Isolation and Bioactive Characterization of Berberis kaschgarica Rupr-Derived Exosome-Like Nanovesicles: Exploring Therapeutic Potential in Atherosclerosis Pathogenesis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
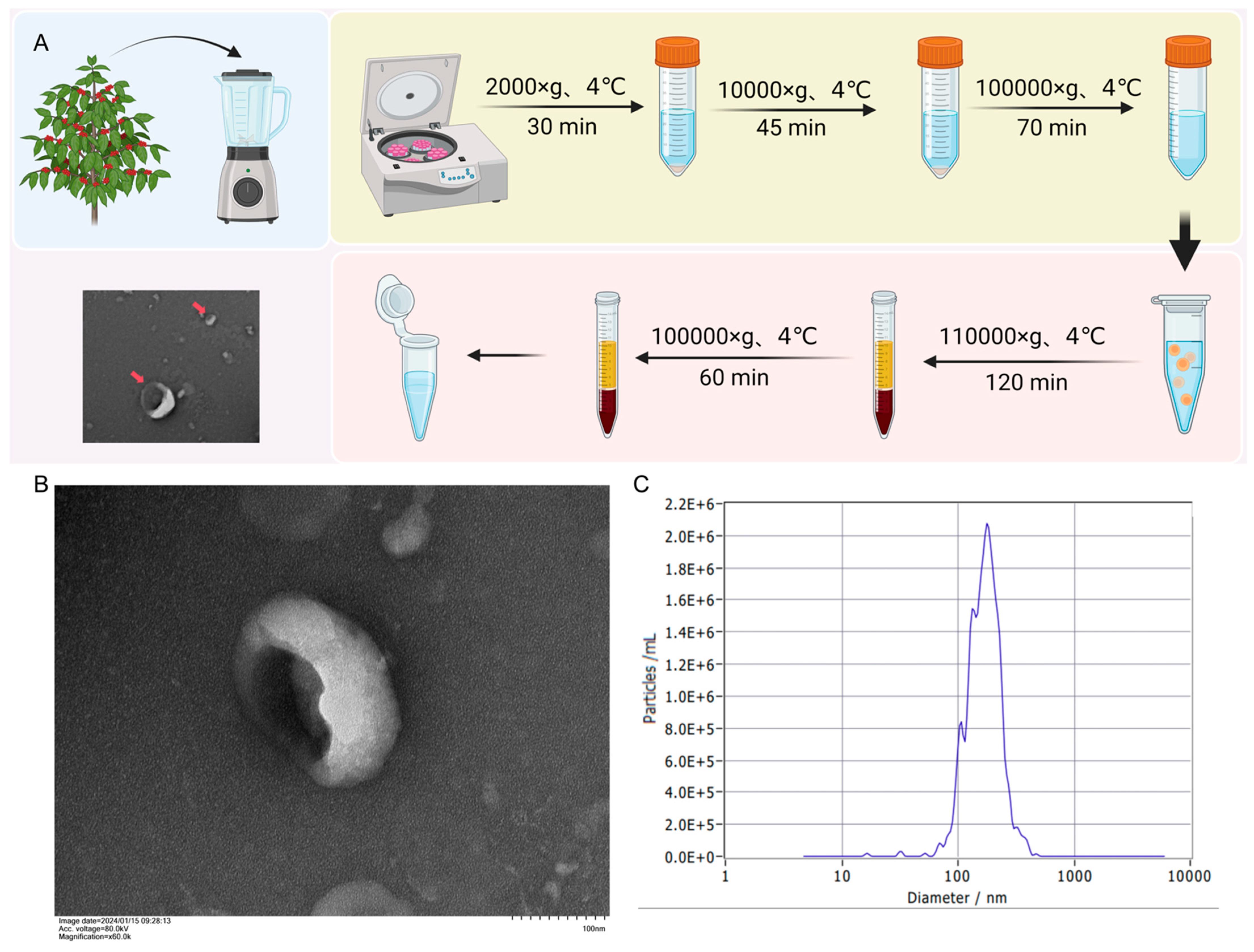
2.2. Isolation of BELNs from Berberis kaschgarica Rupr.
2.3. Transmission Electron Microscopy (TEM) and Nanoparticle Tracking Analysis (NTA)
2.4. LC-MS-Based Lipidomic Analysis
2.5. Protein Determination
2.5.1. Protein Quantification and Analysis
2.5.2. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis
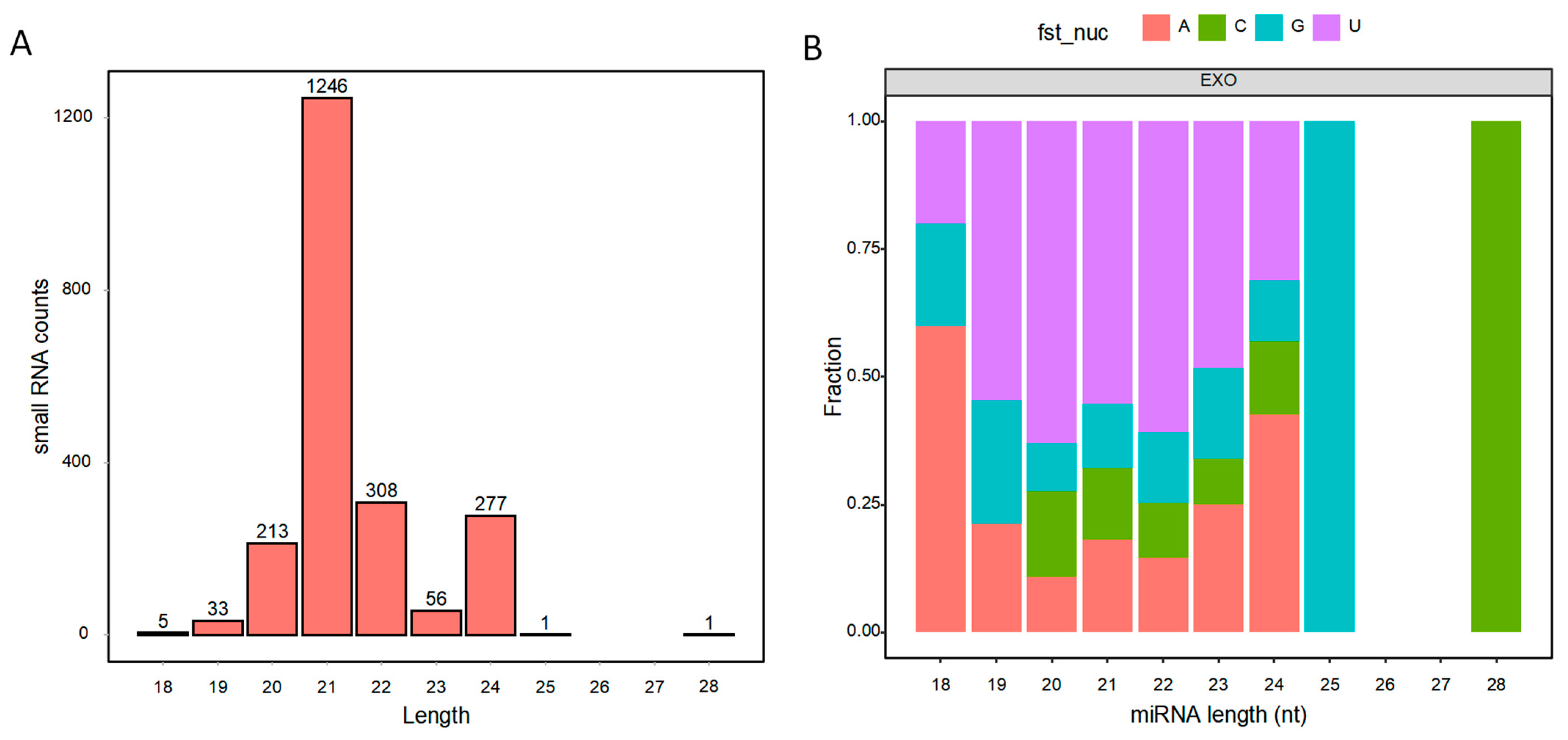
2.6. MiRNA Sequencing for BELNs and Data Analysis
2.7. Endothelial Cell Culture and Viability Assessment
2.8. Oil Red O Staining and Biochemical Indicator Testing
2.9. Statistical Analysis
3. Results
3.1. The Extraction and Structural Characterization of BELNs
3.2. Lipid Composition of BELNs
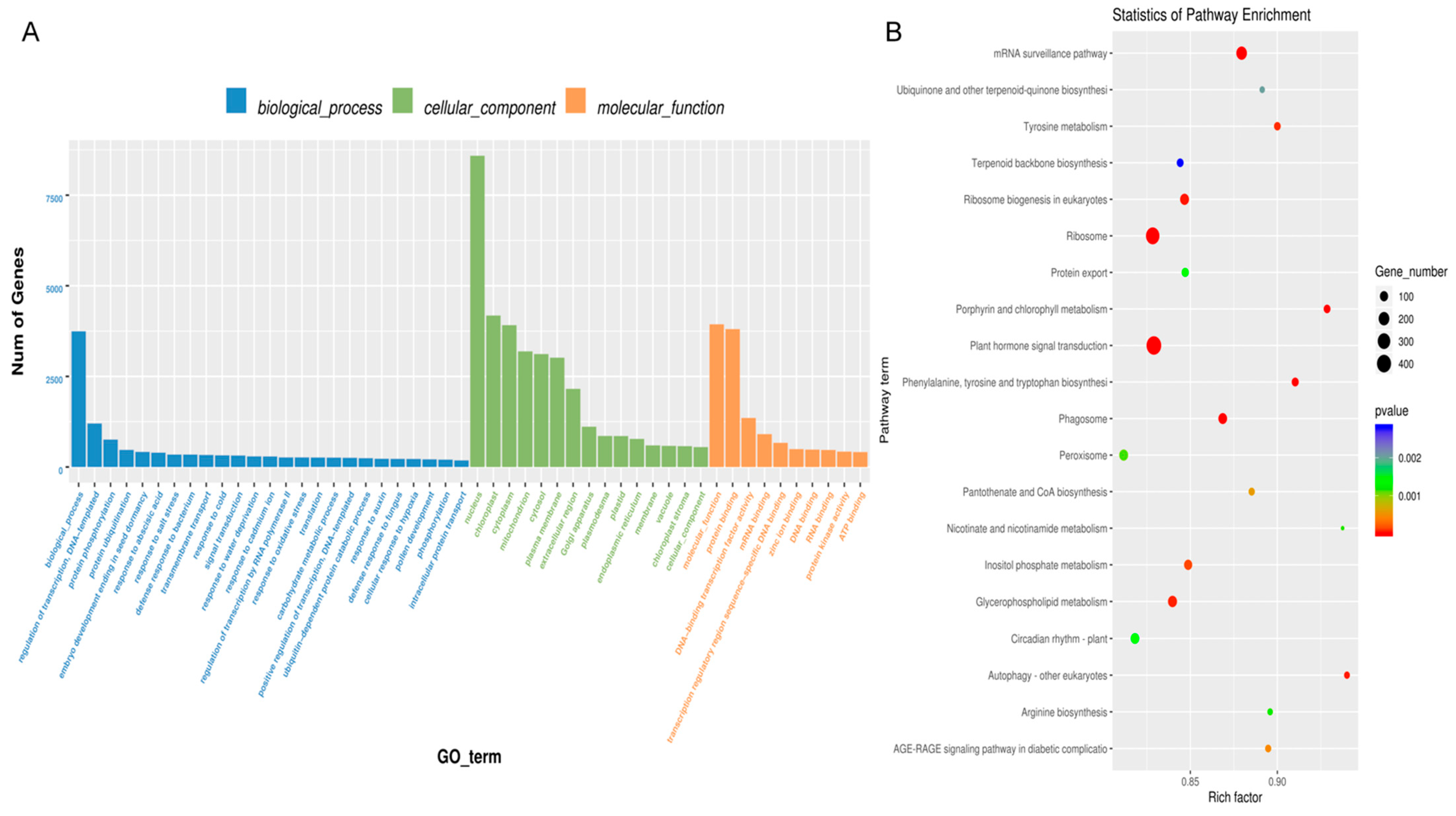
3.3. Proteomic Analyses of BELNs
3.4. Contents of Small RNAs in BELNs
3.5. BELNs Effectively Enhance Cell Viability and Reduce Lipid Deposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BELNs | Berberis kaschgarica Rupr.-derived exosome-like nanovesicles |
| PC | Phosphatidylcholine |
| SPH | Sphingomyelin |
| FA | Fatty acid |
| PG | Phosphatidylglycerol |
| LPC | Lysophosphatidylcholine |
| ELNs | Exosome-like nanoplants |
| TEM | Transmission electron microscopy |
| NTA | Nanoparticle tracking analysis |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Khan, N.; Yusufu, M.; Ahmadova, Z.; Maihemuti, N.; Hailati, S.; Talihat, Z.; Nueraihemaiti, N.; Dilimulati, D.; Baishan, A.; Duan, L.; et al. Optimization of Ultrasound Extraction of Total Anthocyanin from Berberis kaschgarica Rupr. by Response Surface Methodology and Its Antihypertensive Effect. Food Sci. Nutr. 2024, 12, 10699–10715. [Google Scholar] [CrossRef] [PubMed]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoEdeficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, K.L.; Gong, Y.X.; Wang, G.H.; Hu, Z.B.; Liu, L.; Lu, J.; Chen, P.P.; Lu, C.C.; Ruan, X.Z.; et al. Platelet microparticles mediate glomerular endothelial injury in early diabetic nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2671–2695. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Wu, J.; Liu, M.; Li, M.; Sun, Y.; Huang, W.; Li, Y.; Zhang, Y.; Tang, W.; et al. Endothelial SIRT6 is vital to prevent hypertension and associated cardiorenal injury through targeting Nkx3.2-GATA5 Signaling. Circ. Res. 2019, 124, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Trentini, M.; Zanolla, I.; Tiengo, E.; Zanotti, F.; Sommella, E.; Merciai, F.; Campiglia, P.; Licastro, D.; Degasperi, M.; Lovatti, L.; et al. Link between organic nanovescicles from vegetable kingdom and human cell physiology: Intracellular calcium signalling. J. Nanobiotechnol 2024, 22, 68. [Google Scholar] [CrossRef]
- Hwang, J.H.; Park, Y.S.; Kim, H.S.; Kim, D.H.; Lee, S.H.; Lee, C.H.; Lee, S.H.; Kim, J.E.; Lee, S.; Kim, H.M.; et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J. Control. Release 2023, 355, 184–198. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, J.; Jin, C.; Ye, L.; Wang, L.; Gao, Y.; Lv, N.; Zhang, J.; You, F.; Qiao, H.; et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J. Nanobiotechnol 2023, 21, 78. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Sundaram, G.M. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021, 414, 115425. [Google Scholar] [CrossRef]
- Du, J.; Liang, Z.; Xu, J.; Zhao, Y.; Li, X.; Zhang, Y.; Zhao, D.; Chen, R.; Liu, Y.; Joshi, T.; et al. Plant-derived phosphocholine facilitates cellular uptake of anti-pulmonary fibrotic HJT-sRNA-m7. Sci. China Life Sci. 2019, 62, 309–320. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C13–C18. [Google Scholar] [CrossRef]
- Kruk, M.E.; Gage, A.D.; Joseph, N.T.; Danaei, G.; García-Saisó, S.; Salomon, J.A. Mortality due to low-quality health systems in the universal health coverage era: A systematic analysis of amenable deaths in 137 countries. Lancet 2018, 392, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Grechko, A.V.; Myasoedova, V.A.; Orekhov, A.N. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp. Mol. Pathol. 2018, 104, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef]
- Casabonne, D.; Gracia, E.; Espinosa, A.; Bustamante, M.; Benavente, Y.; Robles, C.; Costas, L.; Alonso, E.; Gonzalez-Barca, E.; Tardón, A.; et al. Fruit and vegetable intake and vitamin C transporter gene (SLC23A2) polymorphisms in chronic lymphocytic leukaemia. Eur. J. Nutr. 2017, 56, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.K.; Choi, S.; Lee, Y.R.; Lee, E.O.; Park, M.S.; Park, K.B.; Kim, C.S.; Lim, Y.P.; Park, J.T.; Jeon, B.H. Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and Apo E−/− Mice. Int. J. Mol. Sci. 2018, 19, 816. [Google Scholar] [CrossRef]
- Mititelu, M.; Popovici, V.; Neacșu, S.M.; Musuc, A.M.; Busnatu, Ș.S.; Oprea, E.; Boroghină, S.C.; Mihai, A.; Streba, C.T.; Lupuliasa, D.; et al. Assessment of Dietary and Lifestyle Quality among the Romanian Population in the Post-Pandemic Period. Healthcare 2024, 12, 1006. [Google Scholar] [CrossRef]
- Brondani, J.E.; Comim, F.V.; Flores, L.M.; Martini, L.A.; Premaor, M.O. Fruit and vegetable intake and bones: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0217223. [Google Scholar] [CrossRef]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10 (Suppl. 4), S404–S421. [Google Scholar] [CrossRef] [PubMed]
- López de Las Hazas, M.C.; Tomé-Carneiro, J.; Del Pozo-Acebo, L.; Del Saz-Lara, A.; Chapado, L.A.; Balaguer, L.; Rojo, E.; Espín, J.C.; Crespo, C.; Moreno, D.A.; et al. Therapeutic potential of plant-derived extracellular vesicles as nanocarriers for exogenous miRNAs. Pharmacol. Res. 2023, 198, 106999. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Acebo, L.; López de Las Hazas, M.C.; Tomé-Carneiro, J.; Del, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022, 185, 106472. [Google Scholar] [CrossRef]
- Jackson, K.K.; Mata, C.; Marcus, R.K. A rapid capillary-channeled polymer (C-CP) fiber spin-down tip approach for the isolation of plant-derived extracellular vesicles (PDEVs) from 20 common fruit and vegetable sources. Talanta 2023, 252, 123779. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like nanovesicles isolated from Citrus limon L. have anti-oxidative effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-α-Induced Change on Gene Expression in EA. hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef]
- Yan, W. Qualitative Analysis of Chemical Components in the Fruits of Berberis kaschgarica. J. Tarim Univ. 2009, 21, 19–22. [Google Scholar]
- Abuduwaili, A.; Abudureheman, A.; Subuhati, Y.; Abulizi, A.; Wenting, Z. The Effects of Berberis kaschgarica Rupr. Extract on Blood Lipids and Inflammation-Related Indicators in Atherosclerotic Rats. Cent. S. Pharm. 2022, 20, 1052–1056. [Google Scholar]
- Tayier, R.; Yusupu, M.; Qinwe, X.; Axi, W.; Tuxunjiang, A. Screening of the vasodilatory activity of different extraction fractions from the leaves of Berberis kaschgarica. Propr. Chin. Med. 2021, 43, 612–616. [Google Scholar]
- Yusupu, M.; Wenting, Z.; Sawuti, A.; Dawuti, A.; Simayi, J. The vasorelaxant effect of the aqueous extract of Berberis kaschgarica fruits on isolated thoracic aortic rings of rats. Propr. Chin. Med. 2019, 41, 448–450. [Google Scholar]
- Zhou, L.; Cai, Y.; Yang, L.; Zou, Z.; Zhu, J.; Zhang, Y. Comparative Metabolomics Analysis of Stigmas and Petals in Chinese Saffron (Crocus sativus) by Widely Targeted Metabolomics. Plants 2022, 11, 2427. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, Y.; Wang, Y. Changes of anthocyanin and amino acid metabolites in saffron petals (Crocus sativus L.) during fermentation based on untargeted metabolomics. LWT 2024, 192, 115724. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iran. J. Basic. Med. Sci. 2018, 21, 1091–1099. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Li, X.; Cui, X.; Yang, E.; Chen, N.; Yuan, X.; Zhao, J.; Hou, X.; Kang, C. Phosphatidylcholine-Engineered Exosomes for Enhanced Tumor Cell Uptake and Intracellular Antitumor Drug Delivery. Macromol. Biosci. 2021, 21, e2100042. [Google Scholar] [CrossRef] [PubMed]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef]
- Zheng, H.; Siddharth, S.; Parida, S.; Wu, X.; Sharma, D. Tumor Microenvironment: Key Players in Triple Negative Breast Cancer Immunomodulation. Cancers 2021, 13, 3357. [Google Scholar] [CrossRef]
- Yang, F.; Wu, Y.; Chen, Y.; Xi, J.; Chu, Y.; Jin, J.; Yan, Y. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate liver steatosis by promoting fatty acid oxidation and reducing fatty acid synthesis. JHEP Rep. 2023, 5, 100746. [Google Scholar] [CrossRef]
- Kimura, R.; Shibata, M.; Koeda, S.; Miyagawa, A.; Yamamura, H.; Mizuno, T. Development of New Antimicrobial Agents from Cationic PG-Surfactants Containing Oligo-Lys Peptides. Bioconjug. Chem. 2018, 29, 4072–4082. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.F.; Andrade, L.N.S.; Bustos, S.O.; Chammas, R. Phosphatidylcholine-Derived Lipid Mediators: The Crosstalk Between Cancer Cells and Immune Cells. Front. Immunol. 2022, 13, 768606. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Shu-Fang, L.; Lang-Jun, C.; Jian, W. Differential expression of 15 miRNAs in Salvia miltiorrhiza Bunge under drought stress. Guangdong AGR Sci. 2013, 40, 134–137. [Google Scholar]
- Minutolo, A.; Potestà, M.; Roglia, V.; Cirilli, M.; Iacovelli, F.; Cerva, C.; Fokam, J.; Desideri, A.; Andreoni, M.; Grelli, S.; et al. Plant microRNAs from Moringa oleifera regulate immune response and HIV infection. Front. Pharmacol. 2020, 11, 620038. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Wang, R.; Luo, J.; He, J.; Ye, R.; Xie, M.; Xi, Q.; Jiang, Q.; Sun, J.; et al. Plant MIR156 regulates intestinal growth in mammals by targeting the Wnt/β-catenin pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C434–C448. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Ruan, J.; Yang, C.; Tian, Y.; Lu, B.; Wang, Y. Cross-kingdom regulation of ginseng miRNA156 on immunity and metabolism. Int. Immunopharmacol. 2024, 138, 112577. [Google Scholar] [CrossRef]
- Gismondi, A.; Nanni, V.; Monteleone, V.; Colao, C.; Di Marco, G.; Canini, A. Plant miR171 modulates mTOR pathway in HEK293 cells by targeting GNA12. Mol. Biol. Rep. 2021, 48, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Luo, R.; Li, L.; Liu, X.; Yuan, Y.; Zhu, W.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Mesenchymal stem cells alleviate palmitic acid-induced endothelial-to-mesenchymal transition by suppressing endoplasmic reticulum stress. American journal of physiology. Endocrinol. Metab. 2020, 319, E961–E980. [Google Scholar] [CrossRef]



| Metabolite | Retention Time (min) | m/z | Adducts |
|---|---|---|---|
| DG(16:0_16:0) | 17.99592699 | 586.5405005 | M + NH4 |
| MGDG(18:3_18:3) | 13.91099355 | 797.5174215 | M + Na |
| LPE(18:2) | 2.651805773 | 476.2782655 | M−H |
| LPC(16:0) | 3.221360685 | 540.3306955 | M + HCOO |
| DAP(O-8:0_O-8:0) | 11.19310263 | 391.2842865 | M + H |
| TG(O-15:0_18:0_3:0) | 19.37484361 | 642.6031005 | M + NH4 |
| SPH(d14:1) | 2.143380594 | 244.2271055 | M + H |
| LPC(18:1) | 3.561631005 | 522.3554185 | M + H |
| Cer(d16:0_16:0) | 17.24053772 | 512.5037205 | M + H |
| PC(34:3) | 14.67353089 | 756.5537835 | M + H |
| PC(34:2) | 15.43213762 | 758.5694335 | M + H |
| PC(34:1) | 16.21210249 | 760.5850835 | M + H |
| PC(36:4) | 14.79537964 | 782.5694335 | M + H |
| SPH(d15:0) | 1.594016317 | 244.2634905 | M + H |
| PC(36:3) | 15.55398637 | 784.5850835 | M + H |
| PC(36:2) | 16.3055966 | 786.6007335 | M + H |
| SPH(d14:0) | 1.127579401 | 246.2427555 | M + H |
| SPH(d17:0) | 1.898047996 | 288.2897055 | M + H |
| SPH(d18:0) | 2.33114669 | 302.3053555 | M + H |
| SPH(d20:0) | 3.980294343 | 330.3366555 | M + H |
| MG(O-15:2) | 2.444098446 | 299.2580715 | M + H |
| FA(16:0) | 8.101046132 | 255.2329535 | M−H |
| FA(22:0) | 16.2752598 | 339.3268535 | M−H |
| FA(26:0) | 18.18207962 | 395.3894535 | M−H |
| FA(28:0) | 18.95159637 | 423.4207535 | M−H |
| FA(30:0) | 19.44821288 | 451.4520535 | M−H |
| PG(25:2_22:7) | 12.55670308 | 913.5964115 | M−H |
| PG(25:1_22:7) | 14.89752817 | 915.6120615 | M−H |
| DGDG(20:0_14:2) | 12.73955966 | 915.6050485 | M−H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilimulati, D.; Nueraihemaiti, N.; Baishan, A.; Hailati, S.; Aikebaier, A.; Paerhati, Y.; Zhou, W. Isolation and Bioactive Characterization of Berberis kaschgarica Rupr-Derived Exosome-Like Nanovesicles: Exploring Therapeutic Potential in Atherosclerosis Pathogenesis. Biology 2025, 14, 726. https://doi.org/10.3390/biology14060726
Dilimulati D, Nueraihemaiti N, Baishan A, Hailati S, Aikebaier A, Paerhati Y, Zhou W. Isolation and Bioactive Characterization of Berberis kaschgarica Rupr-Derived Exosome-Like Nanovesicles: Exploring Therapeutic Potential in Atherosclerosis Pathogenesis. Biology. 2025; 14(6):726. https://doi.org/10.3390/biology14060726
Chicago/Turabian StyleDilimulati, Dilihuma, Nuerbiye Nueraihemaiti, Alhar Baishan, Sendaer Hailati, Alifeiye Aikebaier, Yipaerguli Paerhati, and Wenting Zhou. 2025. "Isolation and Bioactive Characterization of Berberis kaschgarica Rupr-Derived Exosome-Like Nanovesicles: Exploring Therapeutic Potential in Atherosclerosis Pathogenesis" Biology 14, no. 6: 726. https://doi.org/10.3390/biology14060726
APA StyleDilimulati, D., Nueraihemaiti, N., Baishan, A., Hailati, S., Aikebaier, A., Paerhati, Y., & Zhou, W. (2025). Isolation and Bioactive Characterization of Berberis kaschgarica Rupr-Derived Exosome-Like Nanovesicles: Exploring Therapeutic Potential in Atherosclerosis Pathogenesis. Biology, 14(6), 726. https://doi.org/10.3390/biology14060726








