Comparative Analysis of Microalgae’s Physiological Responses to Fibrous and Layered Clay Minerals
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Incubation and Mineral Treatments
2.2. Photosynthesis Measurements
2.3. Respiration Rate Assay
2.4. Phosphorus Accumulation Analysis
2.5. Fluorescence Spectroscopy of EPS
2.6. Intra/Extracellular Protein and Polysaccharide Assays
2.7. Functional Groups and Cell Morphology
2.8. Transcriptomic Analysis
2.9. Data Analysis
3. Results
3.1. Photosynthesis Parameters
3.2. P Uptake, Cell Number and CO2 Emission Rate
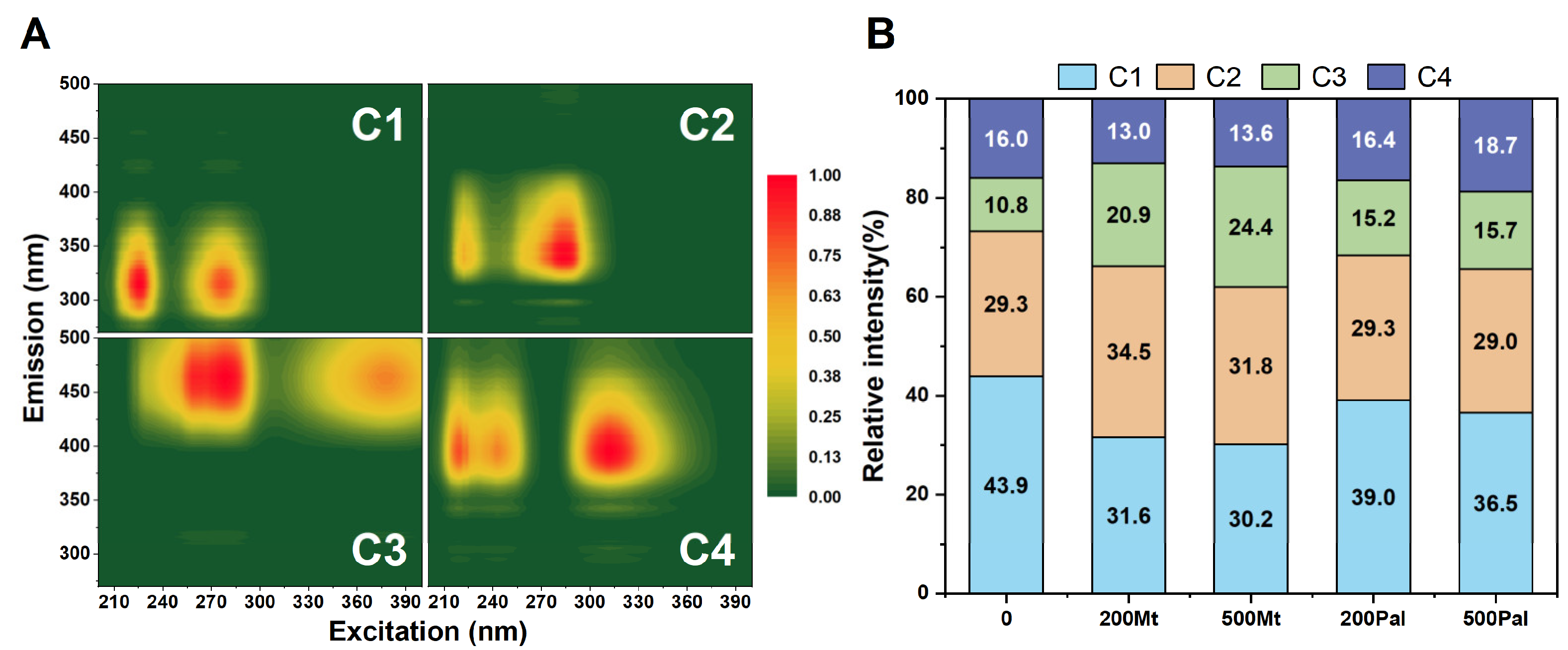
3.3. Three-Dimensional Excitation–Emission Matrix Fluorescence Spectra
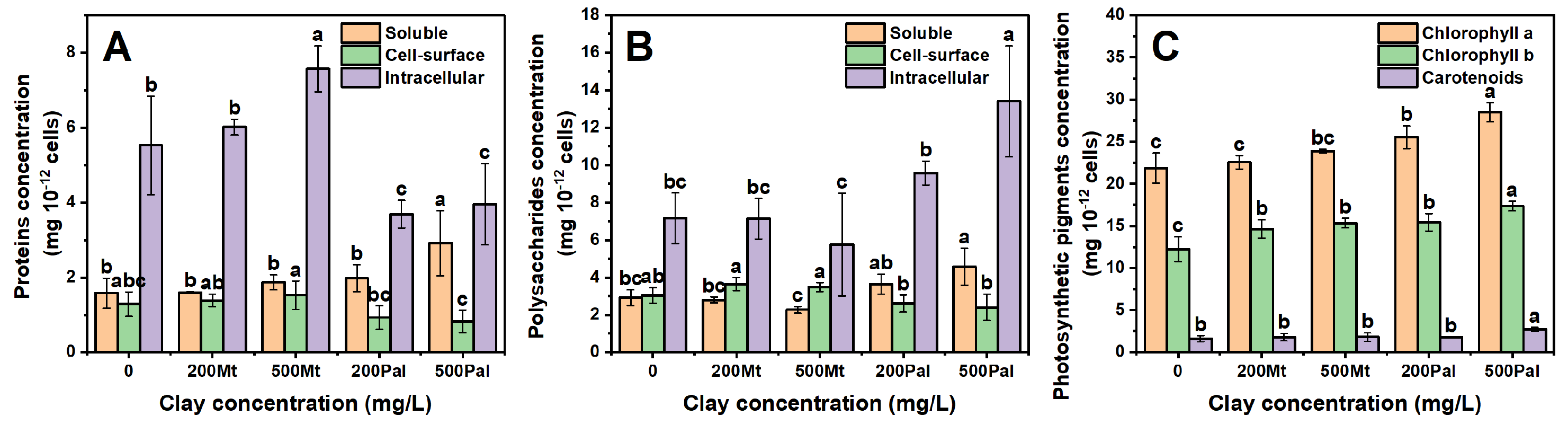
3.4. Polysaccharides, Proteins, Chlorophyll and Carotenoids
3.5. Functional Groups on the Cell Surface
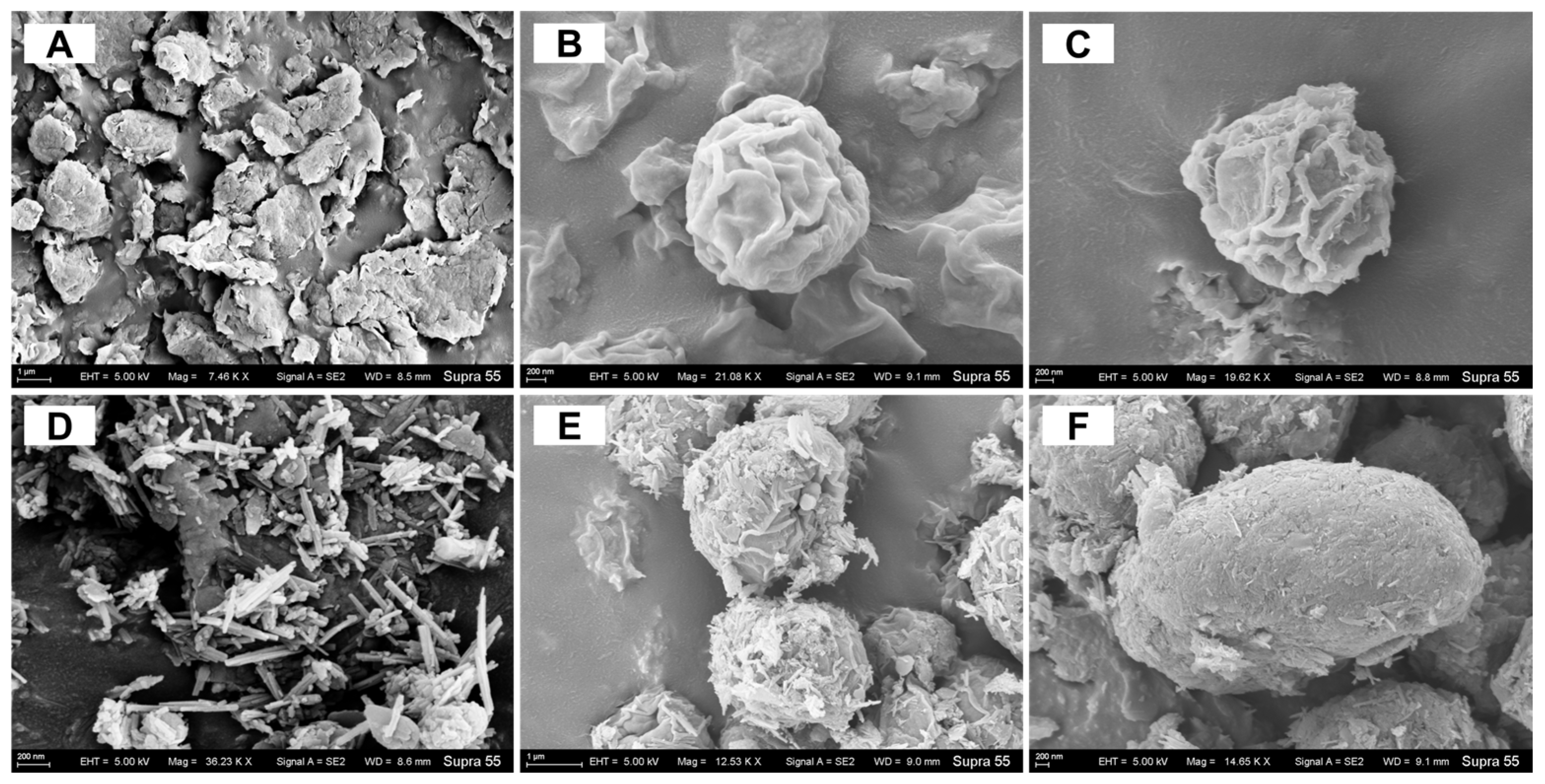
3.6. Cell Morphology
3.7. Annotations of Differentially Expressed Genes (DEGs)
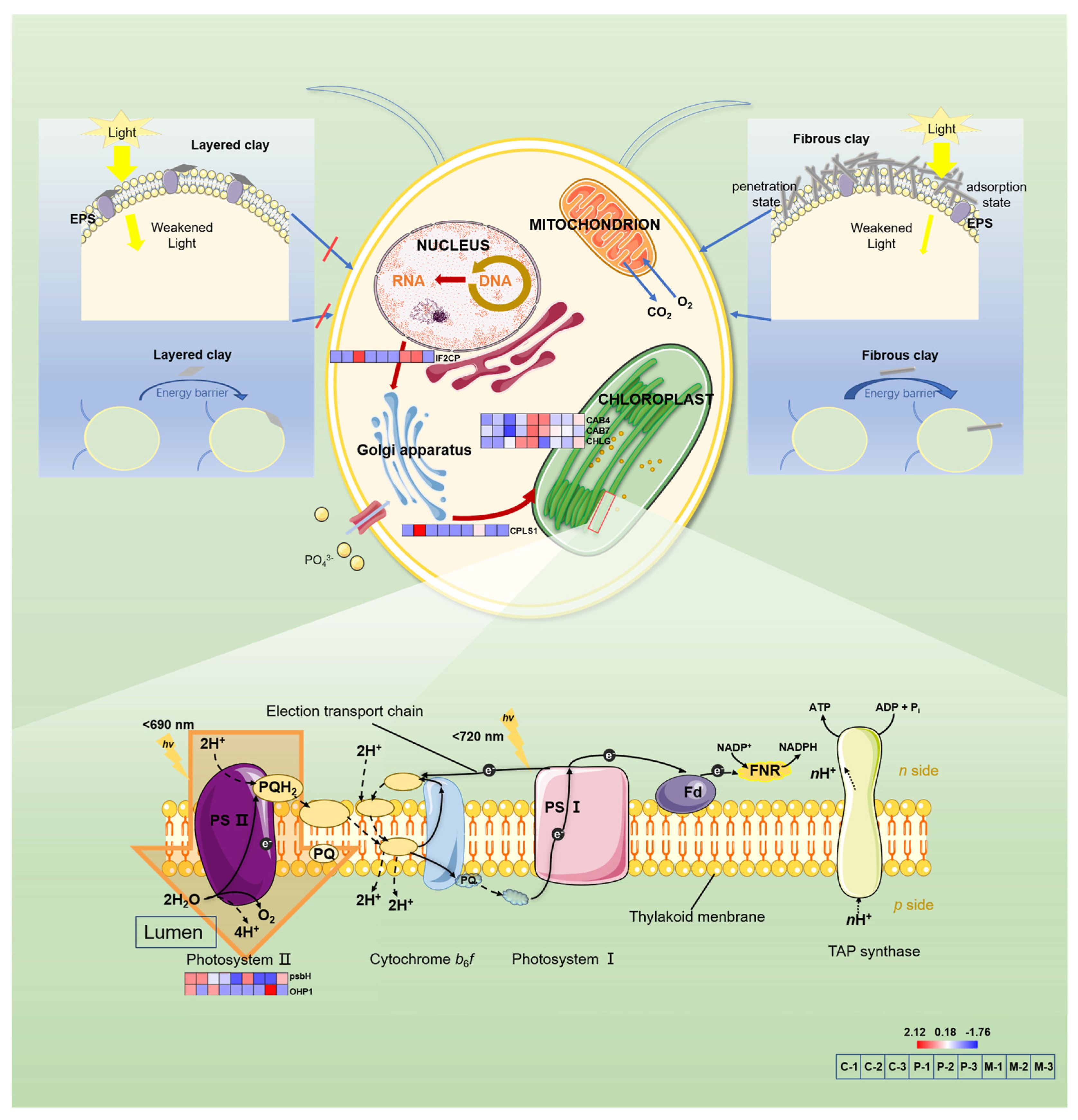
4. Discussion
4.1. Differential Effects of Mt and Pal on Algal Photosynthesis
4.2. Phosphorus Uptake and Metabolic Pathway Responses
4.3. EPS Composition and Functional Group Dynamics
4.4. Cellular Damage and Adaptive Stress Responses
4.5. Environmental Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arrigo, K.R. Marine Microorganisms and Global Nutrient Cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.; Leoni, B. A Critical Review of Interactions between Microplastics, Microalgae and Aquatic Ecosystem Function. Water Res. 2021, 188, 116476. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.J.; Windom, H.; Goldberg, E.D. The Distribution of Clay Minerals in the World Ocean. Deep Sea Res. Oceanogr. Abstr. 1968, 15, 433–459. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between Microorganisms and Clay Minerals: New Insights and Broader Applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Islam, M.R.; Singh, B.; Dijkstra, F.A. Stabilisation of Soil Organic Matter: Interactions between Clay and Microbes. Biogeochemistry 2022, 160, 145–158. [Google Scholar] [CrossRef]
- Ma, W.; Peng, D.; Walker, S.L.; Cao, B.; Gao, C.-H.; Huang, Q.; Cai, P. Bacillus Subtilis Biofilm Development in the Presence of Soil Clay Minerals and Iron Oxides. npj Biofilms Microbiomes 2017, 3, 4. [Google Scholar] [CrossRef]
- Lünsdorf, E.; Abraham, T. ‘Clay Hutches’: A Novel Interaction between Bacteria and Clay Minerals. Environ. Microbiol. 2000, 2, 161–168. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Hu, J.; Zheng, Q.; Zhao, Y.; Lv, G.; Liao, L. Clay Minerals and Clay-Based Materials for Heavy Metals Pollution Control. Sci. Total Environ. 2024, 954, 176193. [Google Scholar] [CrossRef]
- Rivenbark, K.J.; Wang, M.; Lilly, K.; Tamamis, P.; Phillips, T.D. Development and Characterization of Chlorophyll-Amended Montmorillonite Clays for the Adsorption and Detoxification of Benzene. Water Res. 2022, 221, 118788. [Google Scholar] [CrossRef]
- Churchman, G.J.; Gates, W.P.; Theng, B.K.G.; Yuan, G. Chapter 11.1 Clays and Clay Minerals for Pollution Control. In Developments in Clay Science; Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 625–675. [Google Scholar]
- Shemesh, A.; Zvulunov, Y.; Radian, A. Impact of Cocultivation on the Aggregation and Sedimentation Trends of Cyanobacteria with Native and Modified Clay Minerals. Sep. Purif. Technol. 2021, 278, 119179. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yu, Z. An Eco-Environmental Assessment of Harmful Algal Bloom Mitigation Using Modified Clay. Harmful Algae 2021, 107, 102067. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, S.E.; Sengco, M.R.; Anderson, D.M. Using Clay to Control Harmful Algal Blooms: Deposition and Resuspension of Clay/Algal Flocs. Harmful Algae 2005, 4, 123–138. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Han, F.; Wu, Y.; Tian, D.; Su, M.; Wang, S.; Li, Z.; Hu, S. Influences of Multiple Clay Minerals on the Phosphorus Transport Driven by Aspergillus Niger. Appl. Clay Sci. 2019, 177, 12–18. [Google Scholar] [CrossRef]
- Li, Z.; Su, M.; Duan, X.; Tian, D.; Yang, M.; Guo, J.; Wang, S.; Hu, S. Induced Biotransformation of Lead (II) by Enterobacter Sp. in SO4-PO4-Cl Solution. J. Hazard. Mater. 2018, 357, 491–497. [Google Scholar] [CrossRef]
- Tan, J.; Yi, H.; Zhang, Z.; Meng, D.; Li, Y.; Xia, L.; Song, S.; Wu, L.; Sáncheze, R.M.T.; Farías, M.E. Montmorillonite Facilitated Pb(II) Biomineralization by Chlorella Sorokiniana FK in Soil. J. Hazard. Mater. 2022, 423, 127007. [Google Scholar] [CrossRef]
- Jin, J.; Xia, W.; Hu, P.; Yang, H. Efficient Removal of Algae and Turbidity from Water by a Composite Coagulant Composed of a Cationic Starch and Attapulgite. Sci. China Technol. Sci. 2022, 65, 2979–2989. [Google Scholar] [CrossRef]
- Gao, Z.; Peng, X.; Zhang, H.; Luan, Z.; Fan, B. Montmorillonite–Cu(II)/Fe(III) Oxides Magnetic Material for Removal of Cyanobacterial Microcystis Aeruginosa and Its Regeneration. Desalination 2009, 247, 337–345. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Liu, X.; Cai, D.; Feng, H.; Miao, C.; Wang, X.; Wu, Z.; Yu, Z. Flocculation of Harmful Algal Blooms by Modified Attapulgite and Its Safety Evaluation. Water Res. 2011, 45, 2855–2862. [Google Scholar] [CrossRef]
- Plouviez, M.; Bolot, P.; Shilton, A.; Guieysse, B. Phosphorus Uptake and Accumulation in Chlamydomonas Reinhardtii: Influence of Biomass Concentration, Phosphate Concentration, Phosphorus Depletion Time, and Light Supply. Algal Res. 2023, 71, 103085. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Fu, H.; Zhai, S.; Hu, F.; Naveed, S.; Zhang, C.; Ge, Y. Contrasting Detoxification Mechanisms of Chlamydomonas Reinhardtii under Cd and Pb Stress. Chemosphere 2021, 274, 129771. [Google Scholar] [CrossRef]
- Tian, S.; Li, Z.; Wang, Z.; Jiang, E.; Wang, W.; Sun, M. Mineral Composition and Particle Size Distribution of River Sediment and Loess in the Middle and Lower Yellow River. Int. J. Sediment Res. 2021, 36, 392–400. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, Y.; Guan, H.; Sun, D.; Fang, S.; Ma, X.; Wang, Z.; Li, Z.; Zhang, C.; Ge, Y. Contributions of Polysaccharides to Arsenate Resistance in Chlamydomonas Reinhardtii. Ecotoxicol. Environ. Saf. 2022, 229, 113091. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, T.; Zhu, M.; Zhang, L.; Zhang, F.; Jing, E.; Ren, Y.; Wang, Z.; Xin, Z.; Lin, T. Transcriptome and Physiological Analyses for Revealing Genes Involved in Wheat Response to Endoplasmic Reticulum Stress. BMC Plant Biol. 2019, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Jiang, Z.; Jiang, L.; Su, M.; Feng, Z.; Zhang, L.; Wang, S.; Li, Z.; Hu, S. A New Insight into Lead (II) Tolerance of Environmental Fungi Based on a Study of Aspergillus Niger and Penicillium Oxalicum. Environ. Microbiol. 2019, 21, 471–479. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, T.; Sun, Y.; Nong, Y.; Tang, L.; Gu, T.; Wang, S.; Li, Z. Application of Pb(II) to Probe the Physiological Responses of Fungal Intracellular Vesicles. Ecotoxicol. Environ. Saf. 2020, 194, 110441. [Google Scholar] [CrossRef]
- Furnes, H.; McLoughlin, N.; Muehlenbachs, K.; Banerjee, N.; Staudigel, H.; Dilek, Y.; Wit, M.D.; Van Kranendonk, M.; Schiffman, P. Oceanic Pillow Lavas and Hyaloclastites as Habitats for Microbial Life Through Time—A Review. In Links Between Geological Processes, Microbial Activities&Evolution of Life: Microbes and Geology; Dilek, Y., Furnes, H., Muehlenbachs, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–68. ISBN 978-1-4020-8306-8. [Google Scholar]
- Beveridge, T.J. The Bacterial Surface: General Considerations towards Design and Function. Can. J. Microbiol. 2011, 34, 363–372. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.À.; Gago, J.; Ribas-Carbó, M.; Galmés, J. High-Throughput Phenotyping of a Large Tomato Collection under Water Deficit: Combining UAVs’ Remote Sensing with Conventional Leaf-Level Physiologic and Agronomic Measurements. Agric. Water Manag. 2022, 260, 107283. [Google Scholar] [CrossRef]
- Deng, C.; Pan, X.; Wang, S.; Zhang, D. Cu2+ Inhibits Photosystem II Activities but Enhances Photosystem I Quantum Yield of Microcystis Aeruginosa. Biol. Trace Elem. Res. 2014, 160, 268–275. [Google Scholar] [CrossRef]
- Kilian, O.; Steunou, A.S.; Grossman, A.R.; Bhaya, D. A Novel Two Domain-Fusion Protein in Cyanobacteria with Similarity to the CAB/ELIP/HLIP Superfamily: Evolutionary Implications and Regulation. Mol. Plant 2008, 1, 155–166. [Google Scholar] [CrossRef]
- Reuveni, M.; Debbi, A.; Kutsher, Y.; Gelbart, D.; Zemach, H.; Belausov, E.; Levin, I.; Lapidot, M. Tomato Yellow Leaf Curl Virus Effects on Chloroplast Biogenesis and Cellular Structure. Physiol. Mol. Plant Pathol. 2015, 92, 51–58. [Google Scholar] [CrossRef]
- Shah, A.A.; Yasin, N.A.; Mudassir, M.; Ramzan, M.; Hussain, I.; Siddiqui, M.H.; Ali, H.M.; Shabbir, Z.; Ali, A.; Ahmed, S.; et al. Iron Oxide Nanoparticles and Selenium Supplementation Improve Growth and Photosynthesis by Modulating Antioxidant System and Gene Expression of Chlorophyll Synthase (CHLG) and Protochlorophyllide Oxidoreductase (POR) in Arsenic-Stressed Cucumis Melo. Environ. Pollut. 2022, 307, 119413. [Google Scholar] [CrossRef] [PubMed]
- Myouga, F.; Takahashi, K.; Tanaka, R.; Nagata, N.; Kiss, A.Z.; Funk, C.; Nomura, Y.; Nakagami, H.; Jansson, S.; Shinozaki, K. Stable Accumulation of Photosystem II Requires ONE-HELIX PROTEIN1 (OHP1) of the Light Harvesting-Like Family. Plant Physiol. 2018, 176, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Teng, Y.; Xu, J.; Zhang, N.; Ren, W.; Zhao, L.; Christie, P.; Luo, Y. Influence of Kaolinite and Montmorillonite on Benzo[a]Pyrene Biodegradation by Paracoccus AminovoransHPD-2 and the Underlying Interface Interaction Mechanisms. Pedosphere 2022, 32, 246–255. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. The Effects of Phosphorus Supply Limitation on Photosynthesis, Biomass Production, Nutritional Quality, and Mineral Nutrition in Lettuce Grown in a Recirculating Nutrient Solution. Sci. Hortic. 2019, 252, 379–387. [Google Scholar] [CrossRef]
- Fattore, N.; Bellan, A.; Pedroletti, L.; Vitulo, N.; Morosinotto, T. Acclimation of Photosynthesis and Lipids Biosynthesis to Prolonged Nitrogen and Phosphorus Limitation in Nannochloropsis Gaditana. Algal Res. 2021, 58, 102368. [Google Scholar] [CrossRef]
- Loudari, A.; Mayane, A.; Naciri, R.; Zeroual, Y.; Colinet, G.; Oukarroum, A. Root Morphological and Anatomical Responses to Increasing Phosphorus Concentration of Wheat Plants Grown under Salinity. Plant Stress 2022, 6, 100121. [Google Scholar] [CrossRef]
- Xing, D.; Li, X.; Wang, Y.; Deng, S.; Jin, C.; Zhao, Y.; Guo, L. The Comprehensive Impact of Phosphorus Sources on Microalgae Biochemical Metabolism and Phosphorus Transformation. J. Water Process Eng. 2023, 51, 103477. [Google Scholar] [CrossRef]
- Yan, S.; Cai, Y.; Li, H.; Song, S.; Xia, L. Enhancement of Cadmium Adsorption by EPS-Montmorillonite Composites. Environ. Pollut. 2019, 252, 1509–1518. [Google Scholar] [CrossRef]
- Krause, L.; Biesgen, D.; Treder, A.; Schweizer, S.A.; Klumpp, E.; Knief, C.; Siebers, N. Initial Microaggregate Formation: Association of Microorganisms to Montmorillonite-Goethite Aggregates under Wetting and Drying Cycles. Geoderma 2019, 351, 250–260. [Google Scholar] [CrossRef]
- Rodríguez-Beltrán, J.; Elabed, H.; Gaddour, K.; Blázquez, J.; Rodríguez-Rojas, A. Simple DNA Transformation in Pseudomonas Based on the Yoshida Effect. J. Microbiol. Methods 2012, 89, 95–98. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Wei, T.; Wu, S.; Wang, Z.; Zhao, Z.; Zhang, L.; Ge, Y.; Li, Z. Comparative Analysis of Microalgae’s Physiological Responses to Fibrous and Layered Clay Minerals. Biology 2025, 14, 647. https://doi.org/10.3390/biology14060647
Jiang Z, Wei T, Wu S, Wang Z, Zhao Z, Zhang L, Ge Y, Li Z. Comparative Analysis of Microalgae’s Physiological Responses to Fibrous and Layered Clay Minerals. Biology. 2025; 14(6):647. https://doi.org/10.3390/biology14060647
Chicago/Turabian StyleJiang, Zhongquan, Tianyi Wei, Sijia Wu, Zhongyang Wang, Zhonghua Zhao, Lu Zhang, Ying Ge, and Zhen Li. 2025. "Comparative Analysis of Microalgae’s Physiological Responses to Fibrous and Layered Clay Minerals" Biology 14, no. 6: 647. https://doi.org/10.3390/biology14060647
APA StyleJiang, Z., Wei, T., Wu, S., Wang, Z., Zhao, Z., Zhang, L., Ge, Y., & Li, Z. (2025). Comparative Analysis of Microalgae’s Physiological Responses to Fibrous and Layered Clay Minerals. Biology, 14(6), 647. https://doi.org/10.3390/biology14060647










