Simple Summary
Natural products are one of the most important sources of drug discovery, providing abundant structural templates and inspiration for new drug development. They play a vital role in modern pharmaceutical research. Traditional Chinese medicine (TCM), known for its unique therapeutic effects, has been used in China for centuries. Increasing attention has been paid to the study and development of its main active constituents. This review focuses on baicalin, a key bioactive compound derived from TCM, and systematically summarizes recent advances in its pharmacological effects and underlying mechanisms in the treatment of cancer, cardiovascular diseases, neuroprotection, and metabolic disorders, aiming to provide valuable insights and references for future research.
Abstract
Baicalin, a kind of polyphenolic flavonoid, is a major bioactive flavone derived from the root of Scutellaria baicalensis, which has been widely utilized in clinical practice in China for thousands of years. In recent years, it has attracted increasing attention due to its potential therapeutic properties observed in preclinical studies involving various disease models. However, the precise mechanisms underlying its biological activities have not been fully elucidated. This review summarizes recent research progress on the molecular mechanisms through which baicalin exerts its effects, particularly in tumor suppression, cardiovascular protection, neuronal preservation, and glucose and lipid metabolism regulation in murine models. Additionally, we discuss the delivery methods of baicalin and its transformation by intestinal microbiota.
1. Introduction
Traditional Chinese medicines (TCMs) are invaluable resources due to their proven clinical effects and complex components, which confer a wide range of therapeutic functions. However, this complexity also introduces uncertainty about the specific actions of these herbs, and the imprecise target of herbal molecules complicates the explanation of their clinical outcomes. Advances in modern medical science now allow us to isolate the main bioactive components and elucidate the underlying mechanisms. More importantly, TCMs have the potential to provide broad ground for modern drug development [1].
Huangqin (Scutellaria baicalensis), with its bitter taste and mild nature, is one of the most common traditional Chinese herbs. It has been historically employed for removing damp heat, quenching fire, counteracting toxicity, arresting bleeding, and preventing abortion according to traditional Chinese medicine theory. It has also been widely used in Chinese herbal formulas together with other herbs (Table 1).

Table 1.
Multi-herb formulations containing baicalin.
Advances in modern pharmacology have raised expectations for the precise identification of active constituents in TCMs. To date, more than 200 chemical constituents have been isolated and identified from Scutellaria baicalensis, the majority of which are flavonoids and their glycosides [20]. Utilizing chromatographic fingerprint analysis and a backpropagation–artificial neural network model, baicalein, baicalin (Figure 1), wogonoside, and wogonin were determined as the quality control markers of Scutellariae radix and its wine-processed and carbonized products [21]. While all four flavonoids have been studied, this review focuses specifically on baicalin, which is the most abundant constituent, accounting for approximately 25.80% of the methanolic extract of Scutellariae radix and 8.12% of the dry root mass [22]. Given the complexity of herbal extracts, it is often difficult to ascribe specific biological effects to baicalin alone. Thus, in order to provide a more accurate evaluation of baicalin’s pharmacological properties, this review focuses on studies in which baicalin has been isolated and its concentration clearly determined.
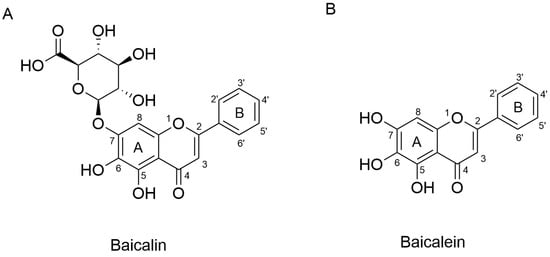
Figure 1.
Chemical structures of baicalin (A) and baicalein (B).
Baicalin is a free-B-ring flavonoid purified from Scutellariae radix through uridine diphosphate glucuronidation. Purified baicalin (baicalein 7-O-glucuronide) is a light yellow-to-yellow powder with a molecular weight of 446.3640. It is barely dissolvable in aqueous buffers, so its oral bioavailability is low. As a study reported, the absolute bioavailability of oral baicalin is 30% [23], and numerous studies have been conducted with the aim of improving its intestinal absorption [24,25].
Baicalin is an important class of flavonoid glycosides, and baicalein is the aglycone of baicalin. Baicalin is metabolized to baicalein via β-glucuronidase produced from intestinal microbiota, indicating that the digestive microbiota plays an important role in the biological activity of baicalin [26]. In fact, bacterial hydrolysis might be the only way and the rate-limiting step for its absorption [27]. Glucuronidation is the main metabolic pathway for baicalin. The absorbed baicalein is widely metabolized in the liver by two phase II metabolic enzymes, UDP glucuronosyltransferase (UGT) and sulfotransferase (SULT). UGT catalyzes the transfer of glucuronic acid to lipophilic substrates [28], and SULT catalyzes the transfer of the sulfate groups. Then, the glucuronides (including baicalin) [29] or sulfates [30] of baicalein enter the enterohepatic circulation (Figure 2). Following this process, baicalin is distributed to different tissues such as the brain, lung, heart, and so on. Oral administration of baicalin exclusively presents in the plasma as baicalein glucuronides/sulfates, which means that the conjugated metabolites of baicalein are in fact responsible for its in vivo effects [31]. In vitro studies on baicalin only partially explain its in vivo effects. Eventually, baicalin is primarily excreted into the bile in the form of glucuronides. Of note, the enterohepatic circulation contributes to the overall systemic disposition of baicalin and its conjugated metabolites [23].
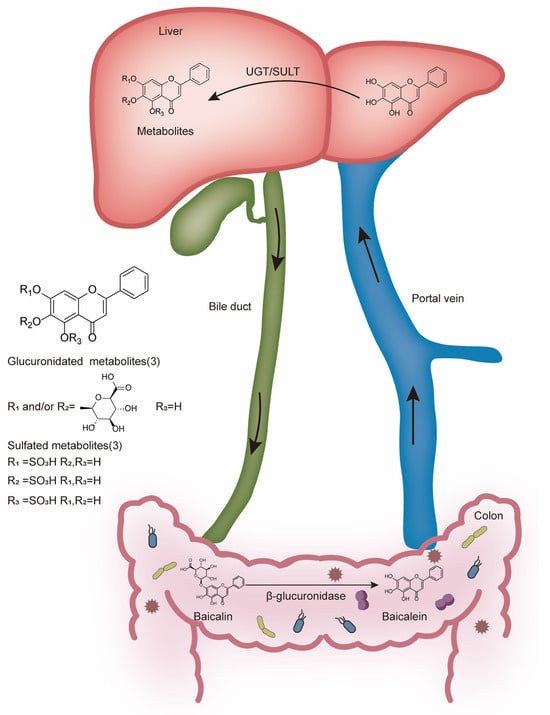
Figure 2.
Metabolism of baicalin in the colon and liver.
Baicalin has been reported to possess multifaceted functions, including anti-inflammatory, anti-bacterial, antiviral, and antiallergic effects. Over the past decade, the antioxidant and anti-inflammatory activity of baicalin has been given considerable attention. Considering the increased attention paid to herbal medicines and the promising effects of baicalin, we discuss the diseases in which baicalin may have beneficial effects. We also addressed the pathways by which baicalin participates in exhibiting its effects (Table 2).

Table 2.
The effects and mechanisms of baicalin.
2. Antitumor Effects
In recent years, there has been an increase in the development of natural anti-cancer compounds due to their therapeutic efficacy, fewer side effects, and lower cost [71]. Early studies focused on formulations containing baicalin. Dating back to the 1990s, Japanese scientists conducted a considerable amount of research on sho-saiko-to, which was the most popular herbal medicine and contained huangqin as the main component, and confirmed its effect on suppressing hepatocellular carcinoma and cholangiocarcinoma in vitro [72,73]. Another study pointed out that sho-saiko-to displayed antitumor and anti-metastatic effects on melanoma in vivo and in vitro [74]. However, formulations make it difficult to attribute the effects directly to baicalin, and studies on purified baicalin have been increasingly focused. The direct anticancer activities of baicalin have been confirmed in prostate cancer, lung cancer, lymphoma, and hepatocellular carcinoma (HCC) [32,33,75,76,77]. And multiple signaling pathways are involved, including apoptosis, the cell cycle, invasion, migration, angiogenesis, autophagy, and immune evasion [78,79]. Sometimes it seems to be controversial across different studies. For example, research conducted by Su’s group tested concentration-dependent cell growth inhibition in response to baicalin (50–200 μmol/L) in breast cancer cells MCF-7 and MDA-MB-231, and the effect was enhanced when in combination with baicalein [80]. While another study found that baicalin (100 μg/mL, 224 μmol/L) enhanced the growth of MCF-7 human breast cancer cells, but baicalein and wogonin significantly inhibited MCF-7 cell growth [81]. These inconsistent findings could be due to the differences in cell line sensitivities, cell states, experimental conditions, or the concentration of baicalin. Such discrepancies emphasize the need to test different drug concentration gradients and multiple cell lines. In most studies, this direct antitumor effect is mainly accomplished through apoptosis, cell cycle arrest, and ferroptosis [41]. Dou et al. illustrated that baicalin treatment dramatically inhibited tumor growth not by inducing apoptosis but through the induction of tumor cellular senescence; moreover, they established decidual protein induced by progesterone as a key node regulating senescence induction, which could be a novel target for cancer treatment [34]. And in a recent study, researchers found that autophagy is also a strategy by which baicalin inhibits tumor growth [82]. Except for the inhibition of cell growth and proliferation, baicalin exerts its antitumor effects by inhibiting migration and invasion [83,84], which plays a vital role in the progression of cancer and is usually associated with poor prognosis. Furthermore, this study also demonstrated that baicalin suppressed HCC cell growth and metastasis by inhibiting ROCK1 signaling, which regulates cell polarity and migration by boosting actomyosin contraction and focal adhesions, and ROCK1 might be a stable and direct target of baicalin [40]. This indicates that baicalin not only acts through broad cytotoxic mechanisms but also targets specific regulatory molecules of metastasis, suggesting good therapeutic selectivity.
Tumor progression is also modulated by the tumor microenvironment (TME), which is the home of cancer cells and serves as a bridge connecting cancer with the entire organism [85]. The TME is populated by many immune cells, of which macrophages are among the most abundant [86]. Generally, macrophages are classified into M1 and M2. M1 is often considered antitumoral, and M2 is typically regarded as pro-tumoral. There are several studies demonstrating that baicalin mediates tumor-associated macrophage (TAM) repolarization and suppression of tumor progression. Feng’s group measured M1/M2-like macrophages in the liver of baicalin-treated mice, and they found that baicalin treatment led to an increase in the M1-like macrophage population while there was a significant reduction in the M2-like population in the liver tissue of the mice. This was further evidenced by the fact that baicalin skewed M2-like macrophages toward the M1-like phenotype, without causing any significant changes in M1-like macrophages in vitro [33]. Also, in non-small cell lung cancer, baicalin effectively inhibited tumor growth in Lewis lung carcinoma tumor-bearing mice and increased the infiltration of M1-type macrophages in the TME [87]. When combined with a nano-complex, the baicalin taken up by TAMs was released to the tumor sites, and it not only killed tumor cells directly but also remodeled the tumor microenvironment, as mentioned above [88]. Except for macrophages, baicalin also induces responses in T and B cells [89]. Nonetheless, studies on its immunomodulatory functions beyond macrophages are inadequate, and more research is needed to clarify whether baicalin can support long-term antitumor immunity.
Despite the considerable advances that have been made in cancer treatment, chemotherapy remains widely used. However, drug resistance is an obstacle to clinical effectiveness. Studies have shown how baicalin works in chemotherapeutic resistance. In breast cancer cell lines, baicalin was able to enhance doxorubicin cytotoxicity via the reactive oxygen species (ROS)/[Ca2+]-mediated intrinsic apoptosis pathway [90]. Consistent with this, baicalin successfully enhanced sensitivity to 5-Fluorouracil and reduced Ehrlich tumor growth (a spontaneous murine mammary adenocarcinoma) via cooperative inhibition of inflammation, angiogenesis, and triggering apoptotic cell death [38]. Also, an efficient in vitro and in vivo study in colorectal cancer showed that baicalin enhanced the effect of 5-fluorouracil-based chemotherapy via inhibition of the CDK-RB pathway [39]. Studies in gastric cancer cell lines have provided evidence that baicalin enhances 5-Fluorouracil by promoting ROS-related ferroptosis in gastric cancer and inhibits drug resistance [42]. These findings support the potential of baicalin as a chemosensitizer, but the safety and pharmacokinetics of baicalin in combination therapy need to be evaluated systematically.
Taken together, baicalin effectively inhibits the growth of various cancer cells and leads to tumor shrinkage. On the one hand, it kills cancer cells and inhibits proliferation; on the other hand, it plays a role in immune cells and remodels the tumor microenvironment. More importantly, when in combination with other chemotherapeutic drugs, 5-fluorouracil, for instance, it alleviates chemotherapy resistance while retaining its own antitumor function at the same time.
3. Cardiovascular Protection Effects
Cardiovascular diseases (CVDs) are the leading cause of death globally. An estimated 17.9 million people died from CVDs in 2019, representing 32% of all global deaths. Baicalin has been reported to improve hyperglycemia-induced dysplasia of the cardiovascular system during early embryo development. In early chick embryos, it rescued hyperglycemia-induced cell proliferative reduction and apoptosis increase, redressed the unbalanced secondary effect of autophagy on heart tube formation, and stabilized the oxidative stresses’ secondary effect on angiogenesis, thus reversing the hyperglycemia-inhibited development of early chick embryos [91]. Herein, we review the protective effects and underlying pharmacological mechanisms of baicalin against CVDs. The available studies suggest that baicalin has great potential for the treatment of CVDs and is worthy of more research.
3.1. Protection of the Heart
Myocardial ischemia is a main factor that leads to the loss of cardiomyocytes. In hypoxia/reoxygenation (H/R)-treated neonatal rat cardiomyocytes, baicalin pretreatment reduced cell death, attenuated oxidative stress, and improved morphological changes; what is more, it suppressed inflammatory cytokine IL-6, increased anti-inflammatory cytokine IL-10 levels, and inhibited the nuclear translocation of NF-κB induced by H/R. These results indicate that baicalin has a positive effect on cardiomyocytes suffering from H/R insults through antioxidation and anti-inflammation mechanisms [92]. Furthermore, the protective effect was verified in vivo. Baicalin improved cardiac function, decreased the area of myocardial infarction, and inhibited the apoptosis of myocardial cells in myocardial ischemia–reperfusion (IR) rats, and the protective mechanism was related to the promotion of NO production and the inhibition of necroptosis in cardiac microvascular endothelial cells through activation of the PI3K-AKT signaling pathway [93]. Zeng et al. conducted a meta-analysis of animal studies on the positive pharmacological effects of baicalin on IR injury, illustrating a chain of preclinical evidence and providing rigorous and systematic support for further clinical research [94].
Another cardiomyocyte disorder is myocardial hypertrophy, which is a compensatory response to a persistent increase in load. In both angiotensin II (Ang II)-induced cardiomyocyte hypertrophy in vitro models and abdominal aortic constriction-induced mouse cardiac hypertrophy in vivo models, baicalin exerted an anti-cardiac hypertrophy effect. Molecular docking experiments indicate that baicalin might directly interact with PSMB5, inhibiting the activation of the proteasome and the degradation of SIRT3, whose downstream signaling pathway has an impact on cardiac hypertrophy [45].
3.2. Regulation of Blood Vessels
The effect of baicalin in regulating blood vessels is reflected in multiple aspects, including anti-atherosclerosis, angiogenesis, and vasodilation. Atherosclerosis is a chronic inflammatory disease of the arterial wall, with an imbalanced lipid metabolism and a maladaptive immune response involved [95]. The excessive proliferation and migration of vascular smooth muscle cells (VSMCs) are important events in the development of atherosclerotic lesions. Baicalin induced growth arrest in platelet-derived growth factor (PDGF)-BB stimulated VSMCs via blockade of the PDGF receptor β-ERK1/2 signaling cascade. In rat carotid arterial balloon-injury models, it also prevented neointimal hyperplasia [96]. Also, Jin’s group reported that baicalin exerted anti-atherosclerosis effects through regulation of the lipid profile. In THP-1 cells, decreased ox-LDL-induced foam cell formation and intracellular lipid accumulation were observed at nontoxic concentrations [43]. Similarly, an in vivo study demonstrated that baicalin decreased lipid accumulation by upregulating the lipolysis-related proteins peroxisome proliferator-activated receptor α(PPARα) and carnitine palmitoyl-transferase1 (CPT1) and suppressing adipogenesis-related proteins sterol-CoA response element binding protein-1c (SREBP-1c) and ACS; in addition, lower expression of LDL-C and TG and higher concentrations of TCH were detected in the baicalin model group compared with the AS group. Except for the anti-adipogenic effect, antioxidant and anti-inflammatory effects also participate in anti-atherosclerotic action [97]. In one study, it was shown that baicalin exhibited potent biological activity to restore the function of endothelial cells and inhibited VSMC proliferation and migration and the release of inflammation markers from activated macrophages [98]. These studies collectively suggest that baicalin exerts multi-target anti-atherogenic potential, likely through a combination of endothelial restoration, lipid regulation, and inflammation suppression.
Angiogenesis plays a critical role in injury caused by ischemia, like stroke and myocardial infarction. Vascular endothelial growth factor (VEGF) is one of the most specific factors that stimulate angiogenesis. Through the activation of the ERRα, baicalin induced VEGF expression and angiogenesis [44]. This underscores baicalin’s therapeutic promise in ischemic cardiovascular conditions by promoting vascular regeneration.
Hypertension, characterized by elevated systemic arterial pressure, represents a major risk factor for cardiovascular diseases and remains the leading cause of premature death worldwide [99]. Vascular constriction/relaxation function directly affects blood pressure. Influx of extracellular Ca2+ regulates the contraction of smooth muscle; the major pathway for this increase is through voltage-dependent Ca2+ channels (VDCCs). Large-conductance Ca2+-activated channels, acting as a negative feedback mechanism, play a central role in the regulation of vascular tone [100]. Baicalin showed VDCC inhibition and BK activation properties by stimulating the cGMP/PKG and cAMP/PKA pathways [101]. Also, baicalin effectively decreased vascular tension in spontaneously hypertensive rats’ (SHR) aortas and lowered their blood pressure. The activated ATP-sensitive potassium channel is, in part, explained by the vasorelaxant effect of baicalin [102]. RNA sequence analysis was conducted to explore the underlying mechanisms of baicalin on hypertension, and the calcium signaling pathway and vascular smooth muscle contraction signaling pathways were found. Through a series of experiments, Peng’s group not only verified that baicalin reversed the elevation of BP, vascular pathological injury, and VSMC proliferation induced by Ang II, but also demonstrated that the mechanism was due to the activation of the MLCK/p-MLC signaling pathway and decreased intracellular calcium release in VSMCs [47]. These findings collectively indicate that baicalin improves hypertension mainly through the synergistic regulation of vascular tone and calcium-dependent contractile machinery.
4. Neurological Disorders
Brain disorders such as cerebral ischemia and neurodegenerative diseases have become the biggest threats to people [103]. Emerging data indicate the potential neuroprotective function of baicalin in the treatment of these diseases. In vitro, baicalin showed protective effects on PC12 cells suffering from colistin sulfate-induced apoptosis [104]. For brain diseases, effective blood–brain barrier (BBB) penetration is a formidable challenge [105]. Evidence from multiple studies supports the potential of baicalin passing through the BBB [106], which is the basis for its effect on the central nervous system. Recently, many studies have focused on borneol–baicalin liposome [107,108]. Borneol is widely used for “waking up” the brain according to TCM theory. As expected, the addition of borneol prolonged the efficacy time of baicalin, improved blood–brain barrier integrity, and better exerted the therapeutic effect on cerebral I/R injury in vivo and in vitro. Improving the bioavailability of baicalin can also be achieved by transforming the method of drug administration. Nose-to-brain drug delivery administration is an alternative way for baicalin to be used to treat brain diseases [109]. Baicalin-loaded ligand-modified nanoparticles were prepared for nose-to-brain delivery, and the effect was significant because baicalin was delivered to the entire brain with little delivery to the peripheral circulation [110].
4.1. Neuroprotection in Ischemic Stroke
Stroke is a leading cause of mortality and disability worldwide. Ischemic stroke caused by arterial occlusion is responsible for the majority of strokes. Increasing knowledge about the exact pathophysiology of stroke is necessary to design suitable drugs. The ischemic cascade responses in cells include reduced availability of glucose and oxygen, increased extracellular levels of glutamate, neuronal calcium influx (mediated via the N-methyl-d-aspartate (NMDA) ion receptor (NMDAR)) and subsequent production of nitric oxide by neuronal nitric oxide synthase (NOS), blood–brain barrier dysfunction, and pro-inflammatory cytokine release from microglia, leading to an inflammatory response; the result of all of these cumulative effects is neuron death. Interruption of these processes may provide a way to prevent, or at least reduce, the ischemic damage. Management of ischemic stroke focuses on rapid restoration of blood flow with intravenous thrombolysis and endovascular thrombectomy, and this is critical to reduce disability [111]. Tissue plasminogen activator is the only FDA-approved drug for the treatment of cerebral ischemia. Together with this, neuroprotective therapy represents another major strategy for ischemic stroke [112]. Advances in the natural flavonoid baicalin are highlighted in improving neuroprotection following brain ischemia injury.
The authors of a previous study assessed the efficacy of baicalin in rat models of cerebral artery occlusion, and neurological deficits, cerebral infarct volume, and pathomorphological change in ischemic brain tissue were assessed. These three indicators were all improved in the baicalin group compared with the ischemia group; also, cerebral infarct volume was similar in the valproic acid (positive control) and baicalin groups [113]. These findings suggest that baicalin appears to be a potential therapeutic agent for the treatment of ischemic stroke. Considerable effort has been made to identify the targets of baicalin and the pathways it regulates. In nuclear magnetic resonance titration experiments, baicalin displayed high PDZ2 binding affinity, which is associated with NMDAR [114]. Gene expression profiling was conducted to identify the differential gene expression and the pharmacological mechanism of baicalin in cerebral ischemia rats. By comparing the differences in infarction areas before and after baicalin treatment, cell signal transduction and protein phosphorylation were significant [115]. After improving the research method, Wang et al. used transcriptome analysis to explore the pure therapeutic mechanisms of baicalin contributing to phenotype variation and the reversal of pathological processes in ischemic stroke mice. According to the analysis, seven specific targeted molecules were found; they are ATF3, BCL2L1, ARF1, FGF12, GRIN1, MAP2K6, and PRKAR1E. Forty-one differentially expressed genes were identified. Based on these genes, a statistically significant network was constructed, and the functions of the target network include cell death, genetic disorder, and immunological disease. Fifty-one canonical pathways and seventy biological functions were identified. This pure mechanistic analysis might provide a clearer outline of the target profiles of baicalin therapies [116].
Glutamate is considered the triggering spark in the cascade of responses of ischemic neuronal damage. Its level was increased with excessive neurological stimulation, causing glutamate-induced neuronal toxicity and excitotoxicity [117]. In the process of glutamate clearance, Na+-dependent high-affinity glutamate transporters (excitatory amino acid transporters, EAATs) remove glutamate from the extracellular space to maintain its extracellular concentration below excitotoxic levels. Of all five EAATs, EAAT2 (GLT-1), the glial-type glutamate transporter, provided the majority of total glutamate uptake. Moreover, glutamine synthetase (GS) in astrocytes was required for the glutamate–glutamine cycle and helped glutamate be repackaged into synaptic vesicles [118]. Baicalin might help with the clearance of glutamate via different mechanisms. It was confirmed to upregulate GLT-1 expression in peri-infarct cortices in hypoxic–ischemic encephalopathy models 24 h after injury and exhibited protective effects [119]. Also, baicalin was found to maintain GS protein stability from 20S-mediated proteasomal degradation in astrocytes [120]. These results provide evidence to support baicalin’s ability to combat glutamate excitotoxicity to prevent ischemic neuronal injury.
The inflammatory response to cerebral ischemia is another important process in stroke pathobiology and neuronal death. 5-lipoxygenase is a key enzyme in the catalytic conversion of arachidonic acid to inflammatory mediator leukotrienes. Studies in oxygen–glucose deprivation-induced neuronal damage indicate that baicalin inhibits 5-lipoxygenase activation mediated by NMDAR and oxidative stress [121,122]. Moreover, Chen’s results demonstrated the neuroprotective effect of baicalin on permanent middle cerebral artery occlusion (pMCAO) rat models, and this protection might be associated with its potent anti-inflammatory and antiapoptotic properties. In further detail, mRNA expression of iNOS and COX-2 in the ischemic brain after pMCAO decreased after baicalin treatment [123]. In another study, the effects of baicalin on the TLR2/4 signaling pathway were investigated. Both in the oxygen glucose deprivation model in vitro and the I/R model in vivo, TLR2/4 responded to the damage, and the expression of its downstream factor, tumor necrosis factor α (TNFα), increased. As for NF-κB, baicalin not only decreased its expression but also inhibited its translocation from the cytoplasm to the nucleus in vitro [124]. These studies show that the protective role of baicalin in ischemic neuronal injury is closely related to its anti-inflammatory properties.
The disruption of the BBB is also a factor that aggravates the brain damage observed in cerebral IR injury. Baicalin has been found to be capable of restoring the barrier function of the BBB in various conditions [125,126]. Therefore, baicalin can preserve brain tissue viability before reperfusion through anti-excitotoxicity and anti-inflammatory effects, protection of the BBB’s integrity properties, etc. (Figure 3).
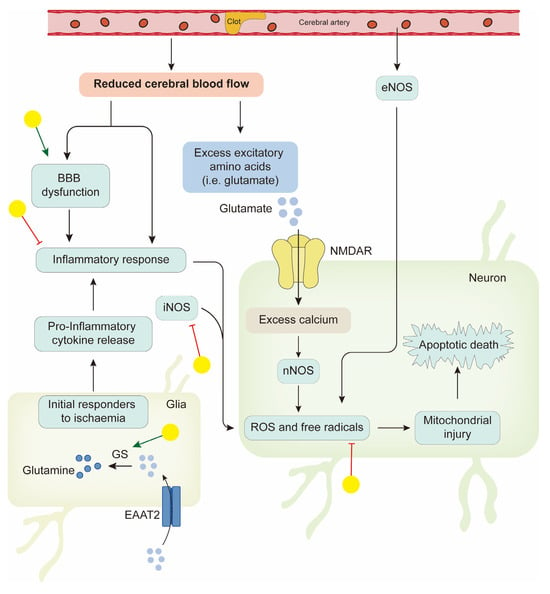
Figure 3.
Neuroprotective effects of baicalin in ischemic stroke. Yellow balls indicate baicalin.
4.2. Neuroprotection in Neurodegenerative Diseases
A wide spectrum of neurodegenerative disorders, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), affect the central nervous system (CNS). Characterized by the progressive degradation of synapses and axons, they eventually lead to neuronal death [127]. Baicalin can act as an iron chelator to protect dopaminergic neurons and delay PD neural degeneration in Parkinson’s disease rats [48]. In C6 glioma cells, baicalin decreased divalent metal transporter1 expression, increased ferroportin1 expression, subsequently lowered the iron concentration, and protected nerve cells [128]. As for AD, the improvement of cognitive function and brain damage and decreased eicosanoid production were observed in mice models of flavocoxid (a mixture of purified baicalin and catechin), and these protective effects could be attributed to its anti-inflammatory and anti-apoptotic properties [49]. Analogous to the BBB, the blood–spinal cord barrier (BSCB) is a specialized protective barrier that plays a crucial role in maintaining the homeostasis and internal environmental stability of the CNS [129]. Disruption of the BSCB is common in neurodegenerative diseases and CNS traumatic injury. In an established spinal cord injury rat model, baicalin evidently restored BSCB integrity. And in SH-SY5Y cell models of excitotoxicity, baicalin showed similar results and significantly promoted PI3K and Akt phosphorylation, rescuing tight junction protein loss and reducing neuronal apoptosis.
4.3. Antidepression
Depression is a major mental health-related disease. Different from other diseases, its onset is mainly in mid-to-late adolescence. It prevents people from reaching their full potential; thus, it is prospectively associated with serious issues, including suicide [130]. Currently, depression is conceptualized as a disorder of neural networks, incorporating changes in widely distributed brain areas. Improving synaptic plasticity and modulators of monoamines (e.g., serotonin, noradrenaline, and dopamine) are effective antidepressants [131]. Immune dysfunction is proposed to be relevant in depression. Numerous studies have confirmed the potential role of baicalin in the reversion of depressive-like behaviors. On the one hand, baicalin protects neurons, promoting neuronal survival, proliferation, maturation, and synaptic plasticity [132,133,134,135]. On the other hand, it alleviates neuroinflammation. Associative evidence is strong, with decreased inflammatory cytokine levels, including interleukin IL-β, IL-6, and TNFα, after baicalin administration [136,137]. In addition, several studies have analyzed the potential protective effects of baicalin in improving spatial learning and memory deficits and controlling primary symptoms of attention-deficit hyperactivity disorder (ADHD), which may be influenced by neuroinflammation, but the mechanism needs further investigation [138].
In addition, demyelinating lesions are common pathologic characteristics of various CNS diseases, and baicalin has been reported to promote myelin production and regeneration by activating the peroxisome PPARγ signaling pathway [50]. New progress in the use of baicalin for epilepsy treatment has also been revealed [139].
As discussed above, baicalin may exhibit neuroprotective effects on multiple CNS disorders via mechanisms involving antioxidant stress [140], anti-apoptotic, anti-inflammatory, and anti-excitotoxic effects, ameliorating BBB disruption and promoting neurogenesis, and cell differentiation. As mentioned above, all of this evidence is from preclinical studies, and clinical application needs further concerted effort.
5. Regulation of Metabolic Disorders
With changes in lifestyle, metabolic diseases have become a significant burden worldwide. Metabolic diseases include hypertension, type 2 diabetes mellitus (T2DM), hyperlipidemia, obesity, and, more recently, non-alcoholic fatty liver disease (NAFLD). Many of these diseases occur in tandem and share common risk factors [141]. For example, dyslipidemia is a common cause of obesity, hyperlipidemia, and NAFLD. The pathophysiology of T2DM and NAFLD can be largely attributable to insulin resistance. Mounting evidence demonstrates that baicalin is linked to metabolic regulation, which may provide useful hints for the treatment of metabolic diseases [142,143,144,145] (Figure 4). To better understand its pharmacological roles, the metabolic effects are separated in the context of lipid metabolism, glucose homeostasis, and cross-talk pathways involving oxidative stress and inflammation.
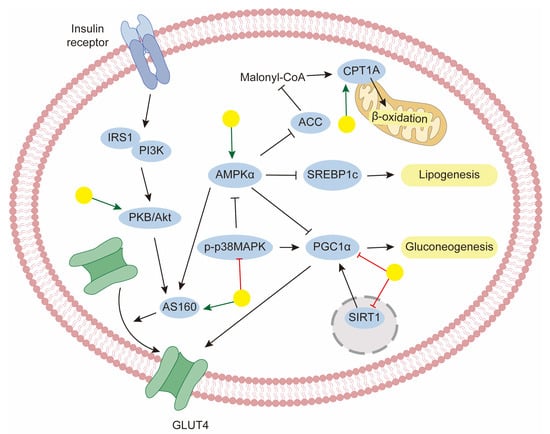
Figure 4.
The mechanism of baicalin for the management of metabolic disorders. Yellow balls indicate baicalin.
5.1. Obesity and NAFLD
Obesity, or being overweight, is a condition of excessive fat deposits that presents a risk to health. NAFLD is considered one of the complications of hyperlipidemia and obesity, characterized by the accumulation of toxic lipid species in the liver, which induces hepatocellular stress, injury, and death [146]. Thus, lipid metabolism is closely linked to the etiologies of obesity and NAFLD. Using a system of 3T3-L1 preadipocytes, baicalin was shown to inhibit the differentiation of preadipocytes into adipocytes. Microarray analyses showed that baicalin modulated adipogenesis and cholesterol biosynthesis pathways, and this was confirmed in 3T3-L1 preadipocytes [52]. This result demonstrated the modulation of adipogenesis in adipocytes by baicalin. Its ability in hepatocytes has also been extensively studied. First, we expect to elucidate the pathways leading to lipo-toxicity. Excessive fatty acids serve as substrates for the generation of lipotoxic species. Lipolysis of triglycerides in adipose tissue is the main source of fatty acids in the liver [147]. The second major source of fatty acids is their synthesis from glucose and fructose through de novo lipogenesis, in which acetyl-CoA carboxylase and SREBP-1c play a positive role. In addition, PPARγ ligands enhance the diversion of fatty acids away from the liver [146].
To regulate the processes discussed above, baicalin has shown promise in modulating key transcriptional factors and enzymes through AMP-activated protein kinase (AMPK) activation, which is the central regulator of lipid metabolism. Cellular energy sensor AMPK activation may improve NAFLD by inhibiting lipid and sterol synthesis and stimulating alterations. In this case, baicalin reduced hepatic lipid levels in high-fat diet (HFD)-fed rats, and this protective effect was mainly associated with significant enhancement of hepatic AMPK activation. Furthermore, effects on circulating lipid levels were also confirmed [148]. In a similar study, Li et al. discovered that baicalin ameliorated HFD-caused lipid accumulation in mouse liver models and found that it upregulated the phosphorylation of AMPK at the Thr172 site, and the protective roles of baicalin against NAFLD were exerted through AMPK-mediated modulation of the SREBP1/Nrf2/NF-κB pathways [56]. Fatty acids in the liver were metabolized either via mitochondrial β-oxidation or through esterification to form triglycerides. CPT1 is the rate-limiting enzyme for the fatty acid oxidation process; thus, increasing its level would be a logical strategy for therapy. Wang’s group performed quantitative chemo-proteomic profiling and identify CPT1A as the direct target of baicalin. Decreasing CPT1A activity impaired the anti-steatosis activity of baicalin in vitro. Furthermore, in the DIO animal model, baicalin ameliorated diet-induced obesity and hepatic steatosis. Further disruption of the predicted binding site of baicalin on CPT1A completely abolished the beneficial effect of baicalin [54]. In addition, CPT1 activity seems to be modulated by AMPK activity. Therefore, CPT1A binding and AMPK activation may underlie baicalin’s therapeutic mechanism. There also exists a study about the herb–drug interaction between baicalin and rosuvastatin, which was used for the clinical treatment of dyslipidemia. To a certain extent, baicalin induced hepatic rosuvastatin uptake and decreased rosuvastatin plasma concentrations [149]. Furthermore, baicalin has significant effectiveness in the treatment of NAFLD-related fibrosis and shows potential in hepatoprotective properties [150].
5.2. Diabetes
Diabetes is defined as a chronic, metabolic disease characterized by elevated levels of blood glucose, which leads, over time, to serious damage to macrovascular systems, the eyes, kidneys, and nerves. It occurs either when the pancreas does not produce enough insulin or when the body becomes resistant to it. Numerous experiments involving baicalin have shown that it can improve diabetes in preclinical animal models. The anti-diabetic effects of baicalin cover the main insulin-sensitive tissues, such as skeletal muscle, adipose tissue, and the liver.
Controlling hyperglycemia is of great importance for diabetes treatment, which can be achieved through an increase in glucose consumption and the inhibition of gluconeogenesis. In this regard, Wang et al. reported that baicalin decreased plasma glucose levels in a dose-dependent manner in a model of streptozotocin–nicotinamide-induced diabetic rats. In fact, they revealed that the protective properties might be exerted by increasing the hepatic glycogen content and glycolysis [151]. In one study, Wang and coworkers evaluated the glucose consumption level in palmitate induced-insulin resistant HepG-2 cells. They found that baicalin significantly increased glucose consumption and downregulated gluconeogenic genes via the AMPK signaling pathway [152]. Similarly, another study also determined that baicalin suppressed gluconeogenic gene expression in the liver of HFD-fed mice. Moreover, in this study, it was shown that this effect of baicalin was dependent on STAT3 acetylation and activity, regulated by the downregulation of SirT1 [143]. Furthermore, the p38MAPK inhibitor has been shown to strengthen the inhibitory effects of baicalin on glucagon-mediated gluconeogenic gene expression, indicating that baicalin suppresses gluconeogenic activity, at least in part, via the downregulation of p38MAPK [142]. The convergence of multiple pathways suggests that baicalin exerts coordinated regulation of hepatic glucose production.
Another strategy for the treatment of diabetes is the improvement of insulin resistance. It is the decreased sensitivity of target tissues to glucose uptake in response to insulin. In normal conditions, glucose transporter isoform 4 (GLUT4) responds to insulin signals and translocates from the cytoplasm to the cell surface, facilitating the storage of glucose. Several studies have indicated the protective effects of baicalin on the upregulation of GLUT4 levels. Yang et al. found that baicalin promoted glucose disposal in adipocytes dependent on increased AMPK phosphorylation, which subsequently enhanced AS160 phosphorylation, resulting in increased GLUT4 translocation to the plasma membrane [153]. Similar conclusions were also confirmed in myotubes [144,154]. In addition, activated insulin signaling pathways were also reported after baicalin administration in DIO mice [155].
Chronic hyperglycemia leads to complications of diabetes, consisting of microvascular diseases, such as retinopathy, neuropathy, and nephropathy, and macrovascular diseases, including coronary heart disease and cerebrovascular disorders. Excess mitochondrial superoxide production explains the pathobiology of diabetic complications, with inflammation also being involved [156]. Baicalin is an efficient antioxidant through the increased expression of antioxidant enzyme activities in type 2 diabetic rats [157]. In diabetes-associated cognitive impairment, the antioxidant and anti-inflammatory defense of baicalin was mediated through the KEAP1-Nrf2 axis [158]. Its protective effects were also confirmed in hyperglycemia-induced malformation of the cardiovascular system [91] and diabetes-associated kidney disease [58,59]. These studies emphasize the potential of baicalin not only as a hypoglycemic agent but also as a systemic protector against diabetes-related end-organ damage. In addition, one study reported that baicalin suppressed the progression of type 2 diabetes-induced liver tumors through the reversal of high glucose concentration-induced JAK2/STAT1/caspase-3 inhibition [159].
Glucose metabolism and lipid metabolism have a clear and complex biological link. For example, diabetes can lead to disorder in bone–fat balance, and the progression of diabetic nephropathy is usually due to the obstruction of fatty acid oxidation in the renal tubules [59,160]. Baicalin often targets the skeletal muscle, the adipose tissue, and the liver to exert its beneficial effects on glucose and lipid metabolism [145]. In animal models, it has shown great effects in reducing body weight, decreasing hyperglycemia, and mediating dyslipidemia. Taken together, baicalin holds strong translational potential, yet further validation in clinical applications is necessary.
6. Discussion
In this review, we summarize the current knowledge regarding the pharmacological effects and associated mechanisms of baicalin in tumors, cardiovascular diseases, and neurological and metabolic disorders. From these studies, we conclude that many of the biological effects of baicalin are attributed to its potent anti-inflammatory and antioxidant capacities [161,162,163] (Figure 5). ROS are closely linked to a variety of oxidative stress-related diseases such as diabetes, Alzheimer’s disease, and Parkinson’s disease. Ferroptosis, a recently discovered type of programmed cell death characterized by the overproduction of ROS [164], is also relevant here. Baicalin can play a protective role in various tissues by scavenging ROS and inhibiting ferroptosis [122,164]. The ortho-dihydroxyl groups in ring A of baicalin contribute significantly to its radical scavenging ability [165,166,167]. As for its anti-inflammatory properties, baicalin modulates various inflammatory signaling pathways, including STING, the NLRP3 inflammasome, TLRs, and NF-κB. Extensive studies have been conducted using different inflammatory disease models, demonstrating that baicalin acts on diverse immune cell types, especially macrophages, T cells, and mast cells. Most experiments have examined the effects on macrophages. Robust evidence supports the anti-inflammatory effect of baicalin in LPS-induced inflammation in macrophages, wherein it decreases the expression of pro-inflammatory proteins and genes [168,169,170]. Similarly, in various animal models of inflammatory diseases, baicalin shows protective effects by modulating the Th17/Treg paradigm, reducing pro-inflammatory cytokine levels, and increasing Treg cell and related cytokine levels [69,171,172]. Mast cells, which play a critical role in allergic reactions, are also influenced by baicalin. For instance, in ovalbumin-induced allergic rhinitis guinea pigs, oral administration of baicalin improved histological changes in the nasal mucosa and decreased serum levels of histamine and other inflammatory markers [64]. The broad anti-inflammatory and antioxidant activities noted in pharmacological studies also indicate its non-selectivity. Conflicting results are observed across different disease models, particularly between tumor models and other disease models. For example, in studies using HCC tumor supernatant-derived TAMs or BMDM-derived macrophages polarized to M1-like or M2-like phenotypes, baicalin treatment skewed M2-like macrophages toward an M1-like phenotype without significantly affecting M1-like macrophages [33]. These findings, along with others showing baicalin-induced M1-type polarization [87], contradict its known anti-inflammatory effects. Regarding ROS, baicalin has been shown to increase ROS levels to inhibit tumors [42] but decrease peroxide-induced oxidative stress to protect tissues [70,140]. This bidirectional regulatory profile suggests that baicalin may act through a complex network of molecular targets. Therefore, we need to further elucidate its differential manifestations across various cells, tissues, and disease states.
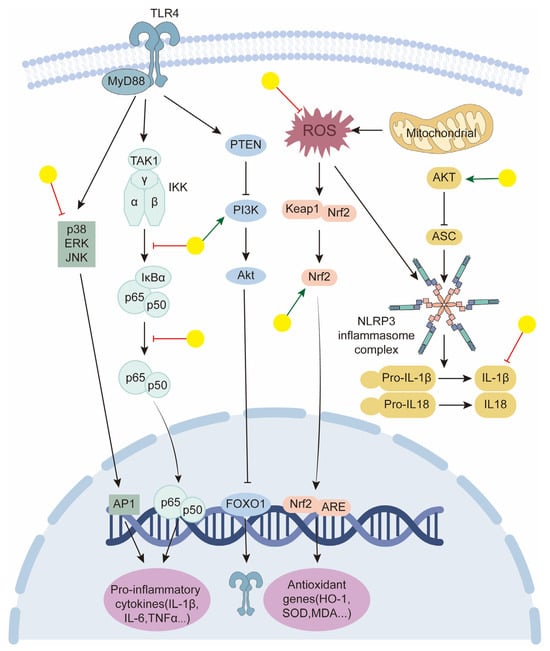
Figure 5.
Schematic representation of baicalin targeting in key signaling pathways to promote anti-inflammatory and antioxidant effects. Yellow balls indicate baicalin.
To date, most studies have focused on phenotypic observations and explored mechanisms to some extent but lack a systematic exploration of the structure–activity relationship of baicalin. Notably, Wang et al. [54] employed activity-based protein profiling (ABPP), successfully identifying carnitine palmitoyl-transferase 1 (CPT1) as the specific molecular target of baicalin. Based on this finding, they conducted further target-oriented structural modifications, representing a valuable attempt to elucidate its precise molecular mechanisms. From a medicinal chemistry perspective, structure optimization strategies based on molecular target characteristics hold great promise. Molecular design, aimed at enhancing target selectivity, improving pharmacokinetic properties, and reducing toxicity, is a feasible and valuable approach for advancing baicalin as a candidate for innovative drug development.
Moreover, while numerous studies have reported promising biological functions, the majority of them are based on in vitro or murine models. Challenges such as a lack of precise molecular target identification and an incomplete understanding of long-term effects hinder the clinical development of baicalin. This highlights the necessity of critically assessing the current preclinical evidence and the underlying mechanisms. Also, well-designed clinical trials are needed to validate baicalin’s therapeutic efficacy, particularly with regard to key aspects such as oral bioavailability, human pharmacokinetics, and long-term safety. It should be noted that a higher dose of drug exposure is required in vivo to achieve an equivalent effect in vitro due to reasons such as oral bioavailability and drug metabolism. In animal models, various strategies have been proposed to enhance its drug transport and administration methods. Interestingly, a combination of acupuncture with the oral administration of Scutellaria baicalensis Georgi extracts significantly improved baicalin absorption in normal rats [173]. Additionally, novel drug delivery systems have attracted attention in the pharmaceutical field. For example, Labrasol, a penetration enhancer, has been shown to increase the corneal permeability and bioavailability of baicalin following topical administration in rabbits [174]. Low-molecular-weight chitosans have also been found to enhance baicalin’s transdermal delivery [175]. Regarding pharmacokinetics, the discrepancy between in vitro and in vivo active forms is often overlooked, and the metabolized components may induce toxicity. Therefore, it is necessary to clearly explain the true components of the drug after metabolism in the body. The toxicity of TCM has always been a concern, with liver injury reported as a proven adverse effect of flavocoxid (which includes baicalin and catechins) [176]. However, another study found no toxicological changes or mortality after oral administration of fermented Scutellariae radix extract at a dose of up to 2000 mg/kg in rats or dogs [177]. Experimental data also suggest that baicalin exerts hepatoprotective activity against various hepatotoxic insults, including drug-induced hepatotoxicity [178], liver injury [179,180], hepatic fibrosis [181,182], non-alcoholic steatohepatitis [55], and cirrhosis [183].
Therefore, future studies on baicalin should focus on the following aspects:
- Identifying specific molecular targets in different diseases;
- Exploring the dose–response relationship between baicalin and the tissue-specific response mechanism under multi-organ and multi-pathological conditions to delineate its tissue selectivity;
- Standardizing baicalin preparations, clarifying its pharmacokinetic characteristics, and exploring its application in combination therapies to bridge the gap between bench and bedside;
- Optimizing chemical structure or delivery systems to enhance its oral bioavailability, stability, and overall pharmacological efficacy.
7. Conclusions
Baicalin’s versatile beneficial functions in human diseases are increasingly recog-nized, further clinical studies are needed to identify the mechanisms involved in specific human pathological conditions. Moreover, while validating its efficacy through scientific and modern methods, we aspire to unearth its more potent pharmacological actions and, when necessary, employ advanced techniques to structurally modify it, thereby enhancing its value and therapeutic potential.
Author Contributions
Conceptualization, D.H. and J.C.; methodology, X.Q.; validation, D.H., J.C. and X.C.; investigation, X.Q.; writing—original draft preparation, X.Q.; writing—review and editing, S.L.; supervision, R.H., J.X., S.L. and X.C.; funding acquisition, D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 82274317, 82474287, and 82070136, and the Natural Science Foundation of Hubei Province, grant numbers 2024AFD264 and ZY2023M020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TCM | traditional Chinese medicine |
| HCC | hepatocellular carcinoma |
| TME | tumor microenvironment |
| TAM | tumor-associated macrophage |
| ROS | reactive oxygen species |
| CVDs | cardiovascular diseases |
| H/R | hypoxia/reoxygenation |
| IR | ischemia–reperfusion |
| Ang II | angiotensin II |
| AAC | abdominal aortic constriction |
| VSMCs | vascular smooth muscle cells |
| PDGF | platelet-derived growth factor |
| VEGF | vascular endothelial growth factor |
| VDCCs | voltage-dependent Ca2+ channels |
| BK | large-conductance Ca2+-activated channels |
| BBB | Blood–brain barrier |
| NMDA | N-methyl-d-aspartate |
| NOS | nitric oxide synthase |
| GS | glutamine synthetase |
| pMCAO | permanent middle cerebral artery occlusion |
| TNFα | tumor necrosis factor α |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| CNS | central nervous system. |
| BSCB | blood–spinal cord barrier |
| PPAR | peroxisome proliferator-activated receptor |
| T2DM | type 2 diabetes mellitus |
| NAFLD | non-alcoholic fatty liver disease |
| SREBP-1c | sterol-CoA response element binding protein-1c |
| AMPK | AMP-activated protein kinase |
| HFD | high-fat diet |
| CPT1 | carnitine palmitoyl-transferase1 |
| GLUT4 | glucose transporter isoform 4 |
| DARTS | drug affinity responsive target stability |
References
- Corson, T.W.; Crews, C.M. Molecular Understanding and Modern Application of Traditional Medicines: Triumphs and Trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kase, Y.; Saitoh, K.; Makino, B.; Hashimoto, K.; Ishige, A.; Komatsu, Y. Relationship between the antidiarrhoeal effects of Hange-Shashin-To and its active components. Phytother. Res. 1999, 13, 468–473. [Google Scholar] [CrossRef]
- Yang, H.J.; Ma, J.Y.; Weon, J.B.; Lee, B.; Ma, C.J. Qualitative and quantitative simultaneous determination of six marker compounds in Soshiho-tang by HPLC-DAD-ESI-MS. Arch. Pharm. Res. 2012, 35, 1785–1791. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, M.; Song, C.; Wang, C.; Lin, X.; Liu, Y. Expressions of apoptosis-related proteins in rats with focal cerebral ischemia after Angong Niuhuang sticker point application. Neural Regen. Res. 2012, 7, 2347–2353. [Google Scholar]
- Zhu, H.; Qian, Z.; He, F.; Liu, M.; Pan, L.; Zhang, Q.; Tang, Y. Novel pharmacokinetic studies of the Chinese formula Huang-Lian-Jie-Du-Tang in MCAO rats. Phytomedicine 2013, 20, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Liu, J.; Qian, G.; Fu, C. Quality evaluation of Huaijiao pill by chromatographic fingerprint and simultaneous determination of its major bioactive components. J. Pharm. Anal. 2016, 6, 249–255. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Yang, X.; Fu, C.; Zou, L.; Zhang, J. Traditional Chinese medicine Gegen Qinlian decoction ameliorates irinotecan chemotherapy-induced gut toxicity in mice. Biomed. Pharmacother. 2019, 109, 2252–2261. [Google Scholar] [CrossRef]
- Su, H.X.; Yao, S.; Zhao, W.F.; Li, M.J.; Liu, J.; Shang, W.J.; Xie, H.; Ke, C.Q.; Hu, H.C.; Gao, M.N.; et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef]
- Wen, Y.; Han, C.; Liu, T.; Wang, R.; Cai, W.; Yang, J.; Liang, G.; Yao, L.; Shi, N.; Fu, X.; et al. Chaiqin chengqi decoction alleviates severity of acute pancreatitis via inhibition of TLR4 and NLRP3 inflammasome: Identification of bioactive ingredients via pharmacological sub-network analysis and experimental validation. Phytomedicine 2020, 79, 153328. [Google Scholar] [CrossRef]
- Wu, X.; Shen, A.; Bao, L.; Wu, M.; Lin, X.; Wang, H.; Chen, Y.; Cai, Q.; Lin, S.; Zhou, X.; et al. Qingda granules attenuate hypertensive cardiac remodeling and inflammation in spontaneously hypertensive rats. Biomed. Pharmacother. 2020, 129, 110367. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Z.; Chen, R.; Lei, B.; Liu, B.; Jiang, H.; Chen, Z.; Cai, X.; Guo, X.; Zhou, M.; et al. Effect of Jinzhen granule on two coronaviruses: The novel SARS-CoV-2 and the HCoV-229E and the evidences for their mechanisms of action. Phytomedicine 2022, 95, 153874. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.B.; Cao, H.H.; Fang, X.C.; Liu, S.H.; Zou, L.F.; Yu, J.H.; Zuo, J.P.; Zhao, W.; Lu, Z.B.; et al. Liang-Ge-San inhibits dengue virus serotype 2 infection by reducing caveolin1-induced cytoplasmic heat shock protein 70 translocation into the plasma membrane. Phytomedicine 2023, 119, 154977. [Google Scholar] [CrossRef]
- Liu, S.; Ding, P.; Wu, M.; Zhu, Z.; Tao, J.; Wang, J.; Xue, Z.; Wang, R. Screening quality markers (Q-markers) of Xiaoer Chaige Tuire Oral Liquid by in vitro sequential metabolism and in vivo biopharmaceutical analysis. Phytomedicine 2023, 116, 154844. [Google Scholar] [CrossRef]
- Ma, H.; Fu, W.; Yu, H.; Xu, Y.; Xiao, L.; Zhang, Y.; Wu, Y.; Liu, X.; Chen, Y.; Xu, T. Exploration of the anti-inflammatory mechanism of Lanqin oral solution based on the network pharmacology analysis optimized by Q-markers selection. Comput. Biol. Med. 2023, 154, 106607. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, C.; Zheng, Y.; Zhang, S.; Yan, J.; Cheng, F.; Wang, X.; Wang, Q.; Li, C. Refined Qingkailing protects the in vitro neurovascular unit against oxygen-glucose deprivation and re-oxygenation-induced injury. Biomed. Pharmacother. 2023, 167, 115580. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Jin, X.; Ma, Q.; Li, H.; Zhou, Q.; Chen, W. Bu-Shen-Ning-Xin decoction ameliorates premature ovarian insufficiency by suppressing oxidative stress through rno_circRNA_012284/rno_miR-760-3p/HBEGF pathway. Phytomedicine 2024, 133, 155920. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, G.; Wu, Y.; Hua, Y.; Ding, W.; Han, X.; Liu, B.; Zhou, C.; Li, A. Protective effects of Wenqingyin on sepsis-induced acute lung injury through regulation of the receptor for advanced glycation end products pathway. Phytomedicine 2024, 129, 155654. [Google Scholar] [CrossRef]
- Ma, S.; Wei, T.; Zhang, B.; Zhang, Y.; Lai, J.; Qu, J.; Liu, J.; Yin, P.; Shang, D. Integrated pharmacokinetic properties and tissue distribution of multiple active constituents in Qing-Yi Recipe: A comparison between granules and decoction. Phytomedicine 2024, 129, 155645. [Google Scholar] [CrossRef]
- Lai, M.; Sun, S.; Zuo, T.; Li, L.; Zhao, Q.; Li, W.; Zheng, J.; Hong, M. Sanfeng Tongqiao Dripping Pills alleviate House Dust Mite-induced allergic rhinitis in mice by inhibiting Th2 differentiation and repairing the nasal epithelial barrier. Phytomedicine 2024, 132, 155899. [Google Scholar] [CrossRef]
- Ma, W.; Liu, T.; Ogaji, O.D.; Li, J.; Du, K.; Chang, Y. Recent advances in Scutellariae Radix: A comprehensive review on ethnobotanical uses, processing, phytochemistry, pharmacological effects, quality control and influence factors of biosynthesis. Heliyon 2024, 10, e36146. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Wang, L.; Xiong, Z.-Y.; Gao, W.; Li, P.; Li, H.-J. Discovery of discriminatory quality control markers for Chinese herbal medicines and related processed products by combination of chromatographic analysis and chemometrics methods: Radix Scutellariae as a case study. J. Pharm. Biomed. Anal. 2017, 138, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bochoráková, H.; Paulová, H.; Slanina, J.; Musil, P.; Táborská, E. Main flavonoids in the root of Scutellaria baicalensis cultivated in Europe and their comparative antiradical properties. Phytother. Res. 2003, 17, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Chen, X.; Zhong, D. Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 2005, 78, 140–146. [Google Scholar] [CrossRef]
- Wu, H.; Long, X.; Yuan, F.; Chen, L.; Pan, S.; Liu, Y.; Stowell, Y.; Li, X. Combined use of phospholipid complexes and self-emulsifying microemulsions for improving the oral absorption of a BCS class IV compound, baicalin. Acta Pharm. Sin. B 2014, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Guo, J.; Zheng, X.; Wu, J.; Zhou, Y.; Yu, Y.; Ye, Y.; Zhang, L.; Zhao, L. Preparation, pharmacokinetics and biodistribution of baicalin-loaded liposomes. Int. J. Nanomed. 2014, 9, 3623–3630. [Google Scholar]
- Kim, Y.S.; Kim, J.J.; Cho, K.H.; Jung, W.S.; Moon, S.K.; Park, E.K.; Kim, D.H. Biotransformation of ginsenoside Rb1, crocin, amygdalin, geniposide, puerarin, ginsenoside Re, hesperidin, poncirin, glycyrrhizin, and baicalin by human fecal microflora and its relation to cytotoxicity against tumor cells. J. Microbiol. Biotechnol. 2008, 18, 1109–1114. [Google Scholar]
- Kim, D.H.; Jung, E.A.; Sohng, I.S.; Han, J.A.; Kim, T.H.; Han, M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharm. Res. 1998, 21, 17–23. [Google Scholar] [CrossRef]
- Mano, E.C.C.; Scott, A.L.; Honorio, K.M. UDP-glucuronosyltransferases: Structure, Function and Drug Design Studies. Curr. Med. Chem. 2018, 25, 3247–3255. [Google Scholar] [CrossRef]
- Kang, M.J.; Ko, G.S.; Oh, D.G.; Kim, J.S.; Noh, K.; Kang, W.; Yoon, W.K.; Kim, H.C.; Jeong, H.G.; Jeong, T.C. Role of metabolism by intestinal microbiota in pharmacokinetics of oral baicalin. Arch. Pharm. Res. 2014, 37, 371–378. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014, 354, 5–11. [Google Scholar] [CrossRef]
- Lai, M.Y.; Hsiu, S.L.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol. 2003, 55, 205–209. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, J.; Zheng, J.; Li, J.; Wei, T.; Zheng, Z.; Chen, Y. Down-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin. J. Exp. Clin. Cancer Res. 2012, 31, 48. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Man, K.; Tsao, S.W.; Che, C.M.; Feng, Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015, 6, e1942. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.; Su, M.; Zhou, Y.; Mao, K.; Li, C.; Peng, G.; Zhou, C.; Shen, B.; Dou, J. Baicalin induces cellular senescence in human colon cancer cells via upregulation of DEPP and the activation of Ras/Raf/MEK/ERK signaling. Cell Death Dis. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Zakki, S.A.; Cui, Z.G.; Sun, L.; Feng, Q.W.; Li, M.L.; Inadera, H. Baicalin Augments Hyperthermia-Induced Apoptosis in U937 Cells and Modulates the MAPK Pathway via ROS Generation. Cell Physiol. Biochem. 2018, 45, 2444–2460. [Google Scholar] [CrossRef]
- Kong, N.; Chen, X.; Feng, J.; Duan, T.; Liu, S.; Sun, X.; Chen, P.; Pan, T.; Yan, L.; Jin, T.; et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin. B 2021, 11, 4045–4054. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Jiang, Z.B.; Xu, C.; Meng, W.Y.; Liu, P.; Zhang, Y.Z.; Xie, C.; Xu, J.Y.; Xie, Y.J.; Liang, T.L.; et al. Andrographolide suppresses non-small-cell lung cancer progression through induction of autophagy and antitumor immune response. Pharmacol. Res. 2022, 179, 106198. [Google Scholar] [CrossRef]
- Shehatta, N.H.; Okda, T.M.; Omran, G.A.; Abd-Alhaseeb, M.M. Baicalin; a promising chemopreventive agent, enhances the antitumor effect of 5-FU against breast cancer and inhibits tumor growth and angiogenesis in Ehrlich solid tumor. Biomed Pharmacother. 2022, 146, 112599. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Zhou, Z.; Chung, J.; Zhang, G.; Chang, J.; Parise, R.A.; Chu, E.; Schmitz, J.C. Scutellaria baicalensis enhances 5-fluorouracil-based chemotherapy via inhibition of proliferative signaling pathways. Cell Commun. Signal 2023, 21, 147. [Google Scholar] [CrossRef]
- Sun, J.; Yang, X.; Sun, H.; Huang, S.; An, H.; Xu, W.; Chen, W.; Zhao, W.; He, C.; Zhong, X.; et al. Baicalin inhibits hepatocellular carcinoma cell growth and metastasis by suppressing ROCK1 signaling. Phytother. Res. 2023, 37, 4117–4132. [Google Scholar] [CrossRef]
- Wen, R.J.; Dong, X.; Zhuang, H.W.; Pang, F.X.; Ding, S.C.; Li, N.; Mai, Y.X.; Zhou, S.T.; Wang, J.Y.; Zhang, J.F. Baicalin induces ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4 regulatory axis. Phytomedicine 2023, 116, 154881. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Khan, S.U.; Yan, J.; Lu, J.; Yang, C.; Tong, Q. Baicalin enhances the efficacy of 5-Fluorouracil in gastric cancer by promoting ROS-mediated ferroptosis. Biomed. Pharmacother. 2023, 164, 114986. [Google Scholar] [CrossRef] [PubMed]
- He, X.W.; Yu, D.; Li, W.L.; Zheng, Z.; Lv, C.L.; Li, C.; Liu, P.; Xu, C.Q.; Hu, X.F.; Jin, X.P. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 2016, 83, 257–264. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, J.; Mori, T.; Smith-Powell, L.; Synold, T.W.; Chen, S.; Wen, W. Baicalin increases VEGF expression and angiogenesis by activating the ERRα/PGC-1αpathway. Cardiovasc. Res. 2011, 89, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Jiang, S.; Huang, C.; Shen, A.; Zhang, X.; Yang, W.; Xiao, Y.; Gao, S.; Du, R.; Zheng, G.; et al. Baicalin inhibits pressure overload-induced cardiac hypertrophy by regulating the SIRT3-dependent signaling pathway. Phytomedicine 2023, 114, 154747. [Google Scholar] [CrossRef]
- Huang, X.; Wu, P.; Huang, F.; Xu, M.; Chen, M.; Huang, K.; Li, G.P.; Xu, M.; Yao, D.; Wang, L. Baicalin attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A(2A) receptor-induced SDF-1/CXCR4/PI3K/AKT signaling. J. Biomed. Sci. 2017, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, Y.; Chu, J.; Wu, M.; Yan, M.; Wang, D.; Xie, Q.; Ali, F.; Fang, Y.; Wei, L.; et al. Baicalin attenuates angiotensin II-induced blood pressure elevation and modulates MLCK/p-MLC signaling pathway. Biomed. Pharmacother. 2021, 143, 112124. [Google Scholar] [CrossRef]
- Xiong, P.; Chen, X.; Guo, C.; Zhang, N.; Ma, B. Baicalin and deferoxamine alleviate iron accumulation in different brain regions of Parkinson’s disease rats. Neural Regen. Res. 2012, 7, 2092–2098. [Google Scholar]
- Bitto, A.; Giuliani, D.; Pallio, G.; Irrera, N.; Vandini, E.; Canalini, F.; Zaffe, D.; Ottani, A.; Minutoli, L.; Rinaldi, M.; et al. Effects of COX1-2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm. Res. 2017, 66, 389–398. [Google Scholar] [CrossRef]
- Ai, R.S.; Xing, K.; Deng, X.; Han, J.J.; Hao, D.X.; Qi, W.H.; Han, B.; Yang, Y.N.; Li, X.; Zhang, Y. Baicalin Promotes CNS Remyelination via PPARγ Signal Pathway. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1142. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, X.; Bi, X.Y.; Yang, H.; Zhang, Q. Baicalin attenuates blood-spinal cord barrier disruption and apoptosis through PI3K/Akt signaling pathway after spinal cord injury. Neural Regen. Res. 2022, 17, 1080–1087. [Google Scholar] [PubMed]
- Lee, H.; Kang, R.; Hahn, Y.; Yang, Y.; Kim, S.S.; Cho, S.H.; Chung, S.I.; Yoon, Y. Antiobesity effect of baicalin involves the modulations of proadipogenic and antiadipogenic regulators of the adipogenesis pathway. Phytother. Res. 2009, 23, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.X.; Liu, D.H.; Ma, Y.; Liu, J.F.; Wang, Y.; Du, Z.Y.; Wang, X.; Shen, J.K.; Peng, H.L. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol. Sin. 2009, 30, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liang, K.; Zhao, S.; Jia, W.; Liu, Y.; Wu, H.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S.; et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2018, 115, E5896–E5905. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Deng, X.; Zhang, N.; Liu, B.; Xin, S.; Li, G.; Xu, K. Baicalin attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, inflammation and fibrosis in mice. Life Sci. 2018, 192, 46–54. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Hao, Z.; Sun, N.; Guo, J.; Zheng, X.; Sun, P.; Yin, W.; Fan, K.; Li, H. Baicalin ameliorates high fat diet-induced nonalcoholic fatty liver disease in mice via adenosine monophosphate-activated protein kinase-mediated regulation of SREBP1/Nrf2/NF-κB signaling pathways. Phytother. Res. 2023, 37, 2405–2418. [Google Scholar] [CrossRef]
- Liu, W.J.; Chen, W.W.; Chen, J.Y.; Sun, Y.B.; Chang, D.; Wang, C.X.; Xie, J.D.; Lin, W.; Li, S.H.; Xu, W.; et al. Baicalin attenuated metabolic dysfunction-associated fatty liver disease by suppressing oxidative stress and inflammation via the p62-Keap1-Nrf2 signalling pathway in db/db mice. Phytother. Res. 2023, 39, 1663–1678. [Google Scholar] [CrossRef]
- Nam, J.E.; Jo, S.Y.; Ahn, C.W.; Kim, Y.S. Baicalin attenuates fibrogenic process in human renal proximal tubular cells (HK-2) exposed to diabetic milieu. Life Sci. 2020, 254, 117742. [Google Scholar] [CrossRef]
- Hu, H.; Li, W.; Hao, Y.; Peng, Z.; Zou, Z.; Liang, W. Baicalin ameliorates renal fibrosis by upregulating CPT1α-mediated fatty acid oxidation in diabetic kidney disease. Phytomedicine 2024, 122, 155162. [Google Scholar] [CrossRef]
- Li, Z.; Xia, X.; Zhang, S.; Zhang, A.; Bo, W.; Zhou, R. Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed. Pharmacother. 2009, 63, 120–128. [Google Scholar] [CrossRef]
- Ma, C.; Ma, Z.; Fu, Q.; Ma, S. Anti-asthmatic effects of baicalin in a mouse model of allergic asthma. Phytother. Res. 2014, 28, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhai, L.; Peng, J.; Wu, H.; Bian, Z.; Xiao, H. Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomed. Pharmacother. 2021, 141, 111931. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, J.S.; Choi, J.S.; Nam, Y.J.; Han, J.H.; Byun, H.D.; Song, M.J.; Oh, J.S.; Kim, S.G.; Choi, Y. Identification and Characterization of Baicalin as a Phosphodiesterase 4 Inhibitor. Phytother. Res. 2016, 30, 144–151. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Wang, H.; Sui, H.H.; Li, L.; Zhou, C.L.; Huang, J.J. Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic rhinitis guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflamm. Res. 2016, 65, 603–612. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, S.; He, W.X.; Lu, J.L.; Xu, Y.J.; Yang, J.Y.; Liu, D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017, 186, 125–132. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Gao, Z.; Yu, C.; Zhang, L. Baicalin alleviates IL-1β-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed. Pharmacother. 2018, 99, 184–190. [Google Scholar] [CrossRef]
- Ji, W.; Liang, K.; An, R.; Wang, X. Baicalin protects against ethanol-induced chronic gastritis in rats by inhibiting Akt/NF-κB pathway. Life Sci. 2019, 239, 117064. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Yang, J.; Li, M. Baicalin ameliorates lupus autoimmunity by inhibiting differentiation of Tfh cells and inducing expansion of Tfr cells. Cell Death Dis. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, S.; Wang, Y.; Zhu, J.; Li, X. Potential mechanism of oral baicalin treating psoriasis via suppressing Wnt signaling pathway and inhibiting Th17/IL-17 axis by activating PPARγ. Phytother. Res. 2022, 36, 3969–3987. [Google Scholar] [CrossRef]
- Picciolo, G.; Mannino, F.; Irrera, N.; Minutoli, L.; Altavilla, D.; Vaccaro, M.; Oteri, G.; Squadrito, F.; Pallio, G. Reduction of oxidative stress blunts the NLRP3 inflammatory cascade in LPS stimulated human gingival fibroblasts and oral mucosal epithelial cells. Biomed. Pharmacother. 2022, 146, 112525. [Google Scholar] [CrossRef]
- Braicu, C.; Zanoaga, O.; Zimta, A.-A.; Tigu, A.B.; Kilpatrick, K.L.; Bishayee, A.; Nabavi, S.M.; Berindan-Neagoe, I. Natural compounds modulate the crosstalk between apoptosis- and autophagy-regulated signaling pathways: Controlling the uncontrolled expansion of tumor cells. Semin. Cancer Biol. 2022, 80, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Sawabu, N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994, 86, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Mizoguchi, A.; Fukuda, K.; Haramaki, M.; Ogasawara, S.; Momosaki, S.; Kojiro, M. The herbal medicine sho-saiko-to inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Res. 1994, 54, 448–454. [Google Scholar]
- Kato, M.; Liu, W.; Yi, H.; Asai, N.; Hayakawa, A.; Kozaki, K.; Takahashi, M.; Nakashima, I. The herbal medicine Sho-saiko-to inhibits growth and metastasis of malignant melanoma primarily developed in ret-transgenic mice. J. Investig. Dermatol. 1998, 111, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef]
- Chen, S.; Ruan, Q.; Bedner, E.; Deptala, A.; Wang, X.; Hsieh, T.C.; Traganos, F.; Darzynkiewicz, Z. Effects of the flavonoid baicalin and its metabolite baicalein on androgen receptor expression, cell cycle progression and apoptosis of prostate cancer cell lines. Cell Prolif. 2001, 34, 293–304. [Google Scholar] [CrossRef]
- Wu, Y.X.; Sato, E.; Kimura, W.; Miura, N. Baicalin and scutellarin are proteasome inhibitors that specifically target chymotrypsin-like catalytic activity. Phytother. Res. 2013, 27, 1362–1367. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Meena, A.; Luqman, S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Wang, S.; Zhang, H.; Lu, Y.Y.; Wang, X.F.; Motoo, Y.; Su, S.B. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar] [CrossRef]
- Wang, C.Z.; Li, X.L.; Wang, Q.F.; Mehendale, S.R.; Yuan, C.S. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine 2010, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, X.; Lin, D.; Zhang, L.; Wu, Y.; Chang, Y.; Jin, M.; Huang, G. Baicalin induces cell death of non-small cell lung cancer cells via MCOLN3-mediated lysosomal dysfunction and autophagy blockage. Phytomedicine 2024, 133, 155872. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Ge, A.; Chen, Z.; Bao, T.; Long, Z.; Ge, J.; Huang, L. Investigating the regulation mechanism of baicalin on triple negative breast cancer’s biological network by a systematic biological strategy. Biomed. Pharmacother. 2019, 118, 109253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, B.; Sun, J.; Lu, L.; Liu, L.; Qiu, J.; Li, Q.; Yan, C.; Jiang, S.; Mohammadtursun, N.; et al. Scutellaria Flavonoids Effectively Inhibit the Malignant Phenotypes of Non-small Cell Lung Cancer in an Id1-dependent Manner. Int. J. Biol. Sci. 2019, 15, 1500–1513. [Google Scholar] [CrossRef]
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, L.; Zhu, J.; Zhang, Y.; Yang, R.; Yan, J.; Huang, R.; Zheng, C.; Xiao, W.; Huang, C.; et al. Predicting the herbal medicine triggering innate anti-tumor immunity from a system pharmacology perspective. Biomed. Pharmacother. 2021, 143, 112105. [Google Scholar] [CrossRef]
- Han, S.; Wang, W.; Wang, S.; Yang, T.; Zhang, G.; Wang, D.; Ju, R.; Lu, Y.; Wang, H.; Wang, L. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 2021, 11, 2892–2916. [Google Scholar] [CrossRef]
- Gong, S.Q.; Sun, W.; Wang, M.; Fu, Y.Y. Role of TLR4 and TCR or BCR against baicalin-induced responses in T and B cells. Int. Immunopharmacol. 2011, 11, 2176–2180. [Google Scholar] [CrossRef]
- Lin, M.Y.; Cheng, W.T.; Cheng, H.C.; Chou, W.C.; Chen, H.I.; Ou, H.C.; Tsai, K.L. Baicalin Enhances Chemosensitivity to Doxorubicin in Breast Cancer Cells via Upregulation of Oxidative Stress-Mediated Mitochondria-Dependent Apoptosis. Antioxidants 2021, 10, 1506. [Google Scholar] [CrossRef]
- Wang, G.; Liang, J.; Gao, L.R.; Si, Z.P.; Zhang, X.T.; Liang, G.; Yan, Y.; Li, K.; Cheng, X.; Bao, Y.; et al. Baicalin administration attenuates hyperglycemia-induced malformation of cardiovascular system. Cell Death Dis. 2018, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, X.D.; Davey, A.K.; Wang, J. The anti-inflammatory effect of baicalin on hypoxia/reoxygenation and TNF-alpha induced injury in cultural rat cardiomyocytes. Phytother. Res. 2010, 24, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, Q.; Qi, J.; Yu, H.; Wang, C.; Wang, X.; Ren, Y.; Yang, F. Promoting effect of baicalin on nitric oxide production in CMECs via activating the PI3K-AKT-eNOS pathway attenuates myocardial ischemia-reperfusion injury. Phytomedicine 2019, 63, 153035. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Jiang, L.; Yan, Q.; Zhou, C.; Guo, X.; Chen, T.; Ma, S.; Luo, Y.; Hu, C.; Yang, F.; et al. Evidence construction of baicalin for treating myocardial ischemia diseases: A preclinical meta-analysis. Phytomedicine 2022, 107, 154476. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Dong, L.H.; Wen, J.K.; Miao, S.B.; Jia, Z.; Hu, H.J.; Sun, R.H.; Wu, Y.; Han, M. Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRβ-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res. 2010, 20, 1252–1262. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Fan, L.; Zhang, W.; Wang, T.; Du, Y.; Bai, X. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways. Biomed. Pharmacother. 2018, 97, 1673–1679. [Google Scholar] [CrossRef]
- Paudel, K.R.; Kim, D.W. Microparticles-Mediated Vascular Inflammation and its Amelioration by Antioxidant Activity of Baicalin. Antioxidants 2020, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Daghbouche-Rubio, N.; Lopez-Lopez, J.R.; Perez-Garcia, M.T.; Cidad, P. Vascular smooth muscle ion channels in essential hypertension. Front. Physiol. 2022, 13, 1016175. [Google Scholar] [CrossRef]
- Lin, Y.L.; Dai, Z.K.; Lin, R.J.; Chu, K.S.; Chen, I.J.; Wu, J.R.; Wu, B.N. Baicalin, a flavonoid from Scutellaria baicalensis Georgi, activates large-conductance Ca2+-activated K+ channels via cyclic nucleotide-dependent protein kinases in mesenteric artery. Phytomedicine 2010, 17, 760–770. [Google Scholar] [CrossRef]
- Ding, L.; Jia, C.; Zhang, Y.; Wang, W.; Zhu, W.; Chen, Y.; Zhang, T. Baicalin relaxes vascular smooth muscle and lowers blood pressure in spontaneously hypertensive rats. Biomed. Pharmacother. 2019, 111, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhang, S.; Li, C.; Liu, Y.; Zhao, J.; Wang, Y.; Yang, Y.; Zhang, L. 5-Lipoxygenase as an emerging target against age-related brain disorders. Ageing Res. Rev. 2021, 69, 101359. [Google Scholar] [CrossRef]
- Jiang, H.; Lv, P.; Li, J.; Wang, H.; Zhou, T.; Liu, Y.; Lin, W. Baicalin inhibits colistin sulfate-induced apoptosis of PC12 cells. Neural Regen. Res. 2013, 8, 2597–2604. [Google Scholar]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Huang, X.; Chen, W. The Effects of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging Dis. 2017, 8, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Wan, J.; Yang, Q.; Xiang, Y.; Ni, L.; Long, Y.; Cui, M.; Ci, Z.; Tang, D.; et al. Preparation, Characterization and in vivo Study of Borneol-Baicalin-Liposomes for Treatment of Cerebral Ischemia-Reperfusion Injury. Int. J. Nanomed. 2020, 15, 5977–5989. [Google Scholar] [CrossRef]
- Long, Y.; Liu, S.; Wan, J.; Zhang, Y.; Li, D.; Yu, S.; Shi, A.; Li, N.; He, F. Brain targeted borneol-baicalin liposome improves blood-brain barrier integrity after cerebral ischemia-reperfusion injury via inhibiting HIF-1α/VEGF/eNOS/NO signal pathway. Biomed. Pharmacother. 2023, 160, 114240. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Ma, C.; Li, T.; Yang, L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022, 29, 1282–1298. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, L.; Wang, L.P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef]
- Dai, J.; Qiu, Y.M.; Ma, Z.W.; Yan, G.F.; Zhou, J.; Li, S.Q.; Wu, H.; Jin, Y.C.; Zhang, X.H. Neuroprotective effect of baicalin on focal cerebral ischemia in rats. Neural Regen. Res. 2018, 13, 2129–2133. [Google Scholar] [PubMed]
- Tang, W.; Sun, X.; Fang, J.S.; Zhang, M.; Sucher, N.J. Flavonoids from Radix Scutellariae as potential stroke therapeutic agents by targeting the second postsynaptic density 95 (PSD-95)/disc large/zonula occludens-1 (PDZ) domain of PSD-95. Phytomedicine 2004, 11, 277–284. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, Z.; Zhang, X.Y.; Ying, K.; Liu, J.X.; Wang, Y.Y. Gene expression profile induced by oral administration of baicalin and gardenin after focal brain ischemia in rats. Acta Pharmacol. Sin. 2005, 26, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Q.; Liu, Q.; Xu, W.J.; Yu, Y.N.; Zhang, Y.Y.; Li, B.; Liu, J.; Wang, Z. Pure mechanistic analysis of additive neuroprotective effects between baicalin and jasminoidin in ischemic stroke mice. Acta Pharmacol. Sin. 2018, 39, 961–974. [Google Scholar] [CrossRef]
- Kostandy, B.B. The role of glutamate in neuronal ischemic injury: The role of spark in fire. Neurol. Sci. 2012, 33, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Shigeri, Y.; Seal, R.P.; Shimamoto, K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res. Brain Res. Rev. 2004, 45, 250–265. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Li, Y.L.; Ao, Z.B.; Wen, Z.L.; Chen, Q.W.; Huang, Z.G.; Xiao, B.; Yan, X.H. Baicalin protects neonatal rat brains against hypoxic-ischemic injury by upregulating glutamate transporter 1 via the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neural Regen. Res. 2017, 12, 1625–1631. [Google Scholar]
- Song, X.; Gong, Z.; Liu, K.; Kou, J.; Liu, B.; Liu, K. Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation. Redox Biol. 2020, 34, 101559. [Google Scholar] [CrossRef]
- Ge, Q.F.; Hu, X.; Ma, Z.Q.; Liu, J.R.; Zhang, W.P.; Chen, Z.; Wei, E.Q. Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons. Pharmacol. Res. 2007, 55, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T.; Zhang, W.P.; Fang, S.H.; Lu, Y.B.; Zhang, L.H.; Qi, L.L.; Huang, X.Q.; Huang, X.J.; Wei, E.Q. Baicalin attenuates oxygen-glucose deprivation-induced injury by inhibiting oxidative stress-mediated 5-lipoxygenase activation in PC12 cells. Acta Pharmacol. Sin. 2010, 31, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.K.; Yang, W.Z.; Shi, S.S.; Wang, C.H.; Chen, C.M. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem. Res. 2009, 34, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yuan, Z.Y.; Wang, Y.G.; Wan, H.J.; Hu, J.; Chai, Y.S.; Lei, F.; Xing, D.M.; Du, L.J. Role of baicalin in regulating Toll-like receptor 2/4 after ischemic neuronal injury. Chin. Med. J. 2012, 125, 1586–1593. [Google Scholar]
- Chen, H.; Guan, B.; Chen, X.; Chen, X.; Li, C.; Qiu, J.; Yang, D.; Liu, K.J.; Qi, S.; Shen, J. Baicalin Attenuates Blood-Brain Barrier Disruption and Hemorrhagic Transformation and Improves Neurological Outcome in Ischemic Stroke Rats with Delayed t-PA Treatment: Involvement of ONOO(-)-MMP-9 Pathway. Transl. Stroke Res. 2018, 9, 515–529. [Google Scholar] [CrossRef]
- Chen, H.S.; Chen, X.; Li, W.T.; Shen, J.G. Targeting RNS/caveolin-1/MMP signaling cascades to protect against cerebral ischemia-reperfusion injuries: Potential application for drug discovery. Acta Pharmacol. Sin. 2018, 39, 669–682. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Guo, C.; Chen, X.; Xiong, P. Baicalin suppresses iron accumulation after substantia nigra injury: Relationship between iron concentration and transferrin expression. Neural Regen. Res. 2014, 9, 630–636. [Google Scholar]
- Kumar, H.; Ropper, A.E.; Lee, S.H.; Han, I. Propitious Therapeutic Modulators to Prevent Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury. Mol. Neurobiol. 2017, 54, 3578–3590. [Google Scholar] [CrossRef]
- Herrman, H.; Kieling, C.; Mcgorry, P.; Horton, R.; Sargent, J.; Patel, V. Reducing the global burden of depression: A Lancet-World Psychiatric Association Commission. Lancet 2019, 393, e42–e43. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, J.; Zou, D.; Yang, L.; Wang, Y. Baicalin influences the dendritic morphology of newborn neurons in the hippocampus of chronically stressed rats. Neural Regen. Res. 2013, 8, 496–505. [Google Scholar] [PubMed]
- Yu, H.Y.; Yin, Z.J.; Yang, S.J.; Ma, S.P.; Qu, R. Baicalin Reverses Depressive-Like Behaviours and Regulates Apoptotic Signalling Induced by Olfactory Bulbectomy. Phytother. Res. 2016, 30, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, Z.; Liu, K.; Li, Y.; Liu, D.; Xu, L.; Deng, X.; Qu, R.; Ma, Z.; Ma, S. Baicalin exerts antidepressant effects through Akt/FOXG1 pathway promoting neuronal differentiation and survival. Life Sci. 2019, 221, 241–248. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, G.; Yang, F.; Guan, Z.; Zhang, Z.; Zhao, J.; Liu, Y.; Chu, L.; Pei, L. Baicalin regulates depression behavior in mice exposed to chronic mild stress via the Rac/LIMK/cofilin pathway. Biomed. Pharmacother. 2019, 116, 109054. [Google Scholar] [CrossRef]
- Guo, L.T.; Wang, S.Q.; Su, J.; Xu, L.X.; Ji, Z.Y.; Zhang, R.Y.; Zhao, Q.W.; Ma, Z.Q.; Deng, X.Y.; Ma, S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflam. 2019, 16, 95. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, F.; Guan, X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-kB signaling pathway in olfactory bulbectomized rats. Phytother. Res. 2019, 33, 1480–1489. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, J.; Han, X.; Ma, B.; Yuan, H.; Song, Y. Baicalin regulates the dopamine system to control the core symptoms of ADHD. Mol. Brain 2019, 12, 11. [Google Scholar] [CrossRef]
- Wu, J.; Cao, M.; Peng, Y.; Dong, B.; Jiang, Y.; Hu, C.; Zhu, P.; Xing, W.; Yu, L.; Xu, R.; et al. Research progress on the treatment of epilepsy with traditional Chinese medicine. Phytomedicine 2023, 120, 155022. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Xu, H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef]
- Fang, P.; Sun, Y.; Gu, X.; Shi, M.; Bo, P.; Zhang, Z.; Bu, L. Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC-1α pathway. Phytomedicine 2019, 64, 153074. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Lou, M.; Xia, W.; Liu, Q.; Xie, G.; Liu, L.; Liu, B.; Yang, J.; Qin, M. Baicalin regulates SirT1/STAT3 pathway and restrains excessive hepatic glucose production. Pharmacol. Res. 2018, 136, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Han, S.; Wang, M.; Han, L.; Huang, Y.; Bo, P.; Fang, P.; Zhang, Z. Baicalin protects against insulin resistance and metabolic dysfunction through activation of GALR2/GLUT4 signaling. Phytomedicine 2022, 95, 153869. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. The Anti-Diabetic Potential of Baicalin: Evidence from Rodent Studies. Int. J. Mol. Sci. 2023, 25, 431. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Lancaster, G.I. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. J. Physiol. 2016, 594, 267–279. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising influences of Scutellaria baicalensis and its two active constituents, baicalin, and baicalein, against metabolic syndrome: A review. Phytother. Res. 2021, 35, 3558–3574. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, W.; Guo, D.; Tan, Z.R.; Xu, P.; Li, Q.; Liu, Y.Z.; Zhang, L.; He, T.Y.; Hu, D.L.; et al. The effect of herbal medicine baicalin on pharmacokinetics of rosuvastatin, substrate of organic anion-transporting polypeptide 1B1. Clin. Pharmacol. Ther. 2008, 83, 471–476. [Google Scholar] [CrossRef]
- Li, J.Z.; Chen, N.; Ma, N.; Li, M.R. Mechanism and Progress of Natural Products in the Treatment of NAFLD-Related Fibrosis. Molecules 2023, 28, 7936. [Google Scholar] [CrossRef]
- Li, H.T.; Wu, X.D.; Davey, A.K.; Wang, J. Antihyperglycemic effects of baicalin on streptozotocin—Nicotinamide induced diabetic rats. Phytother. Res. 2011, 25, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, H.; Cao, S.; Chen, Q.; Cui, M.; Wang, Z.; Li, D.; Zhou, J.; Wang, T.; Qiu, F.; et al. Baicalin and its metabolites suppresses gluconeogenesis through activation of AMPK or AKT in insulin resistant HepG-2 cells. Eur. J. Med. Chem. 2017, 141, 92–100. [Google Scholar] [CrossRef]
- Yang, L.L.; Xiao, N.; Liu, J.; Liu, K.; Liu, B.; Li, P.; Qi, L.W. Differential regulation of baicalin and scutellarin on AMPK and Akt in promoting adipose cell glucose disposal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yu, M.; Min, W.; Wan, D.; Han, S.; Shan, Y.; Wang, R.; Shi, M.; Zhang, Z.; Bo, P. Effect of baicalin on GLUT4 expression and glucose uptake in myotubes of rats. Life Sci. 2018, 196, 156–161. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, X.; Zhang, H.; Cheong, M.S.; Chen, X.; Farag, M.A.; Cheang, W.S.; Xiao, J. Baicalin ameliorates insulin resistance and regulates hepatic glucose metabolism via activating insulin signaling pathway in obese pre-diabetic mice. Phytomedicine 2023, 124, 155296. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications—A unified mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Waisundara, V.Y.; Siu, S.Y.; Hsu, A.; Huang, D.; Tan, B.K. Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci. 2011, 88, 1016–1025. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, C.; Liu, W.; Chen, J.; Sun, Y.; Chang, D.; Wang, H.; Xu, W.; Lu, J.J.; Zhou, X.; et al. Upregulation of Nrf2 signaling: A key molecular mechanism of Baicalin’s neuroprotective action against diabetes-induced cognitive impairment. Biomed. Pharmacother. 2024, 174, 116579. [Google Scholar] [CrossRef]
- Jiang, H.; Yao, Q.; An, Y.; Fan, L.; Wang, J.; Li, H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m6A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase3 pathway. Phytomedicine 2022, 94, 153823. [Google Scholar] [CrossRef]
- Qin, W.; Shang, Q.; Shen, G.; Li, B.; Zhang, P.; Zhang, Y.; Zhao, W.; Chen, H.; Liu, H.; Xie, B.; et al. Restoring bone-fat equilibrium: Baicalin’s impact on P38 MAPK pathway for treating diabetic osteoporosis. Biomed. Pharmacother. 2024, 175, 116571. [Google Scholar] [CrossRef]
- Yang, R.; Wang, R.; Xu, A.; Zhang, J.; Ma, J. Mitigating neurodegenerative diseases: The protective influence of baicalin and baicalein through neuroinflammation regulation. Front. Pharmacol. 2024, 15, 1425731. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.B.; Vago, J.P.; Beltrami, V.A.; Araújo, J.M.D.; Grespan, R.; Teixeira, M.M.; Pinho, V. Biochanin A as a modulator of the inflammatory response: An updated overview and therapeutic potential. Pharmacol. Res. 2022, 180, 106246. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef] [PubMed]
- Živanović, N.; Lesjak, M.; Simin, N.; Srai, S.K.S. Beyond Mortality: Exploring the Influence of Plant Phenolics on Modulating Ferroptosis-A Systematic Review. Antioxidants 2024, 13, 334. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhu, Z.Q.; Hu, T.X.; Zhu, D.Y. Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects. Acta Pharmacol. Sin. 2002, 23, 667–672. [Google Scholar] [PubMed]
- Altavilla, D.; Squadrito, F.; Bitto, A.; Polito, F.; Burnett, B.P.; Di Stefano, V.; Minutoli, L. Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophages. Br. J. Pharmacol. 2009, 157, 1410–1418. [Google Scholar] [CrossRef]
- Dai, S.X.; Zou, Y.; Feng, Y.L.; Liu, H.B.; Zheng, X.B. Baicalin down-regulates the expression of macrophage migration inhibitory factor (MIF) effectively for rats with ulcerative colitis. Phytother. Res. 2012, 26, 498–504. [Google Scholar] [CrossRef]
- An, H.J.; Lee, J.Y.; Park, W. Baicalin Modulates Inflammatory Response of Macrophages Activated by LPS via Calcium-CHOP Pathway. Cells 2022, 11, 3076. [Google Scholar] [CrossRef]
- Zou, Y.; Dai, S.X.; Chi, H.G.; Li, T.; He, Z.W.; Wang, J.; Ye, C.G.; Huang, G.L.; Zhao, B.; Li, W.Y.; et al. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch. Pharm. Res. 2015, 38, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiao, Z.; Yan, H.; Lu, X.; Zhang, X.; Luo, L.; Long, C.; Zhu, Y. Baicalin suppresses Th1 and Th17 responses and promotes Treg response to ameliorate sepsis-associated pancreatic injury via the RhoA-ROCK pathway. Int. Immunopharmacol. 2020, 86, 106685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qu, F.; Burrows, E.; Yu, Y.; Nan, R. Acupuncture can improve absorption of baicalin from extracts of Scutellaria baicalensis Georgi in rats. Phytother. Res. 2009, 23, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.; Li, J.; Liu, R.; Shu, L.; Jin, J. Effects of Labrasol on the corneal drug delivery of baicalin. Drug Deliv. 2009, 16, 399–404. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Liu, H.; Yang, Q.; Yao, K.; Wang, X.; Wang, L.; Yang, X. Effect of Low Molecular Weight Chitosans on Drug Permeation through Mouse Skin: 1. Transdermal Delivery of Baicalin. J. Pharm. Sci. 2010, 99, 2991–2998. [Google Scholar] [CrossRef]
- Chalasani, N.; Vuppalanchi, R.; Navarro, V.; Fontana, R.; Bonkovsky, H.; Barnhart, H.; Kleiner, D.E.; Hoofnagle, J.H. Acute liver injury due to flavocoxid (Limbrel), a medical food for osteoarthritis: A case series. Ann. Intern. Med. 2012, 156, 857–860. [Google Scholar] [CrossRef]
- Kim, M.S.; Ham, S.H.; Kim, J.H.; Shin, J.E.; Oh, J.; Kim, T.W.; Yun, H.I.; Lim, J.H.; Jang, B.S.; Cho, J.H. Single-dose oral toxicity of fermented Scutellariae Radix extract in rats and dogs. Toxicol. Res. 2012, 28, 263–268. [Google Scholar] [CrossRef]
- Hwang, J.M.; Wang, C.J.; Chou, F.P.; Tseng, T.H.; Hsieh, Y.S.; Hsu, J.D.; Chu, C.Y. Protective effect of baicalin on tert-butyl hydroperoxide-induced rat hepatotoxicity. Arch. Toxicol. 2005, 79, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Gong, L.K.; Wang, H.; Xiao, Y.; Wu, X.F.; Zhang, Y.H.; Xue, X.; Qi, X.M.; Ren, J. Baicalin protects mouse from Concanavalin A-induced liver injury through inhibition of cytokine production and hepatocyte apoptosis. Liver Int. 2007, 27, 582–591. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, S.; Huang, Z.; Hu, F.; Zhang, T.; Wei, M.; Bai, Q.; Lu, B.; Ji, L. Baicalin promotes liver regeneration after acetaminophen-induced liver injury by inducing NLRP3 inflammasome activation. Free Radic. Biol. Med. 2020, 160, 163–177. [Google Scholar] [CrossRef]
- Yang, M.D.; Chiang, Y.M.; Higashiyama, R.; Asahina, K.; Mann, D.A.; Mann, J.; Wang, C.C.; Tsukamoto, H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor γ in hepatic stellate cells for their antifibrotic effect. Hepatology 2012, 55, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yin, X.; Yuan, J.; Liang, Z.; Song, J.; Li, Y.; Peng, C.; Hylands, P.J.; Zhao, Z.; Xu, Q. Antifibrotic activities of Scutellariae Radix extracts and flavonoids: Comparative proteomics reveals distinct and shared mechanisms. Phytomedicine 2022, 100, 154049. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, R.A.; Zaghloul, A.M.; El-Kashef, D.H. Hepatoprotective effect of Baicalin against thioacetamide-induced cirrhosis in rats: Targeting NOX4/NF-κB/NLRP3 inflammasome signaling pathways. Life Sci. 2022, 295, 120410. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).