Polysaccharide Supplements from Millettia speciosa Champ. ex Benth Enhance Growth and Meat Quality in Wenchang Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Experimental Design
2.3. Growth Performance Testing
2.4. Slaughtering Performance Testing
2.5. Meat Quality
- (1)
- The color of the breast and thigh muscles is measured within 45 min of slaughter using a spectrophotometer (TS7700, 3nh, Shenzhen Threenh Technology co., LTD, Shenzhen, China). The average color value is calculated after three repeated measurements.
- (2)
- The pH of the breast and thigh muscles is measured 45 min and 24 h after slaughter using a Testo 205 pH meter, with the average value calculated from three repeated measurements.
- (3)
- To calculate cooking loss, we trimmed the meat to 2.0 cm × 1 cm × 0.5 cm, recorded its initial weight (m1), wrapped it in foil, heated it in a water bath, let it cool and absorb the surface moisture, and recorded the final weight (m2). The calculation formula is as follows:
- (4)
- To measure drip loss, we trimmed the meat to 2.0 cm × 1 cm × 0.5 cm and recorded the weight as m1. The meat sample was suspended in a conical bottle with a thin thread. The meat sample could not touch the conical bottle. The mouth of the bottle was sealed by self-sealing film and placed in the refrigerator at 4 °C. After 24 h, the liquid on the surface of the meat sample was dried with filter paper and weighed as m2, and the dripping loss was calculated. The calculation formula is as follows:
- (5)
- A 4.0 cm × 1 cm × 0.5 cm meat column was stripped of tendons, fat, and sarcolemma, and cut vertically with a muscle tenderness meter (C-LM36, Tenovo, Beijing, China) to measure shear force. This process was repeated 10 times and the average value is recorded.
2.6. Muscle Hematoxylin and Eosin (H&E) Staining
2.7. Routine Muscle Nutrient Testing
2.8. Detection of Amino Acid Contents in Muscle
2.9. Detection of Fatty Acid Contents in Muscle
2.10. mRNA Expression Analysis
2.11. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Slaughtering Performance
3.3. Meat Quality Traits
3.4. Conventional Intramuscular Nutrients
3.5. Amino Acid Contents in Muscle
3.6. Fatty Acid Contents in Muscle
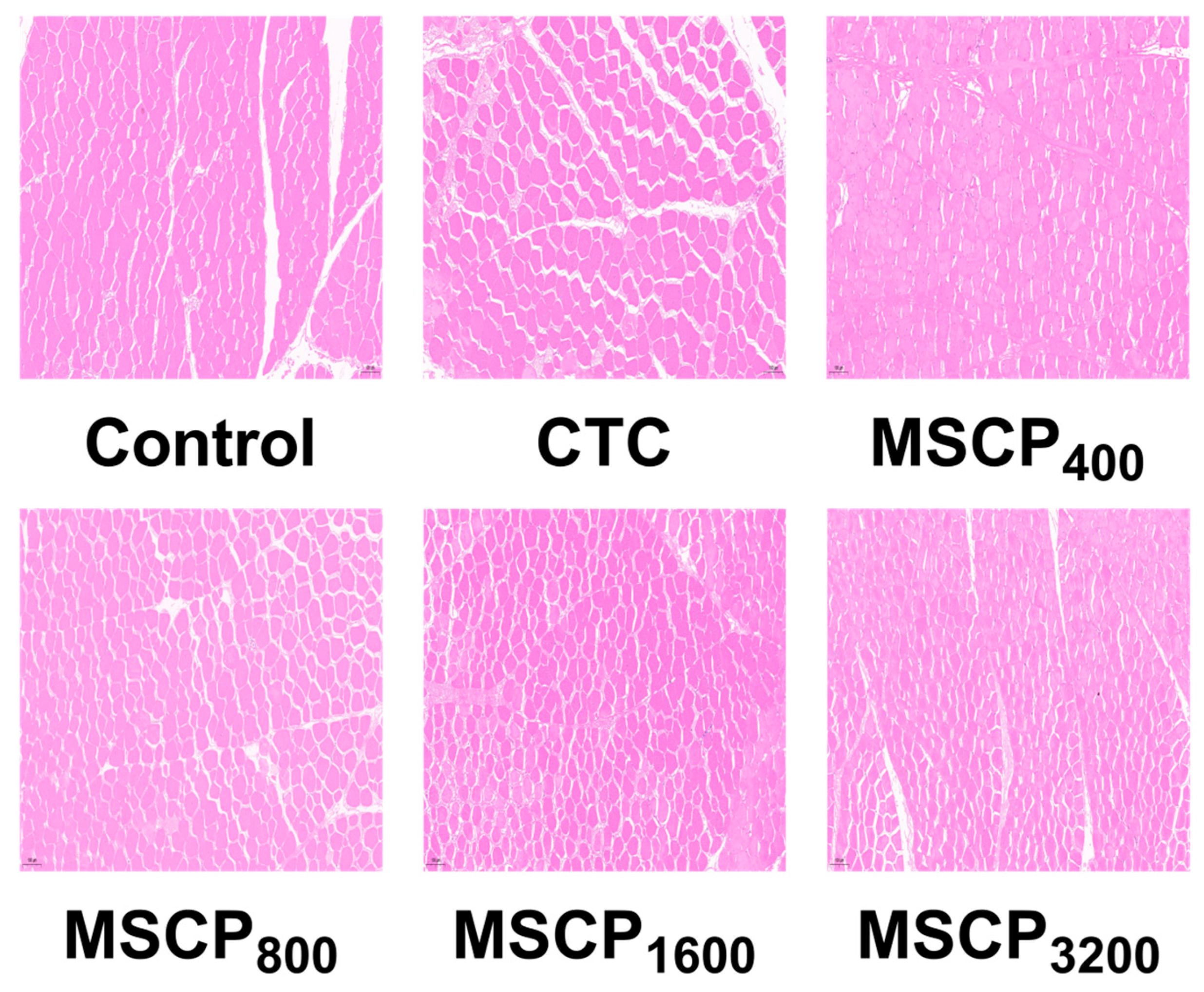
3.7. Muscle Morphology
3.8. Muscle Development-Related Genes Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, S.K.; Xiao, X.; Dai, Z.L.; Zhao, G.L.; Cui, Z.C.; Wu, Y.P.; Yang, C.M. Effects of Bacillus licheniformis on growth performance, immune and antioxidant functions, and intestinal microbiota of broilers. Poult. Sci. 2024, 103, 103210. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Kamegawa, M.; Katafuchi, A.; Ohtsuka, A.; Ijiri, D. Effects of pre-slaughter fasting on antemortem skeletal muscle protein degradation levels and postmortem muscle free amino acid concentrations in broiler chickens. Poult. Sci. 2023, 103, 103307. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Bell, J.A.; Fujita, S.; Dreyer, H.C.; Glynn, E.L.; Volpi, E.; Rasmussen, B.B. Amino acids are necessary for the insulin-induced activation of mTOR/S6K1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin. Nutr. 2008, 27, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef]
- Liu, T.; Ruan, S.; Mo, Q.; Zhao, M.; Wang, J.; Ye, Z.; Chen, L.; Feng, F. Integrated Serum Metabolome and Gut Microbiome to Decipher Chicken Amino Acid Improvements Induced by Medium-Chain Monoglycerides. Metabolites 2023, 13, 208. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.K.; Kim, K.E.; Kim, Y.R.; Kim, E.J.; An, B.K. Effects of Lupin Kernel (Lupinus angustifolius) and Faba Bean (Vicia faba) on Growth Performance and Hepatic Fatty Acid Profiles in Broiler Chicks. Braz. J. Poult. Sci. 2023, 25, 1–8. [Google Scholar] [CrossRef]
- Sujatha, T.; Abhinaya, S.; Sunder, J.; Thangapandian, M.; Kundu, A. Efficacy of early chick nutrition with Aloe vera and Azadirachta indica on gut health and histomorphometry in chicks. Vet. World 2017, 10, 569–573. [Google Scholar] [CrossRef]
- Lu, Y.; Ge, S.H.; Zhang, H.L.; Lu, W.; Bao, X.L.; Pan, S.L.; Pang, Q.H. Metabolomic and microbiome analysis of the protective effects of Puerarin against Salmonella Enteritidis Infection in chicks. BMC Vet. Res. 2023, 19, 242. [Google Scholar] [CrossRef]
- Zhao, X.N.; Wang, X.F.; Liao, J.B.; Guo, H.Z.; Yu, X.D.; Liang, J.L.; Zhang, X.; Su, Z.R.; Zhang, X.J.; Zeng, H.F. Antifatigue Effect of Millettiae speciosae Champ (Leguminosae) Extract in Mice. Trop. J. Pharm. Res. 2015, 14, 479–485. [Google Scholar] [CrossRef]
- Wang, M.Y.; Ma, W.Y.; Wang, Q.L.; Yang, Q.; Yan, X.X.; Tang, H.; Li, Z.Y.; Li, Y.Y.; Feng, S.X.; Wang, Z.N. Flavonoid-enriched extract from Millettia speciosa Champ prevents obesity by regulating thermogenesis and lipid metabolism in high-fat diet-induced obese C57BL/6 mice. Food Sci. Nutr. 2022, 10, 445–459. [Google Scholar] [CrossRef]
- Chen, X.G.; Sun, W.J.; Xu, B.C.; Wu, E.Y.; Cui, Y.; Hao, K.Y.; Zhang, G.Y.; Zhou, C.C.; Xu, Y.P.; Li, J.; et al. Polysaccharides From the Roots of Millettia Speciosa Champ Modulate Gut Health and Ameliorate Cyclophosphamide-Induced Intestinal Injury and Immunosuppression. Front. Immunol. 2021, 12, 766296. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, Y.J.; Chen, X.; Luo, S.Y.; Pu, L.; Li, F.Z.; Zong, M.H.; Lou, W.Y. A novel polysaccharide from the roots of Millettia Speciosa Champ: Preparation, structural characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2020, 145, 547–557. [Google Scholar] [CrossRef]
- He, Y.Z.; Li, J.; Wang, F.F.; Na, W.; Tan, Z. Dynamic Changes in the Gut Microbiota and Metabolites during the Growth of Hainan Wenchang Chickens. Animals 2023, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Jiang, Q.; Dai, J.; Dai, W.; Kang, X.; Xu, T.; Zheng, X.; Fu, A.; Xing, Z.; et al. Supplementation of dietary areca nut extract modulates the growth performance, cecal microbiota composition, and immune function in Wenchang chickens. Front. Vet. Sci. 2023, 10, 1278312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, R.; Liu, Q.; Gong, Y.; Ou, Y.; Qi, Q.; Xie, Y.; Wang, X.; Hu, C.; Jiang, S.; et al. Effects of dietary supplementation with dandelion tannins or soybean isoflavones on growth performance, antioxidant function, intestinal morphology, and microbiota composition in Wenchang chickens. Front. Vet. Sci. 2022, 9, 1073659. [Google Scholar] [CrossRef] [PubMed]
- NY/T3645-2020. Available online: https://codeofchina.com/standard/NYT3645-2020.html (accessed on 17 June 2025).
- NY/T1871-2010. Available online: http://down.foodmate.net/standard/sort/5/26877.html (accessed on 17 June 2025).
- Zhou, T.; Wu, Y.; Bi, Y.; Bai, H.; Jiang, Y.; Chen, G.; Chang, G.; Wang, Z. MYOZ1 Gene Promotes Muscle Growth and Development in Meat Ducks. Genes 2022, 13, 1574. [Google Scholar] [CrossRef]
- NY/T823/2020. Available online: http://down.foodmate.net/standard/sort/5/82806.html (accessed on 17 June 2025).
- GB 5009.5-2016. Available online: https://www.codeofchina.com/standard/GB5009.257-2016.html?gad_source=1&gad_campaignid=22309047764&gbraid=0AAAAADzApimsLnS5JCBuUKEf-sgohQMAf&gclid=EAIaIQobChMI9fiooJyHjgMVupKDBx0wShgGEAAYASAAEgJGGvD_BwE (accessed on 17 June 2025).
- GB 5009.6-2016. Available online: https://www.codeofchina.com/standard/GB5009.6-2016.html?gad_source=1&gad_campaignid=22309047764&gbraid=0AAAAADzApimsLnS5JCBuUKEf-sgohQMAf&gclid=EAIaIQobChMI35m2tpyHjgMV7KuDBx312yHkEAAYASAAEgIruvD_BwE (accessed on 17 June 2025).
- Qi, Q.; Hu, C.; Zhang, H.; Sun, R.; Liu, Q.; Ouyang, K.; Xie, Y.; Li, X.; Wu, W.; Liu, Y.; et al. Dietary Supplementation with Putrescine Improves Growth Performance and Meat Quality of Wenchang Chickens. Animals 2023, 13, 1564. [Google Scholar] [CrossRef]
- GB 5009.168/2016. Available online: https://www.chinesestandard.net/PDF.aspx/GB5009.168-2016 (accessed on 17 June 2025).
- Reppert, E.J.; Reif, K.E.; Montgomery, S.R.; Magnin, G.; Zhang, Y.; Martin-Jimenez, T.; Olson, K.C.; Coetzee, J.F. Determination of plasma-chlortetracycline (CTC) concentrations in grazing beef cattle fed one of four FDA approved free-choice CTC-medicated minerals. Transl. Anim. Sci. 2020, 4, txaa048. [Google Scholar] [CrossRef]
- Ziólkowski, H.; Jasiecka-Mikolajczyk, A.; Madej-Smiechowska, H.; Janiuk, J.; Zygmuntowicz, A.; Dabrowski, M. Comparative pharmacokinetics of chlortetracycline, tetracycline, minocycline, and tigecycline in broiler chickens. Poult. Sci. 2020, 99, 4750–4757. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Q.; Huang, B.; Chen, M.; Liang, Z.; Zhang, Z.; Zhang, J. Utilizing Integrated UHPLC-Q-Exactive Orbitrap-MS, Multivariate Analysis, and Bioactive Evaluation to Distinguish between Wild and Cultivated Niudali (Millettia speciosa Champ.). Molecules 2024, 29, 806. [Google Scholar] [CrossRef]
- Zheng, W.D.; Guan, Y.L.; Wu, B. Effects of Yupingfeng Polysaccharides as Feed Supplement on Immune Function and Intestinal Microbiome in Chickens. Microorganisms 2023, 11, 2774. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, C.; Xiao, Y.; Zheng, S.; Jiang, Q.; Chen, J. Effects of Polysaccharides-Rich Extract from Gracilaria lemaneiformis on Growth Performance, Antioxidant Capacity, Immune Function, and Meat Quality in Broiler Chickens. J. Poult. Sci. 2023, 60, 2023018. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yu, Y.; Wang, B.; Ding, M.; Tian, Y.; Jiang, R.; Sun, G.; Han, R.; Kang, X.; Yan, F.; et al. Dietary supplementation with pseudostellaria heterophylla polysaccharide enhanced immunity and changed mRNA expression of spleen in chicks. Dev. Comp. Immunol. 2024, 151, 105094. [Google Scholar] [CrossRef]
- Aldemir, R. The Effects of Adding Juniper Berry in Broiler Diets on Performance, Parameters of Serum, Carcass, Histopathology and Jejunum Villi Lengths. Braz. J. Poult. Sci. 2022, 24, 1–6. [Google Scholar] [CrossRef]
- Liu, X.F.; Ma, A.J.; Zhi, T.X.; Hong, D.; Chen, Z.; Li, S.T.; Jia, Y.M. Dietary Effect of Brevibacillus laterosporus S62-9 on Chicken Meat Quality, Amino Acid Profile, and Volatile Compounds. Foods 2023, 12, 288. [Google Scholar] [CrossRef]
- Nemauluma, M.F.D.; Manyelo, T.G.; Ng’ambi, J.W.; Kolobe, S.D.; Malematja, E. Effects of bee pollen inclusion on performance and carcass characteristics of broiler chickens. Poult. Sci. 2023, 102, 102628. [Google Scholar] [CrossRef]
- Suliman, G.M.; Hussein, E.O.S.; Al-Owaimer, A.N.; Alhotan, R.A.; Al-Garadi, M.A.; Mahdi, J.M.H.; Ba-Awadh, H.A.; Qaid, M.M.; Swelum, A.A.A. Betaine and nano-emulsified vegetable oil supplementation for improving carcass and meat quality characteristics of broiler chickens under heat stress conditions. Front. Vet. Sci. 2023, 10, 1147020. [Google Scholar] [CrossRef]
- Dang, D.X.; Cho, S.; Wang, H.; Seok, W.J.; Ha, J.H.; Kim, I.H. Quercetin extracted from Sophora japonica flower improves growth performance, nutrient digestibility, cecal microbiota, organ indexes, and breast quality in broiler chicks. Anim. Biosci. 2022, 35, 577–586. [Google Scholar] [CrossRef]
- Fathi, M.; Hosayni, M.; Alizadeh, S.; Zandi, R.; Rahmati, S.; Rezaee, V. Effects of black cumin (Nigella Sativa) seed meal on growth performance, blood and biochemical indices, meat quality and cecal microbial load in broiler chickens. Livest. Sci. 2023, 274, 105272. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Liu, X.; Wang, Y.; Yang, W.; Zhao, W.; Zhao, G.; Cui, H.; Wen, J. Identification of characteristic aroma compounds in chicken meat and their metabolic mechanisms using gas chromatography-olfactometry, odor activity values, and metabolomics. Food Res. Int. 2024, 175, 113782. [Google Scholar] [CrossRef]
- Kokoszynski, D.; Zochowska-Kujawska, J.; Kotowicz, M.; Piatek, H.; Wlodarczyk, K.; Arpásová, H.; Biesiada-Drzazga, B.; Wegner, M.; Saleh, M.; Imanski, M. The Effects of Slaughter Age and Sex on Carcass Traits, Meat Quality, and Leg Bone Characteristics of Farmed Common Pheasants (Phasianus colchicus L.). Animals 2024, 14, 1050. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Y.; Lu, Z.; Zhang, Y.; Zhang, T. Effect of pre-slaughter fasting time on carcass yield, blood parameters and meat quality in broilers. Anim. Biosci. 2024, 37, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Chaiwang, N.; Marupanthorn, K.; Krutthai, N.; Wattanakul, W.; Jaturasitha, S.; Arjin, C.; Sringarm, K.; Setthaya, P. Assessment of nucleic acid content, amino acid profile, carcass, and meat quality of Thai native chicken. Poult. Sci. 2023, 102, 103067. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Xu, M.; Huang, Q.; Zhang, D.; Lin, Z.; Wang, Y.; Liu, Y. Nutrition and Flavor Evaluation of Amino Acids in Guangyuan Grey Chicken of Different Ages, Genders and Meat Cuts. Animals 2023, 13, 1235. [Google Scholar] [CrossRef]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Ashayerizadeh, O.; Dastar, B.; Shargh, M.S.; Soumeh, E.A.; Jazi, V. Effects of black pepper and turmeric powder on growth performance, gut health, meat quality, and fatty acid profile of Japanese quail. Front. Physiol. 2023, 14, 1218850. [Google Scholar] [CrossRef]
- Ceylan, N.; Yenice, E.; Yavas, I.; Çenesiz, A.A.; Toprak, N.N.; Çiftçi, I. Comparative effects of medium-chain fatty acids or phytobiotics-based feed additives on performance, caecum microbiota, volatile fatty acid production and intestinal morphology of broilers. Vet. Med. Sci. 2023, 9, 2719–2730. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhao, Z.T.; Meng, Z.; Yi, H.; Ye, X.M.; Qi, A.; Ouyang, K.H.; Wang, W.J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020, 155, 61–70. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Li, Y.; Li, D.; Shi, L.; Chen, J. Effect of Cage and Floor Housing Systems on Muscle Fiber Characteristics, Carcass Characteristics, and Meat Quality of Slow-Growing Meat-Type Chickens. Agriculture 2023, 13, 365. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, J.; Jin, Y.; Chang, Y.; Shi, M.; Miao, Z. Effects of Chinese yam Polysaccharides on the Muscle Tissues Development-Related Genes Expression in Breast and Thigh Muscle of Broilers. Genes 2022, 14, 6. [Google Scholar] [CrossRef]
- Huang, S.C.; Cao, Q.Q.; Cao, Y.B.; Yang, Y.R.; Xu, T.T.; Yue, K.; Liu, F.; Tong, Z.X.; Wang, X.B. Morinda officinalis polysaccharides improve meat quality by reducing oxidative damage in chickens suffering from tibial dyschondroplasia. Food Chem. 2021, 344, 128688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Li, M.Y.; Xu, S.S.; Sun, J.Y.; Liu, G.J. Expression of Myostatin (Mstn) and Myogenin (Myog) Genes in Zi And Rhine Goose and Their Correlation with Carcass Traits. Braz. J. Poult. Sci. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Mao, H.G.; Wang, M.T.; Ke, Z.J.; Wang, J.B.; Raza, S.H.A.; Dong, X.G.; An, J.; Yin, Z.Z.; Qi, L.L. Association of variants and expression levels of MYOD1 gene with carcass and muscle characteristic traits in domestic pigeons. Anim. Biotechnol. 2023, 34, 4927–4937. [Google Scholar] [CrossRef]
- Ren, H.Y.; Wei, Z.Y.; Li, X.; Wang, Q.; Chen, H.; Lan, X.Y. Goat MyoD1: mRNA expression, InDel and CNV detection and their associations with growth traits. Gene 2023, 866, 147348. [Google Scholar] [CrossRef]
- Hai, C.; Bai, C.; Yang, L.; Wei, Z.; Wang, H.; Ma, H.; Ma, H.; Zhao, Y.; Su, G.; Li, G. Effects of Different Generations and Sex on Physiological, Biochemical, and Growth Parameters of Crossbred Beef Cattle by Myostatin Gene-Edited Luxi Bulls and Simmental Cows. Animals 2023, 13, 3216. [Google Scholar] [CrossRef]
- Liu, C.-L.; Na, R.-S.; Guang-Xin, E.; Han, Y.-G.; Zeng, Y.; Wang, X.; Cheng, S.-Z.; Yang, B.-G.; Guo, Z.-H.; Huang, Y.-F. Genetic Diversity Identification and Haplotype Distribution of Myostatin Gene in Goats. Indian J. Anim. Res. 2023, 57, 273–281. [Google Scholar] [CrossRef]

| Ingredients Composition (%) | Control |
|---|---|
| Corn | 72.1 |
| Bran | 4.5 |
| Soybean meal | 17.1 |
| Soybean oil | 3.0 |
| CaHPO4 | 1.0 |
| Limestone | 1.0 |
| Premix * | 1.0 |
| NaCl | 0.3 |
| Nutrient level (dry mater basis, %) | |
| ME, MJ/kg | 14.80 |
| Crude protein | 14.50 |
| Crude Fat | 6.04 |
| Crude fiber | 2.44 |
| Calcium | 0.69 |
| Phosphorous | 0.51 |
| Available + Phosphorus | 0.38 |
| Lysine | 0.65 |
| Methionine | 0.39 |
| Arginine | 0.87 |
| Gene | Primer Sequences (5′→3′) | Accession | TM °C |
|---|---|---|---|
| β-actin | F: CATTGTCCACCGCAAATGCT R: AAGCCATGCCAATCTCGTCT | L08165.1 | 60 |
| MYOG | F: TTTTCCCGGAGCAGAGGTTT R: GGTCGATGGACACGGTTTTG | NM_204184.2 | 60 |
| MYOD1 | F: GCCCTCGCTCCAACTGCTCC; R: GCTGCCTTTTGGAGTTTGCG | NM_204214.3 | 60 |
| MSTN | F: TTTTGGATGGGACTGGATTATAGCACCT R: GCCTCTGGGATTTGCTTGGTGTACC | NM_001001461.2 | 60 |
| Tratis 1 | Groups 2 | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| IBW/g | 1476.04 ± 8.26 | 1476.04 ± 10.39 | 1473.96 ± 6.95 | 1475.00 ± 8.91 | 1475.00 ± 4.45 | 1478.12 ± 4.31 | 0.919 |
| FBW/g | 2055.42 ± 57.84 b | 2161.67 ± 29.91 a | 2127.29 ± 44.85 a | 2130.95 ± 39.30 a | 2120.00 ± 20.04 a | 2109.58 ± 74.22 a | 0.004 |
| ADG/g | 14.49 ± 1.56 b | 17.14 ± 0.79 ab | 16.33 ± 1.14 a | 16.87 ± 1.70 a | 16.41 ± 0.92 a | 15.79 ± 1.85 a | 0.007 |
| ADFI/g | 70.97 ± 9.42 b | 95.15 ± 0.75 a | 93.04 ± 3.13 a | 93.08 ± 0.86 a | 95.15 ± 10.50 a | 91.09 ± 1.83 a | <0.001 |
| FCR | 5.59 ± 1.16 | 5.56 ± 0.27 | 5.55 ± 0.52 | 5.33 ± 0.30 | 5.58 ± 0.11 | 5.67 ± 0.39 | 0.942 |
| Tratis 1 | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| Dressing percentage% | 92.73 ± 2.51 | 92.34 ± 1.67 | 93.39 ± 4.48 | 92.74 ± 4.62 | 94.57 ± 1.30 | 95.37 ± 1.46 | 0.297 |
| Semi-eviscerated percentage% | 79.95 ± 3.51 | 81.97 ± 4.63 | 83.46 ± 5.58 | 84.27 ± 3.02 | 81.34 ± 1.30 | 82.36 ± 1.40 | 0.298 |
| Eviscerated percentage% | 68.75 ± 3.84 | 69.46 ± 0.47 | 70.27 ± 3.63 | 71.39 ± 2.51 | 70.14 ± 2.16 | 70.72 ± 3.41 | 0.623 |
| Breast muscle percentage% | 12.35 ± 1.44 | 13.15 ± 0.96 | 13.01 ± 1.25 | 13.09 ± 1.34 | 13.67 ± 1.27 | 12.89 ± 1.67 | 0.585 |
| Thigh muscle percentage% | 16.53 ± 1.75 | 16.58 ± 1.44 | 16.35 ± 1.41 | 16.31 ± 1.93 | 16.89 ± 1.39 | 16.24 ± 2.71 | 0.986 |
| Abdominal fat rate% | 9.19 ± 1.88 | 9.32 ± 1.99 | 9.16 ± 1.85 | 8.51 ± 1.66 | 9.12 ± 1.63 | 7.59 ± 1.82 | 0.406 |
| Tratis 1 | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| pH45 min | 5.97 ± 0.18 | 5.94 ± 0.23 | 6.20 ± 0.28 | 6.09 ± 0.30 | 6.00 ± 0.23 | 5.98 ± 0.10 | 0.389 |
| pH24 h | 5.82 ± 0.05 bc | 5.76 ± 0.15 c | 6.03 ± 0.06 a | 6.06 ± 0.20 a | 5.96 ± 0.19 ab | 5.96 ± 0.14 ab | 0.014 |
| Drop loss at 24 h/% | 2.10 ± 0.84 | 3.19 ± 0.49 | 2.31 ± 0.82 | 2.03 ± 0.42 | 2.08 ± 0.47 | 2.20 ± 0.28 | 0.090 |
| Cooking loss/% | 17.73 ± 5.21 | 16.40 ± 2.94 | 12.12 ± 1.26 | 15.05 ± 1.05 | 16.82 ± 1.50 | 17.52 ± 3.50 | 0.063 |
| Shear force/N | 14.16 ± 2.40 | 12.48 ± 2.23 | 13.02 ± 1.96 | 11.87 ± 2.14 | 11.67 ± 2.49 | 12.57 ± 2.04 | 0.452 |
| Lightness (L *) | 49.91 ± 1.86 | 48.66 ± 2.50 | 48.98 ± 3.05 | 47.96 ± 2.69 | 48.71 ± 2.33 | 48.99 ± 3.52 | 0.334 |
| Redness (a *) | 0.61 ± 0.15 | 0.83 ± 0.19 | 0.70 ± 0.22 | 0.81 ± 0.22 | 0.74 ± 0.34 | 0.67 ± 0.27 | 0.989 |
| Yellowness (b *) | 12.46 ± 2.37 a | 12.02 ± 1.51 a | 9.89 ± 2.24 b | 10.22 ± 1.76 b | 9.46 ± 2.76 b | 8.89 ± 2.99 b | <0.001 |
| Tratis 1 | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| pH45 min | 5.76 ± 0.27 | 5.95 ± 0.53 | 6.13 ± 0.36 | 6.28 ± 0.49 | 5.84 ± 0.30 | 5.80 ± 0.12 | 0.312 |
| pH24 h | 5.61 ± 0.22 b | 5.62 ± 0.02 b | 5.69 ± 0.13 ab | 5.71 ± 0.07 ab | 5.86 ± 0.23 a | 5.92 ± 0.13 a | 0.021 |
| Drop loss 24 h/% | 1.88 ± 0.44 | 1.93 ± 0.90 | 1.86 ± 0.69 | 2.30 ± 0.59 | 1.68 ± 0.65 | 1.94 ± 0.30 | 0.731 |
| Cooking loss/% | 27.10 ± 2.01 b | 31.66 ± 1.41 a | 21.42 ± 4.20 c | 28.34 ± 3.24 ab | 30.49 ± 2.55 ab | 30.53 ± 3.68 ab | 0.001 |
| Shear force/N | 29.53 ± 1.22 a | 28.52 ± 3.91 a | 28.55 ± 2.56 a | 23.77 ± 1.93 b | 23.55 ± 4.31 b | 29.61 ± 4.76 a | 0.015 |
| Lightness (L *) | 47.83 ± 2.68 | 49.69 ± 2.74 | 49.79 ± 2.80 | 48.13 ± 1.98 | 48.49 ± 2.97 | 49.54 ± 3.48 | 0.068 |
| Redness (a *) | 6.67 ± 3.56 | 7.30 ± 3.26 | 7.20 ± 2.21 | 8.28 ± 2.32 | 7.80 ± 2.20 | 7.24 ± 1.66 | 0.407 |
| Yellowness (b* ) | 15.04 ± 3.63 | 15.49 ± 2.79 | 14.02 ± 2.92 | 14.43 ± 3.75 | 13.45 ± 3.45 | 12.69 ± 5.60 | 0.136 |
| Tratis | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| Moiture (%) | 68.69 ± 1.15 ab | 67.97 ± 1.21 b | 69.70 ± 0.79 a | 69.10 ± 1.17 ab | 69.34 ± 0.55 a | 69.91 ± 1.21 a | 0.036 |
| Crude Protein (g/kg) | 732.18 ± 95.73 a | 611.73 ± 139.61 b | 693.44 ± 94.09 b | 778.02 ± 114.36 a | 770.99 ± 34.54 a | 783.84 ± 16.44 a | 0.024 |
| Intramuscular fat (%) | 3.98 ± 1.47 b | 10.14 ± 2.69 a | 6.94 ± 2.01 b | 5.88 ± 2.86 b | 5.76 ± 3.20 b | 5.62 ± 2.05 b | 0.005 |
| Tratis | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| TAA | 309.05 ± 137.50 | 341.25 ± 126.79 | 290.71 ± 188.43 | 433.21 ± 173.73 | 264.11 ± 116.44 | 274.79 ± 96.07 | 0.365 |
| EAA | 96.49 ± 42.16 | 122.40 ± 54.83 | 97.22 ± 71.43 | 165.52 ± 71.64 | 89.26 ± 46.40 | 93.09 ± 42.02 | 0.182 |
| NEAA | 212.56 ± 95.65 | 218.85 ± 72.77 | 193.50 ± 117.25 | 267.69 ± 102.33 | 174.85 ± 71.27 | 181.71 ± 55.22 | 0.508 |
| FAA | 179.13 ± 81.28 | 192.64 ± 69.01 | 160.76 ± 96.28 | 218.23 ± 82.99 | 146.50 ± 59.77 | 152.18 ± 46.97 | 0.554 |
| EAA/TAA | 0.31 ± 0.01 b | 0.35 ± 0.04 ab | 0.31 ± 0.02 b | 0.38 ± 0.02 a | 0.33 ± 0.04 b | 0.33 ± 0.04 b | 0.016 |
| EAA/NEAA | 0.46 ± 0.02 b | 0.54 ± 0.08 ab | 0.46 ± 0.09 b | 0.61 ± 0.04 a | 0.49 ± 0.09 b | 0.50 ± 0.08 b | 0.013 |
| Tratis | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| PUFAs | 3068.65 ± 290.76 | 3571.86 ± 405.99 | 3584.58 ± 325.14 | 3333.60 ± 753.02 | 3164.20 ± 434.07 | 3513.31 ± 239.02 | 0.223 |
| MUFAs | 206.63 ± 38.53 | 247.19 ± 43.22 | 242.60 ± 70.61 | 210.28 ± 113.58 | 235.16 ± 58.11 | 250.98 ± 58.80 | 0.796 |
| SFAs | 2481.39 ± 234.09 | 2938.60 ± 277.92 | 2848.23 ± 252.45 | 2706.92 ± 689.47 | 2633.64 ± 424.53 | 2862.52 ± 283.36 | 0.370 |
| PUFAs/SFAs | 1.23 ± 0.03 | 1.21 ± 0.06 | 1.26 ± 0.02 | 1.24 ± 0.04 | 1.21 ± 0.04 | 1.23 ± 0.05 | 0.342 |
| n-6/n-3 PUFAs | 1.32 ± 0.03 | 1.30 ± 0.07 | 1.34 ± 0.03 | 1.31 ± 0.03 | 1.29 ± 0.03 | 1.32 ± 0.05 | 0.434 |
| Tratis | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | CTC | MSCP400 | MSCP800 | MSCP1600 | MSCP3200 | ||
| Diameter/μm | 69.71 ± 3.49 b | 73.82 ± 3.94 a | 66.39 ± 2.30 c | 65.56 ± 3.44 c | 66.07 ± 3.92 c | 65.88 ± 2.42 c | <0.001 |
| Density root/mm2 | 453.00 ± 49.18 b | 393.74 ± 42.12 c | 494.47 ± 43.69 a | 522.30 ± 63.85 a | 513.24 ± 65.49 a | 505.53 ± 55.44 a | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-H.; Liu, J.; Feng, X.; Liu, Q.-W.; Sun, R.-P.; Wu, W.; Ouyang, K.; Yuan, J.-L.; Zhang, Y.; Wang, X.-P.; et al. Polysaccharide Supplements from Millettia speciosa Champ. ex Benth Enhance Growth and Meat Quality in Wenchang Chickens. Biology 2025, 14, 755. https://doi.org/10.3390/biology14070755
Liu Y-H, Liu J, Feng X, Liu Q-W, Sun R-P, Wu W, Ouyang K, Yuan J-L, Zhang Y, Wang X-P, et al. Polysaccharide Supplements from Millettia speciosa Champ. ex Benth Enhance Growth and Meat Quality in Wenchang Chickens. Biology. 2025; 14(7):755. https://doi.org/10.3390/biology14070755
Chicago/Turabian StyleLiu, Yu-Hang, Jie Liu, Xin Feng, Quan-Wei Liu, Rui-Ping Sun, Wei Wu, Kun Ouyang, Jing-Li Yuan, Yan Zhang, Xiu-Ping Wang, and et al. 2025. "Polysaccharide Supplements from Millettia speciosa Champ. ex Benth Enhance Growth and Meat Quality in Wenchang Chickens" Biology 14, no. 7: 755. https://doi.org/10.3390/biology14070755
APA StyleLiu, Y.-H., Liu, J., Feng, X., Liu, Q.-W., Sun, R.-P., Wu, W., Ouyang, K., Yuan, J.-L., Zhang, Y., Wang, X.-P., Zhao, G.-P., & Wei, L.-M. (2025). Polysaccharide Supplements from Millettia speciosa Champ. ex Benth Enhance Growth and Meat Quality in Wenchang Chickens. Biology, 14(7), 755. https://doi.org/10.3390/biology14070755






