Aquatic Organisms in Response to Salinity Stress: Ecological Impacts, Adaptive Mechanisms, and Resilience Strategies
Simple Summary
Abstract
1. Introduction
2. Ecological Consequences of Salinity Stress on Aquatic Organisms
2.1. Salinity Ranges in Aquatic Environment
2.2. Effect of Salinity Variability on Species Distribution and Biodiversity
2.3. Climate Change Implications on Salinity Fluctuations
3. Organismal Adaptations to Salinity Stress
3.1. Adaptive Strategies for Salinity Tolerance in Aquatic Organisms
3.2. Behavioral Adaptations to Salinity Fluctuations in Aquatic Organisms
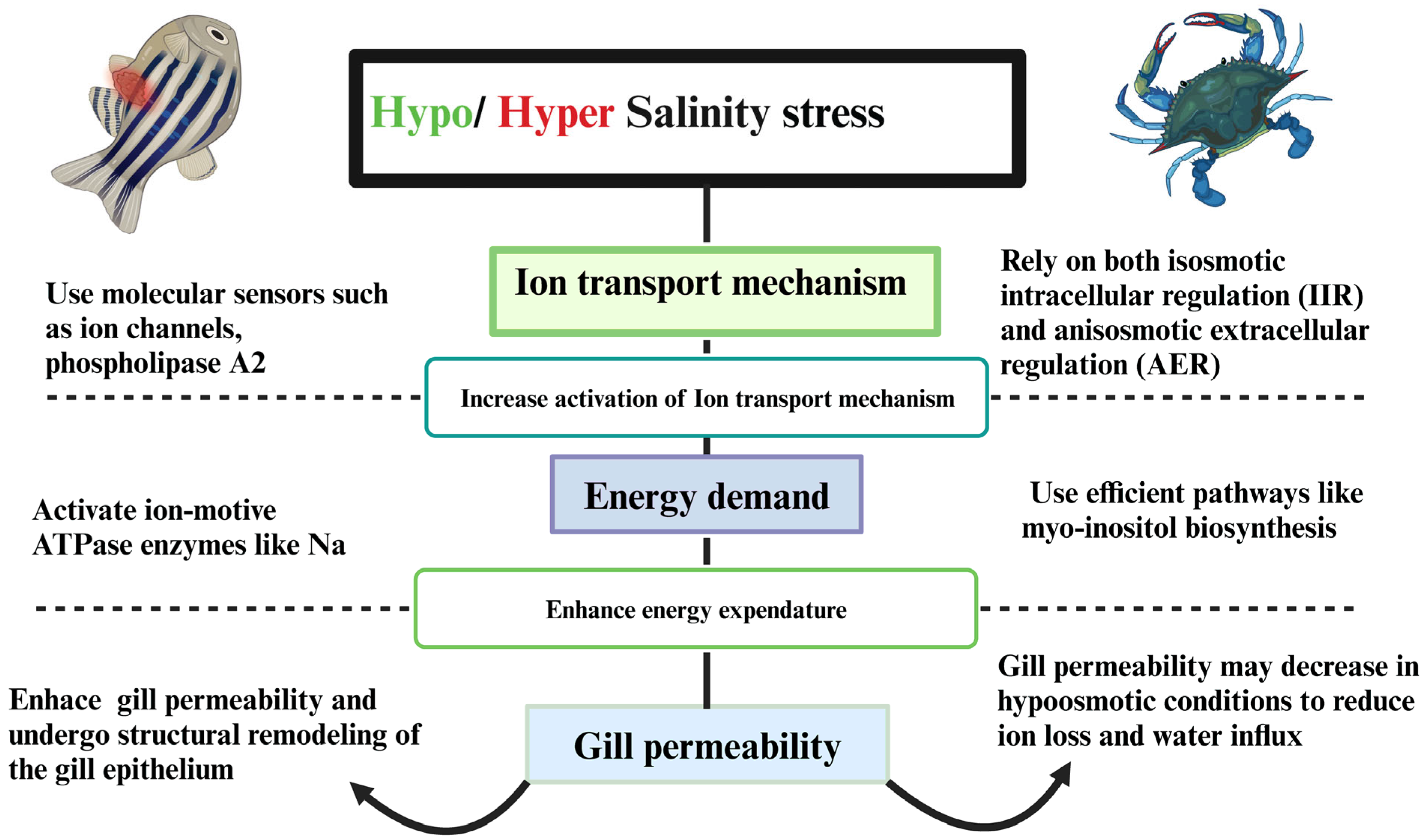
3.3. Osmotic Stress and Ion-Transport Mechanism Response in Fluctuating Salinity Environments
3.4. Oxidative Stress Response in Aquatic Organisms Due to Salinity Stress
| Species | Salinity Type | Duration | Enzyme Activity | References |
|---|---|---|---|---|
| Brachionus koreanus | High | 3–24 h | ROS and GST activity elevated | [83] |
| Crassostrea gigas | High/Low | 4 months | Elevated level of CAT, whereas no effect observed at SOD | [84] |
| Litopenaeus vannamei | High/Low | 24 h | Activities of SOD, GPx, and CAT decreased at both lower and higher salinities | [85] |
| Eriocheir sinensis | High/Low | 24–72 h | At higher salinities, GSH-Px activity is enhanced; works synergistically with SOD and CAT to mitigate lipid peroxidation damage | [86] |
| Procambarus clarkiis | Low | 48 h | Antioxidants like CAT and GPX-PX decrease, while MDA levels increase | [87] |
| Scylla olivacea | Medium | Varied | Increase in gill SOD activities helps cope with elevated ROS levels from high metabolic demands | [88] |
| Lates calcarifer | High/Low | 8 weeks | GPX and CAT activities increase in high salinity and decrease in low salinity | [89] |
| Oreochromis niloticus | Medium/Low | 4 weeks | The activity of SOD, GPX, and CAT elevates under medium salinity alongside hypoxia | [90] |
| Dicentrarchus labrax, Chanos chanos | High/Low | 4 weeks | SOD level of activity elevated in both fresh and saline water | [91] |
| Cyprinus carpio | Medium/Low | 8 weeks | Exposure to medium salinity reduced lysozyme activity, SOD, CAT, GPx, and increased MDA levels before and after heat stress | [92] |
3.5. Molecular Responses to Salinity Stress
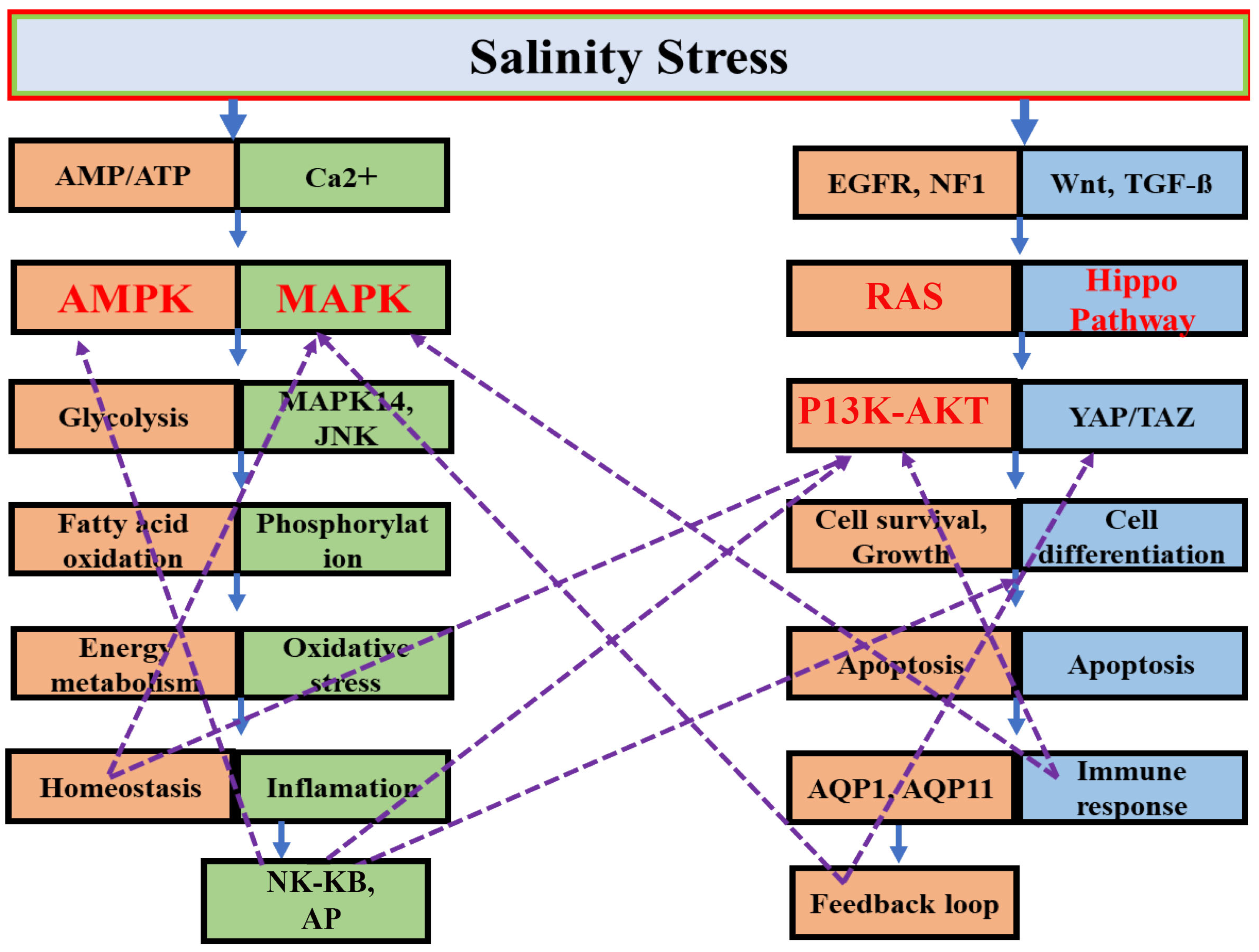
3.5.1. Key Signaling Pathways
MAPK Signaling Pathway Response in Aquatic Species Under Salinity Stress
PI3K-AKT Signaling Pathways in Response to Salinity Stress
AMPK Signaling Pathway and Energy Regulation in Response to Salinity Stress
The Role of the Hippo Signaling Pathway in Salinity Stress
3.5.2. Stress-Responsive Genes
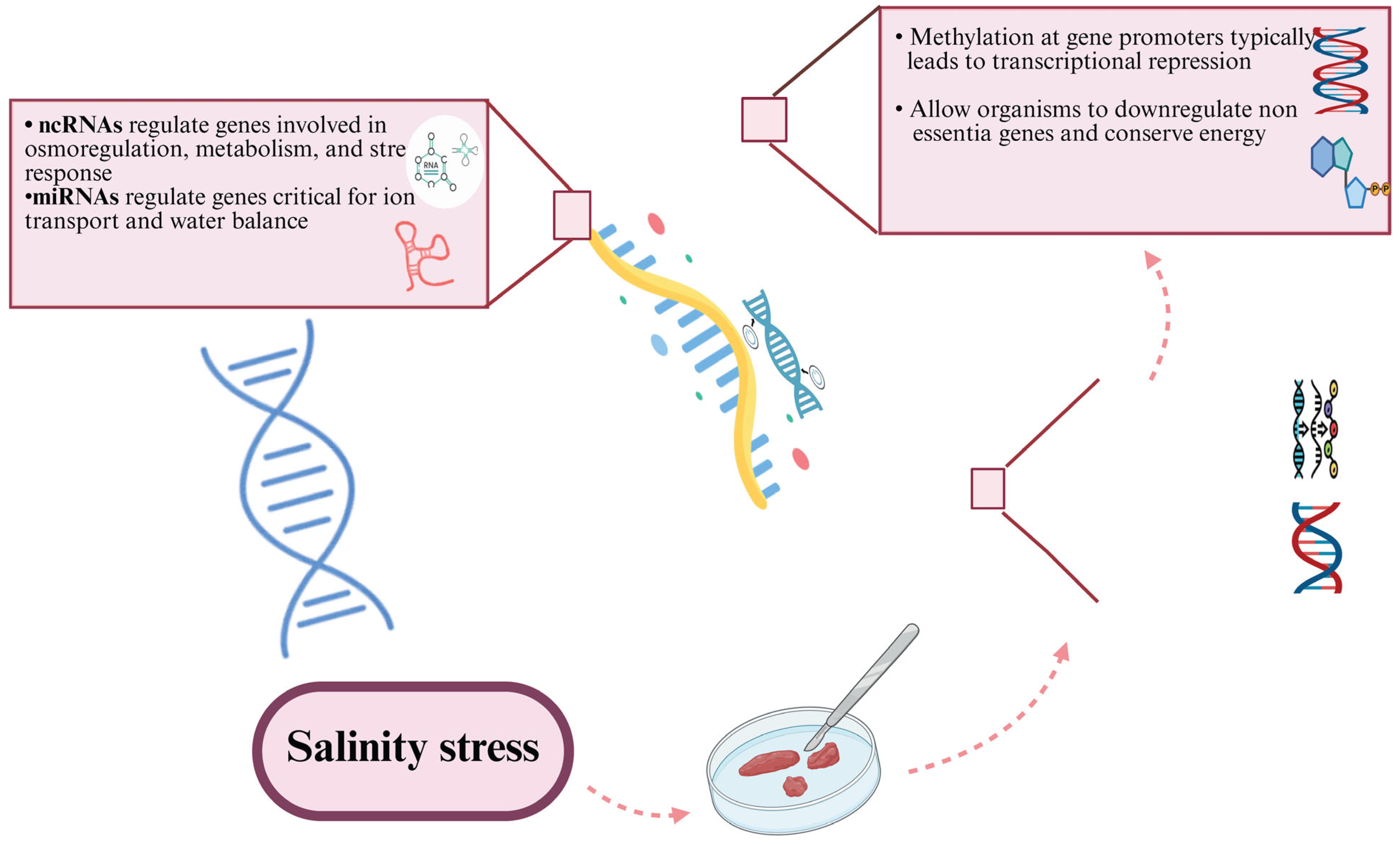
3.5.3. Epigenetic Modifications Under Salinity Fluctuations
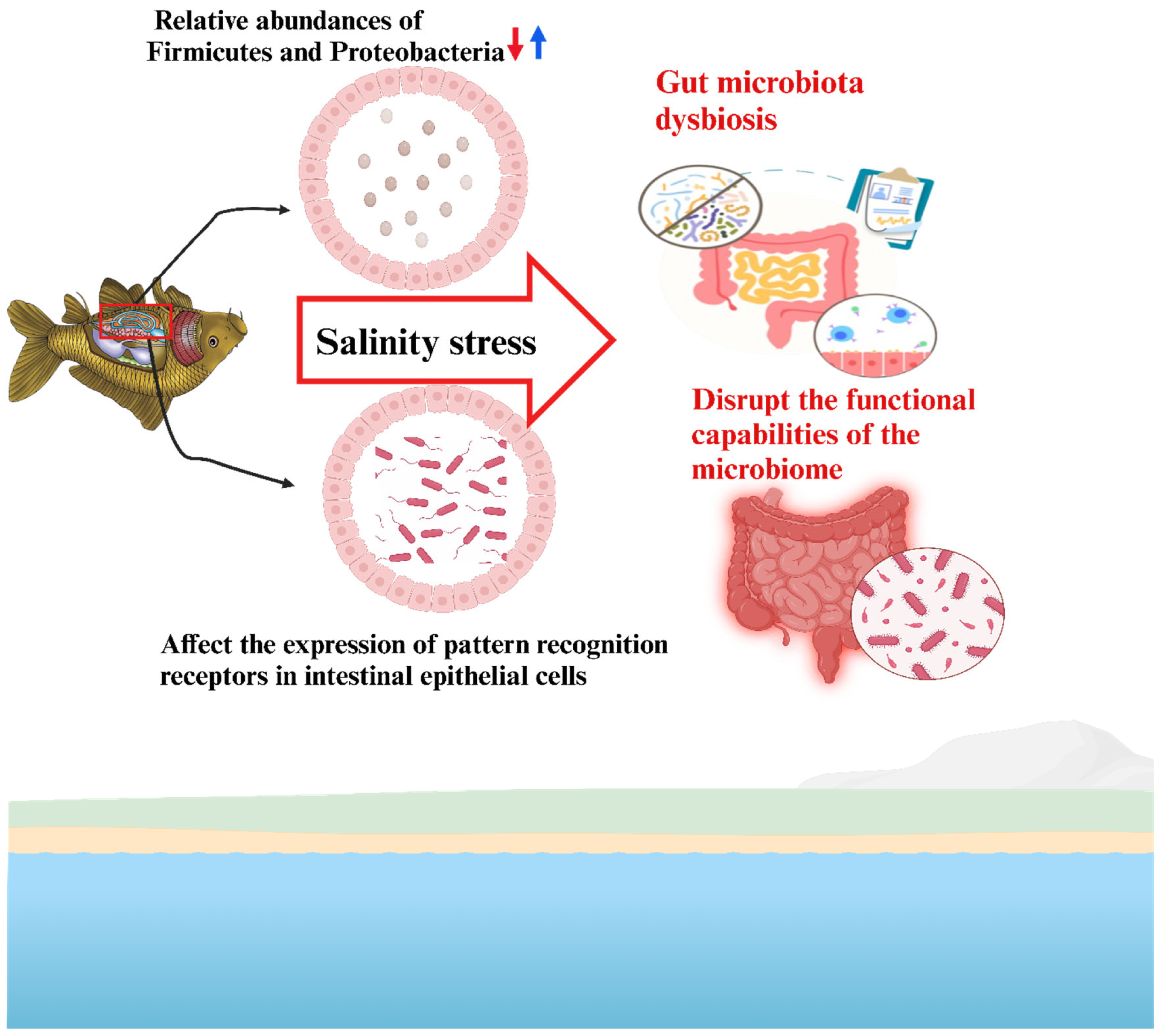
4. Microbiome Interactions and Response Under Salinity Stress
5. Genetic Diversity and Resilience of Aquatic Species
6. Strategies for Mitigating Salinity Stress in Aquatic Organisms
6.1. Nutritional Intervention
6.2. Development of Salinity-Resistant Varieties
7. Shortcomings
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gattoni, K.; Gendron, E.M.S.; Borgmeier, A.; McQueen, J.P.; Mullin, P.G.; Powers, K.; Powers, T.O.; Porazinska, D.L. Context-Dependent Role of Abiotic and Biotic Factors Structuring Nematode Communities Along Two Environmental Gradients. Mol. Ecol. 2022, 31, 3903–3916. [Google Scholar] [CrossRef] [PubMed]
- Brauner, C.J.; Gonzalez, R.J.; Wilson, J.M. Extreme Environments: Hypersaline, Alkaline, and Ion-Poor Waters. Fish Physiol. 2012, 32, 435–476. [Google Scholar]
- Bal, A.; Panda, F.; Pati, S.G.; Das, K.; Agrawal, P.K.; Paital, B. Modulation of Physiological Oxidative Stress and Antioxidant Status by Abiotic Factors Especially Salinity in Aquatic Organisms: Redox Regulation under Salinity Stress. Comp. Biochem. Physiol. Part—C Toxicol. Pharmacol. 2021, 241, 108–941. [Google Scholar]
- Seale, A.P.; Cao, K.; Chang, R.J.A.; Goodearly, T.R.; Malintha, G.H.T.; Merlo, R.S.; Peterson, T.L.; Reighard, J.R. Salinity Tolerance of Fishes: Experimental Approaches and Implications for Aquaculture Production. Rev. Aquac. 2024, 16, 1351–1373. [Google Scholar] [CrossRef]
- Su, M.; Liu, N.; Zhang, Z.; Zhang, J. Osmoregulatory Strategies of Estuarine Fish Scatophagus argus in Response to Environmental Salinity Changes. BMC Genom. 2022, 23, 545. [Google Scholar] [CrossRef]
- Williams, L. The Physiological and Behavioural Response of Mytilus Mussels to Salinity in a Changing Ocean. Ph.D. Thesis, University of Exeter, Exeter, UK, 2024. [Google Scholar]
- Kroeker, K.J.; Sanford, E. Ecological Leverage Points: Species Interactions Amplify the Physiological Effects of Global Environmental Change in the Ocean. Ann. Rev. Mar. Sci. 2022, 14, 75–103. [Google Scholar] [CrossRef]
- Röthig, T.; Trevathan-Tackett, S.M.; Voolstra, C.R.; Ross, C.; Chaffron, S.; Durack, P.J.; Warmuth, L.M.; Sweet, M. Human-induced Salinity Changes Impact Marine Organisms and Ecosystems. Glob. Change Biol. 2023, 29, 4731–4749. [Google Scholar] [CrossRef]
- McNamara, J.C.; Faria, S.C. Evolution of Osmoregulatory Patterns and Gill Ion Transport Mechanisms in the Decapod Crustacea: A Review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 997–1014. [Google Scholar] [CrossRef]
- Anger, K. Neotropical Macrobrachium (Caridea: Palaemonidae):On the Biology, Origin, and Radiation of Freshwater-Invading Shrimp. J. Crustac. Biol. 2013, 33, 151–183. [Google Scholar] [CrossRef]
- Xu, W.B.; Zhang, Y.M.; Li, B.Z.; Lin, C.Y.; Chen, D.Y.; Cheng, Y.X.; Guo, X.L.; Dong, W.R.; Shu, M.A. Effects of Low Salinity Stress on Osmoregulation and Gill Transcriptome in Different Populations of Mud Crab Scylla paramamosain. Sci. Total Environ. 2023, 867, 161522. [Google Scholar] [CrossRef]
- Pini, J.; Planes, S.; Rochel, E.; Lecchini, D.; Fauvelot, C. Genetic Diversity Loss Associated to High Mortality and Environmental Stress during the Recruitment Stage of a Coral Reef Fish. Coral Reefs 2011, 30, 399–404. [Google Scholar] [CrossRef]
- Leeuwis, R.H.J.; Gamperl, A.K. Adaptations and Plastic Phenotypic Responses of Marine Animals to the Environmental Challenges of the High Intertidal Zone. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2022; pp. 625–679. [Google Scholar]
- Rivera-Ingraham, G.A.; Barri, K.; Boël, M.; Farcy, E.; Charles, A.-L.; Geny, B.; Lignot, J.-H. Osmoregulation and Salinity-Induced Oxidative Stress: Is Oxidative Adaptation Determined by Gill Function? J. Exp. Biol. 2016, 219, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chaiyapechara, S.; Uengwetwanit, T.; Arayamethakorn, S.; Bunphimpapha, P.; Phromson, M.; Jangsutthivorawat, W.; Tala, S.; Karoonuthaisiri, N.; Rungrassamee, W. Understanding the Host-Microbe-Environment Interactions: Intestinal Microbiota and Transcriptomes of Black Tiger Shrimp Penaeus monodon at Different Salinity Levels. Aquaculture 2022, 546, 737371. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Huang, W.-T.; Wu, P.-L.; Kumar, R.; Wang, H.-C.; Lu, H.-P. Low Salinity Stress Increases the Risk of Vibrio parahaemolyticus Infection and Gut Microbiota Dysbiosis in Pacific White Shrimp. BMC Microbiol. 2024, 24, 275. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Q.; Sun, C.; Song, C.; Liu, M.; Zhou, Q.; Zheng, X.; Liu, X. Response of Microbiota and Immune Function to Different Hypotonic Stress Levels in Giant Freshwater Prawn Macrobrachium rosenbergii Post-Larvae. Sci. Total Environ. 2022, 844, 157258. [Google Scholar] [CrossRef]
- Wang, L.Y.; Gu, Y.Y.; Zhang, Z.M.; Sun, A.L.; Shi, X.Z.; Chen, J.; Lu, Y. Contaminant Occurrence, Mobility and Ecological Risk Assessment of Phthalate Esters in the Sediment-Water System of the Hangzhou Bay. Sci. Total Environ. 2021, 770, 144705. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Dittmann, S.R.; Whitfield, A.K.; Withers, K.; Hoeksema, S.D.; Potter, I.C. Hypersalinity: Global Distribution, Causes, and Present and Future Effects on the Biota of Estuaries and Lagoons. In Coasts and Estuaries: The Future; Elsevier: Amsterdam, The Netherlands, 2019; pp. 523–546. [Google Scholar]
- Telesh, I.V.; Khlebovich, V.V. Principal Processes within the Estuarine Salinity Gradient: A Review. Mar. Pollut. Bull. 2010, 61, 149–155. [Google Scholar] [CrossRef]
- Serrano, M.A.; Cobos, M.; Magaña, P.J.; Díez-Minguito, M. Sensitivity of Iberian Estuaries to Changes in Sea Water Temperature, Salinity, River Flow, Mean Sea Level, and Tidal Amplitudes. Estuar. Coast. Shelf Sci. 2020, 236, 106624. [Google Scholar] [CrossRef]
- Slade, J.H.; VanReken, T.M.; Mwaniki, G.R.; Bertman, S.; Stirm, B.; Shepson, P.B. Aerosol Production from the Surface of the Great Lakes. Geophys. Res. Lett. 2010, 37, L18807. [Google Scholar] [CrossRef]
- Abbas, M.S.; Yang, Y.; Zhang, Q.; Guo, D.; Godoi, A.F.L.; Godoi, R.H.M.; Geng, H. Salt Lake Aerosol Overview: Emissions, Chemical Composition and Health Impacts under the Changing Climate. Atmosphere 2024, 15, 212. [Google Scholar] [CrossRef]
- Li, G.; Cheng, L.; Zhu, J.; Trenberth, K.E.; Mann, M.E.; Abraham, J.P. Increasing Ocean Stratification over the Past Half-Century. Nat. Clim. Change 2020, 10, 1116–1123. [Google Scholar] [CrossRef]
- Hughes, D.J.; Alderdice, R.; Cooney, C.; Kühl, M.; Pernice, M.; Voolstra, C.R.; Suggett, D.J. Coral Reef Survival under Accelerating Ocean Deoxygenation. Nat. Clim. Change 2020, 10, 296–307. [Google Scholar] [CrossRef]
- Matela, M.; Obolewski, K. Structural Diagnosis of Benthic Invertebrate Communities in Relation to Salinity Gradient in Baltic Coastal Lake Ecosystems Using Biological Trait Analysis. Sci. Rep. 2022, 12, 12750. [Google Scholar] [CrossRef]
- Stickney, R.R. Tilapia Tolerance of Saline Waters: A Review. Fish-Culturist 1986, 48, 161–167. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of Elevated Temperature on Osmoregulation and Stress Responses in Atlantic Salmon Salmo salar Smolts in Fresh Water and Seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef]
- Ingersoll, C.G.; Dwyer, F.J.; Burch, S.A.; Nelson, M.K.; Buckler, D.R.; Hunn, J.B. The Use of Freshwater and Saltwater Animals to Distinguish between the Toxic Effects of Salinity and Contaminants in Irrigation Drain Water. Environ. Toxicol. Chem. 1992, 11, 503–511. [Google Scholar] [CrossRef]
- Day, J.W.; Crump, B.C.; Kemp, W.M.; Yáñez-Arancibia, A. Introduction to Estuarine Ecology. Estuar. Ecol. 2012, 1–18. [Google Scholar] [CrossRef]
- Iglesias, M.C.-A. A Review of Recent Advances and Future Challenges in Freshwater Salinization. Limnetica 2020, 39, 185–211. [Google Scholar]
- Zhang, C.; Zhou, L.; Ye, J.; Wang, L.; Huang, K.; Zhai, Q. Effects of Acute Salinity Stress on the Serum Osmolality, Serum Ion Concentrations, and ATPase Activity in Gill Filaments of Japanese Seabass (Lateolabrax japonicus) Fed with Diets Containing Different Magnesium Levels. J. Fish. China 2012, 36, 1425. [Google Scholar] [CrossRef]
- Perschbacher, P.W. Historical Use of Tilapia in Intensive Co-culture. In Tilapia in Intensive Co-Culture; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 36–49. [Google Scholar]
- Yue, G.H.; Ma, K.Y.; Xia, J.H. Status of Conventional and Molecular Breeding of Salinity-tolerant Tilapia. Rev. Aquac. 2024, 16, 271–286. [Google Scholar] [CrossRef]
- Getz, E.; Eckert, C. Effects of Salinity on Species Richness and Community Composition in a Hypersaline Estuary. Estuaries Coasts 2023, 46, 2175–2189. [Google Scholar] [CrossRef]
- Balushkina, E.V.; Golubkov, S.M.; Golubkov, M.S.; Litvinchuk, L.F.; Shadrin, N.V. Effect of Abiotic and Biotic Factors on the Structural and Functional Organization of the Saline Lake Ecosystems. Zh. Obshch. Biol. 2009, 70, 504–514. [Google Scholar] [PubMed]
- Golubkov, S.M.; Shadrin, N.V.; Golubkov, M.S.; Balushkina, E.V.; Litvinchuk, L.F. Food Chains and Their Dynamics in Ecosystems of Shallow Lakes with Different Water Salinities. Russ. J. Ecol. 2018, 49, 442–448. [Google Scholar] [CrossRef]
- Mohebbi, F. The Brine Shrimp Artemia and Hypersaline Environments Microalgal Composition: A Mutual Interaction. Int. J. Aquat. Sci. 2010, 1, 19–27. [Google Scholar]
- Saccò, M.; White, N.E.; Harrod, C.; Salazar, G.; Aguilar, P.; Cubillos, C.F.; Meredith, K.; Baxter, B.K.; Oren, A.; Anufriieva, E. Salt to Conserve: A Review on the Ecology and Preservation of Hypersaline Ecosystems. Biol. Rev. 2021, 96, 2828–2850. [Google Scholar] [CrossRef]
- Redón, S.; Vasileva, G.P.; Georgiev, B.B.; Gajardo, G. Exploring Parasites in Extreme Environments of High Conservational Importance: Artemia franciscana (Crustacea: Branchiopoda) as Intermediate Host of Avian Cestodes in Andean Hypersaline Lagoons from Salar de Atacama, Chile. Parasitol. Res. 2020, 119, 3377–3390. [Google Scholar] [CrossRef]
- Strang, C.I.; Bosker, T. The Impact of Climate Change on Marine Mega-Decapod Ranges: A Systematic Literature Review. Fish. Res. 2024, 280, 107165. [Google Scholar] [CrossRef]
- Wu, F.; Xie, Z.; Lan, Y.; Dupont, S.; Sun, M.; Cui, S.; Huang, X.; Huang, W.; Liu, L.; Hu, M.; et al. Short-Term Exposure of Mytilus Coruscus to Decreased PH and Salinity Change Impacts Immune Parameters of Their Haemocytes. Front. Physiol. 2018, 9, 166. [Google Scholar] [CrossRef]
- Kelly, M.W.; DeBiasse, M.B.; Villela, V.A.; Roberts, H.L.; Cecola, C.F. Adaptation to Climate Change: Trade-offs among Responses to Multiple Stressors in an Intertidal Crustacean. Evol. Appl. 2016, 9, 1147–1155. [Google Scholar] [CrossRef]
- Morales-Marín, L.A.; Rokaya, P.; Sanyal, P.R.; Sereda, J.; Lindenschmidt, K.E. Changes in Streamflow and Water Temperature Affect Fish Habitat in the Athabasca River Basin in the Context of Climate Change. Ecol. Modell. 2019, 407, 108718. [Google Scholar] [CrossRef]
- Mitra, A.; Abdel-Gawad, F.K.; Bassem, S.; Barua, P.; Assisi, L.; Parisi, C.; Temraz, T.A.; Vangone, R.; Kajbaf, K.; Kumar, V. Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture. Water 2023, 15, 725. [Google Scholar] [CrossRef]
- Sohaib Ahmed Saqib, H.; Yuan, Y.; Shabi Ul Hassan Kazmi, S.; Li, S.; Zheng, H.; Zhang, Y.; Ikhwanuddin, M.; Ma, H. Salinity Gradients Drove the Gut and Stomach Microbial Assemblages of Mud Crabs (Scylla paramamosain) in Marine Environments. Ecol. Indic. 2023, 151, 110315. [Google Scholar] [CrossRef]
- Shields, J.D. Climate Change Enhances Disease Processes in Crustaceans: Case Studies in Lobsters, Crabs, and Shrimps. J. Crustac. Biol. 2019, 39, 673–683. [Google Scholar] [CrossRef]
- Gibson, D.; Riecke, T.V.; Catlin, D.H.; Hunt, K.L.; Weithman, C.E.; Koons, D.N.; Karpanty, S.M.; Fraser, J.D. Climate Change and Commercial Fishing Practices Codetermine Survival of a Long-lived Seabird. Glob. Change Biol. 2023, 29, 324–340. [Google Scholar] [CrossRef]
- Hakim, M.M.; Ganai, N.A.; Ahmad, S.M.; Asmi, O.; Akram, T.; Hussain, S.; Gora, A.H. Nutrigenomics: Omics Approach in Aquaculture Research to Mitigate the Deficits in Conventional Nutritional Practices. J. Entomol. Zool. Stud. 2018, 6, 582–587. [Google Scholar]
- Yoshizaki, G.; Yazawa, R. Application of Surrogate Broodstock Technology in Aquaculture. Fish. Sci. 2019, 85, 429–437. [Google Scholar] [CrossRef]
- Evans, T.G.; Kültz, D. The Cellular Stress Response in Fish Exposed to Salinity Fluctuations. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion Transporters and Osmoregulation in the Kidney of Teleost Fishes as a Function of Salinity. Front. Physiol. 2021, 12, 664588. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Z.; Zhao, R.; Sun, J.; Hao, P.; Zhang, L.; Wang, X.; Cui, Y.; Liu, F.; Wang, R. Effects of High-Salinity on the Expression of Aquaporins and Ion Transport-Related Genes in Chinese Shrimp (Fenneropenaeus chinensis). Aquac. Rep. 2023, 30, 101577. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Wang, C.; Zhao, N.; Zhang, Z.; Deng, Z.; Cui, Y.; Wang, R.; Li, Y. Aquaporins in Pacific White Shrimp (Litopenaeus vannamei): Molecular Characterization, Expression Patterns, and Transcriptome Analysis in Response to Salinity Stress. Front. Mar. Sci. 2022, 9, 817868. [Google Scholar] [CrossRef]
- Jaffer, Y.D.; Bhat, I.A.; Mir, I.N.; Bhat, R.A.H.; Sidiq, M.J.; Jana, P. Adaptation of Cultured Decapod Crustaceans to Changing Salinities: Physiological Responses, Molecular Mechanisms and Disease Implications. Rev. Aquac. 2024, 16, 1520–1543. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; El-Nab, S.; Awad, E.; Nofal, A.I. DNA Barcoding Identifies a Unique Haplotype of Nile Tilapia Oreochromis niloticus Thriving in Egyptian Freshwater and Brackish Water Lakes. Int. J. Ecotoxicol. Ecobiol. 2017, 2, 172–177. [Google Scholar] [CrossRef]
- Dawood, M.; Madkour, K.; Mugwanya, M.; Kimera, F.; Sewilam, H. Growth Performances, Body Composition, and Blood Variables of Nile Tilapia (Oreochromis niloticus) Juveniles Grown in a Recirculating Aquaculture System under Different Water Salinity Levels. J. Appl. Aquac. 2024, 36, 612–626. [Google Scholar] [CrossRef]
- Losee, J.P.; Palm, D.; Claiborne, A.; Madel, G.; Persson, L.; Quinn, T.P.; Brodin, T.; Hellström, G. Anadromous Trout from Opposite Sides of the Globe: Biology, Ocean Ecology, and Management of Anadromous Brown and Cutthroat Trout. Rev. Fish Biol. Fish. 2024, 34, 461–490. [Google Scholar] [CrossRef]
- Yancey, P.H.; Siebenaller, J.F. Co-Evolution of Proteins and Solutions: Protein Adaptation versus Cytoprotective Micromolecules and Their Roles in Marine Organisms. J. Exp. Biol. 2015, 218, 1880–1896. [Google Scholar] [CrossRef]
- Glover, C.N.; Wood, C.M.; Goss, G.G. Drinking and Water Permeability in the Pacific Hagfish. Eptatretus stoutii. J. Comp. Physiol. B 2017, 187, 1127–1135. [Google Scholar] [CrossRef]
- Herrera, I.; de Carvalho-Souza, G.F.; González-Ortegón, E. Physiological Responses of the Invasive Blue Crabs Callinectes Sapidus to Salinity Variations: Implications for Adaptability and Invasive Success. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 297, 111709. [Google Scholar] [CrossRef]
- Gutiérrez, J.S. Living in Environments with Contrasting Salinities: A Review of Physiological and Behavioural Responses in Waterbirds. Ardeola 2014, 61, 233–256. [Google Scholar] [CrossRef]
- Scarlett, K.R.; Lovin, L.M.; Steele, W.B.; Kim, S.; Brooks, B.W. Identifying Behavioral Response Profiles of Two Common Larval Fish Models to a Salinity Gradient. Arch. Environ. Contam. Toxicol. 2022, 83, 180–192. [Google Scholar] [CrossRef]
- Tietze, S.M.; Gerald, G.W. Trade-Offs between Salinity Preference and Antipredator Behaviour in the Euryhaline Sailfin Molly Poecilia latipinna. J. Fish Biol. 2016, 88, 1918–1931. [Google Scholar] [CrossRef]
- Chow, S.C.; Ching, L.Y.; Wong, A.M.F.; Wong, C.K.C. Cloning and Regulation of Expression of the Na+-Cl−-Taurine Transporter in Gill Cells of Freshwater Japanese Eels. J. Exp. Biol. 2009, 212, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Marshall, W.S. Principles and Patterns of Osmoregulation and Euryhalinity in Fishes. Fish Physiol. 2012, 32, 1–44. [Google Scholar]
- Breves, J.P.; Hasegawa, S.; Yoshioka, M.; Fox, B.K.; Davis, L.K.; Lerner, D.T.; Takei, Y.; Hirano, T.; Grau, E.G. Acute Salinity Challenges in Mozambique and Nile Tilapia: Differential Responses of Plasma Prolactin, Growth Hormone and Branchial Expression of Ion Transporters. Gen. Comp. Endocrinol. 2010, 167, 135–142. [Google Scholar] [CrossRef]
- Yang, Y.; Ni, J.; Niu, D.; Zheng, G.; Li, Y. Physiological Response of the Razor Clam Sinonovacula constricta Exposed to Hyposalinity Stress. Aquac. Fish. 2024, 9, 663–673. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, L.; Zhao, X.; Cheng, Y. Effects of Salinity Stress on Osmotic Pressure, Free Amino Acids, and Immune-Associated Parameters of the Juvenile Chinese Mitten Crab. Eriocheir sinensis. Aquaculture 2022, 549, 737776. [Google Scholar] [CrossRef]
- Zikos, A.; Seale, A.P.; Lerner, D.T.; Grau, E.G.; Korsmeyer, K.E. Effects of Salinity on Metabolic Rate and Branchial Expression of Genes Involved in Ion Transport and Metabolism in Mozambique Tilapia (Oreochromis mossambicus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 178, 121–131. [Google Scholar] [CrossRef]
- Scott, G.R.; Richards, J.G.; Forbush, B.; Isenring, P.; Schulte, P.M. Changes in Gene Expression in Gills of the Euryhaline Killifish Fundulus heteroclitus after Abrupt Salinity Transfer. Am. J. Physiol.—Cell Physiol. 2004, 287, C300–C309. [Google Scholar] [CrossRef]
- Zhou, T.; Meng, Q.; Sun, R.; Xu, D.; Zhu, F.; Jia, C.; Zhou, S.; Chen, S.; Yang, Y. Structure and Gene Expression Changes of the Gill and Liver in Juvenile Black Porgy (Acanthopagrus schlegelii) under Different Salinities. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 50, 101228. [Google Scholar] [CrossRef]
- Anufriieva, E.V.; Shadrin, N.V. General Patterns of Salinity Influence on the Energy Balance of Aquatic Animals in Hypersaline Environment. Biol. Bull. Rev. 2023, 13, 420–430. [Google Scholar] [CrossRef]
- Hasan-Nataj-Niazi, E.; Agh, N.; Noori, F.; Atashbar, B.; Van Stappen, G. Substitution of Microalgae by Bioflocs as a Food Source for the Brine Shrimp Artemia Franciscana. Aquac. Res. 2022, 53, 4374–4387. [Google Scholar] [CrossRef]
- Ern, R.; Esbaugh, A.J. Effects of Salinity and Hypoxia-Induced Hyperventilation on Oxygen Consumption and Cost of Osmoregulation in the Estuarine Red Drum (Sciaenops ocellatus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 222, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Moss, W.E.; Olson, N.; Chau, K.F.; Chang, Y.; Johnson, K.E. Feasting in Fresh Water: Impacts of Food Concentration on Freshwater Tolerance and the Evolution of Food× Salinity Response during the Expansion from Saline into Fresh Water Habitats. Evol. Appl. 2013, 6, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Waqas, W.; Yuan, Y.; Ali, S.; Zhang, M.; Shafiq, M.; Ali, W.; Chen, Y.; Xiang, Z.; Chen, R.; Ikhwanuddin, M.; et al. Toxic Effects of Heavy Metals on Crustaceans and Associated Health Risks in Humans: A Review. Environ. Chem. Lett. 2024, 22, 1391–1411. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Anwar, T.N.; Das, K.; Paital, B. Influence of Anthropogenic Activities on Redox Regulation and Oxidative Stress Responses in Different Phyla of Animals in Coastal Water via Changing in Salinity. Water 2022, 14, 4026. [Google Scholar] [CrossRef]
- Lal, M.; Kumari, A.; Pooja; Sheokand, S. Reactive Oxygen Species, Reactive Nitrogen Species and Oxidative Metabolism under Waterlogging Stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Wiley: Hoboken, NJ, USA, 2019; pp. 777–812. [Google Scholar]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Antioxidant Defenses and Oxidative Stress Parameters in Tissues of Mud Crab (Scylla serrata) with Reference to Changing Salinity. Comp. Biochem. Physiol.—C Toxicol. Pharmacol. 2010, 151, 142–151. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Y.; Tang, Y.; Zhu, F.; Jiang, J.; Zhou, J.; Li, Q.; Meng, Q.; Zhang, Z. Effects of Different Salinity Reduction Intervals on Osmoregulation, Anti-Oxidation and Apoptosis of Eriocheir sinensis Megalopa. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 291, 111593. [Google Scholar] [CrossRef]
- Lee, M.-C.; Park, J.C.; Kim, D.-H.; Kang, S.; Shin, K.-H.; Park, H.G.; Han, J.; Lee, J.-S. Interrelationship of Salinity Shift with Oxidative Stress and Lipid Metabolism in the Monogonont Rotifer Brachionus Koreanus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 214, 79–84. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Delisle, L.; Petton, B.; Corporeau, C.; Pernet, F. Metabolism of the Pacific Oyster, Crassostrea gigas, Is Influenced by Salinity and Modulates Survival to the Ostreid Herpesvirus OsHV-1. Biol. Open 2018, 7, bio028134. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.N.; Wang, A.L.; Wang, J.M.; Sun, R.Y. Effects of Dietary Vitamin E Supplementation on Antioxidant Enzyme Activities in Litopenaeus vannamei (Boone, 1931) Exposed to Acute Salinity Changes. Aquaculture 2007, 265, 351–358. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Q.; Zhang, D.M.; Lei, X.Y.; Wang, S.; Wan, J.W.; Liu, H.J.; Chen, Y.K.; Zhao, Y.L.; Wang, G.Q.; et al. Effects of Acute Salinity Stress on Osmoregulation, Antioxidant Capacity and Physiological Metabolism of Female Chinese Mitten Crabs (Eriocheir sinensis). Aquaculture 2022, 552, 737989. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.M.; Xu, W.B.; Chen, D.Y.; Li, B.W.; Cheng, Y.X.; Guo, X.L.; Dong, W.R.; Shu, M.A. The Effects of Salinities Stress on Histopathological Changes, Serum Biochemical Index, Non-Specific Immune and Transcriptome Analysis in Red Swamp Crayfish Procambarus clarkii. Sci. Total Environ. 2022, 840, 156502. [Google Scholar] [CrossRef] [PubMed]
- Boonsanit, P.; Pairohakul, S. Effects of Salinity on Haemolymph Osmolality, Gill Na+/K+ ATPase and Antioxidant Enzyme Activities in the Male Mud Crab Scylla olivacea (Herbst, 1796). Mar. Biol. Res. 2021, 17, 86–97. [Google Scholar] [CrossRef]
- Mozanzadeh, M.T.; Safari, O.; Oosooli, R.; Mehrjooyan, S.; Najafabadi, M.Z.; Hoseini, S.J.; Saghavi, H.; Monem, J. The Effect of Salinity on Growth Performance, Digestive and Antioxidant Enzymes, Humoral Immunity and Stress Indices in Two Euryhaline Fish Species: Yellowfin Seabream (Acanthopagrus latus) and Asian Seabass (Lates calcarifer). Aquaculture 2021, 534, 736329. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Long Term Salinity Disrupts the Hepatic Function, Intestinal Health, and Gills Antioxidative Status in Nile Tilapia Stressed with Hypoxia. Ecotoxicol. Environ. Saf. 2021, 220, 112412. [Google Scholar] [CrossRef]
- Chang, C.H.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.Y.; Lorin-Nebel, C.; Lee, T.H. Effects of Temperature and Salinity on Antioxidant Responses in Livers of Temperate (Dicentrarchus labrax) and Tropical (Chanos chanos) Marine Euryhaline Fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Alkafafy, M.; Sewilam, H. The Antioxidant Responses of Gills, Intestines and Livers and Blood Immunity of Common Carp (Cyprinus carpio) Exposed to Salinity and Temperature Stressors. Fish Physiol. Biochem. 2022, 48, 397–408. [Google Scholar] [CrossRef]
- Park, M.S.; Shin, H.S.; Choi, C.Y.; Kim, N.N.; Park, D.W.; Kil, G.S.; Lee, J. Effect of Hypoosmotic and Thermal Stress on Gene Expression and the Activity of Antioxidant Enzymes in the Cinnamon Clownfish, Amphiprion melanopus. Anim. Cells Syst. 2011, 15, 219–225. [Google Scholar] [CrossRef]
- Andreyeva, A.; Gostyukhina, O.; Gavruseva, T.; Sigacheva, T.; Tkachuk, A.; Podolskaya, M.; Chelebieva, E.; Kladchenko, E. Mediterranean Mussels (Mytilus galloprovincialis) Under Salinity Stress: Effects on Antioxidant Capacity and Gill Structure. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2024, 343, 184–196. [Google Scholar] [CrossRef]
- Bayir, A.; Sirkecioglu, A.N.; Bayir, M.; Haliloglu, H.I.; Kocaman, E.M.; Aras, N.M. Metabolic Responses to Prolonged Starvation, Food Restriction, and Refeeding in the Brown Trout, Salmo trutta: Oxidative Stress and Antioxidant Defenses. Comp. Biochem. Physiol.—B Biochem. Mol. Biol. 2011, 159, 191–196. [Google Scholar] [CrossRef]
- Xu, Z.; Gan, L.; Li, T.; Xu, C.; Chen, K.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Transcriptome Profiling and Molecular Pathway Analysis of Genes in Association with Salinity Adaptation in Nile Tilapia Oreochromis niloticus. PLoS ONE 2015, 10, e0136506. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Q.; Jin, S.; Huang, B.; Wang, Z.; Chen, G. Identification of Mapk Genes, and Their Expression Profiles in Response to Low Salinity Stress, in Cobia (Rachycentron canadum). Comp. Biochem. Physiol. Part—B Biochem. Mol. Biol. 2024, 271, 110950. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, W.; Chen, X.; Wang, M.; Zhong, L.; Liu, J.; Bian, W.; Zhang, S. Genome-Wide Identification and Expression Analysis of Mitogen-Activated Protein Kinase (MAPK) Genes in Response to Salinity Stress in Channel Catfish (Ictalurus punctatus). J. Fish Biol. 2022, 101, 972–984. [Google Scholar] [CrossRef]
- Chu, P.; Luo, S.; Wang, H.; Zhang, K.; Wen, X.; Yin, S.; Wang, T. Interactive Effects of Temperature and Salinity on the Apoptosis, Antioxidant Enzymes, and MAPK Signaling Pathway of Juvenile Pufferfish (Takifugu fasciatus). Aquac. Rep. 2023, 29, 101483. [Google Scholar] [CrossRef]
- Jin, M.; Luo, J.; Zhu, T.; Fang, F.; Xie, S.; Lu, J.; Betancor, M.B.; Tocher, D.R.; Zhou, Q. Examination of Role of the AMP-Activated Protein Kinase (Ampk) Signaling Pathway during Low Salinity Adaptation in the Mud Crab, Scylla paramamosain, with Reference to Glucolipid Metabolism. Aquaculture 2024, 593, 741329. [Google Scholar] [CrossRef]
- Cui, W.; Ma, A.; Wang, X. Response of the PI3K-AKT Signalling Pathway to Low Salinity and the Effect of Its Inhibition Mediated by Wortmannin on Ion Channels in Turbot Scophthalmus maximus. Aquac. Res. 2020, 51, 2676–2686. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Xu, Z.; Wang, S.; Chen, K.; Wang, X.; Li, T.; Qin, J.G.; Chen, L. Molecular Characterization and Expression of AMP-Activated Protein Kinase in Response to Low-Salinity Stress in the Pacific White Shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. Part—B Biochem. Mol. Biol. 2016, 198, 79–90. [Google Scholar] [CrossRef]
- Farhadi, A.; Liu, Y.; Xu, C.; Wang, X.; Li, E. The Role of the Renin-Angiotensin System (RAS) in Salinity Adaptation in Pacific White Shrimp (Litopenaeus vannamei). Front. Endocrinol. 2022, 13, 1089419. [Google Scholar] [CrossRef]
- Wang, H.; Tang, L.; Wei, H.; Lu, J.; Mu, C.; Wang, C. Transcriptomic Analysis of Adaptive Mechanisms in Response to Sudden Salinity Drop in the Mud Crab, Scylla paramamosain. BMC Genom. 2018, 19, 421. [Google Scholar] [CrossRef]
- Li, Y.D.; Si, M.R.; Jiang, S.G.; Yang, Q.B.; Jiang, S.; Yang, L.S.; Huang, J.H.; Chen, X.; Zhou, F.L.; Li, E.C. Transcriptome and Molecular Regulatory Mechanisms Analysis of Gills in the Black Tiger Shrimp Penaeus monodon under Chronic Low-Salinity Stress. Front. Physiol. 2023, 14, 1118341. [Google Scholar] [CrossRef]
- Luo, Z.; Huang, W.; Wang, G.; Sun, H.; Chen, X.; Luo, P.; Liu, J.; Hu, C.; Li, H.; Shu, H. Identification and Characterization of P38MAPK in Response to Acute Cold Stress in the Gill of Pacific White Shrimp (Litopenaeus vannamei). Aquac. Rep. 2020, 17, 100365. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, X.W.; Zechen, E.; Liu, Y.; Chen, S. Genome-Wide Identification of the MAPK Gene Family in Turbot and Its Involvement in Abiotic and Biotic Stress Responses. Front. Mar. Sci. 2022, 9, 1005401. [Google Scholar] [CrossRef]
- Lee, K.; Eun, H.S.; Ho, S.K.; Jae, H.Y.; Hay, J.H.; Mi, S.J.; Sang, M.L.; Kyung, E.K.; Min, C.K.; Moo, J.C.; et al. Regulation of MAPK Phosphatase 1 (AtMKP1) by Calmodulin in Arabidopsis. J. Biol. Chem. 2008, 283, 23581–23588. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wen, H.; Qi, X.; Zhang, X.; Li, Y. Identification of Mapk Gene Family in Lateolabrax maculatus and Their Expression Profiles in Response to Hypoxia and Salinity Challenges. Gene 2019, 684, 20–29. [Google Scholar] [CrossRef]
- Tao, S.; Li, X.; Wang, J.; Bai, Y.; Wang, J.; Yang, Y.; Zhao, Z. Examination of the Relationship of Carbonate Alkalinity Stress and Ammonia Metabolism Disorder-Mediated Apoptosis in the Chinese Mitten Crab, Eriocheir sinensis: Potential Involvement of the ROS/MAPK Signaling Pathway. Aquaculture 2024, 579, 740179. [Google Scholar] [CrossRef]
- Gao, G.; Liang, G.; Wei, H.; Li, X.; Chen, Y.; Wang, H.; Wang, C.; Mu, C.; Liu, S. Involvement of the JNK Signaling Pathway in Regulating Yolk Accumulation in the Swimming Crab, Portunus trituberculatus. Aquaculture 2022, 551, 737890. [Google Scholar] [CrossRef]
- Yu, J.S.L.; Cui, W. Proliferation, Survival and Metabolism: The Role of PI3K/AKT/MTOR Signalling in Pluripotency and Cell Fate Determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Le, O.T.T.; Nguyen, T.T.N.; Lee, S.Y. Phosphoinositide Turnover in Toll-like Receptor Signaling and Trafficking. BMB Rep. 2014, 47, 361–368. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Chu, K.-F.; Yang, W.-K.; Lee, T.-H. Na+, K+-ATPase Β1 Subunit Associates with A1 Subunit Modulating a “Higher-NKA-in-Hyposmotic Media” Response in Gills of Euryhaline Milkfish, Chanos chanos. J. Comp. Physiol. B 2017, 187, 995–1007. [Google Scholar] [CrossRef]
- Liu, Y.; Li, E.; Xu, C.; Su, Y.; Qin, J.G.; Chen, L.; Wang, X. Brain Transcriptome Profiling Analysis of Nile Tilapia (Oreochromis niloticus) under Long-Term Hypersaline Stress. Front. Physiol. 2018, 9, 219. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, E.; Li, T.; Xu, C.; Wang, X.; Lin, H.; Qin, J.G.; Chen, L. Transcriptome and Molecular Pathway Analysis of the Hepatopancreas in the Pacific White Shrimp Litopenaeus vannamei under Chronic Low-Salinity Stress. PLoS ONE 2015, 10, e0131503. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Nie, M.; Wang, L.; Xiong, Y.; Wang, F.; Wang, L.; Xiao, P.; Wu, Z.; Liu, Y.; You, F. Energy Response and Modulation of AMPK Pathway of the Olive Flounder Paralichthys olivaceus in Low-Temperature Challenged. Aquaculture 2018, 484, 205–213. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, L.; Jiang, W.; Liu, Y.; Jiang, J.; Kuang, S.; Li, S.; Tang, L.; Tang, W.; Zhou, X.; et al. Dietary Vitamin A Improved the Flesh Quality of Grass Carp (Ctenopharyngodon idella) in Relation to the Enhanced Antioxidant Capacity through Nrf2/Keap 1a Signaling Pathway. Antioxidants 2022, 11, 148. [Google Scholar] [CrossRef]
- Misra, J.R.; Irvine, K.D. The Hippo Signaling Network and Its Biological Functions. Annu. Rev. Genet. 2018, 52, 65–87. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.-A.; Geng, R.; Deng, H.; Niu, S.; Zuo, H.; Weng, S.; He, J.; Xu, X. White Spot Syndrome Virus (WSSV) Inhibits Hippo Signaling and Activates Yki To Promote Its Infection in Penaeus vannamei. Microbiol. Spectr. 2023, 11, e0236322. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Cheng, X.; Li, W.; Lyu, S.; Chen, X.; Li, Q.; Wang, H. Molecular Evolution of Transforming Growth Factor-β (TGF-β) Gene Family and the Functional Characterization of Lamprey TGF-Β2. Front. Immunol. 2022, 13, 836226. [Google Scholar] [CrossRef]
- Riley, S.E.; Feng, Y.; Hansen, C.G. Hippo-Yap/Taz Signalling in Zebrafish Regeneration. npj Regen. Med. 2022, 7, 9. [Google Scholar] [CrossRef]
- Huang, D.; Li, X.; Sun, L.; Huang, P.; Ying, H.; Wang, H.; Wu, J.; Song, H. Regulation of Hippo Signalling by P38 Signalling. J. Mol. Cell Biol. 2016, 8, 328–337. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.-L. Mechanisms of Hippo Pathway Regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wang, G.; Lin, S.; Zeng, X.; Wang, Y.; Zhang, Z. PI3K-AKT Signaling Pathway Is Involved in Hypoxia/Thermal-Induced Immunosuppression of Small Abalone Haliotis diversicolor. Fish Shellfish Immunol. 2016, 59, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, F.; Fan, H.; Jiang, S.; Yang, Q.; Huang, J.; Yang, L.; Chen, X.; Zhang, W.; Jiang, S. Molecular Technology for Isolation and Characterization of Mitogen-Activated Protein Kinase Kinase 4 from Penaeus monodon, and the Response to Bacterial Infection and Low-Salinity Challenge. J. Mar. Sci. Eng. 2022, 10, 1642. [Google Scholar] [CrossRef]
- Li, X.; Shen, Y.; Bao, Y.; Wu, Z.; Yang, B.; Jiao, L.; Zhang, C.; Tocher, D.R.; Zhou, Q.; Jin, M. Physiological Responses and Adaptive Strategies to Acute Low-Salinity Environmental Stress of the Euryhaline Marine Fish Black Seabream (Acanthopagrus schlegelii). Aquaculture 2022, 554, 738117. [Google Scholar] [CrossRef]
- Shang, X.; Xu, W.; Zhang, Y.; Sun, Q.; Li, Z.; Geng, L.; Teng, X. Transcriptome Analysis Revealed the Mechanism of Luciobarbus capito (L. capito) Adapting High Salinity: Antioxidant Capacity, Heat Shock Proteins, Immunity. Mar. Pollut. Bull. 2023, 192, 115017. [Google Scholar] [CrossRef]
- Accili, D.; Arden, K.C. FoxOs at the Crossroads of Cellular Metabolism, Differentiation, and Transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef]
- Birrer, S.C.; Reusch, T.B.H.; Roth, O. Salinity Change Impairs Pipefish Immune Defence. Fish Shellfish Immunol. 2012, 33, 1238–1248. [Google Scholar] [CrossRef]
- Ching, B.; Chen, X.L.; Yong, J.H.A.; Wilson, J.M.; Hiong, K.C.; Sim, E.W.L.; Wong, W.P.; Lam, S.H.; Chew, S.F.; Ip, Y.K. Increases in Apoptosis, Caspase Activity and Expression of P53 and Bax, and the Transition between Two Types of Mitochondrion-Rich Cells, in the Gills of the Climbing Perch, Anabas testudineus, during a Progressive Acclimation from Freshwater to Seawater. Front. Physiol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, L.; Zhang, L.; Hao, P.; Wang, X.; Wang, B.; Song, G.; Cui, Y.; Liu, F.; Wang, R. RNAi-Mediated Knockdown of the Aquaporin 4 Gene Impairs Salinity Tolerance and Delays the Molting Process in Pacific White Shrimp, Litopenaeus vannamei. Aquac. Rep. 2024, 35, 101974. [Google Scholar] [CrossRef]
- Zhou, J.; Li, N.; Wang, H.; Mu, C.; Wang, C. Transcriptome Analysis Reveals Hepatopancreatic Genes and Pathways Associated with Metabolism Responding to Water Salinities during the Overwintering of Mud Crab Scylla paramamosain. Aquac. Res. 2021, 52, 3212–3225. [Google Scholar] [CrossRef]
- Esmaeili, N.; Ma, H.; Kadri, S.; Tocher, D.R. Protein and Lipid Nutrition in Crabs. Rev. Aquac. 2024, 16, 1499–1519. [Google Scholar] [CrossRef]
- Whitehead, A.; Roach, J.L.; Zhang, S.; Galvez, F. Salinity- and Population-Dependent Genome Regulatory Response during Osmotic Acclimation in the Killifish (Fundulus heteroclitus) Gill. J. Exp. Biol. 2012, 215, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Makvandi-Nejad, S.; Moghadam, H. Genetics and Epigenetics in Aquaculture Breeding. In Epigenetics in Aquaculture; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 439–449. [Google Scholar]
- Guo, J.; Lv, J.; Sun, D.; Liu, P.; Gao, B. Elucidating the Role of DNA Methylation in Low-Salinity Adaptation of the Swimming Crab Portunus Trituberculatus. Aquaculture 2024, 595, 741707. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Kong, L.; Yu, H. DNA Methylation Changes Detected by Methylation-Sensitive Amplified Polymorphism in the Pacific Oyster (Crassostrea gigas) in Response to Salinity Stress. Genes Genom. 2017, 39, 1173–1181. [Google Scholar] [CrossRef]
- Jeremias, G.; Barbosa, J.; Marques, S.M.; De Schamphelaere, K.A.C.; Van Nieuwerburgh, F.; Deforce, D.; Gonçalves, F.J.M.; Pereira, J.L.; Asselman, J. Transgenerational Inheritance of DNA Hypomethylation in Daphnia magna in Response to Salinity Stress. Environ. Sci. Technol. 2018, 52, 10114–10123. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, D.-H.; Lee, J.-S. A Review of Environmental Epigenetics in Aquatic Invertebrates. Mar. Pollut. Bull. 2024, 208, 117011. [Google Scholar] [CrossRef]
- Pierron, F.; Lorioux, S.; Héroin, D.; Daffe, G.; Etcheverria, B.; Cachot, J.; Morin, B.; Dufour, S.; Gonzalez, P. Transgenerational Epigenetic Sex Determination: Environment Experienced by Female Fish Affects Offspring Sex Ratio. Environ. Pollut. 2021, 277, 116864. [Google Scholar] [CrossRef]
- Heckwolf, M.J.; Meyer, B.S.; Döring, T.; Eizaguirre, C.; Reusch, T.B.H. Transgenerational Plasticity and Selection Shape the Adaptive Potential of Sticklebacks to Salinity Change. Evol. Appl. 2018, 11, 1873–1885. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.; Zhou, T.; Li, Q.; Lin, Z. Genome-Wide Methylome and Transcriptome Dynamics Provide Insights into Epigenetic Regulation of Kidney Functioning of Large Yellow Croaker (Larimichthys crocea) during Low-Salinity Adaption. Aquaculture 2023, 571, 739410. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Liu, Y.; Qiao, F.; Chen, L.; Liu, W.T.; Du, Z.; Li, E. Response of Gut Microbiota to Salinity Change in Two Euryhaline Aquatic Animals with Reverse Salinity Preference. Aquaculture 2016, 454, 72–80. [Google Scholar] [CrossRef]
- Schmidt, V.T.; Smith, K.F.; Melvin, D.W.; Amaral-Zettler, L.A. Community Assembly of a Euryhaline Fish Microbiome during Salinity Acclimation. Mol. Ecol. 2015, 24, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, X.; Wu, X.; Yang, D. Temperature Acclimation Alters the Thermal Tolerance and Intestinal Heat Stress Response in a Tibetan Fish Oxygymnocypris stewarti. Front. Microbiol. 2022, 13, 898145. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tan, P.; Yang, L.; Zhu, W.; Xu, D. Effects of Salinity on the Growth, Plasma Ion Concentrations, Osmoregulation, Non-Specific Immunity, and Intestinal Microbiota of the Yellow Drum (Nibea albiflora). Aquaculture 2020, 528, 735470. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Nie, Z.; Wang, J.; Sun, Y.; Xu, G. Integration of Metagenome and Metabolome Analysis Reveals the Correlation of Gut Microbiota, Oxidative Stress, and Inflammation in Coilia nasus under Air Exposure Stress and Salinity Mitigation. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101175. [Google Scholar] [CrossRef]
- Sun, S.; Gong, C.; Deng, C.; Yu, H.; Zheng, D.; Wang, L.; Sun, J.; Song, F.; Luo, J. Effects of Salinity Stress on the Growth Performance, Health Status, and Intestinal Microbiota of Juvenile Micropterus salmoides. Aquaculture 2023, 576, 739888. [Google Scholar] [CrossRef]
- Dehler, C.E.; Secombes, C.J.; Martin, S.A.M. Seawater Transfer Alters the Intestinal Microbiota Profiles of Atlantic Salmon (Salmo salar L.). Sci. Rep. 2017, 7, 13877. [Google Scholar] [CrossRef]
- Kim, J.A.; Park, Y.-S.; Kim, J.-H.; Choi, C.Y. Hyposalinity Elicits Physiological Responses and Alters Intestinal Microbiota in Korean Rockfish Sebastes schlegelii. Fish Physiol. Biochem. 2024, 50, 2315–2326. [Google Scholar] [CrossRef]
- Lai, K.P.; Boncan, D.A.T.; Qin, X.; Chan, T.F.; Tse, W.K.F. Roles and Occurrences of Microbiota in the Osmoregulatory Organs, Gills and Gut, in Marine Medaka upon Hypotonic Stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101285. [Google Scholar] [CrossRef]
- Mkulo, E.M.; Iddrisu, L.; Zhong, J.; Zheng, A.; Jin, M.; Wang, L.; Mpwaga, A.Y.; Zhang, H.; Tang, B.; Zhou, H. Exploring Salinity Adaptation in Teleost Fish, Focusing on Omics Perspectives on Osmoregulation and Gut Microbiota. Front. Mar. Sci. 2025, 12, 1559871. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase System and Its Role in Shrimp Immune Responses against Major Pathogens. Fish Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Hansson, G.C. Immunological Aspects of Intestinal Mucus and Mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zheng, M.; Li, S.; Xie, J.; Fang, W.; Gao, D.; Huang, J.; Lu, J. Response of Gut Microbiota and Immune Function to Hypoosmotic Stress in the Yellowfin Seabream (Acanthopagrus latus). Sci. Total Environ. 2020, 745, 140976. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.K.; Antony, L.; Avunje, S.; Viswanathan, B.; Lalitha, N.; Jangam, A.K.; Kumar, D.; Solanki, H.G.; Reddy, M.A.; Alavandi, S.V. Bioaugmentation with Nitrifying and Denitrifying Microbial Consortia for Mitigation of Nitrogenous Metabolites in Shrimp Ponds. Aquaculture 2021, 541, 736819. [Google Scholar] [CrossRef]
- Champion, C.; Broadhurst, M.K.; Ewere, E.E.; Benkendorff, K.; Butcherine, P.; Wolfe, K.; Coleman, M.A. Resilience to the Interactive Effects of Climate Change and Discard Stress in the Commercially Important Blue Swimmer Crab (Portunus armatus). Mar. Environ. Res. 2020, 159, 105009. [Google Scholar] [CrossRef]
- Pedrosa, J.A.M.; Cocchiararo, B.; Bordalo, M.D.; Rodrigues, A.C.M.; Soares, A.M.V.M.; Barata, C.; Nowak, C.; Pestana, J.L.T. The Role of Genetic Diversity and Past-History Selection Pressures in the Susceptibility of Chironomus riparius Populations to Environmental Stress. Sci. Total Environ. 2017, 576, 807–816. [Google Scholar] [CrossRef]
- Asem, A.; Eimanifar, A.; Van Stappen, G.; Sun, S.C. The Impact of One-Decade Ecological Disturbance on Genetic Changes: A Study on the Brine Shrimp Artemia urmiana from Urmia Lake, Iran. PeerJ 2019, 7, e7190. [Google Scholar] [CrossRef]
- Pauls, S.U.; Nowak, C.; Bálint, M.; Pfenninger, M. The Impact of Global Climate Change on Genetic Diversity within Populations and Species. Mol. Ecol. 2013, 22, 925–946. [Google Scholar] [CrossRef]
- Laurenzano, C.; Costa, T.M.; Schubart, C.D. Contrasting Patterns of Clinal Genetic Diversity and Potential Colonization Pathways in Two Species of Western Atlantic Fiddler Crabs. PLoS ONE 2016, 11, e0166518. [Google Scholar] [CrossRef][Green Version]
- Peres, P.A.; Mantelatto, F.L. Salinity Tolerance Explains the Contrasting Phylogeographic Patterns of Two Swimming Crabs Species along the Tropical Western Atlantic. Evol. Ecol. 2020, 34, 589–609. [Google Scholar] [CrossRef]
- Geletu, T.T.; Zhao, J. Genetic Resources of Nile Tilapia (Oreochromis niloticus linnaeus, 1758) in Its Native Range and Aquaculture. Hydrobiologia 2023, 850, 2425–2445. [Google Scholar] [CrossRef]
- Sunde, J.; Tamario, C.; Tibblin, P.; Larsson, P.; Forsman, A. Erratum: Author Correction: Variation in Salinity Tolerance between and within Anadromous Subpopulations of Pike (Esox lucius). Sci. Rep. 2018, 8, 6813. [Google Scholar]
- Talukdar, A.; Dharmendra Deo, A.; Prasad Sahu, N.; Sardar, P.; Aklakur, M.; Harikrishna, V.; Prakash, S.; Shamna, N.; Jana, P. Effects of Different Levels of Dietary Protein on the Growth Performance, Nutrient Utilization, Digestive Enzymes and Physiological Status of White Shrimp, Litopenaeus vannamei Juveniles Reared in Inland Saline Water. Aquac. Nutr. 2021, 27, 77–90. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, F.J.; Liu, Y.J.; Tian, L.X.; Zhang, Z.H. Dietary Tryptophan Requirements of Juvenile Pacific White Shrimp, Litopenaeus vannamei (Boone) Reared in Low-Salinity Water. Aquac. Int. 2017, 25, 955–968. [Google Scholar] [CrossRef]
- Xie, S.; Wei, D.; Fang, W.; Wan, M.; Guo, T.; Liu, Y.; Yin, P.; Tian, L.; Niu, J. Optimal Dietary Lipid Requirement of Postlarval White Shrimp, Litopenaeus vannamei in Relation to Growth Performance, Stress Tolerance and Immune Response. Aquac. Nutr. 2019, 25, 1231–1240. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, W.; Tan, B.; Wang, F.; Chi, S.; Dong, X.; Zhang, H.; Liu, H.; Zhang, S. Effects of Dietary N-3HUFA on Juvenile White Shrimp, Litopenaeus vannamei: Growth, Feed Utilization, Antioxidant Enzymes Activities and Fatty Acid Compositions. Aquac. Res. 2019, 50, 882–894. [Google Scholar] [CrossRef]
- Wen, M.; Liu, Y.J.; Tian, L.X.; Wang, S. Vitamin D3 Requirement in Practical Diet of White Shrimp, Litopenaeus vannamei, at Low Salinity Rearing Conditions. J. World Aquac. Soc. 2015, 46, 531–538. [Google Scholar] [CrossRef]
- Ebadi, H.; Zakeri, M.; Mousavi, S.M.; Yavari, V.; Souri, M. The Interaction Effects of Dietary Lipid, Vitamin E and Vitamin C on Growth Performance, Feed Utilization, Muscle Proximate Composition and Antioxidant Enzyme Activity of White Leg Shrimp (Litopenaeus vannamei). Aquac. Res. 2021, 52, 2048–2060. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress Management in Aquaculture: A Review of Dietary Interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.J.; Tian, L.X.; Yang, H.J.; Liang, G.Y.; Yue, Y.R.; Xu, D.H. Effects of Dietary Astaxanthin on Growth, Antioxidant Capacity and Gene Expression in Pacific White Shrimp Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 917–927. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Yuan, Y.; Cui, W.; Xiang, Z.; Ye, S.; Ikhwanuddin, M.; Ma, H. Impact and Accumulation of Calcium on Soft-Shell Mud Crab Scylla paramamosain in Recirculating Aquaculture System. Aquaculture 2024, 593, 741323. [Google Scholar] [CrossRef]
- Hossain, M.S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Sony, N.M. Dietary Nucleotide Administration Influences Growth, Immune Responses and Oxidative Stress Resistance of Juvenile Red Sea Bream (Pagrus major). Aquaculture 2016, 455, 41–49. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, H.; Wang, L.; Zhou, Y.; Gabr, H.R. Effects of Salinity on Energy Budget in Pond-Cultured Sea Cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture 2010, 306, 348–351. [Google Scholar] [CrossRef]
- Lund, I.; Skov, P.V.; Hansen, B.W. Dietary Supplementation of Essential Fatty Acids in Larval Pikeperch (Sander Lucioperca); Short and Long Term Effects on Stress Tolerance and Metabolic Physiology. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2012, 162, 340–348. [Google Scholar] [CrossRef]
- Kamalam, B.S.; Medale, F.; Panserat, S. Utilisation of Dietary Carbohydrates in Farmed Fishes: New Insights on Influencing Factors, Biological Limitations and Future Strategies. Aquaculture 2017, 467, 3–27. [Google Scholar] [CrossRef]
- Yousefi, M.; Paktinat, M.; Mahmoudi, N.; Pérez-Jiménez, A.; Hoseini, S.M. Serum Biochemical and Non-Specific Immune Responses of Rainbow Trout (Oncorhynchus mykiss) to Dietary Nucleotide and Chronic Stress. Fish Physiol. Biochem. 2016, 42, 1417–1425. [Google Scholar] [CrossRef]
- Palermo, F.A.; Cardinaletti, G.; Cocci, P.; Tibaldi, E.; Polzonetti-Magni, A.; Mosconi, G. Effects of Dietary Nucleotides on Acute Stress Response and Cannabinoid Receptor 1 MRNAs in Sole, Solea solea. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 477–482. [Google Scholar] [CrossRef]
- Fu, M.; Collins, S.A.; Anderson, D.M. Maxi-GenTM Plus: A Nucleotide-Containing Product That Reduces Stress Indicators and Improves Growth Performance during Smoltification in Atlantic Salmon (Salmo salar). Aquaculture 2017, 473, 20–30. [Google Scholar] [CrossRef]
- Yu, X.; Setyawan, P.; Bastiaansen, J.W.M.; Liu, L.; Imron, I.; Groenen, M.A.M.; Komen, H.; Megens, H.J. Genomic Analysis of a Nile Tilapia Strain Selected for Salinity Tolerance Shows Signatures of Selection and Hybridization with Blue Tilapia (Oreochromis Aureus). Aquaculture 2022, 560, 7385277. [Google Scholar] [CrossRef]
- Minh, H.D.; Thuy, Y.D.; Thanh, L.P.; Minh, T.B.; Nam, S.V.; Thanh, H.D.T.; Bich, H.B.T.; Ngoc, T.N.T.; Quang, H.D.; Kestemont, P. Selective Breeding of Saline-Tolerant Striped Catfish (Pangasianodon hypophthalmus) for Sustainable Catfish Farming in Climate Vulnerable Mekong Delta, Vietnam. Aquac. Rep. 2022, 25, 101263. [Google Scholar] [CrossRef]
- Luo, Z.; Yu, Y.; Zhang, Q.; Bao, Z.; Xiang, J.; Li, F. Comparative Transcriptome Analysis Reveals the Adaptation Mechanism to High Salinity in Litopenaeus vannamei. Front. Mar. Sci. 2022, 9, 864338. [Google Scholar] [CrossRef]
- Fang, J.-F.; Li, Q. The Effects of Inbreeding on Stress Resistance of the Pacific Oyster Crassostrea gigas at Different Temperatures and Salinities. Mar. Biol. Res. 2023, 19, 249–260. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, H.; Zheng, H. Selective Breeding of Edible Bivalves and Its Implication of Global Climate Change. Rev. Aquac. 2020, 12, 2559–2572. [Google Scholar] [CrossRef]
| Specie | Pathway | Salinity Level | Duration | Effects | References |
|---|---|---|---|---|---|
| Oreochromis niloticus | AMPK | 8 and 16 psu | 8 weeks | Mediates energy metabolism under stress, maintaining cellular energy homeostasis. | [96] |
| Rachycentron canadum | MAPK | Acute stress (30 ppt to 10 ppt for one hour), Chronic (30 ppt–10 ppt for 4 days) | 1 h to 28 days | MAPK pathway upregulated under acute salinity stress, inducing apoptosis and inflammation; chronic stress shows varied expression for long-term adaptation. | [97] |
| Ictalurus punctatus | MAPK | 3 and 7 psu | 3 weeks | p38-MAPK mediates gene expression, metabolism, and cellular homeostasis under salinity stress. | [98] |
| Takifugu fasciatus | MAPK | 0, 10, and 20 ppt | 3 days | 20 ppt salinity led to increased oxidative stress, and apoptosis-related gene expression. | [99] |
| Scylla paramamosain | AMPK | 23 and 4 ppt | 6 weeks | Regulates stress responses, gene expression, apoptosis, and oxidative stress management; protects against cell damage via p38-MAPK. | [100] |
| Scophthalmus maximus | PI3K-AKT | 5, 10, and 30 ppt | 24 h | Low saline stress downregulated gene expression, protein content, and AKT1 phosphorylation in gill tissues. | [101] |
| Litopenaeus vannamei | AMPK | 3, 20, and 30 psu | 0 h to 96 h | AMPK elevated during acute stress, indicating higher energy needs; sustained during chronic stress for osmoregulation and energy balance. | [102] |
| Litopenaeus vannamei | RAS | 25 and 3 ppt | Short-term stress (0 h–96 h), Long-term stress (8 weeks) | RAS pathway downregulated under stress, affecting homeostasis, osmoregulation, and blood glucose levels. | [103] |
| Scylla paramamosain | RAS | 3, 20, and 23 ppt | 0 h to 168 h | NKA activity increased under stress to regulate osmotic balance, aided by RAS pathway for improved metabolic activity. | [104] |
| Penaeus monodon | PI3K-AKT | 30, 18, and 3 psu | 45 days | Prolonged low saline stress downregulated RAS pathway, diverting energy from growth to ionic homeostasis and osmoregulation. | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dildar, T.; Cui, W.; Ikhwanuddin, M.; Ma, H. Aquatic Organisms in Response to Salinity Stress: Ecological Impacts, Adaptive Mechanisms, and Resilience Strategies. Biology 2025, 14, 667. https://doi.org/10.3390/biology14060667
Dildar T, Cui W, Ikhwanuddin M, Ma H. Aquatic Organisms in Response to Salinity Stress: Ecological Impacts, Adaptive Mechanisms, and Resilience Strategies. Biology. 2025; 14(6):667. https://doi.org/10.3390/biology14060667
Chicago/Turabian StyleDildar, Tariq, Wenxiao Cui, Mhd Ikhwanuddin, and Hongyu Ma. 2025. "Aquatic Organisms in Response to Salinity Stress: Ecological Impacts, Adaptive Mechanisms, and Resilience Strategies" Biology 14, no. 6: 667. https://doi.org/10.3390/biology14060667
APA StyleDildar, T., Cui, W., Ikhwanuddin, M., & Ma, H. (2025). Aquatic Organisms in Response to Salinity Stress: Ecological Impacts, Adaptive Mechanisms, and Resilience Strategies. Biology, 14(6), 667. https://doi.org/10.3390/biology14060667







