Role and Mechanisms of Angiogenesis in Tumours
Simple Summary
Abstract
1. Introduction
2. Overview
3. Molecular Pathways
3.1. Physiological Angiogenesis
3.2. Angiogenesis in Neoplasms
3.3. Factors Regulating the Process of Neoplastic Angiogenesis
- A specific effect on endothelial cells, meaning that their appearance induces angiogenesis;
- The presence of specific receptors for these factors on endothelial cells;
- Their disappearance inhibits angiogenesis [8].
3.3.1. Vascular Endothelial Growth Factor (VEGF)
- -
- Fibroblast growth factor (FGF);
- -
- Epidermal growth factor (EGF);
- -
- Platelet-derived growth factor (PDGF);
- -
- Transforming growth factor (TGF-β1).
3.3.2. Fibroblast Growth Factor (FGF)
3.3.3. Epidermal Growth Factor (EGF)
3.3.4. Platelet-Derived Growth Factor (PDGF)
3.3.5. Transforming Growth Factor (TGF-β1)
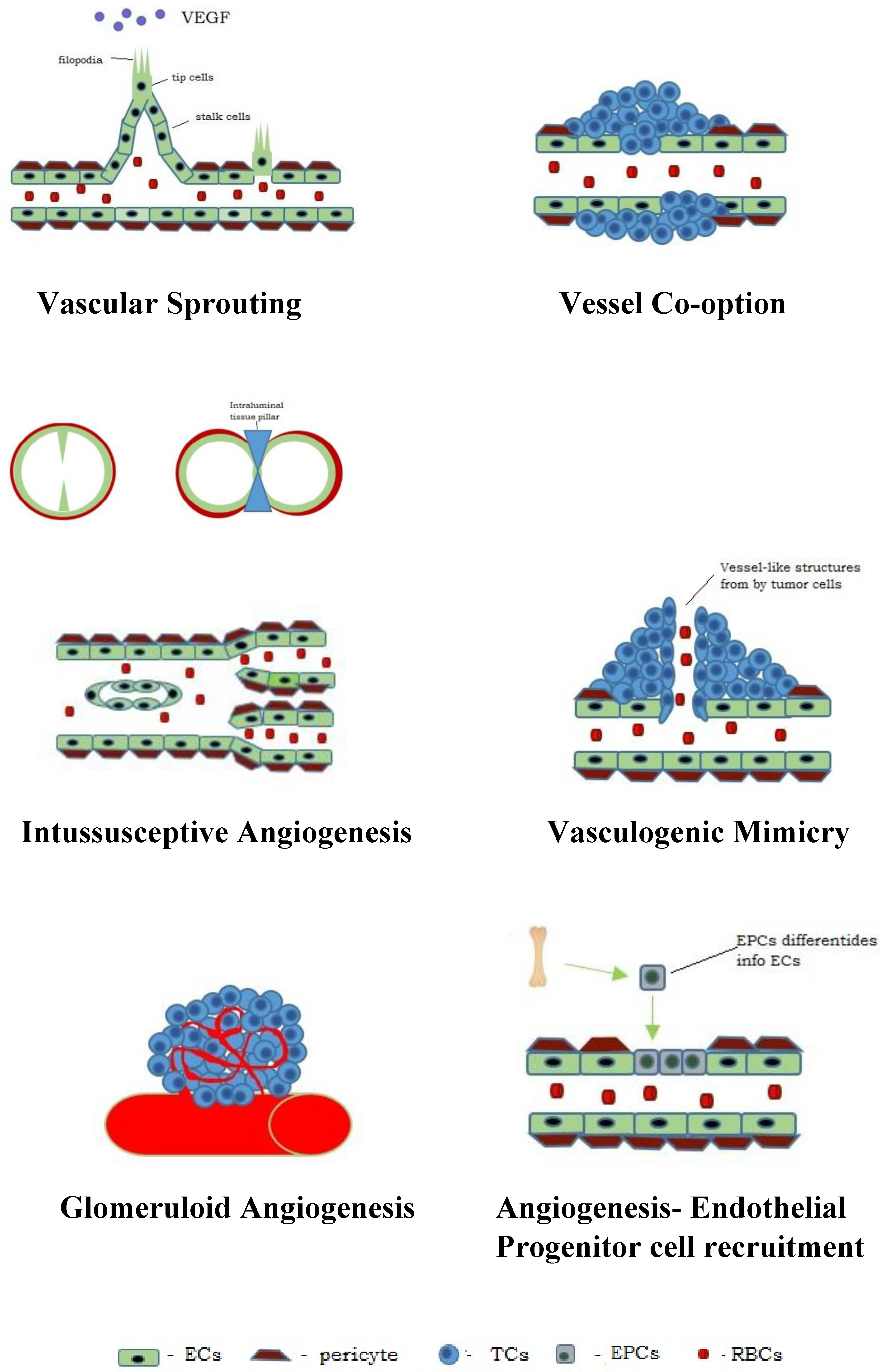
4. Types of Angiogenesis in Neoplasms
4.1. Vascular Sprouting
4.2. Intussusceptive Angiogenesis
4.3. Vessel Co-Option
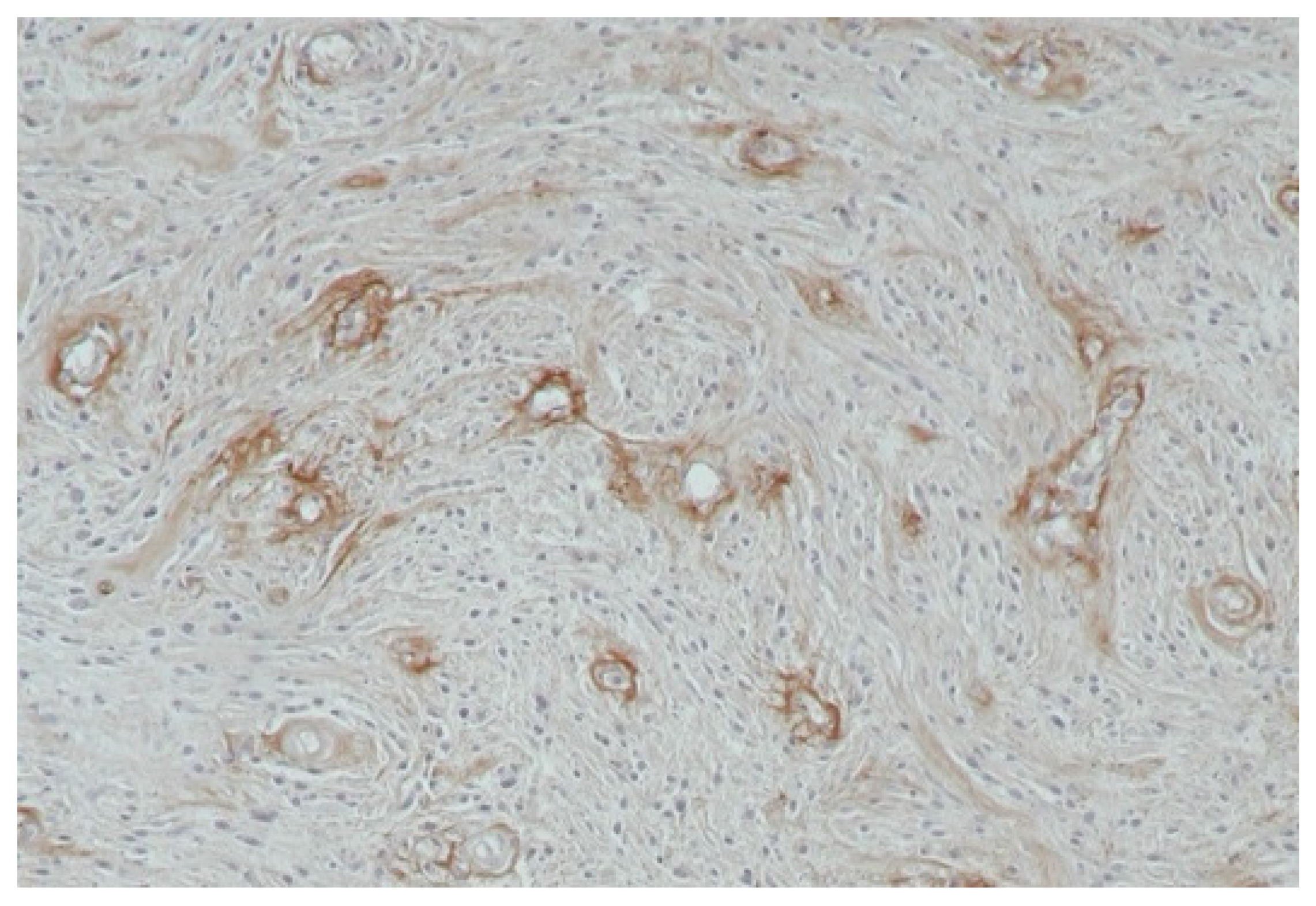
4.4. Vasculogenic Mimicry (VM)
- -
- A lack of vascular endothelial cells on the inner wall of the blood vessel;
- -
- The channels resembling vessels are lined with neoplastic cells;
- -
- The cells lining the channels react positively to PAS staining but negatively to CD31 staining, whereas the endothelial vascular channels are negative in PAS staining but positive in CD31 staining;
- -
- The presence of erythrocytes in the vascular-like channels [87].
4.5. Glomeruloid Angiogenesis
4.6. Endothelial Progenitor Cell Recruitment
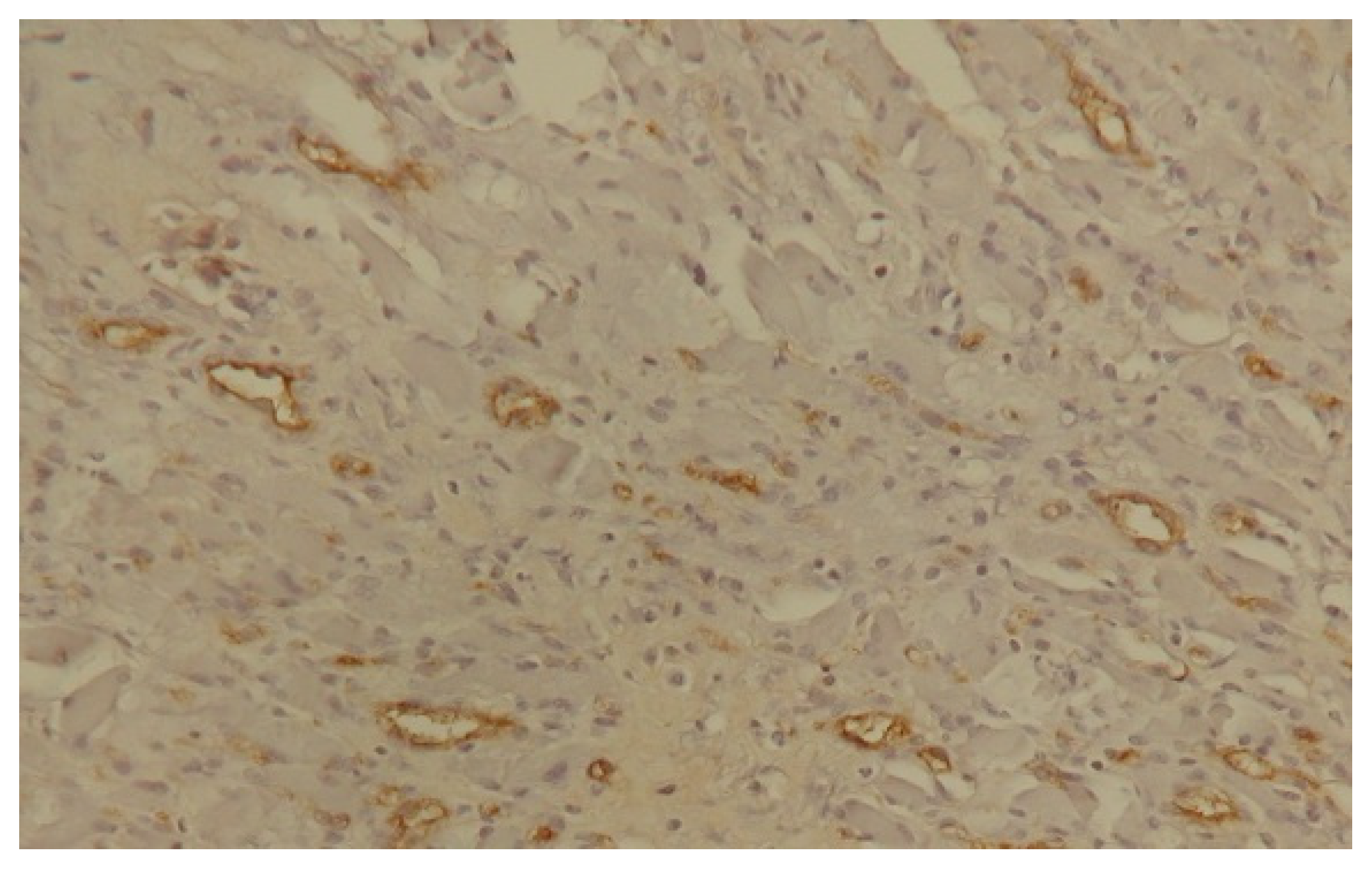
4.7. Characteristics of the Vessels Formed During Neoplastic Angiogenesis
5. Angiogenesis Assessment
6. Clinical Relevance
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanov, A.N.; Chabbarova, Y.R. Mechanisms of Physiological Angiogenesis. J. Evol. Biochem. Physiol. 2023, 59, 914–929. [Google Scholar] [CrossRef]
- Furuya, M.; Nishiyama, M.; Kasuya, Y.; Kimura, S.; Ishikura, H. Pathophysiology of tumor neovascularization. Vasc. Health Risk Manag. 2005, 1, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Shamsi, A.; Mohammad, T.; Islam, A.; Hassan, M.I. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim. Biophys Acta Rev. Cancer 2021, 1876, 188568. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Lorenc, P.; Sikorska, A.; Molenda, S.; Guzniczak, N.; Dams-Kozlowska, H.; Florczak, A. Physiological and tumor-associated angiogenesis: Key factors and therapy targeting VEGF/VEGFR pathway. Biomed. Pharmacother. 2024, 180, 117585. [Google Scholar] [CrossRef]
- Geindreau, M.; Bruchard, M.; Vegran, F. Role of cytokines and chemokines in angiogenesis in a tumor context. Cancers 2022, 14, 2446. [Google Scholar] [CrossRef]
- Tchórzewska, M.; Kowalik, M.; Kuliś, A.; Olejarz, W. Mechanisms leading to angiogenesis in cancers. Biul. Wydz. Farm. WUM 2019, 10, 60–65. [Google Scholar]
- Szala, S.; Jarosz, M. Tumor blood vessels. Postep. Hig. Med. Dosw. 2011, 65, 437–446. [Google Scholar] [CrossRef]
- Sacewicz, I.; Wiktorska, M.; Wysocki, T.; Niewiarowska, J. Mechanisms of cancer angiogenesis. Postep. Hig. Med. Dosw. 2009, 63, 159–168. [Google Scholar]
- Kurzyk, A. Angiogenesis-possibilities, problems and perspectives. Postep. Biochem. 2015, 61, 25–34. [Google Scholar]
- Jarosz, P.; Woźniak, B. Angiogenesis in cancer diseases. Prz. Med. Uniw. Rzesz. Inst. Leków 2012, 4, 498–507. [Google Scholar]
- Sobczyńska-Rak, A.; Silmanowicz, P.; Polkowska, I. Tumor angiogenesis—Factors influencing the development of a tumor vascular network and assessment of neoangiogenesis in histopathological samples. Med. Weter. 2016, 72, 542–548. [Google Scholar] [CrossRef]
- Ziyad, S.; Iruela-Arispe, M.L. Molecular mechanisms of tumor angiogenesis. Genes Cancer 2011, 2, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef]
- Klimczak, A.; Patelski, M.; Trela, P.; Flur, T.; Pietrzak, B.; Czapla, M.; Blida, S. The role of selected proangiogenic factors in growth and development of medulloblastoma. PBK-Postep. Biol. Komorki 2021, 48, 231–248. [Google Scholar]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R. Angiogenesis in health and diseases. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Sujka-Kordowska, P.; Malińska, A.; Nowicki, M. Selected aspects of angiogenesis in retinoblastoma. Postep. Biol. Komorki 2018, 45, 319–334. [Google Scholar]
- Dudley, A.C.; Grifoen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Skóra, J.; Biegus, J.; Pupka, A.; Barć, P.; Sikora, J.; Szyber, P. Molecular basics of angiogenesis. Postep. Hig. Dosw. 2006, 60, 410–415. [Google Scholar]
- Wei, X.; Chen, Y.; Jiang, X.; Peng, M.; Liu, Y.; Mo, Y.; Ren, D.; Hua, Y.; Yu, B.; Zhou, Y.; et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer 2021, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism, Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ốstman, A.; Augsten, M. Cancer-associated fibroblast and tumor growth–bystanders turning into key players. Curr. Opin. Genet. Dev. 2009, 19, 67–73. [Google Scholar] [CrossRef]
- Sobocińska, A.A.; Czarnecka, A.M.; Szczylik, C. Mechanisms of angiogenesis in neoplasia. Postep. Hig. Med. Dosw. 2016, 70, 1166–1181. [Google Scholar]
- King, A.; Balaji, S.; Keswani, S.G.; Crombleholme, T.M. The Role of Stem Cells in Wound Angiogenesis. Adv. Wound Care 2014, 3, 614–625. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Sobczyńska-Rak, A. Correlation between plasma VEGF and angiogenesis of skin and subcutaneous tissue cancer in dogs. Bull. Vet. Inst. Pulawy 2009, 53, 503–506. [Google Scholar]
- Zajkowska, M.; Szmitkowski, M.; Ławicki, S.; Lubowicka, E. VEGF family factors and their receptors in the diagnostics of breast cancer. J. Lab. Diagn. 2019, 54, 105–112. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Halper, J. Growth Factors as Active Participants in Carcinogenesis: A Perspective. Vet. Pathol. 2010, 47, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Paavonsalo, S.; Heinolainen, K.; Lackman, M.H.; Ranta, A.; Hemanthakumar, K.A.; Kubota, Y.; Alitalo, K. Interplay of vascular endothelial growth factor receptors in organ-specific vessel maintenance. J. Exp. Med. 2022, 219, e20210565. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Li, P.; Ferrara, N. Vascular heterogeneity: VEGF receptors make blood vessels special. J. Exp. Med. 2022, 219, e20212539. [Google Scholar] [CrossRef]
- Ahmad, A.; Nawaz, M.I. Molecular mechanism of VEGF and its role in pathological angiogenesis. J. Cell. Biochem. 2022, 123, 1938–1965. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Łojko, A.; Thielemann, A.; Kopczyński, Z. Vascular endothelial growth factor (VEGF) and selected clinicopathological parameters in breast carcinoma. Wspolcz. Onkol. 2007, 11, 300–304. [Google Scholar]
- Camuzi, D.; de Amorim, Í.S.S.; Ribeiro Pinto, L.F.; Oliveira Trivilin, L.; Mencalha, A.L.; Soares Lima, S.C. Regulation Is in the Air: The Relationship between Hypoxia and Epigenetics in Cancer. Cells 2019, 8, 300. [Google Scholar] [CrossRef]
- Huang, X.L.; Khan, M.I.; Wang, J.; Ali, R.; Ali, S.W.; Zahra, Q.U.; Kazmi, A.; Lolai, A.; Huang, Y.L.; Hussain, A.; et al. Role of receptor tyrosine kinases mediated signal transduction pathways in tumor growth and angiogenesis-New insight and futuristic vision. Int. J. Biol. Macromol. 2021, 180, 739–752. [Google Scholar] [CrossRef]
- Sadłecki, P.; Walentowicz-Sadłecka, M.; Grabiec, M. Angiogenesis in neoplastic processes. Przeg Menopauz 2010, 1, 28–31. [Google Scholar]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Schmidt, M.; Paes, K.; De Maziere, A.; Smyczek, T.; Yang, S.; Gray, A.; French, D.; Kasman, I.; Klumperman, J.; Rice, D.S.; et al. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development 2007, 134, 2913–2923. [Google Scholar] [CrossRef] [PubMed]
- Kilvaer, T.; Rakaee, M.; Hellevik, T.; Vik, J.; Petris, L.; Dønnem, T.; Strell, C.; Ostman, A.; Busund, L.; Martinez-Zubiaurre, I. Differential prognostic impact of platelet-derived growth factor receptor expression in NSCLC. Sci. Rep. 2019, 9, 10163. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, D.; Yao, Y.; Wang, M. PDGF signaling in cancer progression. Int. J. Clin. Exp. Med. 2017, 10, 9918–9929. [Google Scholar]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Ahn, J.B.; Rha, S.Y.; Shin, S.J.; Jeung, H.C.; Kim, T.S.; Zhang, X.; Park, K.H.; Noh, S.H.; Roh, J.K.; Chung, H.C. Circulating endothelial progenitor cells (EPC) for tumor vasculogenesis in gastric cancer patients. Cancer Lett. 2010, 288, 124–132. [Google Scholar] [CrossRef]
- Felcht, M.; Thomas, M. Angiogenesis in malignant melanoma. J. Dtsch. Dermatol. Ges. 2015, 13, 125–136. [Google Scholar] [CrossRef]
- Ferda, J.; Frölich, M.; Ferdová, E.; Heidenreich, F.; Charvát, R.; Mírka, H. Neovascularization, vascular mimicry and molecular exchange: The imaging of tumorous tissue aggressiveness based on tissue perfusion. Eur. J. Radiol. 2023, 163, 110797. [Google Scholar] [CrossRef]
- Fouladzadeh, A.; Dorraki, M.; Min, K.K.M.; Cockshell, M.P.; Thompson, E.J.; Verjans, J.W.; Allison, A.; Bonder, C.S.; Abbott, D. The development of tumour vascular networks. Commun. Biol. 2021, 4, 1111. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Pavani, K.; Nikarika, R.; Sumantran, V.N. Intussusceptive angiogenesis as a key therapeutic target for cancer therapy. Life Sci. 2020, 252, 117670. [Google Scholar] [CrossRef]
- Zduńska, M.; Ziółkowska, S. The role of angiogenesis in multiple myeloma treatment. Diagn. Lab. 2020, 56, 1–6. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Mahfouz, R.A.; Makarem, J.A.; Shamseddine, A. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol. Dis. 2007, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Auguste, P.; Lemiere, S.; Larrieu-Lahargue, F.; Bikfalvi, A. Molecular mechanisms of tumor vascularization. Crit. Rev. Oncol. Hematol. 2005, 54, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Knopik-Skrocka, A.; Kręplewska, P.; Jarmołowska-Jurczyszyn, D. Tumor blood vessels and vasculogenic mimicry—Current knowledge and searching for new cellular/molecular targets of anti-angiogenic therapy. Adv. Cell Biol. 2017, 5, 50–71. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivee, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Francis, C.R.; Kushner, E.J. Trafficking in blood vessel development. Angiogenesis 2022, 25, 291–305. [Google Scholar] [CrossRef]
- Hofmann, J.J.; Iruela-Arispe, M.L. Notch signaling in blood vessels. Who is talking to whom about what? Circ. Res. 2007, 100, 1556–1568. [Google Scholar] [CrossRef]
- Pontes-Quero, S.; Fernández-Chacón, M.; Luo, W.; Lunella, F.F.; Casquero-Garcia, V.; Garcia-Gonzalez, I.; Hermoso, A.; Rocha, S.F.; Bansal, M.; Benedito, R. High mitogenic stimulation arrests angiogenesis. Nat. Commun. 2019, 10, 2016. [Google Scholar] [CrossRef]
- Hillen, F.; Griffioen, A.W. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Pandita, A.; Ekstrand, M.; Bjursten, S.; Zhao, Z.; Fogelstrand, P.; Le Gal, K.; Ny, L.; Bergo, M.O.; Karlsson, J.; Nilsson, J.A.; et al. Intussusceptive angiogenesis in human metastatic malignant melanoma. Am. J. Pathol. 2021, 191, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Pezzella, F. Overview on the Different Patterns of Tumor Vascularization. Cells 2021, 10, 639. [Google Scholar] [CrossRef]
- Djonov, V.G.; Kurz, H.; Burri, P.H. Optimality in the developing vascular system: Branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev. Dyn. 2002, 224, 391–402. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Donnem, T.; Reynolds, A.R.; Kuczynski, E.A.; Gatter, K.; Vermeulen, P.B.; Kerbel, R.S.; Harris, A.L.; Pezzella, F. Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer 2018, 18, 323–336. [Google Scholar] [CrossRef]
- Döme, B.; Hendrix, M.J.; Paku, S.; Tovari, J.; Timar, J. Alternative vascularization mechanisms in cancer. Pathology and therapeutic implications. Am. J. Pathol. 2007, 170, 1–15. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef]
- Spiliopoulos, K.; Peschos, D.; Batistatou, A.; Ntountas, I.; Agnantis, N.; Kitsos, G. Vasculogenic Mimicry: Lessons from Melanocytic Tumors. In Vivo 2015, 29, 309–317. [Google Scholar]
- Zhang, Z.; Imani, S.; Shasaltaneh, M.D.; Hosseinifard, H.; Zou, L.; Fan, Y.; Wen, Q. The role of vascular mimicry as a biomarker in malignant melanoma: A systematic review and meta-analysis. BMC Cancer 2019, 19, 1134. [Google Scholar] [CrossRef]
- Latacz, E.; Caspani, E.; Barnhill, R.; Lugassy, C.; Verhoef, C.; Grunhagen, D.; Van Laere, S.; Moro, C.F.; Gerling, M.; Dirix, M.; et al. Pathological features of vessel co-option versus sprouting angiogenesis. Angiogenesis 2020, 23, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Dudley, A.C. Models and molecular mechanisms of blood vessel co-option by cancer cells. Angiogenesis 2020, 23, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, P.; Valiente, M. Vascular co-option in brain metastasis. Angiogenesis 2020, 23, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, E.A.; Reynolds, A.R. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis 2020, 23, 55–74. [Google Scholar] [CrossRef]
- Carbonell, W.S.; Ansorge, O.; Sibson, N.; Muschel, R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 2009, 4, e5857. [Google Scholar] [CrossRef]
- Yao, H.; Price, T.T.; Cantelli, G.; Ngo, B.; Warner, M.J.; Olivere, L.; Ridge, S.M.; Jablonski, E.M.; Therrien, J.; Tannheimer, S.; et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 2018, 560, 55–60. [Google Scholar] [CrossRef]
- Seano, G.; Jain, R.K. Vessel co-option in glioblastoma: Emerging insights and opportunities. Angiogenesis 2020, 23, 9–16. [Google Scholar] [CrossRef]
- Angara, K.; Borin, T.F.; Arbab, A.S. Vascular mimicry: A novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma. Transl. Oncol. 2017, 10, 650–660. [Google Scholar] [CrossRef]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”. Front. Oncol. 2019, 9, 803. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Seftor, R.E.B.; Chao, J.T.; Chien, D.S.; Chu, Y.W. Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacol. Ther. 2016, 159, 83–92. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicrin. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.A.; Moinfar, M.; Gohari Moghaddam, K.; Bahadori, M. Practical application of angiogenesis and vasculogenic mimicry in prostatic adenocarcinoma. Arch. Iran. Med. 2010, 13, 498–503. [Google Scholar] [PubMed]
- Liu, Q.; Qiao, L.; Liang, N.; Xie, J.; Zhang, J.; Deng, G.; Luo, H.; Zhang, J. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J. Cell. Mol. Med. 2016, 20, 1761–1779. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Li, X.; Sun, B.; Zhao, X.; Zhang, D.; Zhao, S. A pilot study of vasculogenic mimicry immunohistochemical expression in intraocular melanoma model. Oncol. Rep. 2009, 21, 989–994. [Google Scholar]
- Liu, W.; Xu, G.; Jia, W.; Li, J.; Ma, J.; Chen, K.; Wang, Z.; Ge, Y.; Ren, W.; Yu, J.; et al. Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med. Oncol. 2011, 28, 228–238. [Google Scholar] [CrossRef]
- Xiang, T.; Lin, Y.X.; Ma, W.; Zhang, H.J.; Chen, K.M.; He, G.P.; Zhang, X.; Xu, M.; Feng, Q.S.; Chen, M.Y.; et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat. Commun. 2018, 9, 5009. [Google Scholar] [CrossRef]
- Ge, H.; Luo, H. Overview of advances in vasculogenic mimicry—A potential target for tumor therapy. Cancer Manag. Res. 2018, 10, 2429–2437. [Google Scholar] [CrossRef]
- Wechman, S.L.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Vascular mimicry: Triggers, molecular interactions and in vivo models. Adv. Cancer Res. 2020, 148, 27–67. [Google Scholar]
- Sun, B.; Zhang, D.; Zhao, N.; Zhao, X. Epithelial-to-endothelial transition and cancer stem cells: Two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget 2016, 8, 30502–30510. [Google Scholar] [CrossRef]
- Yao, X.H.; Ping, Y.F.; Bian, X.W. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell 2011, 2, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Wang, W.; Sun, B.C.; Cai, W.J.; Li, L.; Lu, H.H.; Han, C.R.; Zhang, J.M. Vasculogenic mimicry is a key prognostic factor for laryngeal squamous cell carcinoma: A new pattern of blood supply. Chin. Med. J. 2012, 125, 3445–3459. [Google Scholar] [PubMed]
- Yang, J.P.; Liao, Y.D.; Mai, D.M.; Xie, P.; Qiang, Y.Y.; Zheng, L.S.; Wang, M.Y.; Mei, Y.; Meng, D.F.; Xu, L.; et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: A meta-analysis. Angiogenesis 2016, 19, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Van Meir, E.G. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF. Am. J. Pathol. 2001, 158, 789–796. [Google Scholar] [CrossRef]
- Akslen, L.A.; Straume, O.; Geisler, S.; Sorlie, T.; Chi, J.T.; Aas, T.; Borresen-Dale, A.L.; Lonning, P.E. Glomeruloid microvascular proliferation is associated with lack of response to chemotherapy in breast cancer. Br. J. Cancer 2011, 105, 9–12. [Google Scholar] [CrossRef]
- Kandemir, N.O.; Narli, Z.I.; Kalayci, M.; Ozdamar, S.O. A rare pattern of angiogenesis in meningiomas: Glomeruloid microvascular proliferation. Turk. Neurosurg. 2014, 24, 765–769. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into endothelial progenitor cells: Origin, classification, potentials, and prospects. Stem Cells Int. 2018, 18, 9847015. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–977. [Google Scholar] [CrossRef]
- Ghajar, C.M.; George, S.C.; Putnam, A.J. Matrix metalloproteinase control of capillary morphogenesis. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 251–278. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef]
- Sobczyńska-Rak, A. Angiogenesis in skin and subcutaneous tissue of dogs with tumors. Med. Wet. 2009, 2, 110–114. [Google Scholar]
- Sobczyńska-Rak, A.; Polkowska, I.; Śmiech, A.; Sobolewska, E. Angiogenesis in malignant oral-cavity tumours in dogs. Bull. Vet. Inst. Pulawy 2009, 3, 463–466. [Google Scholar]
- Sobczyńska-Rak, A.; Żylińska, B.; Polkowska, I.; Szponder, T. Elevated EGF Levels in the Blood Serum of Dogs with Periodontal Diseases and Oral Tumours. In Vivo 2018, 32, 507–515. [Google Scholar]
- Kopeć, M.; Abramczyk, H. Angiogenesis-a crucial step in breast cancer growth, progression and dissemination by Raman imaging. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2018, 198, 338–345. [Google Scholar] [CrossRef]
- Al-Dissi, A.N.; Haines, D.M.; Singh, B.; Kidney, B.A. Immunohistochemical expression of vascular endothelial growth factor and vascular endothelial growth factor receptor associated with tumor cell proliferation in canine cutaneous squamous cell carcinomas and trichoepitheliomas. Vet. Pathol. 2007, 44, 823–830. [Google Scholar] [CrossRef]
- Al-Dissi, A.N.; Haines, D.M.; Singh, B.; Kidney, B.A. Immunohistochemical expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 in canine simple mammary gland adenocarcinomas. Can. Vet. J. 2010, 51, 1109–1114. [Google Scholar]
- Han, H.; Silverman, J.F.; Santucci, T.S.; Macherey, R.S.; dAmato, T.A.; Tung, M.Y.; Weyant, R.J.; Landreneau, R.J. Vascular Endothelial Growth Factor expression in stage I non-small cell lung cancer correlates with neoangiogenesis and a poor prognosis. Ann. Surg. Oncol. 2001, 8, 72–79. [Google Scholar] [CrossRef]
- Chao, C.; Al-Saleem, T.; Brooks, J.J.; Rogatko, A.; Kraybill, W.G.; Eisenberg, B. Vascular Endothelial Growth Factor and soft tissue sarcomas: Tumor expression correlates with grade. Ann. Surg. Oncol. 2001, 8, 260–267. [Google Scholar] [CrossRef]
- Wąsik-Szczepanek, E.; Koczkodaj, D. Vascular endothelial growth factor (VEGF) and its role in B-cell chronic lymphocytic leukemia. Acta Haematol. Pol. 2008, 39, 197–205. [Google Scholar]
- Shiomitsu, K.; Johnson, C.L.; Malarkey, D.E.; Pruitt, A.F.; Thrall, D.E. Expression of epidermal growth factor receptor and vascular endothelial growth factor in malignant canine epithelial nasal tumours. Vet. Comp. Oncol. 2009, 7, 106–114. [Google Scholar] [CrossRef]
- Campos, A.G.; Campos, J.A.D.B.; Sanches, D.S.; Dagli, M.L.Z.; Matera, J.M. Immunohistochemical evaluation of Vascular Endothelial Growth Factor (VEGF) in splenic hemangiomas and hemangiosarcomas in dogs. Open J. Vet. Med. 2012, 2, 191–195. [Google Scholar] [CrossRef]
- De Queiroz, G.F.; Dagli, M.L.; Fukumasu, H.; Zavala, A.A.; Matera, J.M. Vascular endothelial growth factor expression and microvascular density in soft tissue sarcomas in dogs. J. Vet. Diagn. Investig. 2010, 22, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Wong-Rolle, A.; Wei, H.K.; Zhao, C.; Jin, C. Unexpected guests in the tumor microenvironment: Microbiome in cancer. Protein Cell 2021, 12, 426–435. [Google Scholar] [CrossRef] [PubMed]
| Proangiogenic Factors | Antiangiogenic Factors |
|---|---|
| VEGF—vascular endothelial growth factor FGF—fibroblast growth factor TGF-β—transforming growth factor EGF—epidermal growth factor PDGF—platelet-derived growth factor HGF—hepatocyte growth factor Angiogenin Ang-1—angiopoetin-1 IGF-1—insulin-like growth factor-1 PG-E—prostaglandin-E IL-8—interleukin-8 Proliferin Epo—erythropoetin TNF ά—tumour necrosis factor ά | TIMP—tissue inhibitors of matrix metalloproteinase TSP-1—thrombospondin-1 Angiostatin Endostatin Ang-2—angiopoetin-2 Prolactin (16 kDa fragment) Platelet factors-4 INF α/β—interferon α/β IL-1—interleukin-1 IL-6—interleukin-6 IL-10—interleukin-10 IL-12—interleukin-12 Somatostatin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczyńska-Rak, A.; Żylińska, B.; Nowicka, B.; Rak, E.; Rzepka, T. Role and Mechanisms of Angiogenesis in Tumours. Biology 2025, 14, 756. https://doi.org/10.3390/biology14070756
Sobczyńska-Rak A, Żylińska B, Nowicka B, Rak E, Rzepka T. Role and Mechanisms of Angiogenesis in Tumours. Biology. 2025; 14(7):756. https://doi.org/10.3390/biology14070756
Chicago/Turabian StyleSobczyńska-Rak, Aleksandra, Beata Żylińska, Beata Nowicka, Eryk Rak, and Tomasz Rzepka. 2025. "Role and Mechanisms of Angiogenesis in Tumours" Biology 14, no. 7: 756. https://doi.org/10.3390/biology14070756
APA StyleSobczyńska-Rak, A., Żylińska, B., Nowicka, B., Rak, E., & Rzepka, T. (2025). Role and Mechanisms of Angiogenesis in Tumours. Biology, 14(7), 756. https://doi.org/10.3390/biology14070756






