-Induced Splenocyte Apoptosis in Chronic Stress. Front. Physiol. 2020, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Alfonso, S.; Cousin, X.; Prunet, P.; Bégout, M.; Leguen, I. Global assessment of the response to chronic stress in European sea bass. Aquaculture 2021, 544, 737072. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kobayashi, T.; Hanamatsu, H.; Yokota, I.; Teranishi, Y.; Iwamoto, A.; Kitagawa, M.; Ashida, S.; Sakurai, A.; Matsuo, S.; et al. Tolerable glycometabolic stress boosts cancer cell resilience through altered N-glycosylation and Notch signaling activation. Cell Death Dis. 2024, 15, 53. [Google Scholar] [CrossRef]
- Habelhah, H.; Blackwell, K.; Altaeva, A.; Zhang, L.; Shi, Z. Abstract 1692: TRAF2 phosphorylation plays a critical role in cell adaptation to chronic oxidative stress. Cancer Res. 2010, 70, 1692. [Google Scholar] [CrossRef]
- Habelhah, H.; Zhang, L.; Xialikaer, A.; Blackwell, K. Abstract 3496: TRAF2 protects mammary epithelial and cancer cells from endoplasmic reticulum stress-induced apoptosis. Cancer Res. 2016, 76, 3496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).