Comparison of the Burrowing Ability of Different Groups of Manila Clams (Ruditapes philippinarum)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
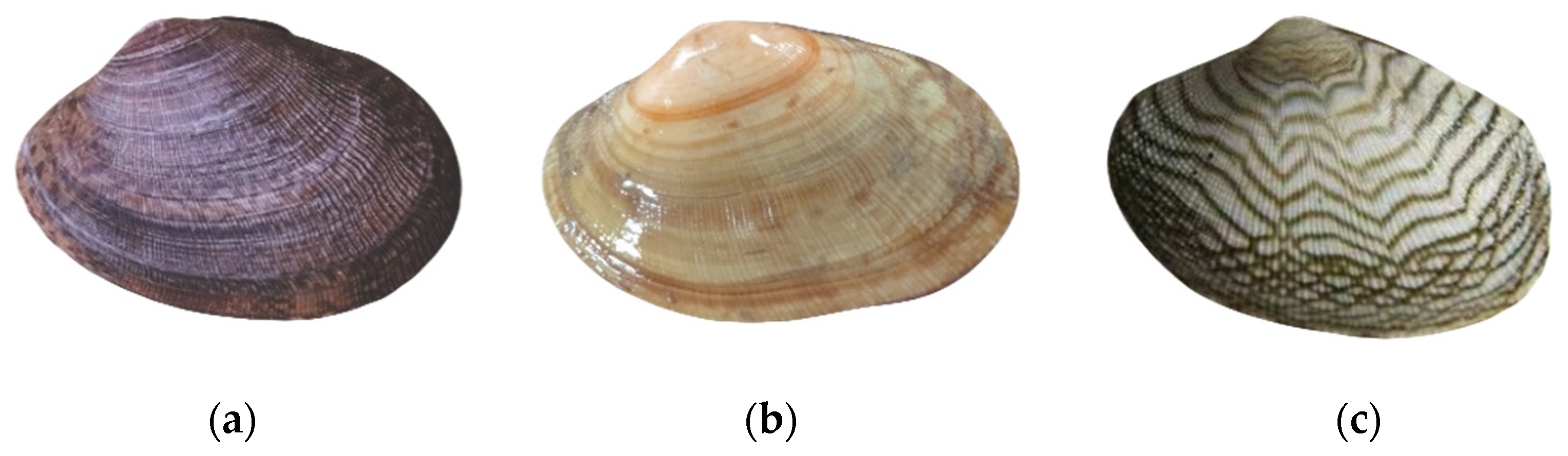
2.1. Materials
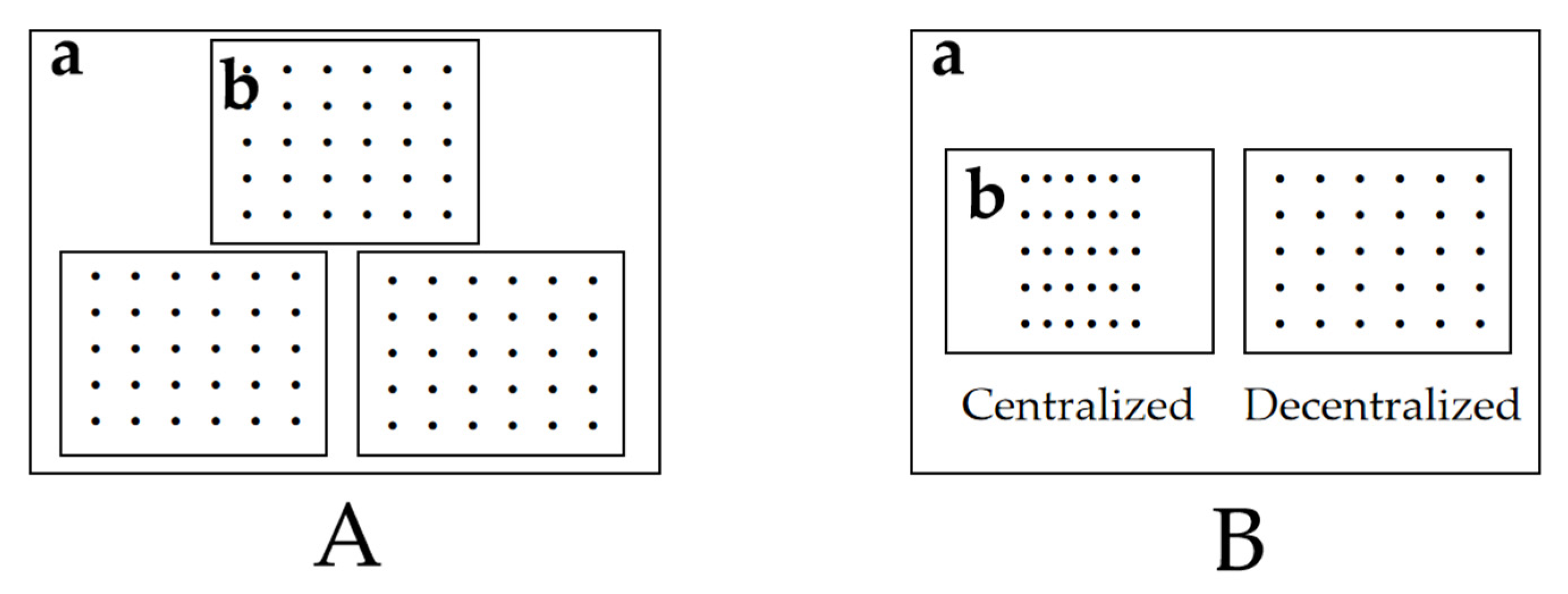
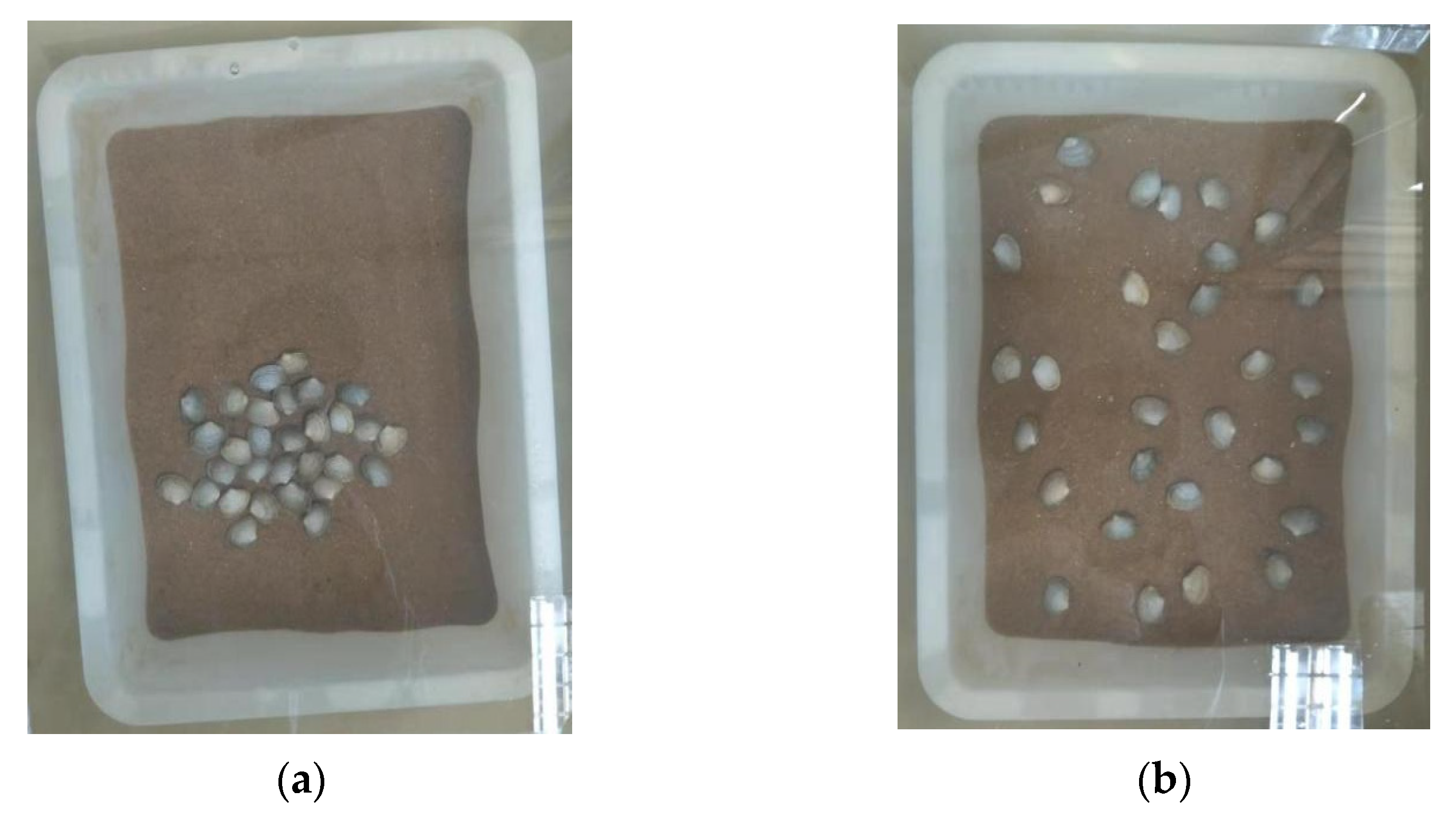
2.2. Test Method
2.3. Data Processing
3. Results
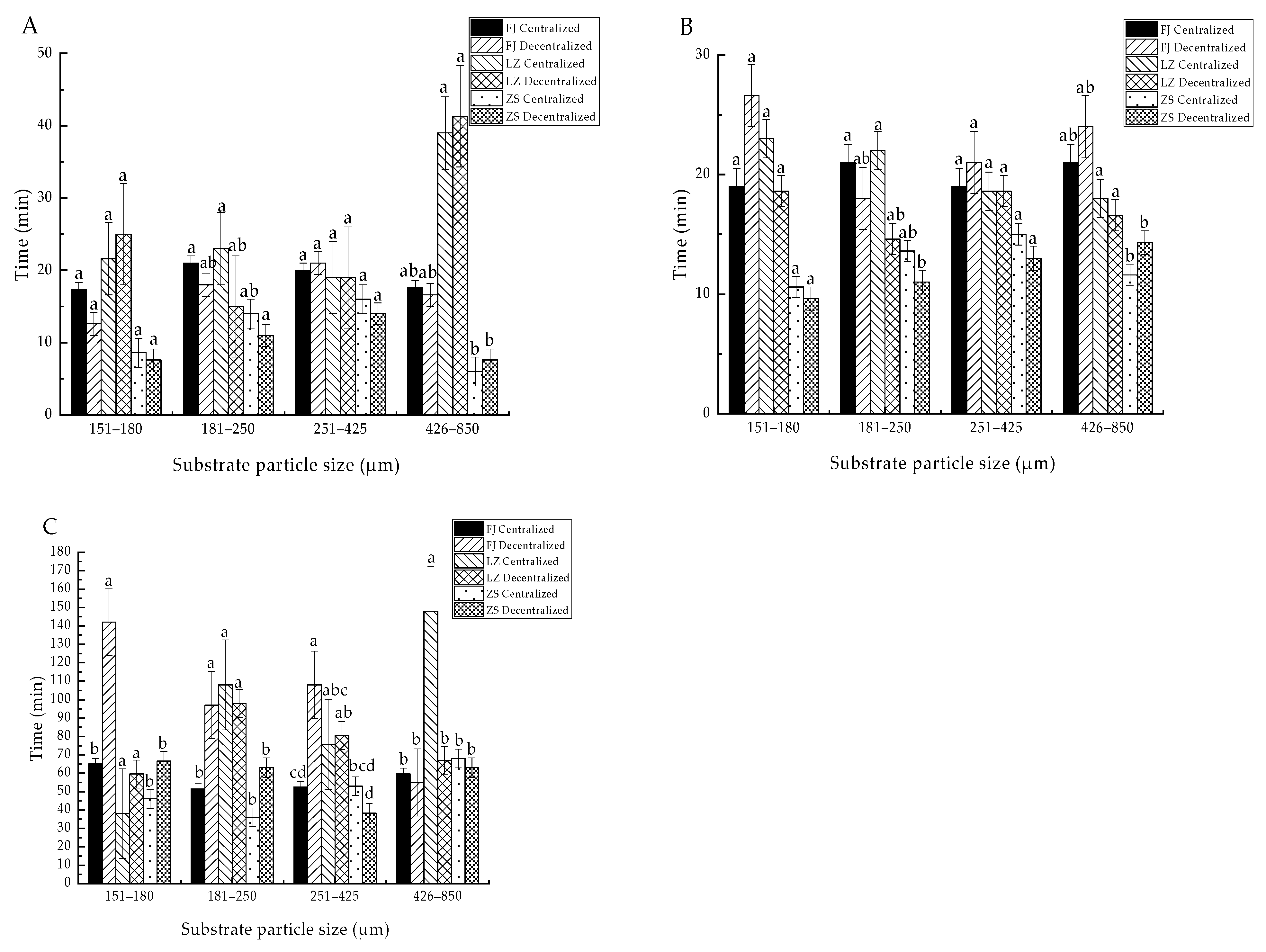
3.1. Effects of Substrate Conditions on ET50 of Clams from Different Groups
3.2. Effect of Substrate Conditions on Burrowing Rate of Clams from Different Groups
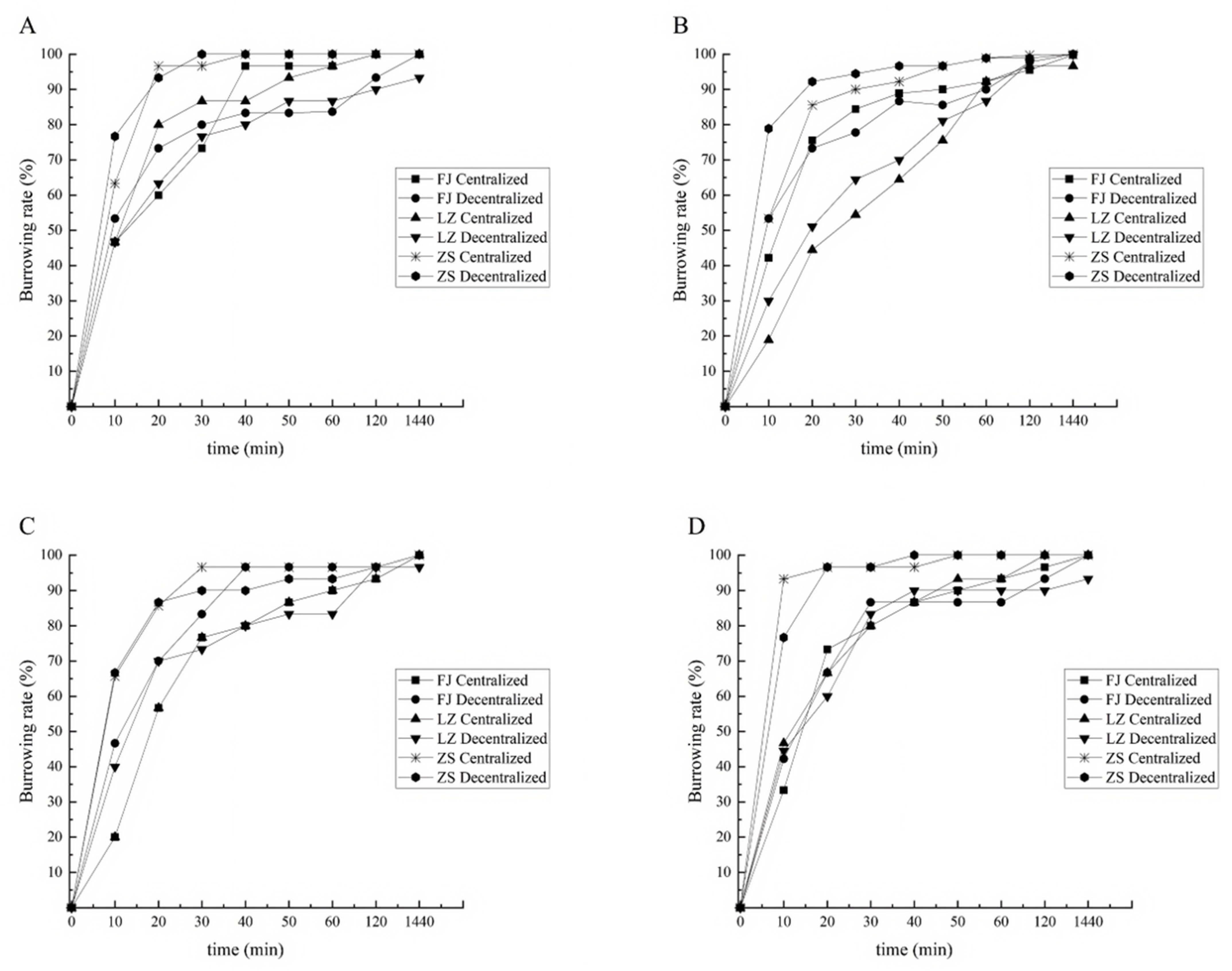
3.2.1. Burrowing Rate of Clams with Shell Length of 1.0 cm
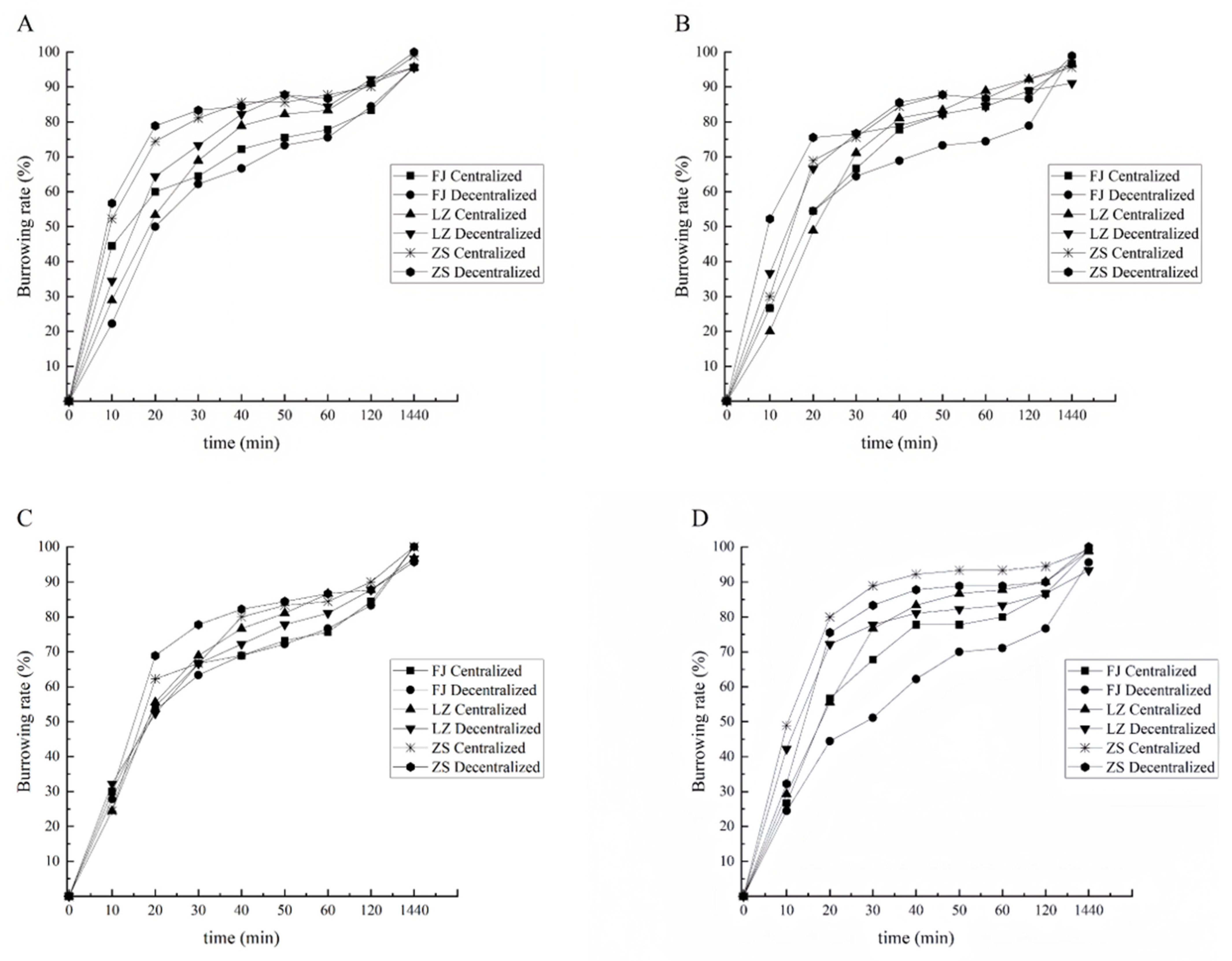
3.2.2. Burrowing Rate of Clams with Shell Length of 1.5 cm
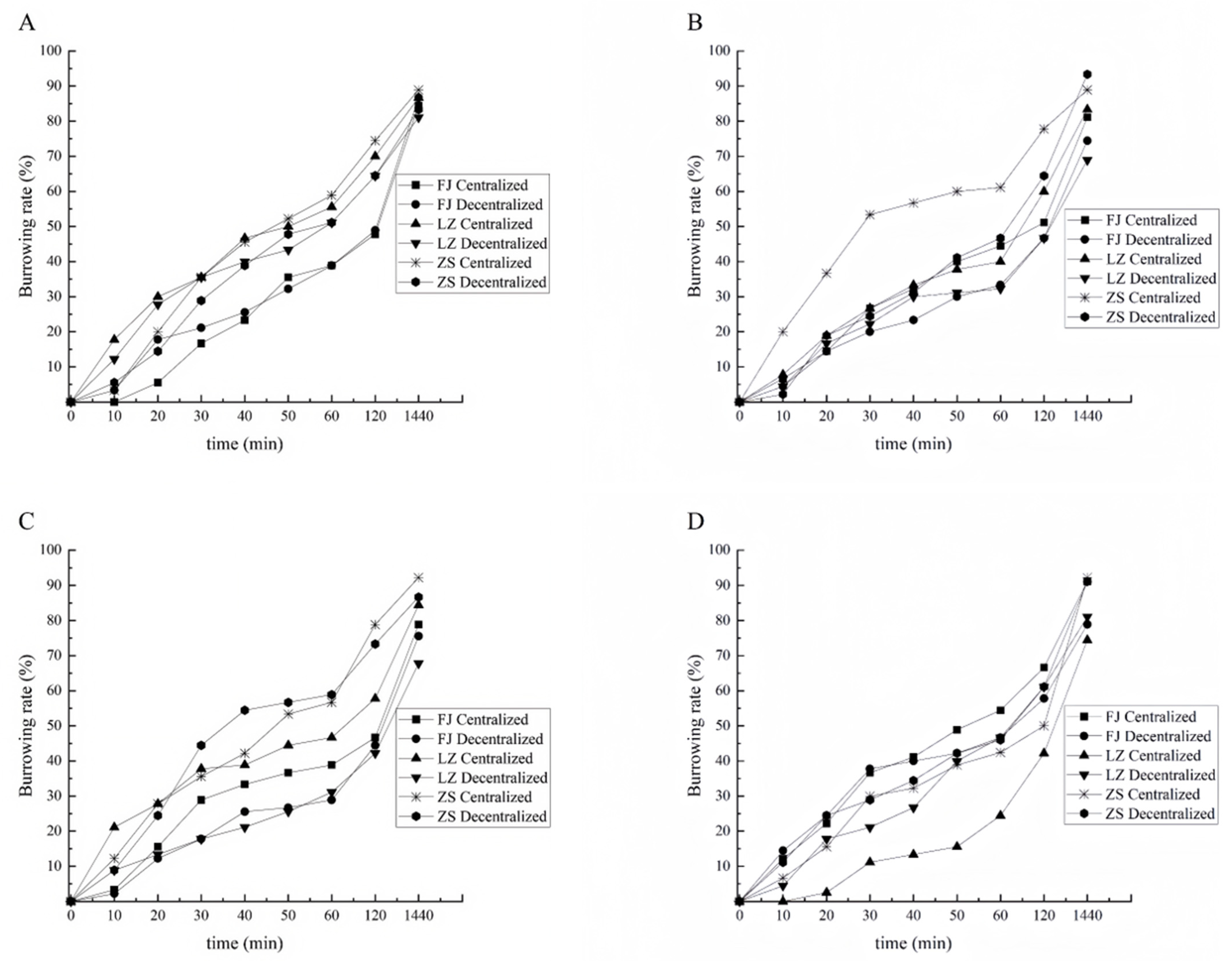
3.2.3. Burrowing Rate of Clams with Shell Length of 2.0 cm
4. Discussion
4.1. Comparison of Burrowing Ability of Different Groups of Clams
4.2. Effect of the Substrate Conditions on the Burrowing Ability of Clams
4.3. The Effect of the Sowing Mode on the Burrowing Ability of Clams
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, S.; Zhang, T.; Tu, K.; Li, L.; Liu, Z.; Wu, B.; Zhou, L.; Sun, X. Population Genetics of Manila Clam (Ruditapes philippinarum) in China Inferred from Microsatellite Markers. Biology 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, O.; Turolla, E.; Lanzoni, M.; Moore, D.; Castaldelli, G. Manila clam and Mediterranean mussel aquaculture is sustainable and a net carbon sink. Sci. Total Environ. 2022, 848, 157508. [Google Scholar] [CrossRef] [PubMed]
- Curtin, T.P.; Volkenborn, N.; Dwyer, I.P.; Aller, R.C.; Zhu, Q.Z.; Gobler, C.J. Buffering muds with bivalve shell significantly increases the settlement, growth, survival, and burrowing of the early life stages of the Northern quahog, Mercenaria mercenaria, and other calcifying invertebrates. Estuar. Coast. Shelf Sci. 2022, 264, 107686. [Google Scholar] [CrossRef]
- Zhang, A.; Yuan, X.T.; Yang, X.L.; Shao, S.L.; Li, J.; Ding, D.W. Temporal and spatial distributions of intertidal macrobenthos in the sand flats of the Shuangtaizi Estuary, Bohai Sea in China. Acta Ecol. Sin. 2016, 36, 172–179. [Google Scholar] [CrossRef]
- Kornijów, R.; Pawlikowski, K.; Jakubowska-Lehrmann, M.; Całkiewicz, J.; Smolarz, K.; Drgas, A.; Białowąs, M. Burrowing behaviour of estuarine clam Rangia cuneata outside its native range. Estuar. Coast. Shelf Sci. 2025, 312, 109046. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, X.; Liu, S.; Shi, W.; Han, Y.; Cheng, G.; Xin, P.; Xue, L.C.; Guang, X.L. Ocean acidification alters the burrowing behaviour, Ca2+/Mg2+-ATPase activity, metabolism, and gene expression of a bivalve species, Sinonovacula constricta. Mar. Ecol. Prog. Ser. 2017, 575, 107–117. [Google Scholar] [CrossRef]
- Kim, B.; Shin, Y.; Choi, N.; Oh, B.; Sohn, S.; Jung, C.; Son, T.; Kang, K. Effects of Size and Environmental Condition on Burrowing of Artificial Seedling of Ark Shell, Scapharca broughtonii (Schrenck). Korean J. Malacol. 2007, 23, 1–8. [Google Scholar]
- Jing, W.W.; Fang, J.H.; Rastrick, S.P.S.; Samuelsen, O.B.; Liang, B.Y.; Strand, Ø.; Fang, J.G.; Jiang, Z.J. CO2-Induced Ocean Acidification Alters the Burrowing Behavior of Manila Clam (Ruditapes philippinarum) by Reversing GABAA Receptor Function. Environ. Sci. Technol. 2023, 57, 8921–8932. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Masanja, F.; Liu, Y.; Zhao, L.Q. Behavioral responses of clams to recurrent marine heatwaves. Mar. Pollut. Bull. 2025, 210, 117362. [Google Scholar] [CrossRef]
- Nakamura, Y. Secretion of a mucous cord for drifting by the clam Meretrix lusoria (Veneridae). Plankton Benthos Res. 2013, 8, 31–37. [Google Scholar] [CrossRef]
- Liang, Z.J.; Xu, J.L.; Ma, S.; Zhao, C.; Zeng, Y.; Wang, A.; Yin, W.H.; Liao, K. Influence and the underlying mechanisms of substrate type on the growth of razor clam Sinonovacula constricta. Aquac. Eng. 2025, 111, 102549. [Google Scholar] [CrossRef]
- Ledoux, T.; Clements, J.; Gallant, D.; Sonier, R.; Miron, G. Burrowing behaviour of soft-shell clams (Mya arenaria) following human disturbance. J. Exp. Mar. Biol. Ecol. 2023, 565, 151916. [Google Scholar] [CrossRef]
- Compton, T.J.; Bodnar, W.; Koolhaas, A.; Dekinga, A.; Holthuijsen, S.; Ten Horn, J.; Piersma, T. Burrowing behavior of a deposit feeding bivalve predicts change in intertidal ecosystem state. Front. Ecol. Evol. 2016, 4, 19. [Google Scholar] [CrossRef]
- Tallqvist, M. Burrowing behaviour of the Baltic clam Macoma balthica: Effects of sediment type, hypoxia and predator presence. Mar. Ecol. Prog. Ser. 2001, 212, 183–191. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hashizume, K.; Koyama, K.; Tamaki, A. Effects of salinity on sand burrowing activity, feeding and growth of the clams Mactra veneriformis, Ruditapes philippinarum and Meretrix lusoria. J. Shellfish. Res. 2005, 24, 1053–1059. [Google Scholar]
- Sakurai, I.; Seto, M.; Nakao, S. Effects of water temperature, salinity and substrata on burrowing behaviors of the three bivalves, Pseudocardium sachalinensis, Mactra chinensis, and Ruditapes philippinarum. Nippon Suisan Gakkaishi 1996, 62, 878–885. [Google Scholar] [CrossRef]
- Takeuchi, S.J.; Yamada, F.; Shirozu, H.J.; Ohashi, S.; Tamaki, A. Burrowing ability as a key trait in the establishment of infaunal bivalve populations following competitive release on an extensive intertidal sandflat. J. Exp. Mar. Biol. Ecol. 2015, 466, 9–23. [Google Scholar] [CrossRef]
- Huo, Z.M.; Rbbani, M.G.; Cui, H.; Xu, L.Q.; Yan, X.W.; Fang, L.; Wang, Y.; Yang, F. Larval development, juvenile survival, and burrowing rate of geoduck clams (Panopea japonica) under different pH conditions. Aquac. Int. 2019, 27, 1331–1342. [Google Scholar] [CrossRef]
- Nie, H.T.; Chen, P.; Huo, Z.M.; Chen, Y.; Hou, X.L.; Yang, F.; Yan, X.W. Effects of temperature and salinity on oxygen consumption and ammonia excretion in different colour strains of the Manila clam, Ruditapes philippinarum. Aquac. Res. 2017, 48, 2778–2786. [Google Scholar] [CrossRef]
- Nel, H.A.; Perissinotto, R.; Taylor, R.H.; CarrascoSalinity, N.K. Tolerance of the Bivalve Solen cylindraceus (Hanley, 1843) (Mollusca: Euheterodonta: Solenidae) in the St Lucia Estuary. Afr. Invertebr. 2011, 52, 1–12. [Google Scholar] [CrossRef]
- Yu, J.; Yin, Z.H.; Zhang, Y.M.; Bi, J.H.; Yan, X.W.; Nie, H.T. Effects of high water temperature on physiology, survival, and resistance to high temperature air-exposure in the Manila clam Ruditapes philippinarum. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 262, 109469. [Google Scholar] [CrossRef]
- Zhang, P.F.; Yongo, E.; Feng, H.; Pan, S.; Sun, A.; Zhou, L.; Guo, Z.Q.; Ke, C. Effects of substrate, temperature, salinity, size and transportation on burrowing capacity of juvenile undulated surf clam Paphia undulata. Aquac. Res. 2022, 53, 2796–2805. [Google Scholar] [CrossRef]
- St-Onge, P.; Miron, G.; Moreau, G. Burrowing behaviour of the softshell clam (Mya arenaria) following erosion and transport. J. Exp. Mar. Biol. Ecol. 2007, 340, 103–111. [Google Scholar] [CrossRef]
- Abarca, A.; Oliva, D.; Toledo, P. Effect of transfer time, temperature, and size on burrowing capacity of juvenile clams, Mulinia edulis, from hatchery. J. World Aquac. Soc. 2018, 50, 774–788. [Google Scholar] [CrossRef]
- Zaklan, S.D.; Ydenberg, R. The body size–burial depth relationship in the infaunal clam Mya arenaria. J. Exp. Mar. Biol. Ecol. 1997, 215, 1–17. [Google Scholar] [CrossRef]
- Levine, T.D.; Hansen, H.B.; Gerald, G.W. Effects of shell shape, size, and sculpture in burrowing and anchoring abilities in the freshwater mussel Potamilus alatus (Unionidae). Biol. J. Linn. Soc. 2014, 111, 136–144. [Google Scholar] [CrossRef]
- Diga, R.; Gilboa, M.; Moskovich, R.; Darmon, N.; Amit, T.; Belmaker, J.; Yahel, G. Invading bivalves replaced native Mediterranean bivalves, with little effect on the local benthic community. Biol. Invasions 2023, 25, 1441–1459. [Google Scholar] [CrossRef]
- Liu, C.F.; Yuan, L.; Yao, J.L.; Wu, H.X.; Xue, J.Z. Mechanisms of intertidal substrate influence on the distribution of two species of Macrophthalminae along the north coast of Hangzhou Bay: Adaptation of morphology and burrowing behaviour to substrate compaction (Decapoda: Ocypodoidea). Mar. Biol. 2024, 171, 220. [Google Scholar] [CrossRef]
- Hyvärinen, H.; Saarinen-Valta, M.; Mäenpää, E.; Taskinen, J. Effect of substrate particle size on burrowing of the juvenile freshwater pearl mussel Margaritifera margaritifera. Hydrobiologia 2021, 848, 1137–1146. [Google Scholar] [CrossRef]
- Sassa, S.; Watabe, Y.; Yang, S.; Tomohiro, K. Burrowing criteria and burrowing mode adjustment in bivalves to varying geoenvironmental conditions in intertidal flats and beaches. PLoS ONE 2011, 6, e25041. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, S.; Li, J.; Fang, J.; Liu, L.; Ma, Z.; Yu, W.; Zhuang, H.; Mao, Y. Influences of substrate grain size on the burrowing behavior of juvenile Meretrix meretrix. Animals 2022, 12, 2094. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, C.; Li, X.Z.; An, D.Z.Y.; Yu, H.R.; Ma, Z.; Liu, F. The effect of substrate grain size on burrowing ability and distribution characteristics of Perinereis aibuhitensis. Acta Oceanol. Sin. 2019, 38, 52–58. [Google Scholar] [CrossRef]
- Tezuka, N.; Kanematsu, M.; Asami, K.; Sakiyama, K.; Hamaguchi, M.; Usuki, H. Effect of salinity and substrate grain size on larval settlement of the asari clam (Manila clam, Ruditapes philippinarum). J. Exp. Mar. Biol. Ecol. 2013, 439, 108–112. [Google Scholar] [CrossRef]
- Huz, R.D.L.; Lastra, M.; López, J. The influence of sediment grain size on burrowing, growth and metabolism of Donax trunculus L. (Bivalvia: Donacidae). J. Sea Res. 2002, 47, 85–95. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.Y.; Zhang, S.; Xing, K.; Sun, J.; Liu, Q.; Lu, Y.J.; Hao, X.P.; Cui, F.; Liu, H.Y. The observation and analysis of burrowing behaviors of ark shell Scapharca subcrenata in different substrates and sowing conditions. Fish. Mod. 2016, 43, 22–26. (In Chinese) [Google Scholar]
- Zhang, K.N.; Zheng, S.; Zhao, C.H.; Liang, J.H.; Sun, X.X. Bioturbation effects and behavioral changes in buried bivalves after exposure to microplastics. J. Hazard. Mater. 2025, 484, 136765. [Google Scholar] [CrossRef]
| Group | Size/cm | Shell Length/mm | Shell Width/mm | Shell Height/mm | Wet Weight/g |
|---|---|---|---|---|---|
| Fujian group | 1.0 | 10.16 ± 0.66 | 3.97 ± 0.34 | 7.10 ± 0.53 | 0.17 ± 0.04 |
| 1.5 | 14.80 ± 0.58 | 5.89 ± 0.57 | 10.50 ± 0.78 | 0.50 ± 0.11 | |
| 2.0 | 20.38 ± 0.88 | 9.14 ± 0.68 | 14.01 ± 0.94 | 1.69 ± 0.28 | |
| Laizhou group | 1.0 | 9.94 ± 0.68 | 3.75 ± 0.23 | 6.80 ± 0.49 | 0.18 ± 0.03 |
| 1.5 | 14.87 ± 0.42 | 5.96 ± 0.35 | 10.34 ± 0.49 | 0.59 ± 0.08 | |
| 2.0 | 20.08 ± 0.86 | 8.34 ± 0.66 | 13.49 ± 0.79 | 1.56 ± 0.25 | |
| Zebra strain group | 1.0 | 10.26 ± 0.60 | 4.22 ± 0.30 | 7.11 ± 0.38 | 0.19 ± 003 |
| 1.5 | 15.05 ± 0.87 | 5.95 ± 0.47 | 10.19 ± 0.54 | 0.61 ± 0.11 | |
| 2.0 | 20.16 ± 0.95 | 9.35 ± 0.61 | 15.07 ± 0.80 | 1.93 ± 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, J.; Zhang, Z.; Wen, J.; Li, Y.; Zhang, H.; Lu, P.; Chen, L. Comparison of the Burrowing Ability of Different Groups of Manila Clams (Ruditapes philippinarum). Biology 2025, 14, 689. https://doi.org/10.3390/biology14060689
Li X, Wang J, Zhang Z, Wen J, Li Y, Zhang H, Lu P, Chen L. Comparison of the Burrowing Ability of Different Groups of Manila Clams (Ruditapes philippinarum). Biology. 2025; 14(6):689. https://doi.org/10.3390/biology14060689
Chicago/Turabian StyleLi, Xiang, Jianing Wang, Zelin Zhang, Jin Wen, Yu Li, Haoyang Zhang, Pan Lu, and Lei Chen. 2025. "Comparison of the Burrowing Ability of Different Groups of Manila Clams (Ruditapes philippinarum)" Biology 14, no. 6: 689. https://doi.org/10.3390/biology14060689
APA StyleLi, X., Wang, J., Zhang, Z., Wen, J., Li, Y., Zhang, H., Lu, P., & Chen, L. (2025). Comparison of the Burrowing Ability of Different Groups of Manila Clams (Ruditapes philippinarum). Biology, 14(6), 689. https://doi.org/10.3390/biology14060689





