Exploring the Bacterial Microbiome of High-Moisture Plant-Based Meat Substituted Soybean Flour with Mung Bean Protein and Duckweed Powder
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Sampling
2.2. Proximate Analysis
2.3. Bacterial Microbiome Analysis
2.3.1. DNA Extraction and 16S rRNA Sequencing
2.3.2. Bioinformatics
2.4. Statistical Analysis
3. Results
3.1. Nutrient Proximate Composition
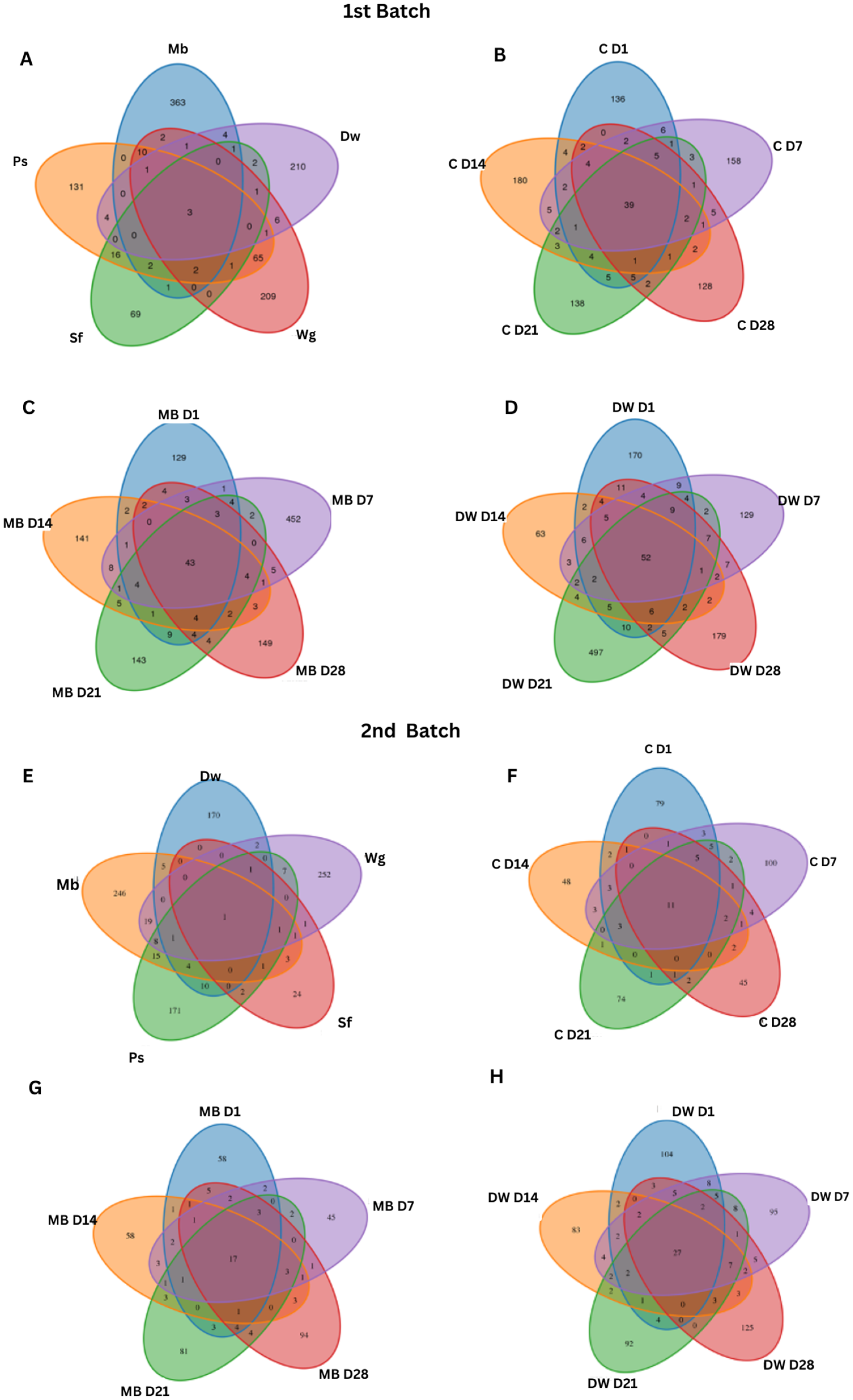
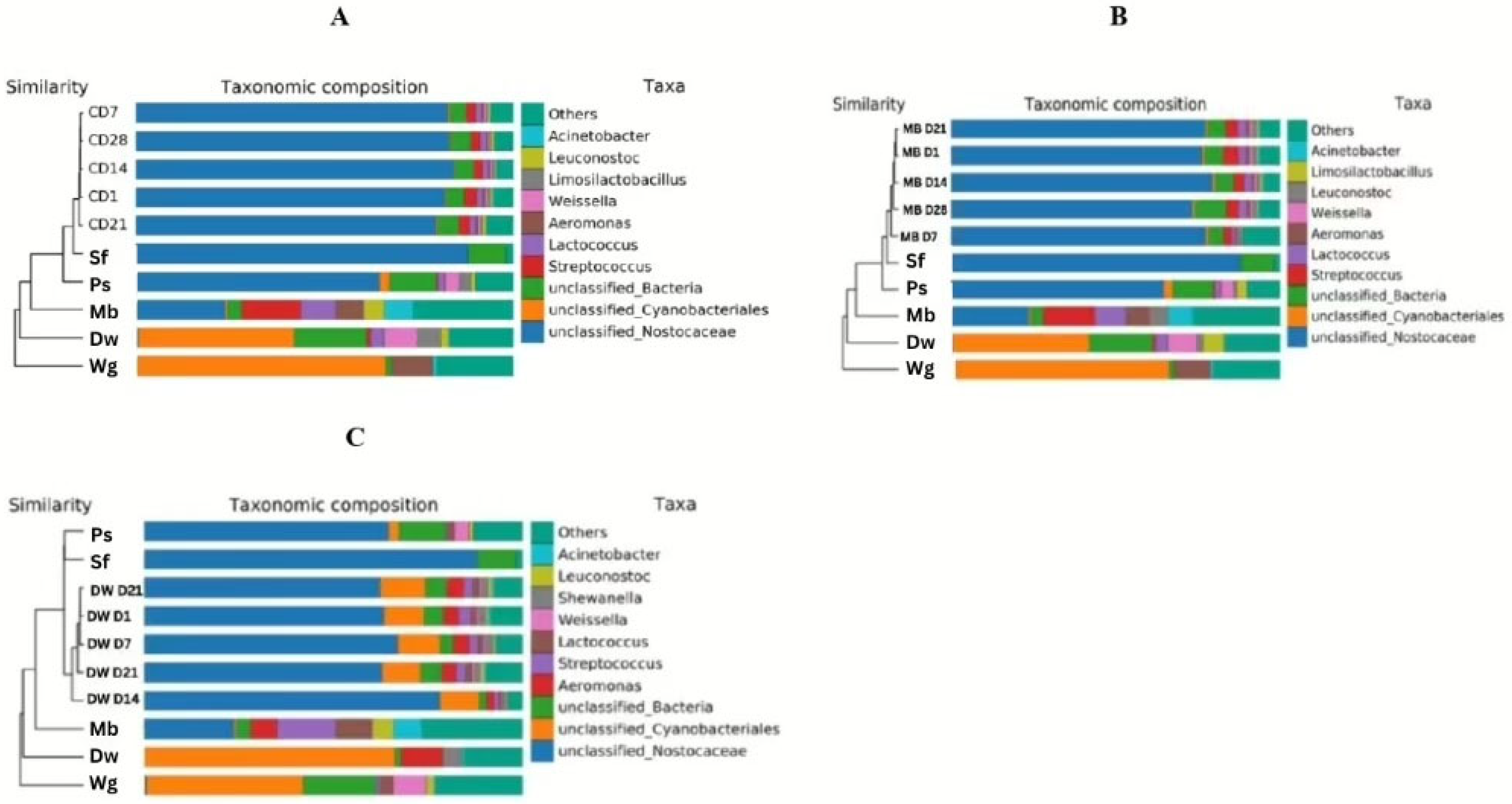
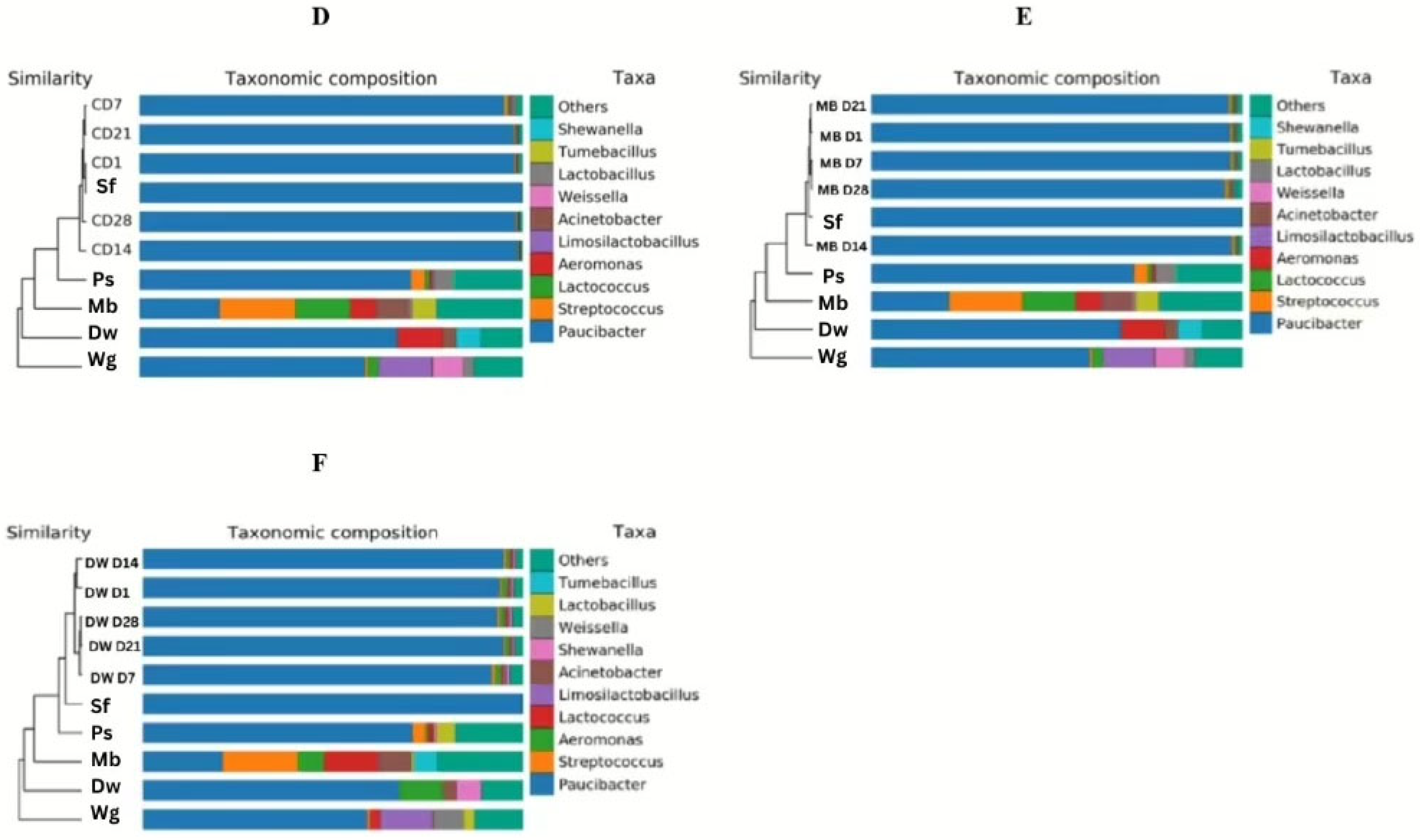
3.2. Bacterial Diversity
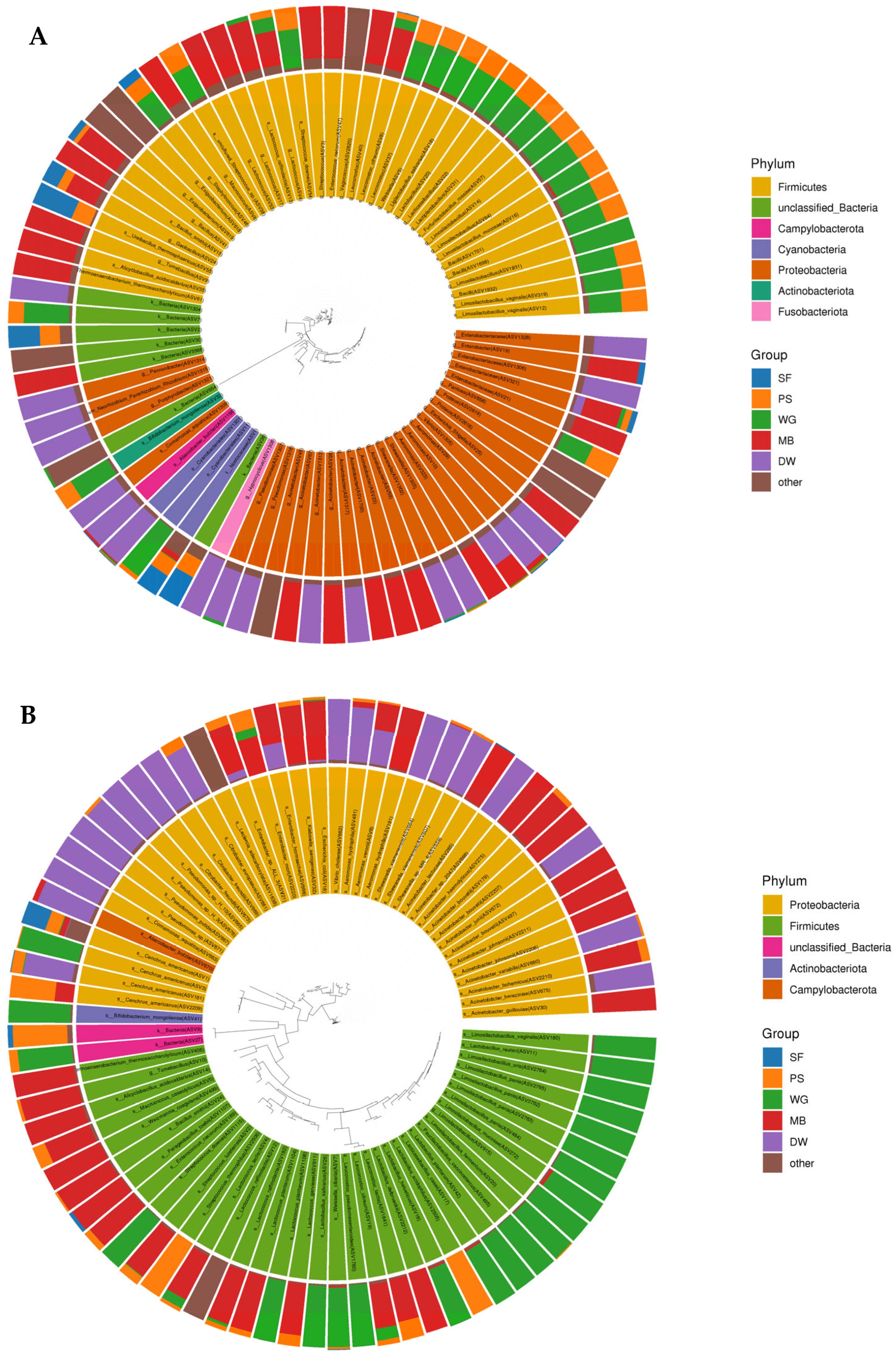
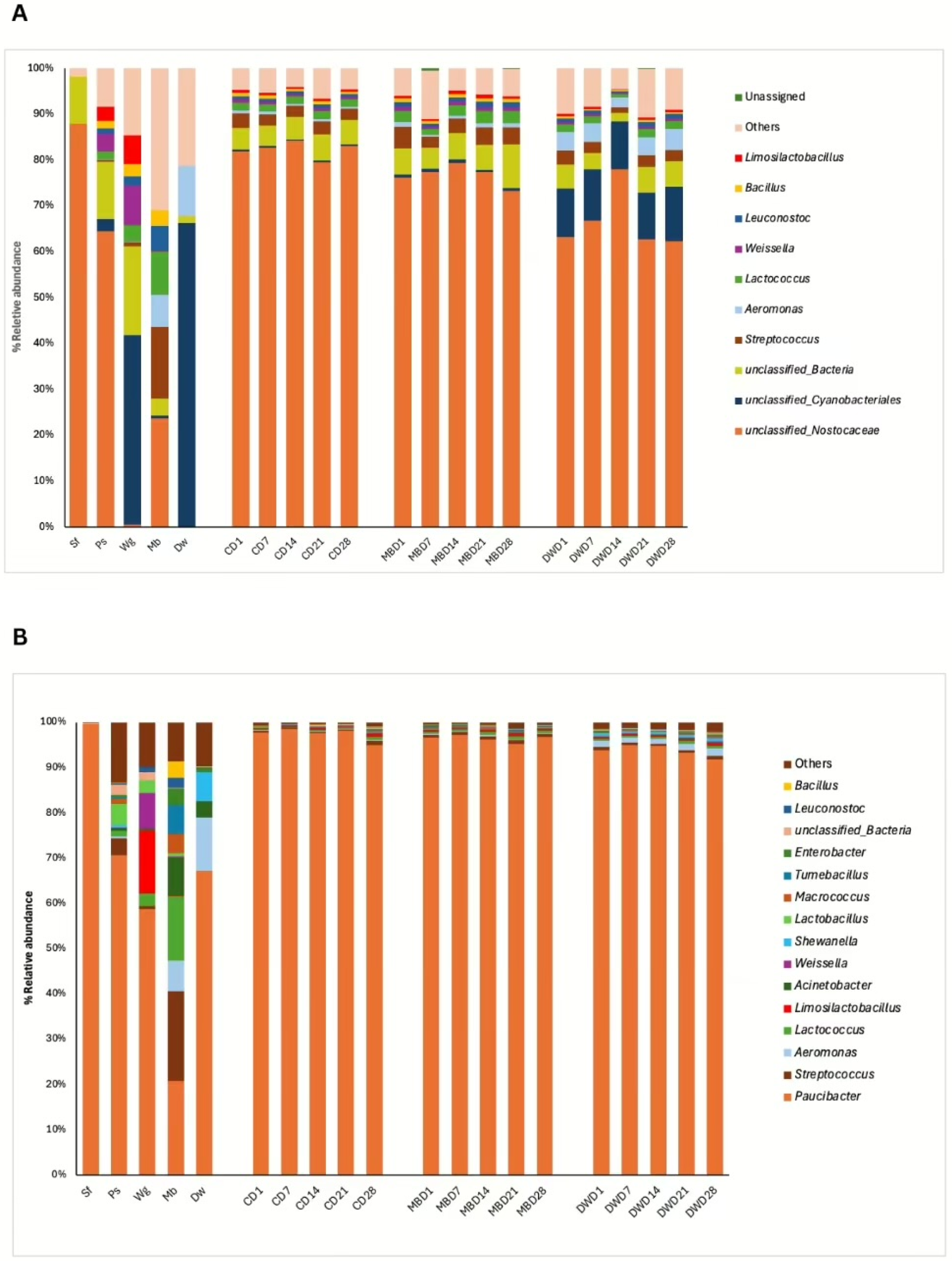
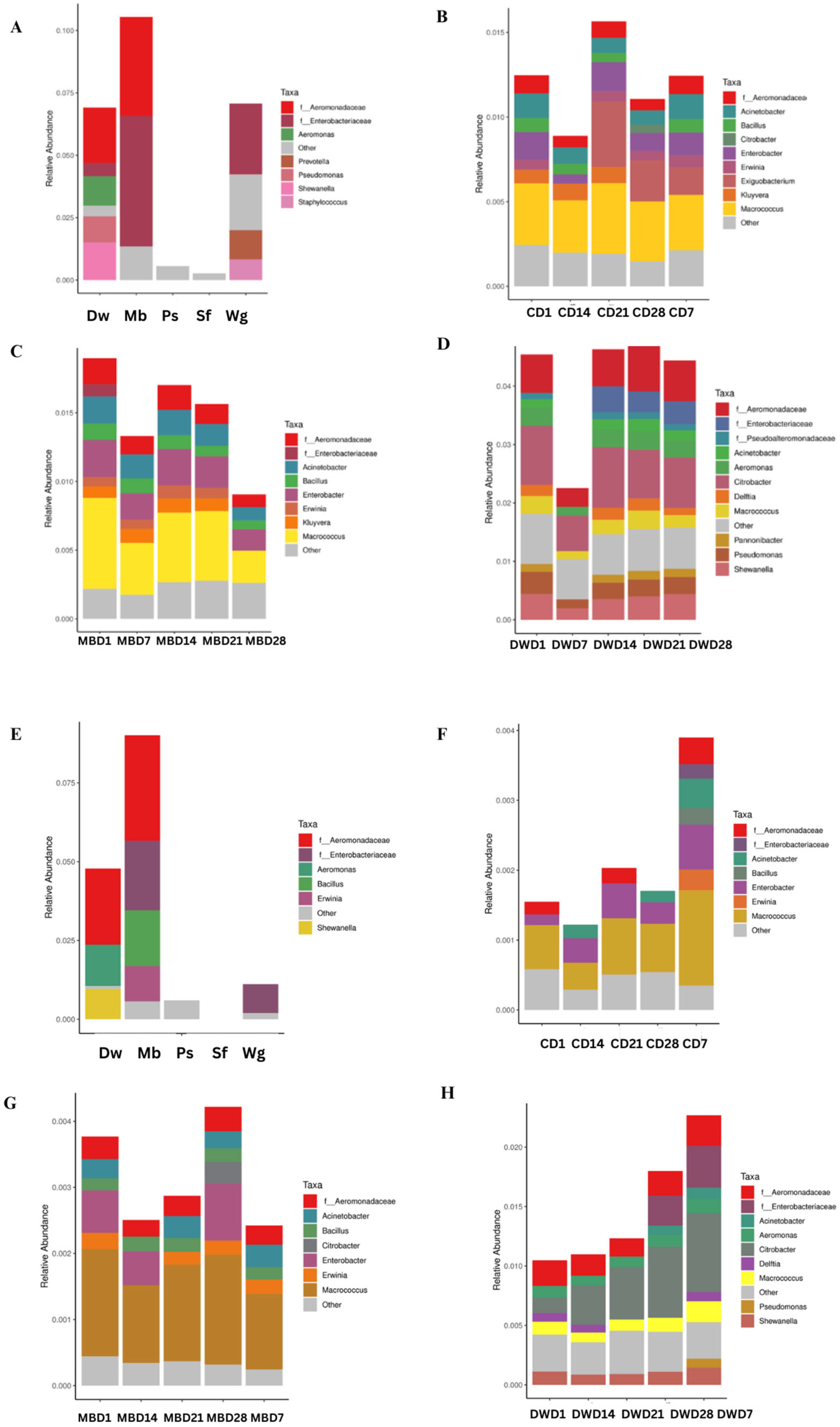
3.3. Bacterial Microbiome of High Moisture Plant-Based Meat (PBM) and the 5 Ingredient Compositions During a Storage Period of 28 Days
3.4. Relationship Between Change in Bacterial Population and Shelf Life of High Moisture Plant-Based Meat (PBM) of Control, Mung Bean Protein, and Duckweed Formulas During a Storage Period of 28 Days
3.5. OTU Predictions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Saari, N. Texturized mung bean protein as a sustainable food source: Effects of extrusion on its physical, textural and protein quality. Innov. Food Sci. Emerg. Technol. 2021, 67, 102591. [Google Scholar] [CrossRef]
- de Beukelaar, M.F.A.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R.H. Duckweed as human food: The influence of meal context and information on duckweed acceptability of Dutch consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Hou, D.Z.; Feng, Q.; Niu, Z.T.; Wang, L.; Yan, Z.; Zhou, S.M. Promising mung bean proteins and peptides: A comprehensive review of preparation technologies, biological activities, and their potential applications. Food Biosci. 2023, 55, 102972. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Yahaya, N.; Hamdan, N.H.; Zabidi, A.R.; Mohamad, A.M.; Suhaimi, M.L.H.; Md Johari, M.A.; Yahya, H. Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses. Future Foods 2022, 5, 100128. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Clark, W.D.; Sharma, J.G.; Goswami, R.K.; Shrivastav, A.K.; Tocher, D.R. Mass production of Lemna minor and its amino acid and fatty acid profiles. Front. Chem. 2018, 6, 479. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef]
- Duangjarus, N.; Chaiworapuek, W.; Rachtanapun, C.; Charoensiddhi, S. Antimicrobial and functional properties of duckweed (Wolffia globosa) protein and peptide extracts prepared by ultrasound-assisted extraction. Foods 2022, 11, 2348. [Google Scholar] [CrossRef]
- Rocha, L.O.; Silva, N.C.C. Editorial: Application of omics-based technologies and the impact on food science. Front. Microbiol. 2023, 14, 1155757. [Google Scholar] [CrossRef] [PubMed]
- Keshri, J.; Krouptiski, Y.; Abu-Fani, L.; Achmon, Y.; Stern Bauer, T.; Zarka, O.; Maler, I.; Pinto, R.; Sela Saldinger, S. Dynamics of bacterial communities in alfalfa and mung bean sprouts during refrigerated conditions. Food Microbiol. 2019, 84, 103261. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, B.; Gerner-Smidt, P.; Allard, M.W.; Leuillet, P.; Winkler, A.; Xiao, Y.; Chaffron, S.; Van Der Vossen, J.; Tang, S.; Katase, M.; et al. The use of next generation sequencing for improving food safety: Translation into practice. Food Microbiol. 2019, 79, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.A.; Panpipat, W.; Chaijan, M.; Shetty, K.; Rawdkuen, S. Role of plant protein on the quality and structure of meat analogs: A new perspective for vegetarian foods. Future Foods 2023, 8, 100280. [Google Scholar] [CrossRef]
- Prasert, W.; Pantoa, T.; Pengpinij, W.; Thidarat, S. Effects of Sacha inchi (Plukenetia volubilis L.) oil and extrusion process conditions on physicochemical properties of fortified omega-3 fibrous high moisture meat analogs. J. Food Process. Preserv. 2022, 46, e17227. [Google Scholar] [CrossRef]
- Hataichanok, S.; Thathong, B.; Somdee, A. Influence of Sacha inchi (Plukenetia volubilis L.) oil and extrusion process parameters on the quality of soya protein-based meat extender: An optimization approach. J. Food Process. Preserv. 2022, 46, e17140. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC INTERNATIONAL (925.10); AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC INTERNATIONAL (991.20); AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC INTERNATIONAL (2003.05); AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC INTERNATIONAL (923.03); AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC International. Methods of Analysis for Nutrition Labeling; Chapter 6, p. 106; AOAC International: Gaithersburg, MD, USA, 1993. [Google Scholar]
- Klinsoda, J.; Vötterl, J.C.; Zebeli, Q.; Metzler-Zebeli, B.U. Lactic acid treatment of cereals and dietary phytase modified fecal microbiome composition without affecting expression of virulence factor genes in growing pigs. Front. Microbiol. 2019, 10, 2345. [Google Scholar] [CrossRef]
- Vatansever, S.; Mehmet, C.; Riaz, M.N. Low- and High-Moisture Extrusion of Pulse Proteins as Plant-Based Meat Ingredients: A Review. Cereal Foods World. 2020, 65, 4. [Google Scholar] [CrossRef]
- Benimana, F.; Huang, Y.; Mohan, A. Chapter 18—Packaging and shelf-life studies of plant-based meat analogs. In Handbook of Plant-Based Meat Analogs, 1st ed.; Ravishankar, G.A., Rao, A.R., Tahergorabi, R., Mohan, A., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 379–394. [Google Scholar]
- Hadi, J.; Brightwell, G. Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, L.J.; Zhang, S.D.; He, X.J.; Li, R.S.; Jin, Q.; Liu, S.G.; Chen, H.J.; Sun, X. High-moisture extrusion technology application in the processing of textured plant protein meat analogues: A review. Food Rev. Int. 2022, 39, 4873–4908. [Google Scholar] [CrossRef]
- Hai, D.; Guo, B.; Qiao, M.; Jiang, H.; Song, L.; Meng, Z.; Huang, X. Evaluating the potential safety risk of plant-based meat analogues by analyzing microbial community composition. Foods 2024, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Wild, F.; Czerny, M.; Janssen, A.M.; Kole, M.; Žunabović, K.; Domig, K.J. The evolution of a plant-based alternative to meat: From niche markets to widely accepted meat alternatives. Agro Food Ind. Hi-Tech 2014, 25, 45–49. [Google Scholar]
- Filho, G.C.; Penna, T.C.; Schaffner, D.W. Microbiological quality of vegetable proteins during the preparation of a meat analog. Ital. J. Food Sci. 2005, 17, 269–283. [Google Scholar]
- Somdee, T.; Thathong, B.; Somdee, A. The removal of cyanobacterial hepatotoxin [Dha7] microcystin-LR via bioaccumulation in water lettuce (Pistia stratiotes L.). Bull. Environ. Contam. Toxicol. 2016, 96, 388–394. [Google Scholar] [CrossRef]
- Prakash, S.; Rivera, J.; Sabillón, L.; Siliveru, K. From wheat grain to flour: A review of potential sources of enteric pathogen contamination in wheat milled products. Crit. Rev. Food Sci. Nutr. 2024, 65, 2965–2975. [Google Scholar] [CrossRef]
- Kormas, K.A.; Lymperopoulou, D.S. Cyanobacterial toxin degrading bacteria: Who are they? Biomed. Res. Int. 2013, 2013, 463894. [Google Scholar] [CrossRef]
- Tóth, A.J.; Dunay, A.; Battay, M.; Illés, C.B.; Bittsánszky, A.; Süth, M. Microbial spoilage of plant-based meat analogues. Appl. Sci. 2021, 11, 8309. [Google Scholar] [CrossRef]
- Bogueva, D.; McClements, D.J. Safety and nutritional risks associated with plant-based meat alternatives. Sustainability 2023, 15, 14336. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zhang, M.; Zheng, H.; Li, L. Preservation of soy protein-based meat analogs by using PLA/PBAT antimicrobial packaging film. Food Chem. 2022, 380, 132022. [Google Scholar] [CrossRef]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-based phenolic molecules as natural preservatives in comminuted meats: A review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef]
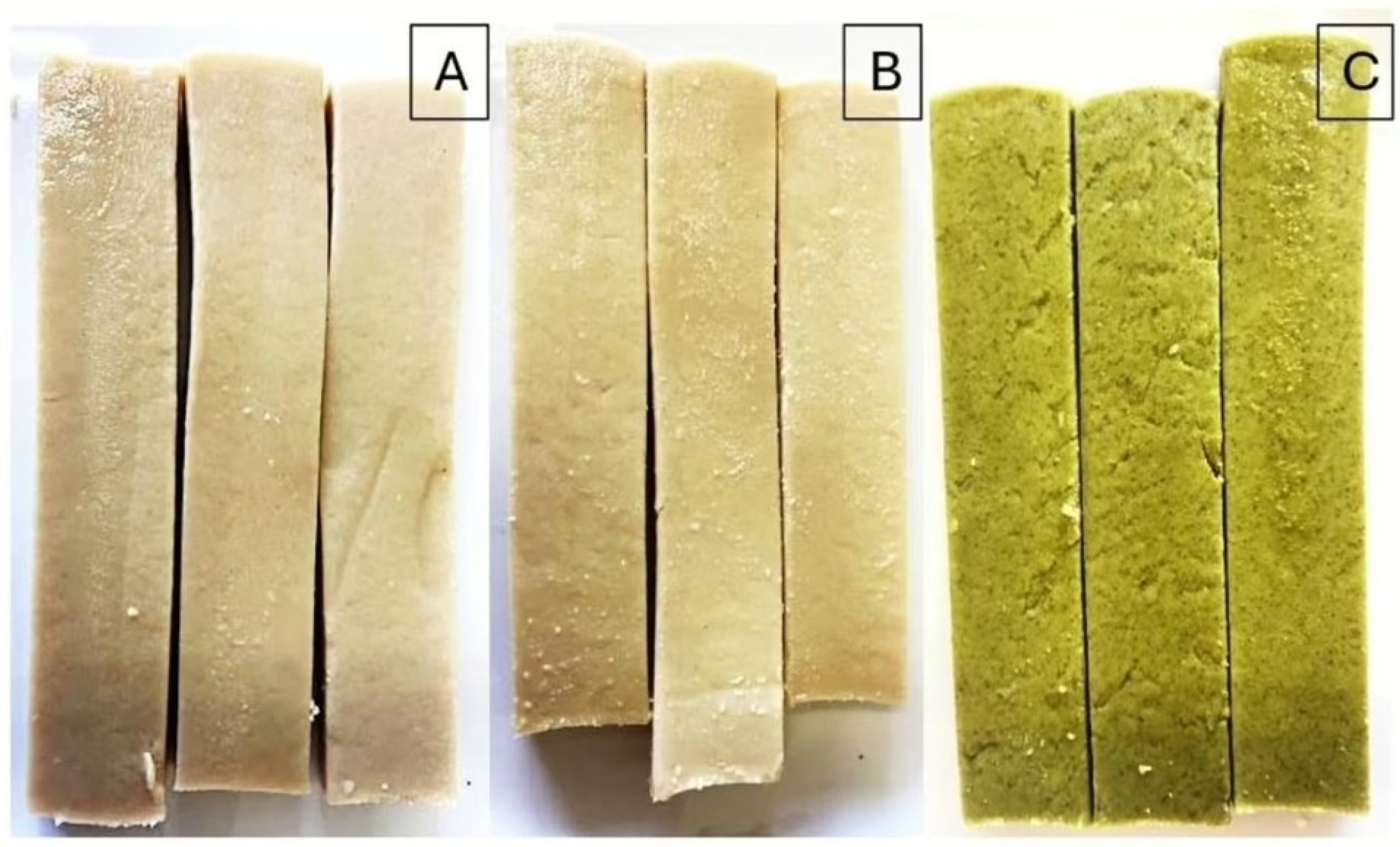
| Items | 20MB-PBM | 30MB-PBM | DW-PBM |
|---|---|---|---|
| Moisture(%) | 62.54 | 68.40 | 61.49 |
| Protein (%) | 26.91 | 21.38 | 25.75 |
| Fat (%) | 1.36 | 2.86 | 3.50 |
| Ash (%) | 1.77 | 1.16 | 1.55 |
| Total carbohydrate(%) | 7.42 | 6.20 | 7.71 |
| Total energy (Kcal/100g) | 149.56 | 136.06 | 165.34 |
| Samples | Diversity Indices (Mean ± SE) | p-Value 1 | ||||
|---|---|---|---|---|---|---|
| Chao1 | Simpson | Shannon | Chao1 | Simpson | Shannon | |
| 1st batch | ||||||
| Individual Ingredients | 252 ± 54.6 a | 0.61 ± 0.06 a | 2.87 ± 0.35 a | 0.719 | 0.005 | 0.015 |
| 20MB-PBM | 224 ± 54.6 a | 0.32 ± 0.06 b | 1.53 ± 0.35 a | <0.01 | <0.01 | <0.01 |
| 30MB-PBM | 287 ± 54.6 a | 0.41 ± 0.06 ab | 1.95 ± 0.35 a | 0.4309 | 0.349 | 0.408 |
| DW-PBM | 323 ± 54.6 a | 0.54 ± 0.06 ab | 2.49 ± 0.35 a | 0.217 | 0.026 | 0.067 |
| 2nd batch | ||||||
| Individual Ingredients | 210 ± 25.8 a | 0.561 ± 0.07 a | 2.74 ± 0.38 a | 0.011 | <0.01 | <0.01 |
| 20MB-PBM | 105 ± 25.8 b | 0.058 ± 0.07 b | 0.34 ± 0.38 b | <0.01 | 0.45 | 0.382 |
| 30MB-PBM | 110 ± 25.8 ab | 0.086 ± 0.07 b | 0.47 ± 0.38 b | 0.893 | 0.798 | 0.813 |
| DW-PBM | 166 ± 25.8 ab | 0.346 ± 0.07 ab | 1.29 ± 0.38 ab | 0.113 | <0.01 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinsoda, J.; Thurakit, T.; Tongkhao, K.; Treesuwan, K.; Yodin, K.; Kantrong, H. Exploring the Bacterial Microbiome of High-Moisture Plant-Based Meat Substituted Soybean Flour with Mung Bean Protein and Duckweed Powder. Biology 2025, 14, 735. https://doi.org/10.3390/biology14060735
Klinsoda J, Thurakit T, Tongkhao K, Treesuwan K, Yodin K, Kantrong H. Exploring the Bacterial Microbiome of High-Moisture Plant-Based Meat Substituted Soybean Flour with Mung Bean Protein and Duckweed Powder. Biology. 2025; 14(6):735. https://doi.org/10.3390/biology14060735
Chicago/Turabian StyleKlinsoda, Jutamat, Theera Thurakit, Kullanart Tongkhao, Khemmapas Treesuwan, Kanokwan Yodin, and Hataichanok Kantrong. 2025. "Exploring the Bacterial Microbiome of High-Moisture Plant-Based Meat Substituted Soybean Flour with Mung Bean Protein and Duckweed Powder" Biology 14, no. 6: 735. https://doi.org/10.3390/biology14060735
APA StyleKlinsoda, J., Thurakit, T., Tongkhao, K., Treesuwan, K., Yodin, K., & Kantrong, H. (2025). Exploring the Bacterial Microbiome of High-Moisture Plant-Based Meat Substituted Soybean Flour with Mung Bean Protein and Duckweed Powder. Biology, 14(6), 735. https://doi.org/10.3390/biology14060735








