Evaluation of Type 2 Diabetes Risk in Individuals With or Without Metabolically Healthy Obesity
Simple Summary
Abstract
1. Introduction
2. Methods
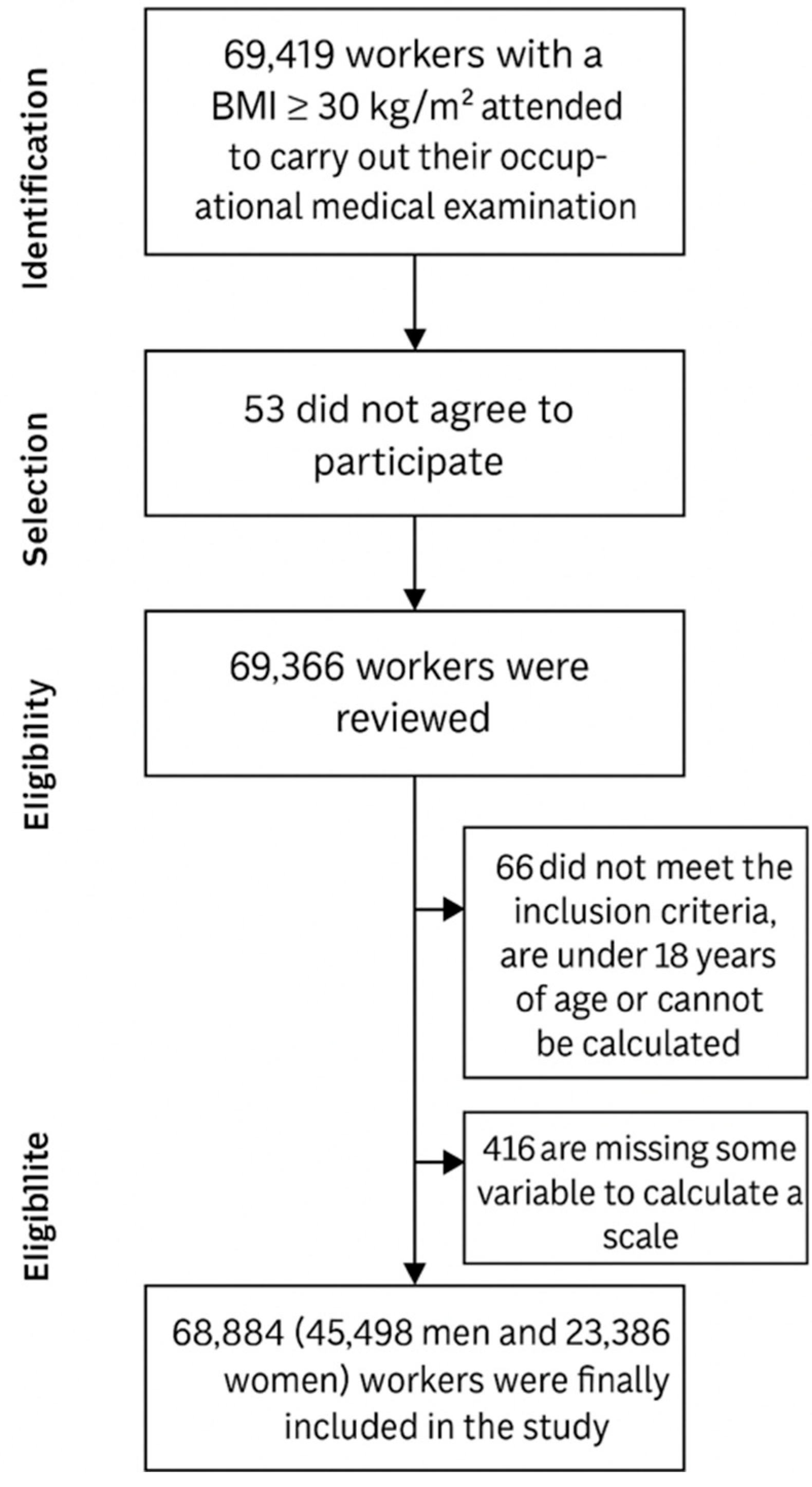
2.1. Study Design and Population
2.2. Inclusion Criteria
- Body mass index (BMI) equal to or greater than 30 kg/m2, indicating obesity;
- Aged between 18 and 69 years;
- Employed by one of the companies involved in the research;
- Provided informed consent to participate.
2.3. Measurement of Variables
2.4. Definition of Metabolically Healthy Obesity (MHO)
- Waist circumference ≥ 88 cm for women and ≥102 cm for men;
- Triglycerides ≥ 150 mg/dL or current treatment for elevated triglycerides;
- HDL-cholesterol < 50 mg/dL in women or <40 mg/dL in men;
- Fasting glucose ≥ 100 mg/dL or ongoing treatment for hyperglycemia;
- Systolic blood pressure ≥ 130 mmHg and/or diastolic ≥85 mmHg, or antihypertensive therapy.
- Participants were categorized into three MHO subgroups:
- Group A: No MetS components present;
- Group B: Presence of one MetS component;
- Group C: Presence of up to two MetS components.
2.5. Sociodemographic and Lifestyle Variables
- Class I: Executives, university-trained professionals, athletes, and artists;
- Class II: Intermediate-level professionals and skilled self-employed workers;
- Class III: Low-skilled laborers.
- Finrisk Score: This tool estimates diabetes risk based on several parameters, including age, gender, body mass index (BMI), waist circumference, levels of physical activity, consumption of fruits and vegetables, use of antihypertensive medication, previous episodes of hyperglycemia, and family history of diabetes. A score exceeding 15 is indicative of a high risk.
- QDiabetes Algorithm: This model incorporates variables such as age, sex, ethnicity, height, weight, fasting blood glucose, smoking status, prior stroke, family history of diabetes, use of blood pressure medications, mental health conditions (including schizophrenia or depression), and use of corticosteroids or statins. Additionally, a history of gestational diabetes or polycystic ovary syndrome is considered. Although this tool does not use a fixed threshold, in this study, a relative risk score of 3 or higher was defined as elevated.
- Canrisk Tool: This score is calculated based on factors such as age, sex, physical activity level, dietary intake of fruits and vegetables, personal history of hypertension or hyperglycemia, family history of diabetes, ethnic background, and educational level. Scores greater than 43 suggest a high probability of developing type 2 diabetes.
- TRAQ-D (Trinidad Risk Assessment Questionnaire for Diabetes): This tool requires input on age, gender, BMI, smoking habits, racial/ethnic background, and family history of diabetes to evaluate individual risk.
- Oman Diabetes Risk Score: This scale utilizes age, waist circumference, BMI, family history of diabetes, and current hypertension status to estimate risk.
2.6. Ethical Considerations
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Future Directions
4.2. Strengths and Limitations
4.2.1. Strengths
4.2.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 30 April 2025).
- López-González, A.A.; Tomás-Gil, P.; Coll Villalonga, J.L.; Martí-Lliteras, P.; González San Miguel, H.M.; Ramírez-Manent, J.I. Correlation between overweight and obesity scales and blood pressure values in 418.343 Spanish workers. Acad. J. Health Sci. 2023, 38, 44–51. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Rey-López, J.P.; de Rezende, L.F.; Pastor-Valero, M.; Tess, B.H. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obes. Rev. 2014, 15, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M. Metabolically healthy obesity across the life course: Epidemiology, determinants, and implications. Ann. N. Y. Acad. Sci. 2017, 1391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Alzamora, T.; Tomás-Gil, P.; Paublini, H.; Pallarés, L.; Ramírez-Manent, J.I.; López-González, A.A. Relationship between different scales of overweight and obesity and heart age values in 139634 spanish workers. Acad. J. Health Sci. 2023, 38, 137–144. [Google Scholar] [CrossRef]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32 (Suppl. S3), S56–S59. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Cadenas-Sánchez, C.; Sui, X.; Blair, S.N.; Lavie, C.J. Role of Fitness in the Metabolically Healthy But Obese Phenotype: A Review and Update. Prog. Cardiovasc. Dis. 2015, 58, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Funtikova, A.N.; Fíto, M.; Schröder, H. Can metabolically healthy obesity be explained by diet, genetics, and inflammation? Mol. Nutr. Food Res. 2015, 59, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Elías-López, D.; Vargas-Vázquez, A.; Mehta, R.; Cruz Bautista, I.; Del Razo Olvera, F.; Gómez-Velasco, D.; Almeda Valdes, P.; Aguilar-Salinas, C.A.; Metabolic Syndrome Study Group. Natural course of metabolically healthy phenotype and risk of developing Cardiometabolic diseases: A three years follow-up study. BMC Endocr. Disord. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, M.; Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, R.; Du, H.; Yang, L.; Chen, Y.; Li, Z.; et al. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: A cohort study. PLoS Med. 2020, 17, e1003351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.A.; Lim, K.; Lee, J.K.; Kang, D.; Shin, S. Metabolically healthy obesity and the risk of all-cause and cardiovascular disease mortality in a Korean population: A prospective cohort study. BMJ Open 2021, 11, e049063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, C.H.; Lee, W.J.; Song, K.H. Metabolically healthy obesity: A friend or foe? Korean J. Intern. Med. 2017, 32, 611–621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, J.A.; Hamer, M.; Sabia, S.; Singh-Manoux, A.; Batty, G.D.; Kivimaki, M. The natural course of healthy obesity over 20 years. J. Am. Coll. Cardiol. 2015, 65, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, Z.; Wei, M.; Feng, J.; Huang, Q.; Liu, Y.; Liu, Z.; Li, X.; Yin, L.; Xia, J. Metabolically healthy obesity, transition from meta-bolic healthy to unhealthy status, and carotid atherosclerosis. Diabetes Metab. Res. Rev. 2024, 40, e3766. [Google Scholar] [CrossRef]
- Tanriover, C.; Copur, S.; Gaipov, A.; Ozlusen, B.; Akcan, R.E.; Kuwabara, M.; Hornum, M.; Van Raalte, D.H.; Kanbay, M. Metabolically healthy obesity: Misleading phrase or healthy phenotype? Eur. J. Intern. Med. 2023, 111, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.D.; Falqueto, H.; Mânica, A.; Zanini, D.; de Oliveira, T.; de Sá, C.A.; Cardoso, A.M.; Manfredi, L.H. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J. Transl. Med. 2021, 19, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavie, C.J.; De Schutter, A.; Patel, D.A.; Milani, R.V. Body composition and fitness in the obesity paradox—Body mass index alone does not tell the whole story. Prev. Med. 2013, 57, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Camhi, S.M.; Waring, M.E.; Sisson, S.B.; Hayman, L.L.; Must, A. Physical activity and screen time in metabolically healthy obese phenotypes in adolescents and adults. J. Obes. 2013, 2013, 984613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsatsoulis, A.; Paschou, S.A. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agius, R.; Pace, N.P.; Fava, S. Phenotyping obesity: A focus on metabolically healthy obesity and metabolically unhealthy normal weight. Diabetes Metab. Res. Rev. 2024, 40, e3725. [Google Scholar] [CrossRef]
- Quinn, R.C.; Campisi, S.C.; McCrindle, B.W.; Korczak, D.J. Adolescent cardiometabolic risk scores: A scoping review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Schick, F.; Häring, H.U. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017, 26, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Johnston, D.G.; Godsland, I.F. Does metabolic health in overweight and obesity persist?—Individual variation and car-diovascular mortality over two decades. Eur. J. Endocrinol. 2016, 175, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Akyea, R.K.; Ntaios, G.; Doehner, W. Obesity, metabolic health and clinical outcomes after incident cardiovascular disease: A nationwide population-based cohort study. J. Cachexia Sarcopenia Muscle 2023, 14, 2653–2662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, C.J.; Chang, Y.C.; Hsu, H.Y.; Tsai, M.C.; Hsu, L.Y.; Hwang, L.C.; Chien, K.L.; Yeh, T.L. Metabolically healthy overweight/obesity and cancer risk: A representative cohort study in Taiwan. Obes. Res. Clin. Pract. 2021, 15, 564–569. [Google Scholar] [CrossRef]
- Najafi, F.; Pasdar, Y.; Nazar, M.M.; Darbandi, M. Association between obesity phenotypes and non-alcoholic fatty liver: A large population- based study. BMC Endocr. Disord. 2024, 24, 96. [Google Scholar] [CrossRef]
- Iqbal, J.; Wu, H.X.; Nawaz, M.A.; Jiang, H.L.; Xu, S.N.; Huang, B.L.; Li, L.; Cai, J.M.; Zhou, H.D. Risk of incident chronic kidney disease in metabolically healthy obesity and metabolically unhealthy normal weight: A systematic review and meta-analysis. Obes. Rev. 2024, 25, e13656. [Google Scholar] [CrossRef]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frühbeck, G.; Toplak, H.; Woodward, E.; Yumuk, V.; Maislos, M.; Oppert, J.M.; Executive Committee of the European Association for the Study of Obesity. Obesity: The gateway to ill health—An EASO position statement on a rising public health, clinical and scientific challenge in Europe. Obes. Facts 2013, 6, 117–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klitgaard, H.B.; Kilbak, J.H.; Nozawa, E.A.; Seidel, A.V.; Magkos, F. Physiological and Lifestyle Traits of Metabolic Dysfunction in the Absence of Obesity. Curr. Diab Rep. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Manzanero, R.Z.; López-González, A.A.; Tomás-Gil, P.; Paublini, H.; Martínez-Jover, A.; Ramírez-Manent, J.I. Determination of cardiometabolic risk scales in 7.962 hotel receptionists. Acad. J. Health Sci. 2024, 39, 113–122. [Google Scholar] [CrossRef]
- Martínez Jover, A.; López González, A.A.; Tomás Gil, P.; Coll Villalonga, J.L.; Martí Lliteras, P.; Ramírez Manent, J.I. Variables influencing the appearance of metabolic syndrome with three different definitions in 418.343 spanish workers. Acad. J. Health Sci. 2023, 38, 129–135. [Google Scholar] [CrossRef]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C.; del Grupo de Determinantes Sociales de la Sociedad Española de Epidemiología. Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011. Gac. Sanit. 2013, 27, 263–272. [Google Scholar] [CrossRef]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of overweight and obesity scales in 386,924 Spanish workers. Acad. J. Health Sci. 2024, 39, 27–35. [Google Scholar] [CrossRef]
- Mestre Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Vallejos, D.; Sastre Alzamora, T.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of cardiometabolic risk scales in 386,924 spanish workers. Acad. J. Health Sci. 2024, 39, 112–121. [Google Scholar] [CrossRef]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of type 2 diabetes risk scales. Acad. J. Health Sci. 2024, 39, 99–106. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gutiérrez-Repiso, C.; Soriguer, F.; Rojo-Martínez, G.; García-Fuentes, E.; Valdés, S.; Goday, A.; Calle-Pascual, A.; López-Alba, A.; Castell, C.; Menéndez, E.; et al. Variable patterns of obesity and cardiometabolic phenotypes and their association with lifestyle factors in the Di@bet.es study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Caleyachetty, R.; Thomas, G.N.; Toulis, K.A.; Mohammed, N.; Gokhale, K.M.; Balachandran, K.; Nirantharakumar, K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J. Am. Coll. Cardiol. 2017, 70, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Perry, I.J. Does inflammation determine metabolic health status in obese and nonobese adults? J. Clin. Endocrinol. Metab. 2013, 98, E1610–E1619. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex. Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vistisen, D.; Witte, D.R.; Tabák, A.G.; Herder, C.; Brunner, E.J.; Kivimäki, M.; Færch, K. Patterns of obesity development before the diagnosis of type 2 diabetes: The Whitehall II cohort study. PLoS Med. 2014, 11, e1001602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stringhini, S.; Sabia, S.; Shipley, M.; Brunner, E.; Nabi, H.; Kivimaki, M.; Singh-Manoux, A. Association of socioeconomic position with health behaviors and mortality. JAMA 2010, 303, 1159–1166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pampel, F.C.; Krueger, P.M.; Denney, J.T. Socioeconomic Disparities in Health Behaviors. Annu. Rev. Sociol. 2010, 36, 349–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Appleton, S.L.; Seaborn, C.J.; Visvanathan, R.; Hill, C.L.; Gill, T.K.; Taylor, A.W.; Adams, R.J.; North West Adelaide Health Study Team. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: A cohort study. Diabetes Care 2013, 36, 2388–2394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortega, F.B.; Lee, D.C.; Katzmarzyk, P.T.; Ruiz, J.R.; Sui, X.; Church, T.S.; Blair, S.N. The intriguing metabolically healthy but obese phenotype: Cardiovascular prognosis and role of fitness. Eur. Heart J. 2013, 34, 389–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefan, N.; Schulze, M.B. Metabolic health and cardiometabolic risk clusters: Implications for prediction, prevention, and treatment. Lancet Diabetes Endocrinol. 2023, 11, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Stamatakis, E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J. Clin. Endocrinol. Metab. 2012, 97, 2482–2488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulze, M.B.; Stefan, N. Metabolically healthy obesity: From epidemiology and mechanisms to clinical implications. Nat. Rev. Endocrinol. 2024, 20, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Obese | Non-Obese | |||||
|---|---|---|---|---|---|---|
| Men n = 45,498 | Women n = 23,386 | Men n = 187,316 | Women n = 130,724 | |||
| Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | |
| Age (years) | 42.9 (10.0) | 42.0 (10.4) | <0.001 | 39.0 (10.3) | 38.8 (1.0) | <0.001 |
| Height (cm) | 173.2 (7.1) | 160.0 (6.7) | <0.001 | 174.1 (7.0) | 161.4 (6.5) | <0.001 |
| Weight (kg) | 99.7 (12.4) | 87.5 (12.3) | <0.001 | 76.6 (9.9) | 61.4 (8.7) | <0.001 |
| BMI (kg/m2) | 33.2 (3.2) | 34.1 (3.9) | <0.001 | 25.3 (3.1) | 23.6 (3.0) | <0.001 |
| Waist (cm) | 96.7 (8.9) | 83.3 (8.8) | <0.001 | 85.5 (7.8) | 72.2 (6.4) | <0.001 |
| Hip (cm) | 108.6 (7.9) | 109.5 (9.3) | <0.001 | 98.0 (7.2) | 95.1 (6.9) | <0.001 |
| Systolic BP (mmHg) | 131.8 (16.2) | 124.0 (15.9) | <0.001 | 122.6 (14.2) | 112.7 (13.9) | <0.001 |
| Diastolic BP (mmHg) | 81.0 (10.7) | 76.9 (11.0) | <0.001 | 74.0 (10.2) | 68.4 (9.6) | <0.001 |
| Total cholesterol (mg/dL) | 204.1 (38.8) | 200.3 (37.4) | <0.001 | 193.9 (38.6) | 192.4 (36.1) | <0.001 |
| HDL-cholesterol (mg/dL) | 48.3 (7.0) | 51.2 (7.1) | <0.001 | 51.7 (6.9) | 54.2 (7.6) | <0.001 |
| LDL-cholesterol (mg/dL) | 124.5 (37.5) | 127.1 (37.0) | <0.001 | 119.5 (37.5) | 121.4 (37.0) | <0.001 |
| Triglycerides (mg/dL) | 158.6 (108.4) | 110.5 (55.8) | <0.001 | 115.3 (80.1) | 84.1 (43.0) | <0.001 |
| Glucose (mg/dL) | 92.3 (14.0) | 89.0 (13.4) | <0.001 | 87.1 (12.4) | 83.3 (11.0) | <0.001 |
| % | % | p-value | % | % | p-value | |
| <30 years | 10.0 | 13.5 | <0.001 | 19.8 | 20.5 | <0.001 |
| 30–39 years | 28.3 | 28.2 | 34.2 | 34.3 | ||
| 40–49 years | 34.5 | 32.3 | 28.5 | 28.9 | ||
| 50–59 years | 22.6 | 21.9 | 14.8 | 14.1 | ||
| 60–69 years | 4.6 | 4.3 | 2.7 | 2.2 | ||
| Elementary school | 63.7 | 64.9 | <0.001 | 60.6 | 49.4 | <0.001 |
| High school | 32.3 | 30.6 | 34.5 | 42.5 | ||
| University | 4.0 | 4.5 | 4.9 | 8.1 | ||
| Social class I | 4.6 | 4.2 | <0.001 | 5.4 | 7.5 | <0.001 |
| Social class II | 15.7 | 21.4 | 17.9 | 35.3 | ||
| Social class III | 79.7 | 74.4 | 76.7 | 57.2 | ||
| No physical activity | 96.5 | 95.3 | <0.001 | 43.4 | 38.5 | <0.001 |
| Yes physical activity | 3.5 | 4.7 | 56.6 | 61.5 | ||
| No Mediterranean diet | 91.8 | 85.1 | <0.001 | 49.1 | 39.4 | <0.001 |
| Yes Mediterranean diet | 8.2 | 14.9 | 50.9 | 60.6 | ||
| Non-smokers | 68.3 | 74.0 | <0.001 | 61.6 | 65.8 | <0.001 |
| Smokers | 31.7 | 26.0 | 38.4 | 34.2 |
| n = 8764 | n = 36,734 | n = 24,264 | n = 21,234 | n = 34,660 | n = 10,838 | ||||
|---|---|---|---|---|---|---|---|---|---|
| MHO (A) | MNHO (A) | MHO (B) | MNHO (B) | MHO (C) | MNHO (C) | ||||
| Men | Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value |
| Finrisk | 8.6 (2.2) | 11.1 (3.8) | <0.001 | 9.1 (2.6) | 12.4 (3.9) | <0.001 | 9.9 (3.2) | 13.2 (3.9) | <0.001 |
| Canrisk | 27.6 (5.8) | 33.4 (8.8) | <0.001 | 29.3 (6.7) | 35.6 (9.3) | <0.001 | 30.7 (7.7) | 37.2 (9.3) | <0.001 |
| Total | 22.5 (10.8) | 27.7 (12.4) | <0.001 | 24.6 (11.7) | 29.0 (12.5) | <0.001 | 25.6 (12.1) | 30.0 (12.4) | <0.001 |
| TRAQ-D | 7.1 (3.1) | 8.8 (3.8) | <0.001 | 7.6 (3.4) | 9.5 (3.8) | <0.001 | 7.9 (3.5) | 10.2 (3.8) | <0.001 |
| Thai | 9.7 (1.9) | 11.6 (2.3) | <0.001 | 10.6 (2.1) | 11.9 (2.3) | <0.001 | 10.9 (2.2) | 12.3 (2.3) | <0.001 |
| Oman | 7.9 (4.2) | 11.1 (4.6) | <0.001 | 9.3 (4.4) | 11.9 (4.7) | <0.001 | 9.9 (4.6) | 12.5 (4.7) | <0.001 |
| QD-score | 2.9 (1.9) | 3.5 (2.4) | <0.001 | 3.0 (2.0) | 3.7 (2.6) | <0.001 | 3.2 (2.2) | 4.0 (2.6) | <0.001 |
| Women | n = 6146 | n = 17,240 | n = 14,446 | n = 8938 | n = 19,976 | n = 3410 | |||
| Finrisk | 9.0 (2.6) | 11.1 (3.6) | <0.001 | 9.6 (2.9) | 12.1 (3.7) | <0.001 | 10.0 (3.1) | 13.5 (3.8) | <0.001 |
| Canrisk | 21.1 (5.7) | 27.1 (8.6) | <0.001 | 23.1 (6.8) | 29.6 (9.1) | <0.001 | 24.4 (7.5) | 32.3 (9.9) | <0.001 |
| Total | 16.1 (9.2) | 22.8 (12.5) | <0.001 | 18.6 (10.8) | 25.0 (13.0) | <0.001 | 19.8 (11.4) | 28.3 (13.3) | <0.001 |
| TRAQ-D | 5.1 (4.0) | 7.1 (4.4) | <0.001 | 5.7 (4.2) | 8.0 (4.4) | <0.001 | 6.2 (4.3) | 8.8 (4.2) | <0.001 |
| Thai | 8.0 (3.2) | 9.8 (3.6) | <0.001 | 8.7 (3.4) | 10.3 (3.6) | <0.001 | 9.1 (3.5) | 10.9 (3.7) | <0.001 |
| Oman | 8.1 (4.7) | 11.2 (5.1) | <0.001 | 9.3 (5.0) | 12.1 (5.0) | <0.001 | 10.0 (5.1) | 12.8 (5.0) | <0.001 |
| QD-score | 4.1 (3.1) | 4.6 (3.3) | <0.001 | 4.2 (3.0) | 5.0 (3.5) | <0.001 | 4.4 (3.2) | 5.2 (3.2) | <0.001 |
| n = 8764 | n = 36,734 | n = 24,264 | n = 21,234 | n = 34,660 | n = 10,838 | ||||
|---|---|---|---|---|---|---|---|---|---|
| MHO (A) | MNHO (A) | MHO (B) | MNHO (B) | MHO (C) | MNHO (C) | ||||
| Men | % | % | p-Value | % | % | p-Value | % | % | p-Value |
| Finrisk high-very high | 1.3 | 15.9 | <0.001 | 2.5 | 24.5 | <0.001 | 7.2 | 30.9 | <0.001 |
| Canrisk high | 18.0 | 47.8 | <0.001 | 28.3 | 57.7 | <0.001 | 35.0 | 64.7 | <0.001 |
| TRAQ-D high-very high | 7.3 | 12.9 | <0.001 | 6.8 | 16.5 | <0.001 | 8.5 | 20.2 | <0.001 |
| QD-score ≥ 3 | 30.1 | 45.1 | <0.001 | 35.1 | 50.2 | <0.001 | 37.7 | 56.7 | <0.001 |
| Women | n = 6146 | n = 17,240 | n = 14,446 | n = 8938 | n = 19,976 | n = 3410 | |||
| Finrisk high-very high | 1.8 | 17.7 | <0.001 | 4.9 | 27.5 | <0.001 | 8.6 | 47.6 | <0.001 |
| Canrisk high | 4.9 | 24.0 | <0.001 | 10.5 | 32.7 | <0.001 | 14.9 | 42.8 | <0.001 |
| TRAQ-D high-very high | 4.0 | 9.4 | <0.001 | 5.4 | 12.2 | <0.001 | 6.9 | 14.1 | <0.001 |
| QD-score ≥ 3 | 58.1 | 67.0 | <0.001 | 60.0 | 72.1 | <0.001 | 62.7 | 76.2 | <0.001 |
| Finrisk High-Very High | Canrisk High | TRAQ-D High-Very High | QD-Score ≥ 3 | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Female | 1 | 1 | 1 | 1 |
| Male | 0.69 (0.66–0.73) | 5.25 (4.98–5.53) | 1.20 (1.13–1.28) | 0.64 (0.58–0.70) |
| <30 years | 1 | 1 | 1 | 1 |
| 30–39 years | 1.57 (1.43–1.73) | 1.28 (1.21–1.35) | 1.18 (1.11–1.25) | 1.35 (1.23–1.47) |
| 40–49 years | 3.57 (3.25–3.92) | 1.88 (1.60–2.16) | 1.72 (1.42–2.03) | 1.49 (1.30–1.69) |
| 50–59 years | 8.16 (7.33–9.09) | 2.22 (1.90–2.52) | 2.10 (1.71–2.50) | 1.90 (1.61–2.20) |
| 60–69 years | 11.65 (9.89–13.71) | 4.30 (3.78–4.88) | 2.94 (2.68–3.22) | 2.33 (1.90–2.77) |
| University | 1 | 1 | 1 | 1 |
| High school | 1.10 (1.05–1.15) | 1.54 (1.45–1.64) | 1.24 (1.14–1.35) | 1.18 (1.12–1.25) |
| Elementary school | 2.26 (2.05–2.52) | 3.28 (2.50–4.06) | 2.02 (1.67–3.37) | 1.60 (1.42–1.79) |
| Social class I | 1 | 1 | 1 | 1 |
| Social class II | 1.05 (1.02–1.08) | 1.19 (1.11–1.27) | 1.30 (1.18–1.42) | 1.23 (1.15–1.31) |
| Social class III | 2.57 (2.17–2.97) | 2.40 (2.01–2.80) | 2.21 (1.81–2.62) | 1.78 (1.58–1.99) |
| Yes physical activity | 1 | 1 | 1 | 1 |
| No physical activity | 6.88 (6.12–7.64) | 5.15 (4.60–5.71) | 2.89 (2.50–3.29) | 4.23 (3.60–4.87) |
| Yes Mediterranean diet | 1 | 1 | 1 | 1 |
| No Mediterranean diet | 4.29 (3.90–4.69) | 2.79 (2.50–3.09) | 1.90 (1.48–2.33) | 2.40 (2.01–2.80) |
| Smokers | 1 | 1 | 1 | 1 |
| Non-smokers | 1.13 (1.08–1.18) | 2.18 (2.04–2.34) | 5.31 (5.01–5.63) | 1.55 (1.38–1.72) |
| MHO (A) | 1 | 1 | 1 | 1 |
| MNHO (A) | 5.74 (4.72–6.76) | 2.17 (2.05–2.29) | 1.41 (1.28–1.55) | 1.42 (1.36–1.49) |
| MHO (B) | 1 | 1 | 1 | 1 |
| MNHO (B) | 3.96 (3.68–4.24) | 2.50 (2.36–2.66) | 1.59 (1.48–1.71) | 1.37 (1.31–1.43) |
| MHO (C) | 1 | 1 | 1 | 1 |
| MNHO (C) | 2.63 (2.48–2.79) | 2.85 (2.40–3.30) | 1.42–1.33–1.52) | 1.57 (1.49–1.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Samuelsson, M.; Tárraga López, P.J.; López-González, Á.A.; Busquets-Cortés, C.; Obrador de Hevia, J.; Ramírez-Manent, J.I. Evaluation of Type 2 Diabetes Risk in Individuals With or Without Metabolically Healthy Obesity. Biology 2025, 14, 608. https://doi.org/10.3390/biology14060608
García Samuelsson M, Tárraga López PJ, López-González ÁA, Busquets-Cortés C, Obrador de Hevia J, Ramírez-Manent JI. Evaluation of Type 2 Diabetes Risk in Individuals With or Without Metabolically Healthy Obesity. Biology. 2025; 14(6):608. https://doi.org/10.3390/biology14060608
Chicago/Turabian StyleGarcía Samuelsson, Miguel, Pedro Juan Tárraga López, Ángel Arturo López-González, Carla Busquets-Cortés, Joan Obrador de Hevia, and José Ignacio Ramírez-Manent. 2025. "Evaluation of Type 2 Diabetes Risk in Individuals With or Without Metabolically Healthy Obesity" Biology 14, no. 6: 608. https://doi.org/10.3390/biology14060608
APA StyleGarcía Samuelsson, M., Tárraga López, P. J., López-González, Á. A., Busquets-Cortés, C., Obrador de Hevia, J., & Ramírez-Manent, J. I. (2025). Evaluation of Type 2 Diabetes Risk in Individuals With or Without Metabolically Healthy Obesity. Biology, 14(6), 608. https://doi.org/10.3390/biology14060608







