Potential Distribution and Cultivation Areas of Argentina anserina (Rosaceae) in the Upper Reaches of the Dadu River and Minjiang River Basin Under Climate Change: Applications of Ensemble and Productivity Dynamic Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Screening of Sample Data
2.2. Selection and Processing of Environmental Variables
2.3. Construction and Expression of the Ensemble Model
2.4. Changes in Ecological Niche
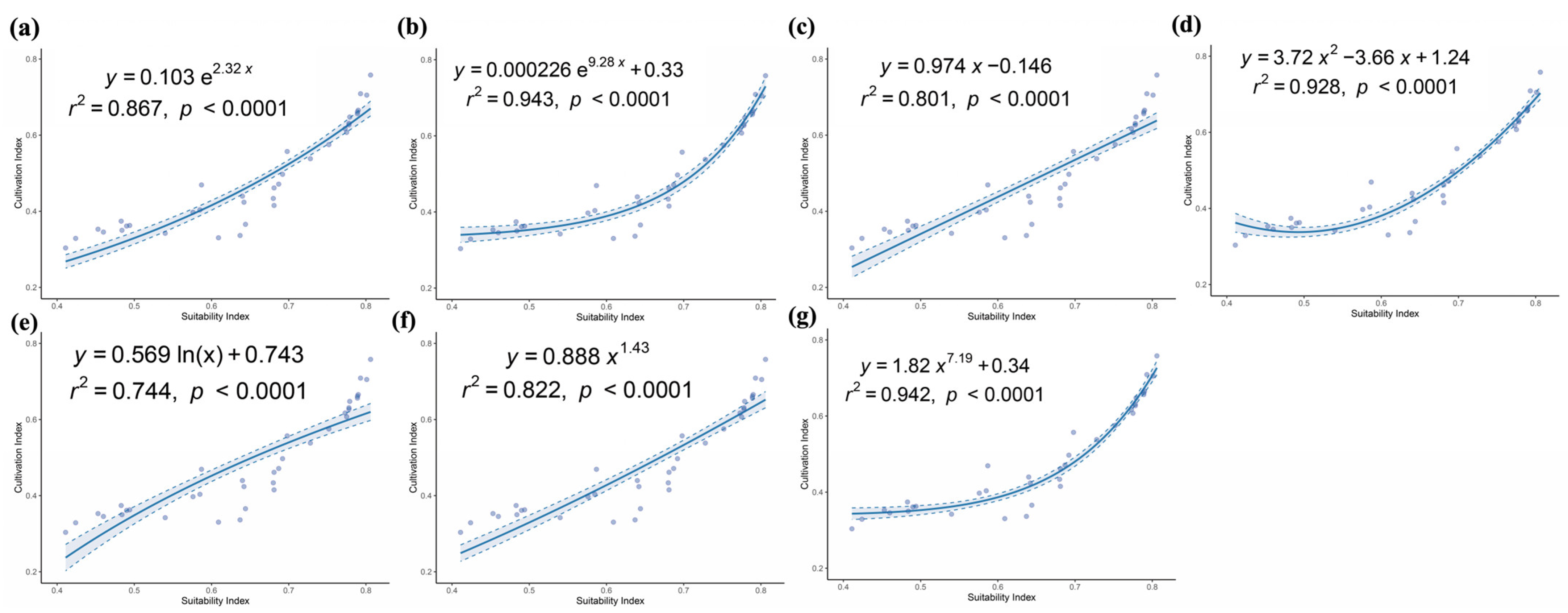
2.5. Establishment of the Relationship Between Cultivation Productivity and Environmental Suitability
3. Results
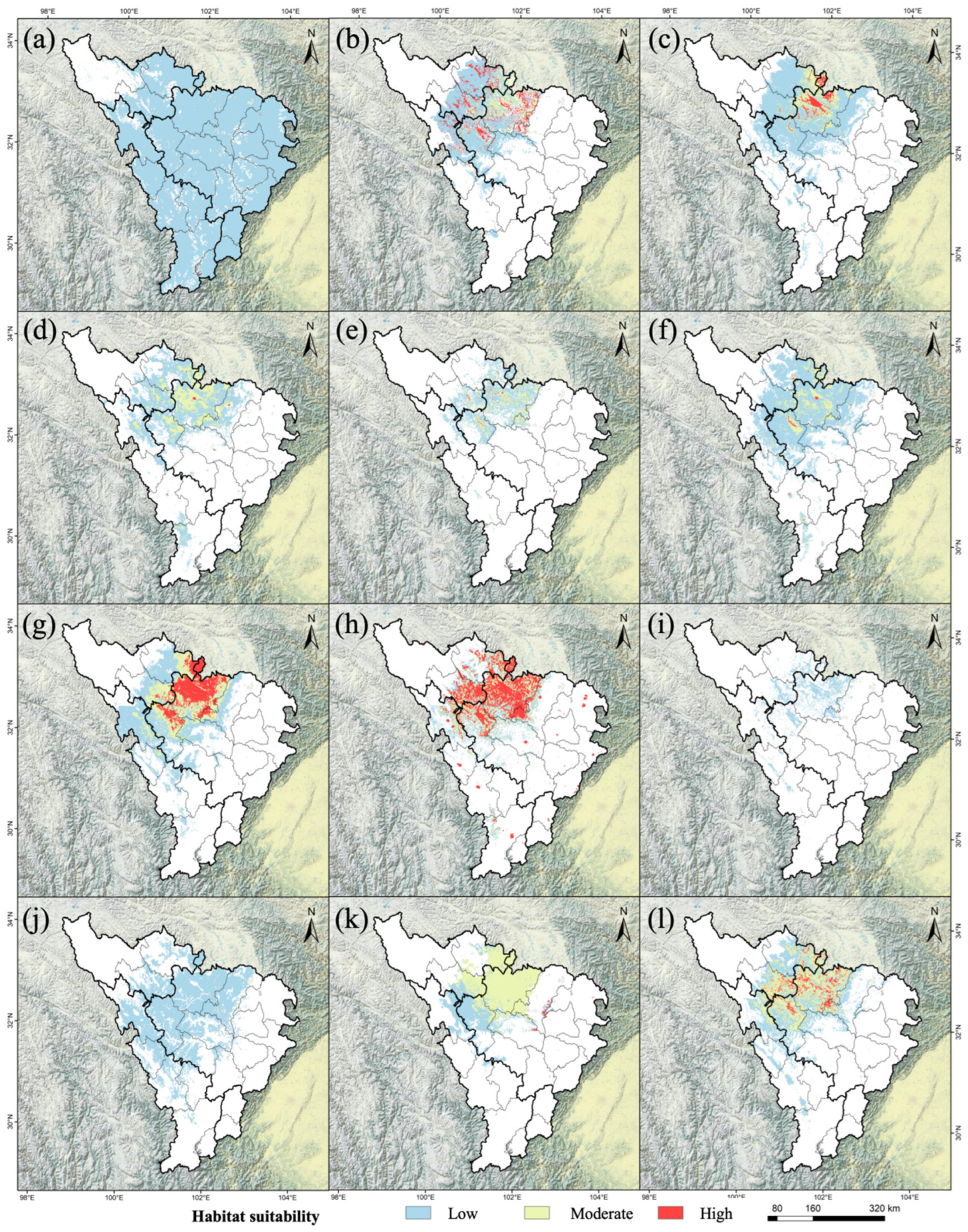
3.1. Prediction Results of Each Model and Model Accuracy Verification
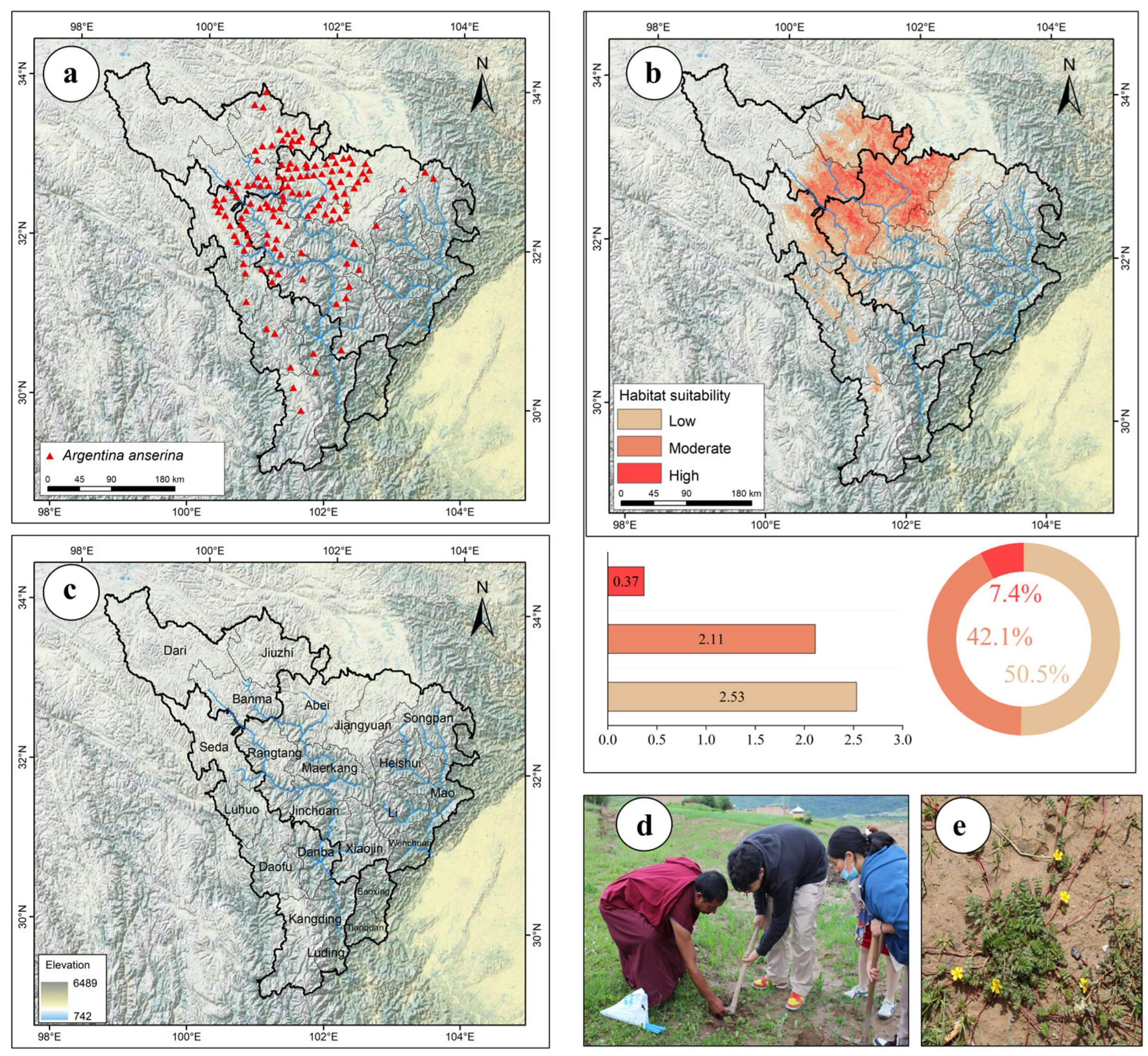
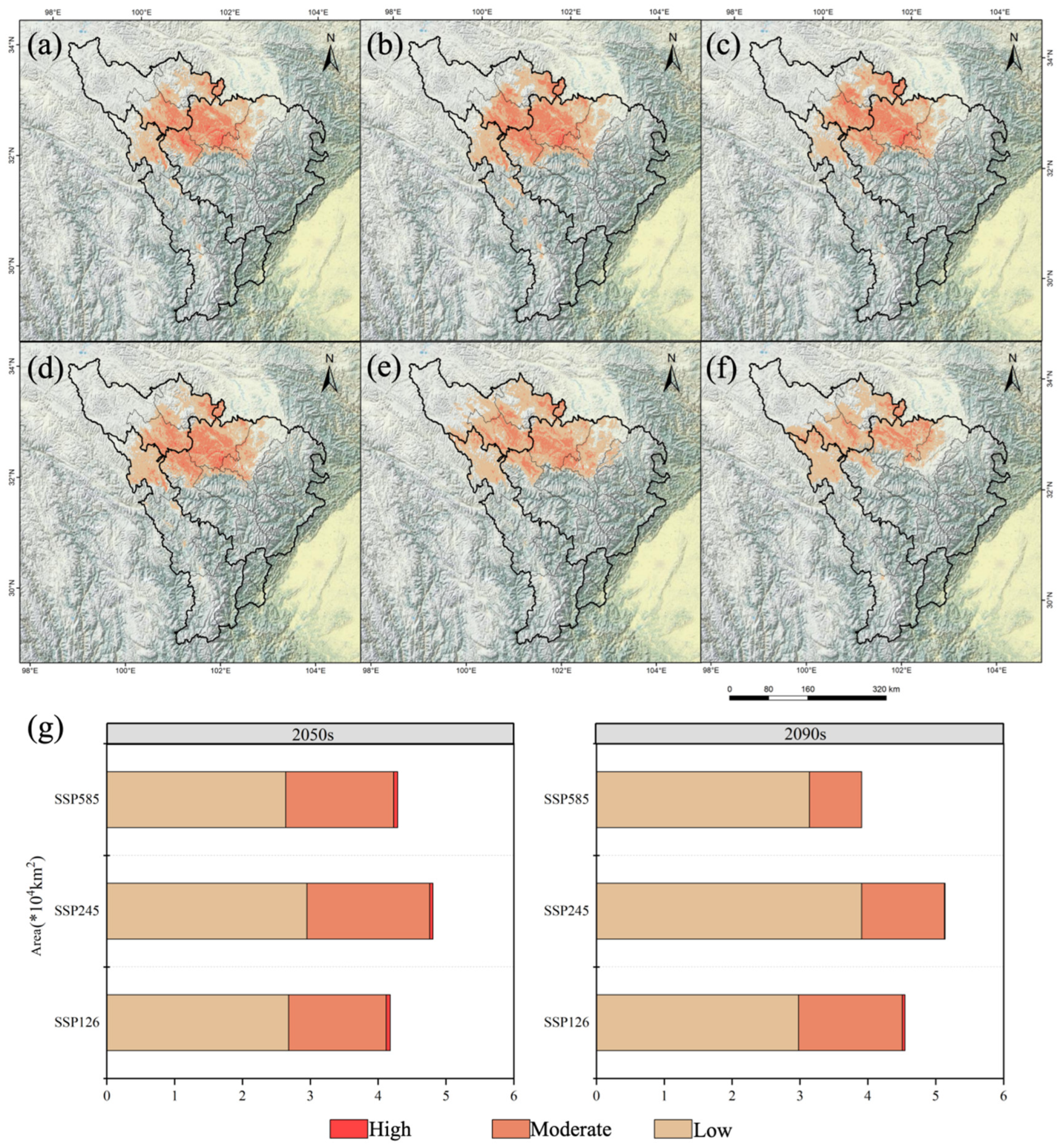
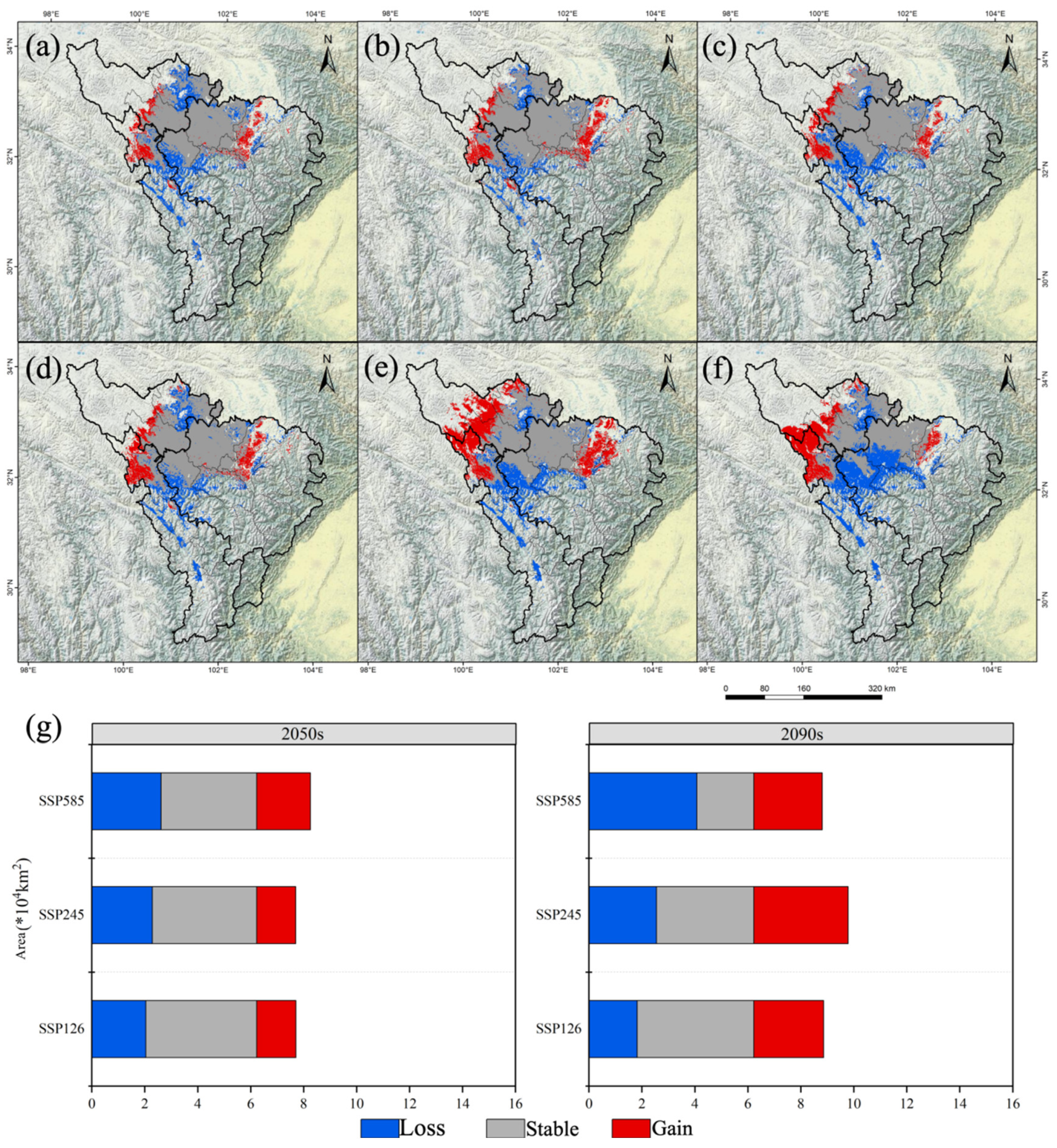
3.2. Potential Distribution Areas and Changes in A. anserina in Different Periods Under the Background of Climate Change
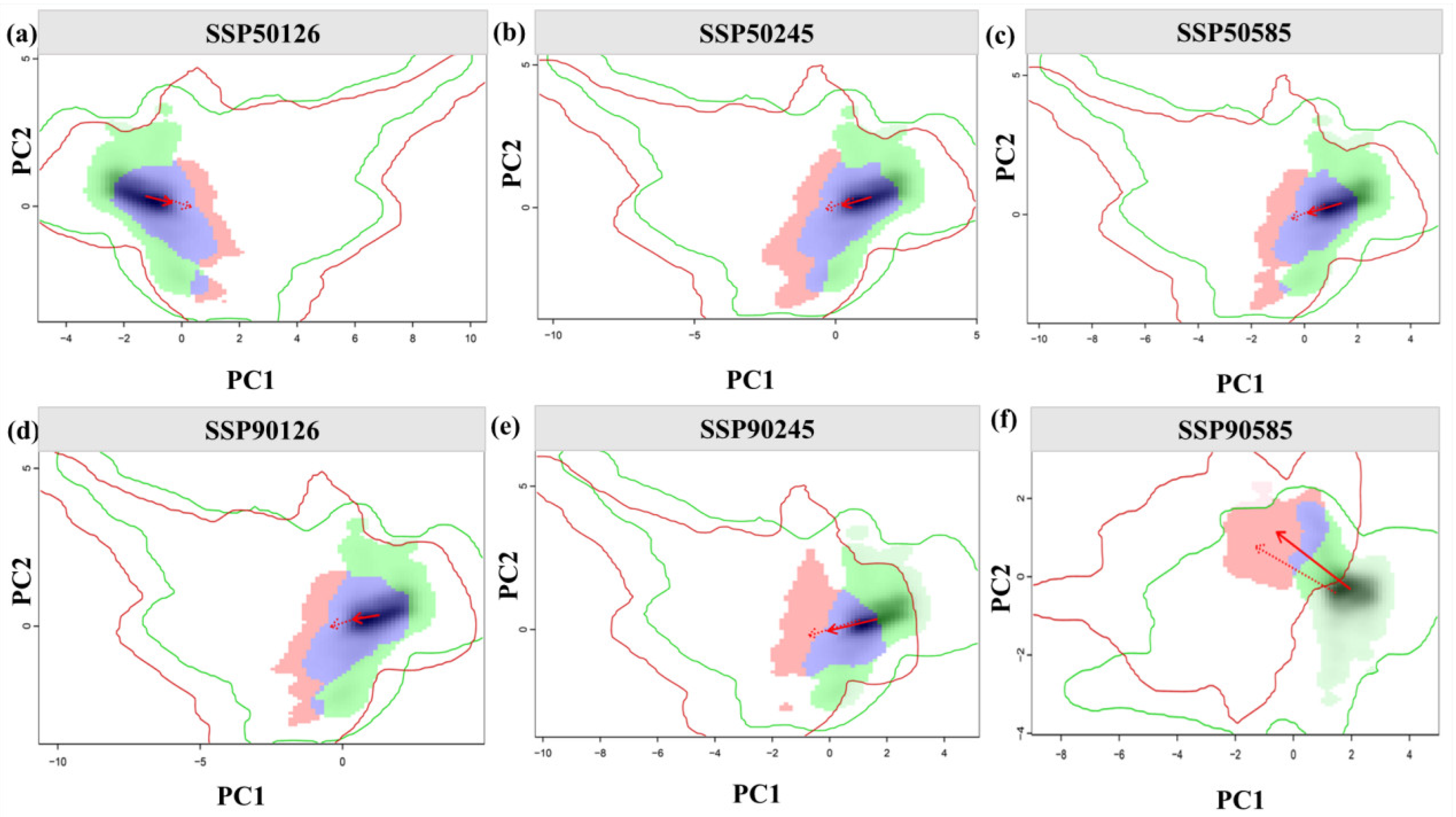
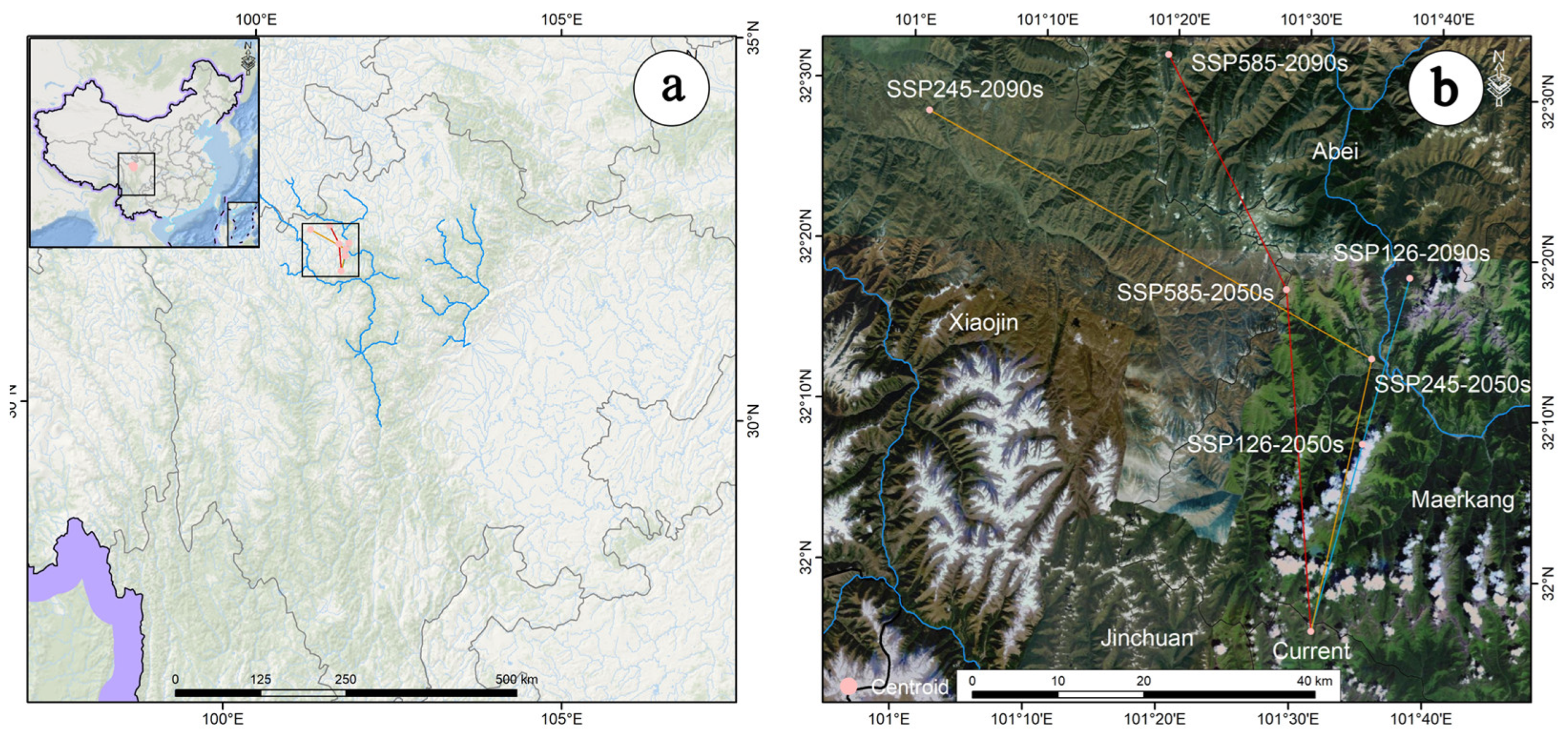
3.3. Analysis of the Ecological Niche Changes and Habitat Centroid Movement Trajectory of A. anserina in the Future Period
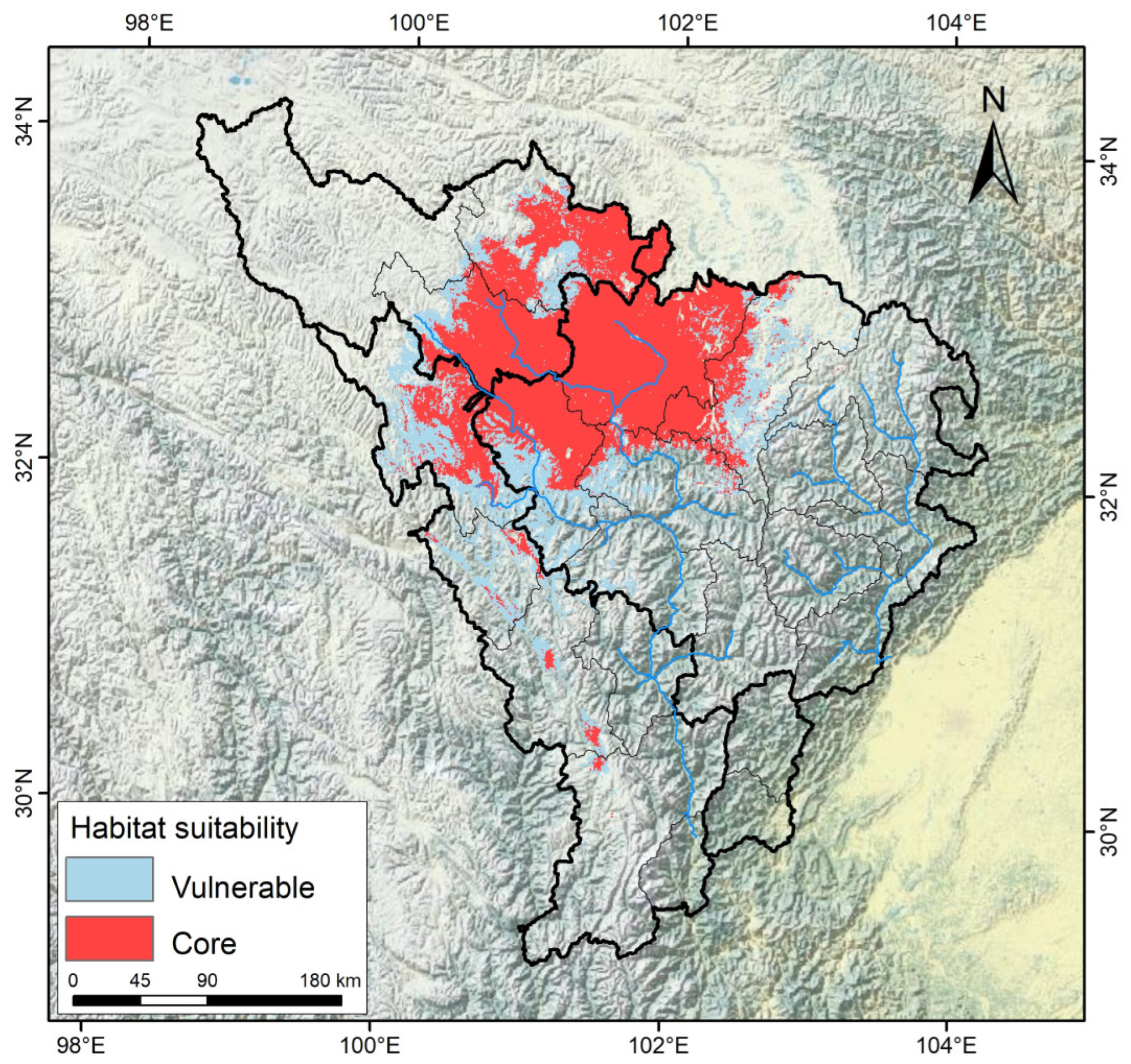
3.4. Delineation of the Potential Cultivation Production Areas of A. anserina
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mi, C.; Ma, L.; Yang, M.; Li, X.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira-Donoso, D.; Harvey, L.P.; Jablonski, D.; et al. Global Protected Areas as Refuges for Amphibians and Reptiles under Climate Change. Nat. Commun. 2023, 14, 1389. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef]
- Chen, F.H.; Dong, G.H.; Zhang, D.J.; Liu, X.Y.; Jia, X.; An, C.B.; Ma, M.M.; Xie, Y.W.; Barton, L.; Ren, X.Y.; et al. Agriculture Facilitated Permanent Human Occupation of the Tibetan Plateau after 3600 B.P. Science 2015, 347, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guan, L.; Zhao, H.; Huang, Y.; Mou, Q.; Liu, K.; Chen, T.; Wang, X.; Zhang, Y.; Wei, B.; et al. Modeling Habitat Suitability of Houttuynia Cordata Thunb (Ceercao) Using MaxEnt under Climate Change in China. Ecol. Inform. 2021, 63, 101324. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; Lawler, J.J. Conserving Biodiversity in the Face of Rapid Climate Change Requires a Shift in Priorities. WIREs Clim. Change 2023, 14, e798. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Su, M.; Gong, W.; Liu, Y.; Yang, J.; Huang, Y.; Zhao, C. Modeling the Potential Distribution of the Energy Tree Species Triadica Sebifera in Response to Climate Change in China. Sci. Rep. 2024, 14, 1220. [Google Scholar] [CrossRef]
- Wan, J.-N.; Wang, S.-W.; Leitch, A.R.; Leitch, I.J.; Jian, J.-B.; Wu, Z.-Y.; Xin, H.-P.; Rakotoarinivo, M.; Onjalalaina, G.E.; Gituru, R.W.; et al. The Rise of Baobab Trees in Madagascar. Nature 2024, 629, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lu, X.; Hu, X.; Zhang, Q.; Ali, M.; Long, C. Dulong People’s Traditional Knowledge of Caryota Obtusa (Arecaceae): A Potential Starch Plant with Emphasis on Its Starch Properties and Distribution Prediction. Econ. Bot. 2023, 77, 63–81. [Google Scholar] [CrossRef]
- Patni, B.; Bhattacharyya, M.; Kumari, A.; Purohit, V.K. Alarming Influence of Climate Change and Compromising Quality of Medicinal Plants. Plant Physiol. Rep. 2022, 27, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Ding, J.; Feng, X.; Chai, X.; Yang, J.; Liu, C.; Zeng, Y.; Zhou, W.; Wang, Y. Identification of Q-Markers from Hedan Tablet by Employing “Spider-Web” Mode and Taking Compounds’ Hepatotoxicity into Account. Chin. Herb. Med. 2022, 14, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zhang, B.; Chen, B.; Duan, D.; Zhou, X.; Chen, J.; Zhang, X. A Multi-Dimensional “Climate-Land-Quality” Approach to Conservation Planning for Medicinal Plants: Take Gentiana Scabra Bunge in China as an Example. Ind. Crops Prod. 2024, 211, 118222. [Google Scholar] [CrossRef]
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; Van Andel, T. Scientists’ Warning on Climate Change and Medicinal Plants. Planta Medica 2020, 86, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A. California Tries to Connect Its Scattered Marine Reserves. Science 2005, 308, 487–488. [Google Scholar] [CrossRef]
- Huang, L.; Li, S.; Huang, W.; Xiang, H.; Jin, J.; Oskolski, A.A. Glacial Expansion of Cold-Tolerant Species in Low Latitudes: Megafossil Evidence and Species Distribution Modelling. Natl. Sci. Rev. 2023, 10, nwad038. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the Current and Future Distribution of Three Coptis Herbs in China under Climate Change Conditions, Using the MaxEnt Model and Chemical Analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tao, X.; Liao, J.; Liu, S.; Xu, L.; Yuan, S.; Zhang, Z.; Wang, F.; Deng, N.; Huang, J.; et al. Predicting Potential Cultivation Region and Paddy Area for Ratoon Rice Production in China Using Maxent Model. Field Crops Res. 2022, 275, 108372. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, F.; Xia, P.; Zhao, G.; Wei, M.; Wei, F.; Han, R. Assessment of Suitable Cultivation Region for Panax Notoginseng under Different Climatic Conditions Using MaxEnt Model and High-Performance Liquid Chromatography in China. Ind. Crops Prod. 2022, 176, 114416. [Google Scholar] [CrossRef]
- Cao, B.; Bai, C.; Zhang, M.; Lu, Y.; Gao, P.; Yang, J.; Xue, Y.; Li, G. Future Landscape of Renewable Fuel Resources: Current and Future Conservation and Utilization of Main Biofuel Crops in China. Sci. Total Environ. 2022, 806, 150946. [Google Scholar] [CrossRef]
- Huang, D.; An, Q.; Huang, S.; Tan, G.; Quan, H.; Chen, Y.; Zhou, J.; Liao, H. Biomod2 Modeling for Predicting the Potential Ecological Distribution of Three Fritillaria Species under Climate Change. Sci. Rep. 2023, 13, 18801. [Google Scholar] [CrossRef]
- Sharma, M.K.; Ram, B.; Chawla, A. Ensemble Modelling under Multiple Climate Change Scenarios Predicts Reduction in Highly Suitable Range of Habitats of Dactylorhiza Hatagirea (D.Don) Soo in Himachal Pradesh, Western Himalaya. S. Afr. J. Bot. 2023, 154, 203–218. [Google Scholar] [CrossRef]
- Xie, C.; Chen, L.; Li, M.; Jim, C.Y.; Liu, D. BIOCLIM Modeling for Predicting Suitable Habitat for Endangered Tree Tapiscia Sinensis (Tapisciaceae) in China. Forests 2023, 14, 2275. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Yang, L.; Chen, K.; Shama, Z.; Jiang, X.; Yang, J.; Zhao, G.; Huang, Y. Prediction of the Potential Distribution and Conservation Strategies of the Endangered Plant Tapiscia Sinensis. Forests 2024, 15, 1677. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Wei, H.; Lyu, C.; Yin, X. Spatial Differentiation of Farmland and Influencing Factors on the Qinghai-Tibet Plateau. Sci. Geogr. Sin. 2023, 43, 379–387. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Li, Y.; Wang, H.; Gao, X.; Zhao, J.; Ma, J.; Ji, T.; Wang, H. Analysis of Nutritional Compositions and Evaluation of Quality in Potentilla anserina L. from Qinghai Different Producing Areas. J. Food Sci. Biotechnol. 2022, 41, 95–111. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Leitch, I.J.; Royal Botanic Gardens Kew Genome Acquisition Lab; Plant Genome Sizing collective; Darwin Tree of Life Barcoding collective; Wellcome Sanger Institute Tree of Life programme; Wellcome Sanger Institute Scientific Operations: DNA Pipelines collective; Tree of Life Core Informatics collective; Darwin Tree of Life Consortium. The Genome Sequence of the Silverweed Cinquefoil, Potentilla anserina L., 1753. Wellcome Open Res. 2023, 8, 464. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ha, J.H.; Kang, H.C.; Seo, W.S.; Bin, B.-H. The Protective Effects of Moisturizer Containing Potentilla anserina Extract in the Topical Treatment of Skin Damage Caused by Masks. Int. J. Mol. Sci. 2023, 24, 14294. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, W. Potentilla anserina L. Attenuates Oleic Acid-Induced Steatosis in HepG2 Cells and Hyperlipidemia in High Fat Diet-Induced Obese Mice. J. Funct. Foods 2023, 109, 105772. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, J.; Liu, D.; Gong, H.; Bai, D.; Sun, W. Potentilla anserina Polysaccharide Alleviates Cadmium-Induced Oxidative Stress and Apoptosis of H9c2 Cells by Regulating the MG53-Mediated RISK Pathway. Chin. J. Nat. Med. 2023, 21, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, H.; Ma, J.; Zhang, Y.; Chen, H.; Wei, T.; Gao, D.; Li, J. Enzyme-Assisted Extraction, Characterization, and in Vitro Antioxidant Activity of Polysaccharides from Potentilla anserina L. Front. Nutr. 2023, 10, 1216572. [Google Scholar]
- Wu, D.; Liu, Z.; Feng, Y.; Tang, F.; Li, S.; Zhang, X.; Li, H.; Liu, Q.; Zhang, L.; Liu, Q.; et al. Development and Characterization of DEC-205 Receptor Targeted Potentilla anserina L Polysaccharide PLGA Nanoparticles as an Antigen Delivery System to Enhance in Vitro and in Vivo Immune Responses in Mice. Int. J. Biol. Macromol. 2023, 224, 998–1011. [Google Scholar] [CrossRef]
- Li, J. Study on the Utilization and the Biological Characteristic of Juema (Potentilla anserina L.) That Is Wild Resource Foliage. Ph.D. Thesis, Northwest Sci-Tech University, Yangling, China, 2004. [Google Scholar]
- Zhan, D. A Study on the Diversity of Ethnic Medicine Culture in Tibetan-Qiang-Yi Corridor from the Perspective of Medical Anthropology. Ph.D. Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2018. [Google Scholar]
- Ai, N. On the Geography and Environment of the Tibetan-Yi Corridor. J. Southwest Univ. Natl. (Humanit. Soc. Sci. Ed.) 2007, 2, 15–16. [Google Scholar]
- Hua, X.; Yan, J.; Wang, Q.; Zhang, Y. Comparative analysis on influencing factors of cultivated land use intensity in valley and middle mountain area of upper Dadu River watershed. Trans. Chin. Soc. Agric. Eng. 2013, 29, 234–244. [Google Scholar] [CrossRef]
- Yang, L.; Tian, S.; Fan, N.; Song, L. The spatial coupling relationship between settlements and cultivated land in the upper reaches of the Min River. J. Xi’an Univ. Technol. 2024, 2, 1–10. [Google Scholar]
- Liu, M.; Su, M.-M.; Cai, K.; Qi, P.-S.; Gong, W.; Chen, D.-C.; He, L.; Wu, H.-W.; Deng, D.-Z.; Huang, Y. Distributional Response of Paeonia decomposita to Climate Change and Conservation Strategies. Pol. J. Environ. Stud. 2025, 34, 3657–3669. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Huang, Y.; Zhang, J.; Mou, Q.; Qiu, J.; Wang, R.; Li, Y.; Zhang, D. Simulation of Potential Suitable Distribution of Original Species of Fritillariae Cirrhosae Bulbus in China under Climate Change Scenarios. Environ. Sci. Pollut. Res. 2022, 29, 22237–22250. [Google Scholar] [CrossRef]
- Li, S.-Y.; Miao, L.-J.; Jiang, Z.-H.; Wang, G.-J.; Gnyawali, K.R.; Zhang, J.; Zhang, H.; Fang, K.; He, Y.; Li, C. Projected Drought Conditions in Northwest China with CMIP6 Models under Combined SSPs and RCPs for 2015–2099. Adv. Clim. Change Res. 2020, 11, 210–217. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z.; Li, X. Moderate Warming Will Expand the Suitable Habitat of Ophiocordyceps Sinensis and Expand the Area of O. Sinensis with High Adenosine Content. Sci. Total Environ. 2021, 787, 147605. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R. Coordinate Cleaner: Standardized Cleaning of Occurrence Records from Biological Collection Databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L. Spatiotemporal Dataset on Chinese Population Distribution and Its Driving Factors from 1949 to 2013. Sci. Data 2016, 3, 160047. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968; ISBN 0-691-08062-3. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Sun, J.; Jiao, W.; Wang, Q.; Wang, T.; Yang, H.; Jin, J.; Feng, H.; Guo, J.; Feng, L.; Xu, X.; et al. Potential Habitat and Productivity Loss of Populus deltoides Industrial Forest Plantations Due to Global Warming. For. Ecol. Manag. 2021, 496, 119474. [Google Scholar] [CrossRef]
- Alfonso-Corrado, C.; Naranjo-Luna, F.; Clark-Tapia, R.; Campos, J.E.; Rojas-Soto, O.R.; Luna-Krauletz, M.D.; Bodenhorn, B.; Gorgonio-Ramírez, M.; Pacheco-Cruz, N. Effects of environmental changes on the occurrence of Oreommunnea mexicana (Juglandaceae) in a biodiversity hotspot cloud forest. Forests 2017, 8, 261. [Google Scholar] [CrossRef]
- Jiménez-García, D.; Peterson, A.T. Climate change impact on endangered cloud forest tree species in Mexico. Rev. Mex. Biodivers. 2019, 90, e9027812. [Google Scholar] [CrossRef]
- Téllez-Valdés, O.; Dávila-Aranda, P. Protected areas and climate change: A case study of the cacti in the Tehuacán-Cuicatlán Biosphere Reserve, Mexico. Conserv. Biol. 2003, 17, 846–853. [Google Scholar] [CrossRef]
- Guo, B.; Zang, W.; Yang, F.; Han, B.; Chen, S.; Liu, Y.; Yang, X.; He, T.; Chen, X.; Liu, C.; et al. Spatial and Temporal Change Patterns of Net Primary Productivity and Its Response to Climate Change in the Qinghai-Tibet Plateau of China from 2000 to 2015. J. Arid Land 2020, 12, 1–17. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Zhang, J.; Wang, Y.; Ma, R.; Liu, B.; Lu, X.; Jiang, M. Asymmetric Impacts of Diurnal Warming on Vegetation Carbon Sequestration of Marshes in the Qinghai Tibet Plateau. Glob. Biogeochem. Cycles 2022, 36, e2022GB007396. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Y.; Yue, J.; Wang, Z.; Zou, H.; Ji, X.; Zhang, S.; Liu, Z. Prediction of Potential Suitable Areas and Priority Protection for Cupressus Gigantea on the Tibetan Plateau. Plants 2024, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Xue, X.; Wang, T.; Kang, W.; Liao, J.; Liu, S. Spatial and Temporal Differences in Alpine Meadow, Alpine Steppe and All Vegetation of the Qinghai-Tibetan Plateau and Their Responses to Climate Change. Remote Sens. 2021, 13, 669. [Google Scholar] [CrossRef]
- Yang, J.-T.; Jiang, X.; Chen, H.; Jiang, P.; Liu, M.; Huang, Y. Predicting the Potential Distribution of the Endangered Plant Magnolia Wilsonii Using MaxEnt under Climate Change in China. Pol. J. Environ. Stud. 2022, 31, 4435–4445. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, G.; Zhang, S.; Wang, Z.; Wen, X.; Liu, L.; Zhang, C.; Tie, N.; Sa, R. Priority Conservation Area of Larix Gmelinii under Climate Change: Application of an Ensemble Modeling. Front. Plant Sci. 2023, 14, 1177307. [Google Scholar] [CrossRef]
- Huang, X.-M.; Zhao, C.; Cai, K.; Huang, Y. Climatic Changes in the Anthropocene Have Increased the Suitable Habitat Areas of Paeonia Delavayi in China. Pol. J. Environ. Stud. 2023, 32, 4051–4063. [Google Scholar] [CrossRef]
- Lan, R.; Chen, J.; Pan, J.; Chen, R.; Lin, H.; Li, Z.; Xue, Q.; Liu, C.; Huang, Y. Simulation of Potential Suitable Distribution of Endangered Medicinal of Paeonia rockii under Climate Change Scenarios. Pol. J. Environ. Stud. 2023, 32, 2181–2197. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. In Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Thuiller, W. BIOMOD—Optimizing Predictions of Species Distributions and Projecting Potential Future Shifts under Global Change. Glob. Change Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- McLachlan, J.S.; Hellmann, J.J.; Schwartz, M.W. A Framework for Debate of Assisted Migration in an Era of Climate Change. Conserv. Biol. 2007, 21, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Broennimann, O.; Cornuault, J.; Guisan, A. Data integration methods to account for spatial niche truncation effects in regional projections of species distribution. Ecol. Appl. 2021, 31, e02427. [Google Scholar] [CrossRef]
- Chevalier, M.; Zarzo-Arias, A.; Guelat, J.; Mateo, R.G.; Guisan, A. Accounting for the niche truncation to improve spatial and temporal predictions of species distributions. Front. Ecol. Evol. 2022, 10, 944116. [Google Scholar] [CrossRef]
| Environment Variable | Abbreviation | Unit | Contribution Rate % |
|---|---|---|---|
| The highest temperature of the Hottest month | bio5 | °C | 0.12 |
| The lowest temperature of the Coldest month | bio6 | °C | 0.23 |
| Altitude | elev | m | 3.1 |
| Ecological footprint | footprint | gha | 0.36 |
| Land Cover | landcover | / | 0.51 |
| Seasonal dry matter yield | dmps | g/m2/season | 0 |
| Gross primary productivity | gpp | g C/m2/year | 5.32 |
| Annual average temperature | bio1 | °C | 0.8 |
| annual precipitation | bio12 | mm | 13.9 |
| Seasonal variation coefficient of Temperature | bio4 | C of V | 7.51 |
| Annual temperature difference | bio7 | °C | 55.62 |
| Driest monthly precipitation | bio14 | mm | 0.9 |
| Seasonal variation in Precipitation | bio15 | C of V | 10.53 |
| gravel content | t_gravel | 0.4 | |
| Organic carbon content | t_oc | % | 0.52 |
| PH value of water-soaked soil | t_ph_h2o | / | 0.18 |
| Model Code | Model Type |
|---|---|
| a | y = a ∗ exp (b ∗ x) |
| b | y = a ∗ exp (b ∗ x) + c |
| c | y = a ∗ x + b |
| d | y = a ∗ x2 + b ∗ x + c |
| e | y = a ∗ ln(x) + b |
| f | y = a ∗ xb |
| g | y = a ∗ xb + c |
| ANN | CTA | FDA | GAM | GBM | GLM | MARS | Maxent | RF | Ensemble | SRE | XGBOOST | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | 0.80 | 0.82 | 0.83 | 0.81 | 0.93 | 0.85 | 0.85 | 0.82 | 0.85 | 0.99 | 0.7 | 0.86 |
| Kappa | 0.63 | 0.66 | 0.65 | 0.61 | 0.73 | 0.65 | 0.68 | 0.57 | 0.67 | 0.93 | 0.51 | 0.67 |
| TSS | 0.79 | 0.79 | 0.73 | 0.7 | 0.94 | 0.8 | 0.78 | 0.77 | 0.78 | 0.97 | 0.6 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yang, J.; Zhao, G.; Yang, Y. Potential Distribution and Cultivation Areas of Argentina anserina (Rosaceae) in the Upper Reaches of the Dadu River and Minjiang River Basin Under Climate Change: Applications of Ensemble and Productivity Dynamic Models. Biology 2025, 14, 668. https://doi.org/10.3390/biology14060668
Huang Y, Yang J, Zhao G, Yang Y. Potential Distribution and Cultivation Areas of Argentina anserina (Rosaceae) in the Upper Reaches of the Dadu River and Minjiang River Basin Under Climate Change: Applications of Ensemble and Productivity Dynamic Models. Biology. 2025; 14(6):668. https://doi.org/10.3390/biology14060668
Chicago/Turabian StyleHuang, Yi, Jian Yang, Guanghua Zhao, and Yang Yang. 2025. "Potential Distribution and Cultivation Areas of Argentina anserina (Rosaceae) in the Upper Reaches of the Dadu River and Minjiang River Basin Under Climate Change: Applications of Ensemble and Productivity Dynamic Models" Biology 14, no. 6: 668. https://doi.org/10.3390/biology14060668
APA StyleHuang, Y., Yang, J., Zhao, G., & Yang, Y. (2025). Potential Distribution and Cultivation Areas of Argentina anserina (Rosaceae) in the Upper Reaches of the Dadu River and Minjiang River Basin Under Climate Change: Applications of Ensemble and Productivity Dynamic Models. Biology, 14(6), 668. https://doi.org/10.3390/biology14060668






