Comparative Corneal Histomorphometry Between Birds of Different Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection and Processing
2.3. Tissue Preparation
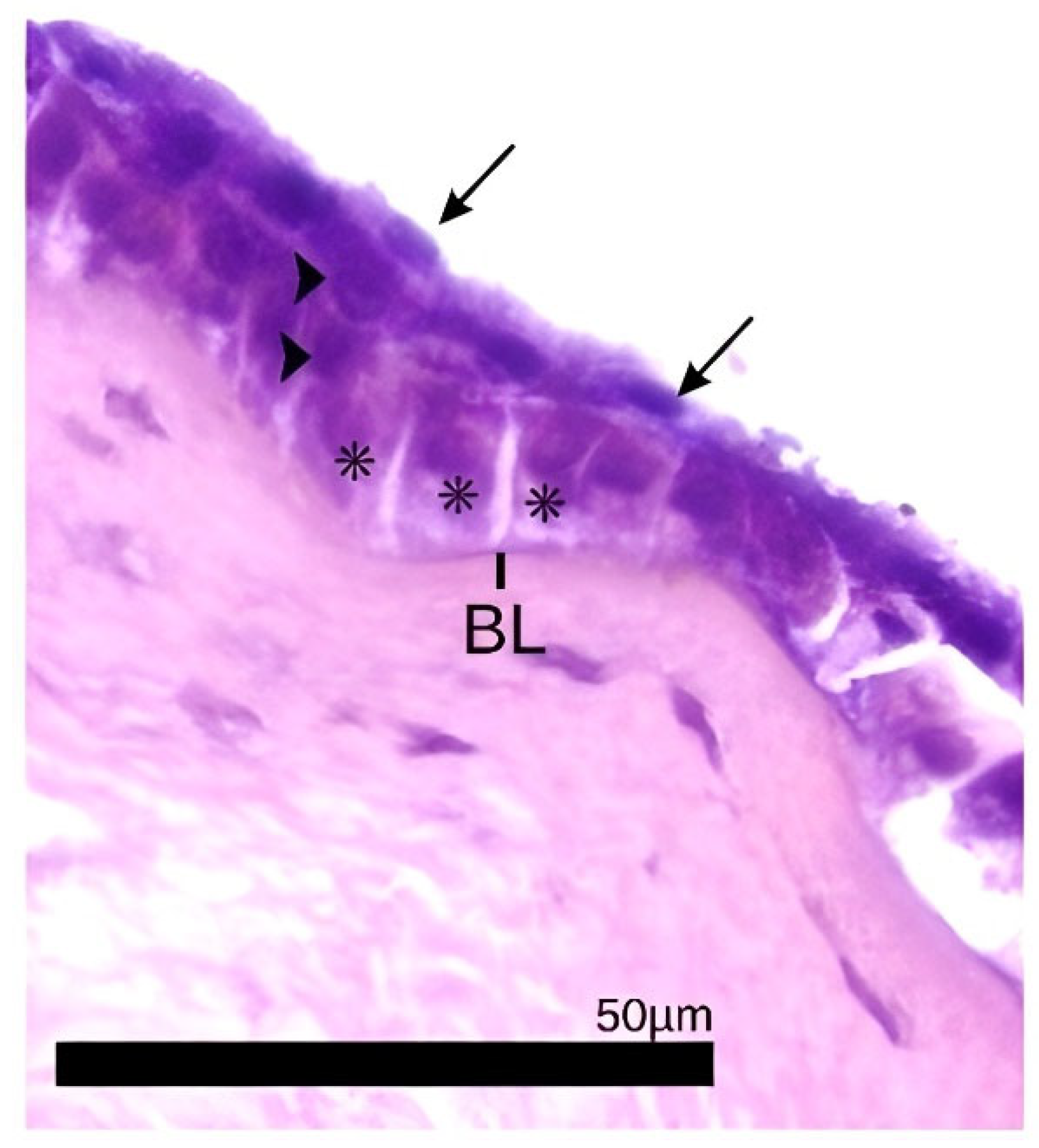
2.4. Histological Analysis and Description
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popova, P.; Malalana, F.; Biddolph, S.; Ramos, T.; Parekh, M.; Chantrey, J.; Ahmad, S. Interspecies Comparative Morphological Evaluation of the Corneal Epithelial Stem Cell Niche: A Pilot Observational Study. J. Vet. Sci. 2022, 23, e62. [Google Scholar] [CrossRef] [PubMed]
- Almubrad, T.; Akhtar, S. Ultrastructure Features of Camel Cornea—Collagen Fibril and Proteoglycans. Vet. Ophthalmol. 2012, 15, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kafarnik, C.; Fritsche, J.; Reese, S. In Vivo Confocal Microscopy in the Normal Corneas of Cats, Dogs and Birds. Vet. Ophthalmol. 2007, 10, 222–230. [Google Scholar] [CrossRef]
- Collin, S.P.; Collin, H.B. A Comparative SEM Study of the Vertebrate Corneal Epithelium. Cornea 2000, 19, 218–230. [Google Scholar] [CrossRef]
- Collin, S.P.; Collin, H.B. The Corneal Epithelial Surface in the Eyes of Vertebrates: Environmental and Evolutionary Influences on Structure and Function. J. Morphol. 2006, 267, 273–291. [Google Scholar] [CrossRef]
- Jessup, A.J.C.; Coroneo, M.T. Examining the Fabric of the Eye: Antoni van Leeuwenhoek, the Draper and Ocular Microscopist. Surv. Ophthalmol. 2024, 70, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Bergmanson, J.P.G. Anatomy and Physiology of the Cornea and Related Structures. In Contact Lenses; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–64. [Google Scholar] [CrossRef]
- Meek, K.M.; Fullwood, N.J. Corneal and Scleral Collagens—A Microscopist’s Perspective. Micron 2001, 32, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.P.; Pierce, K.E.; Ward, D. Avian Vision: A Review of Form and Function with Special Consideration to Birds of Prey. J. Exot. Pet Med. 2007, 16, 69–87. [Google Scholar] [CrossRef]
- Abdelftah, Z.; Gaber, A.R.; Abo-Eleneen, R.E.; El-Bakry, A.M. Microstructure Characteristics of Cornea of Some Birds: A Comparative Study. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 66. [Google Scholar] [CrossRef]
- Gonzalez-Alonso-Alegre, E.M.; Martinez-Nevado, E.; Caro-Vadillo, A.; Rodriguez-Alvaro, A. Central Corneal Thickness and Intraocular Pressure in Captive Black-Footed Penguins (Spheniscus demersus). Vet. Ophthalmol. 2015, 18 (Suppl. S1), 94–97. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhu, X.P.; Wu, J.; Wu, Z.J.; Yin, Y.; Xiao, X.H.; Su, X.; Kong, B.; Pan, S.Y.; Yang, H.; et al. Acellular Ostrich Corneal Stroma Used as Scaffold for Construction of Tissue-Engineered Cornea. Int. J. Ophthalmol. 2016, 9, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Moraes, W. Ocular Morphology, Physiology and Selected Ophthalmic Clinical Tests in the Harpy Eagle (Harpia harpyja). Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2018. [Google Scholar]
- Chard, R.D.; Gundlach, R.H. The Structure of the Eye of the Homing Pigeon. J. Comp. Psychol. 1938, 25, 249–272. [Google Scholar] [CrossRef]
- Meyer, D.B. The Avian Eye and Its Adaptations. In The Visual System in Vertebrates. Handbook of Sensory Physiology; Crescitelli, F., Ed.; Springer: Berlin/Heidelberg, Germany, 1977; Volume 7/5, pp. 549–611. [Google Scholar] [CrossRef]
- Montiani-Ferreira, F.; Cardoso, F.; Petersen-Jones, S. Postnatal Development of Central Corneal Thickness in Chicks of Gallus gallus domesticus. Vet. Ophthalmol. 2004, 7, 37–39. [Google Scholar] [CrossRef]
- Pinto, D.G.; Cruz, G.D.; Teixeira, R.H.F.; Couto, E.P.; de Carvalho, M.P.N. Histological Analysis of the Eyeball of Neotropical Birds of Prey: Caracara plancus, Falco sparverius, Rupornis magnirostris, Megascops choliba, and Athene cunicularia. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 280. [Google Scholar] [CrossRef]
- Werther, K.; Candioto, C.G.; Korbel, R. Ocular Histomorphometry of Free-Living Common Kestrels (Falco tinnunculus). J. Avian Med. Surg. 2017, 31, 319–326. [Google Scholar] [CrossRef]
- de Almeida Lima Massari, C.H.; Silva, A.F.; Magalhães, H.I.R.; Silva, D.R.S.; de Castro Sasahara, T.H.; Miglino, M.A. Using 3D Computed Tomography in the Anatomical Description of the Eye and the Vestibulocochlear Organ of a Blue-and-Yellow Macaw (Ara ararauna) and of a Toucan (Ramphastos toco). Acta Vet. Bras. 2020, 14, 279–285. [Google Scholar] [CrossRef]
- BirdLife International. Ara macao. The IUCN Red List of Threatened Species 2016: e.T22685563A93079992. Available online: http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22685563A93079992.en (accessed on 20 May 2025).
- BirdLife International. Ramphastos toco. The IUCN Red List of Threatened Species 2017: e.T22682164A113557535. Available online: http://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T22682164A113557535.en (accessed on 20 May 2025).
- Calegaro-Marques, C.; Amato, S.B. Urbanization Breaks Up Host–Parasite Interactions: A Case Study on Parasite Community Ecology of Rufous-Bellied Thrushes (Turdus rufiventris) along a Rural–Urban Gradient. PLoS ONE 2014, 9, e103144. [Google Scholar] [CrossRef] [PubMed]
- BirdLife International. Turdus rufiventris. The IUCN Red List of Threatened Species 2016: e.T22708882A94182217. Available online: http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22708882A94182217.en (accessed on 20 May 2025).
- BirdLife International. Pitangus sulphuratus. The IUCN Red List of Threatened Species 2018: e.T22700605A132069895. Available online: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22700605A132069895.en (accessed on 20 May 2025).
- Lago-Paiva, C. Cavity Nesting by Great Kiskadees (Pitangus sulphuratus): Adaptation or Expression of Ancestral Behavior? Auk 1996, 113, 953–955. [Google Scholar] [CrossRef]
- Gasperin, G.; Pizo, M.A. Frugivory and Habitat Use by Thrushes (Turdus spp.) in a Suburban Area in South Brazil. Urban Ecosyst. 2009, 12, 425–436. [Google Scholar] [CrossRef]
- Pérez Orrico, M.L.; Sabater González, M. Ophthalmology of Palaeognathae: Ostriches, Rheas, Emu, Cassowaries, Tinamous, and Kiwis. In Wild and Exotic Animal Ophthalmology; Springer International Publishing: Cham, Switzerland, 2022; pp. 627–648. [Google Scholar] [CrossRef]
- BirdLife International. Rhea americana. The IUCN Red List of Threatened Species 2016: e.T22678073A92754472. Available online: http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22678073A92754472.en (accessed on 20 May 2025).
- Cooke, S.C.; Haskell, L.E.; van Rees, C.B.; Fessl, B. A Review of the Introduced Smooth-Billed Ani Crotophaga ani in Galápagos. Biol. Conserv. 2019, 229, 38–49. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Smooth-Billed Ani (Crotophaga ani) Predation on Butterflies in Mato Grosso, Brazil: Risk Decreases with Increased Group Size. Behav. Ecol. Sociobiol. 2001, 49, 482–492. [Google Scholar] [CrossRef]
- Reavill, D.R.; Dorrestein, G. Psittacines, Coliiformes, Musophagiformes, Cuculiformes. In Pathology of Wildlife and Zoo Animals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 775–798. [Google Scholar] [CrossRef]
- Potier, S.; Mitkus, M.; Kelber, A. Visual Adaptations of Diurnal and Nocturnal Raptors. Semin. Cell Dev. Biol. 2020, 106, 116–126. [Google Scholar] [CrossRef]
- Mitkus, M.; Potier, S.; Martin, G.R.; Duriez, O.; Kelber, A. Raptor Vision. Oxf. Res. Encycl. Neurosci. 2018, 1–39. [Google Scholar] [CrossRef]
- BirdLife International. Nyctibius griseus. The IUCN Red List of Threatened Species 2020: e.T22689646A163600335. 2020. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T22689646A163600335.en (accessed on 20 May 2025).
- Sokolenko, E.; Hilken, G.; Denk, N.; Wyss, F.; Wenker, C.; Hasler, P.W.; Meyer, P. The Eyes of an African Penguin (Spheniscus demersus): General Morphology and Ophthalmopathology. Klin. Monatsbl. Augenheilkd. 2021, 238, 94–98. [Google Scholar] [CrossRef]
- Collin, S.P.; Collin, H.B. Functional Morphology of the Cornea of the Little Penguin Eudyptula minor (Aves). J. Anat. 2021, 239, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Arango, L.J.; Baraldi-Artoni, S.M.; Laus, J.L.; Mendes-Vicenti, F.A.; Pigatto, J.A.T.; Abib, F.C. Ultrastructural Morphology and Morphometry of the Normal Corneal Endothelium of Adult Crossbred Pig. Cienc. Rural 2009, 39, 117–122. [Google Scholar] [CrossRef]
- Moore, B.A.; Fernandez-Juricic, E.; Hawkins, M.G.; Montiani-Ferreira, F.; Lange, R.R. Introduction to Ophthalmology of Aves. In Wild and Exotic Animal Ophthalmology; Springer International Publishing: Cham, Switzerland, 2022; pp. 321–348. [Google Scholar] [CrossRef]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; Blakiston Division, McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Hall, M.I. The Anatomical Relationships between the Avian Eye, Orbit and Sclerotic Ring: Implications for Inferring Activity Patterns in Extinct Birds. J. Anat. 2008, 212, 781–794. [Google Scholar] [CrossRef]
- Coyo, N.; Peña, M.T.; Costa, D.; Ríos, J.; Lacerda, R.; Leiva, M. Effects of Age and Breed on Corneal Thickness, Density, and Morphology of Corneal Endothelial Cells in Enucleated Sheep Eyes. Vet. Ophthalmol. 2015, 19, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Dubielzig, R.R. The Gross and Microscopic Structure of the Golden Eagle (Aquila chrysaetos) Eye. Prog. Vet. Comp. Ophthalmol. 1993, 3, 74–79. [Google Scholar]
- Brooke, M.D.L.; Hanley, S.; Laughlin, S.B. The Scaling of Eye Size with Body Mass in Birds. Proc. R. Soc. B Biol. Sci. 1999, 266, 405–412. [Google Scholar] [CrossRef]
- Murphy, C.J.; Howland, H.C. Owl Eyes: Accommodation, Corneal Curvature and Refractive State. J. Comp. Physiol. A 1983, 151, 277–284. [Google Scholar] [CrossRef]
- Gonçalves, G.C.; Pérez-Merino, P.; Martínez-García, M.C.; Barcía, A.; Merayo-Loves, J. Comparación de Las Características Corneales en Gallina y Codorniz como Modelos Experimentales de Cirugía Refractiva. Arch. Soc. Esp. Oftalmol. 2016, 91, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Mayakkannan, T.; Ramesh, G.; Kumaravel, A.; Venkatesan, S.; Kannan, T.A. Gross and Microanatomical Study of the Cornea in Japanese Quail (Coturnix coturnix japonica). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 599–605. [Google Scholar] [CrossRef]
- Grego, A.L.; de Moraes, W.; Moore, B.A.; Somma, A.T.; Ferreira, T.A.C.; Rodarte-Almeida, A.C.V.; Oriá, A.P.; Montiani-Ferreira, F. The Eye of the Largest Forest Raptor, the Harpy Eagle (Harpia harpyja): Morphologic Observations and Reference Values for Selected Ophthalmic Tests. Vet. Ophthalmol. 2025, 1–13. [Google Scholar] [CrossRef]
- Tozetti, R.A.R.; de Lima Sousa Araújo, R.; Moreira, M.V.L.; de Souza Akiyama, L.C.; Corrêa, J.R.; Moore, B.A.; Galera, P.D. Evaluation of the Common Pauraque (Nyctidromus albicollis) Cornea Using Light and Scanning Electron Microscopy. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2024, 53, e12987. [Google Scholar] [CrossRef]
- Moore, B.A.; Maggs, D.J.; Kim, S.; Motta, M.J.; Bandivadekar, R.; Tell, L.A.; Murphy, C.J. Clinical Findings and Normative Ocular Data for Free-Living Anna’s (Calypte anna) and Black-Chinned (Archilochus alexandri) Hummingbirds. Vet. Ophthalmol. 2019, 22, 13–23. [Google Scholar] [CrossRef]
- Monção-Silva, R.M.; Ofri, R.; Raposo, A.C.S.; Libório, F.A.; Estrela-Lima, A.; Oriá, A.P. Ophthalmic Parameters of Blue-and-Yellow Macaws (Ara ararauna) and Lear’s Macaws (Anodorhynchus leari). Avian Biol. Res. 2016, 9, 240–249. [Google Scholar] [CrossRef]
- Nautscher, N.; Bauer, A.; Steffl, M.; Amselgruber, W.M. Comparative Morphological Evaluation of Domestic Animal Cornea. Vet. Ophthalmol. 2016, 19, 297–304. [Google Scholar] [CrossRef]
- Swamynathan, S.K.; Crawford, M.A.; Robison, W.G.; Kanungo, J.; Piatigorsky, J. Adaptive Differences in the Structure and Macromolecular Compositions of the Air and Water Corneas of the “Four-Eyed” Fish (Anableps anableps). FASEB J. 2003, 17, 1996–2005. [Google Scholar] [CrossRef]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative Observations on Corneas, with Special Reference to Bowman’s Layer and Descemet’s Membrane in Mammals and Amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef]
- Merindano, M.D.; Costa, J.; Canals, M.; Potau, J.M.; Ruano, D. A Comparative Study of Bowman’s Layer in Some Mammals: Relationships with Other Constituent Corneal Structures. Eur. J. Anat. 2002, 6, 133–139. [Google Scholar]
- Wilson, S.E. Bowman’s Layer in the Cornea—Structure, Function and Regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.M.; Rodarte-Almeida, A.C.V.; Santana, M.I.S.; Galera, P.D. Avian Ophthalmic Peculiarities. Ciência Rural 2018, 48, e20170904. [Google Scholar] [CrossRef]
- Willis, A.M.; Wilkie, D.A. Avian Ophthalmology Part 1: Anatomy, Examination, and Diagnostic Techniques. J. Avian Med. Surg. 1999, 13, 160–166. [Google Scholar]
- Bastola, P.; Song, L.; Gilger, B.C.; Hirsch, M.L. Adeno-Associated Virus Mediated Gene Therapy for Corneal Diseases. Pharmaceutics 2020, 12, 767. [Google Scholar] [CrossRef]
- Feizi, S. Corneal Endothelial Cell Dysfunction: Etiologies and Management. Ther. Adv. Ophthalmol. 2018, 10, 251584141881580. [Google Scholar] [CrossRef]
- Tsukahara, N.; Tani, Y.; Lee, E.; Kikuchi, H.; Endoh, K.; Ichikawa, M.; Sugita, S. Microstructure Characteristics of the Cornea in Birds and Mammals. J. Vet. Med. Sci. 2010, 72, 1137–1143. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Crespo-Moral, M.; García-Posadas, L.; López-García, A.; Diebold, Y. Histological and Immunohistochemical Characterization of the Porcine Ocular Surface. PLoS ONE 2020, 15, e0227732. [Google Scholar] [CrossRef]
- Koudouna, E.; Winkler, M.; Mikula, E.; Juhasz, T.; Brown, D.J.; Jester, J.V. Evolution of the Vertebrate Corneal Stroma. Prog. Retin. Eye Res. 2018, 64, 65–76. [Google Scholar] [CrossRef]
- Boote, C.; Elsheikh, A.; Kassem, W.; Kamma-Lorger, C.S.; Hocking, P.M.; White, N.; Inglehearn, C.F.; Ali, M.; Meek, K.M. The Influence of Lamellar Orientation on Corneal Material Behavior: Biomechanical and Structural Changes in an Avian Corneal Disorder. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1243. [Google Scholar] [CrossRef] [PubMed]
- Bergmanson, J.P.G.; Burns, A.; Walker, M. Anatomical Explanation for the Central–Peripheral Thickness Difference in Human Corneas. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4652. [Google Scholar]
- Meek, K.M.; Leonard, D.W. Ultrastructure of the Corneal Stroma: A Comparative Study. Biophys. J. 1993, 64, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.P.; Collin, H.B. A Comparative Study of the Corneal Endothelium in Vertebrates. Clin. Exp. Optom. 1998, 81, 245–254. [Google Scholar] [CrossRef]
- Henriksson, J.T.; Bron, A.J.; Bergmanson, J.P.G. An Explanation for the Central to Peripheral Thickness Variation in the Mouse Cornea. Clin. Exp. Ophthalmol. 2012, 40, 174–181. [Google Scholar] [CrossRef]
- Bergmanson, J.P.G.; Burns, A.R.; Naroo, S.A. Central versus Peripheral Corneal Thickness—A White Spot on the Corneal (Anatomy) Map. Cont. Lens Anterior Eye 2021, 44, 101473. [Google Scholar] [CrossRef]
| Species | Order | Family | Brazilian Popular Names | Popular Names in IUCN Red List |
|---|---|---|---|---|
| Asio stygius (Wagler, 1832) | Strigiformes | Strigidae | Mocho-preto | Stygian Owl |
| Crotophaga ani (Linnaeus, 1758) | Cuculiformes | Cuculidae | Anu-preto | Smooth-billed Ani |
| Pitangus sulphuratus (Linnaeus, 1766) | Passeriformes | Tyrannidae | Bem-te-vi | Great Kiskadee |
| Ramphastos toco (Statius Muller, 1776) | Piciformes | Ramphastidae | Tucanuçu | Toco Toucan |
| Turdus rufiventris (Vieillot, 1818) | Passeriformes | Turdidae | Sabiá-laranjeira | Rufous-bellied Thrush |
| Rhea americana (Linnaeus, 1758) | Rheiformes | Rheidae | Ema | Greater Rhea |
| Ara macao (Linnaeus, 1758) | Psittaciformes | Psitacidae | Araracanga | Scarlet Macaw |
| Nyctibius griseus (Gmelin, 1789) | Nyctibiiformes | Nyctibiidae | Urutau | Common Potoo |
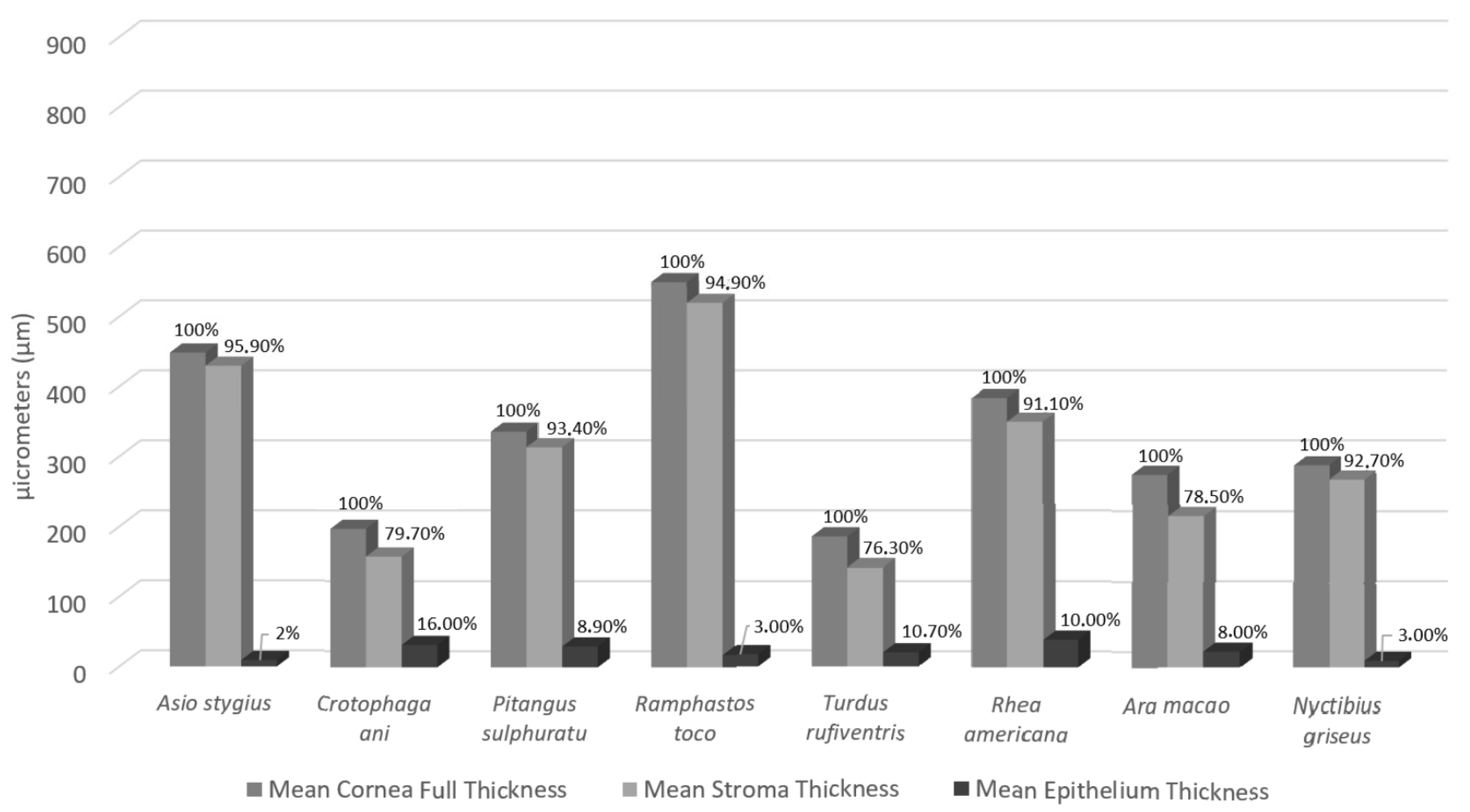
| Cornea Full Thickness | Epithelium | Bowman’s Layer | Stroma | Descemet’s Layer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Central | Peripheral | Central | Peripheral | Central | Peripheral | Central | Peripheral | Central | Peripheral |
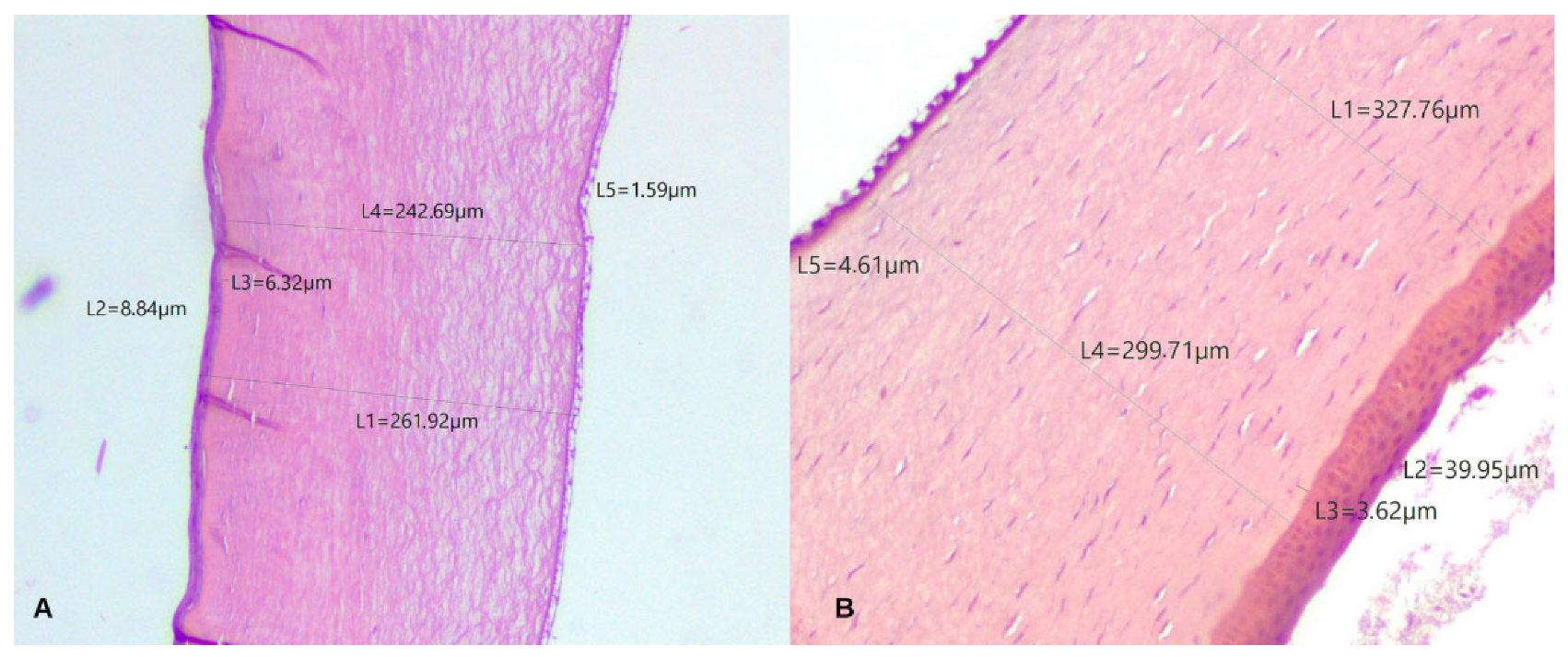
| Asio stygius | 254.76 | 642.2 | 9.53 | 8.91 | 4.3 | 3.02 | 236.08 | 623.19 | 1.92 | 2.33 |
| Crotophaga ani | 172.27 | 220.75 | 37.71 | 26.32 | 4.24 | 4.04 | 131.39 | 182.40 | 1.59 | 1.27 |
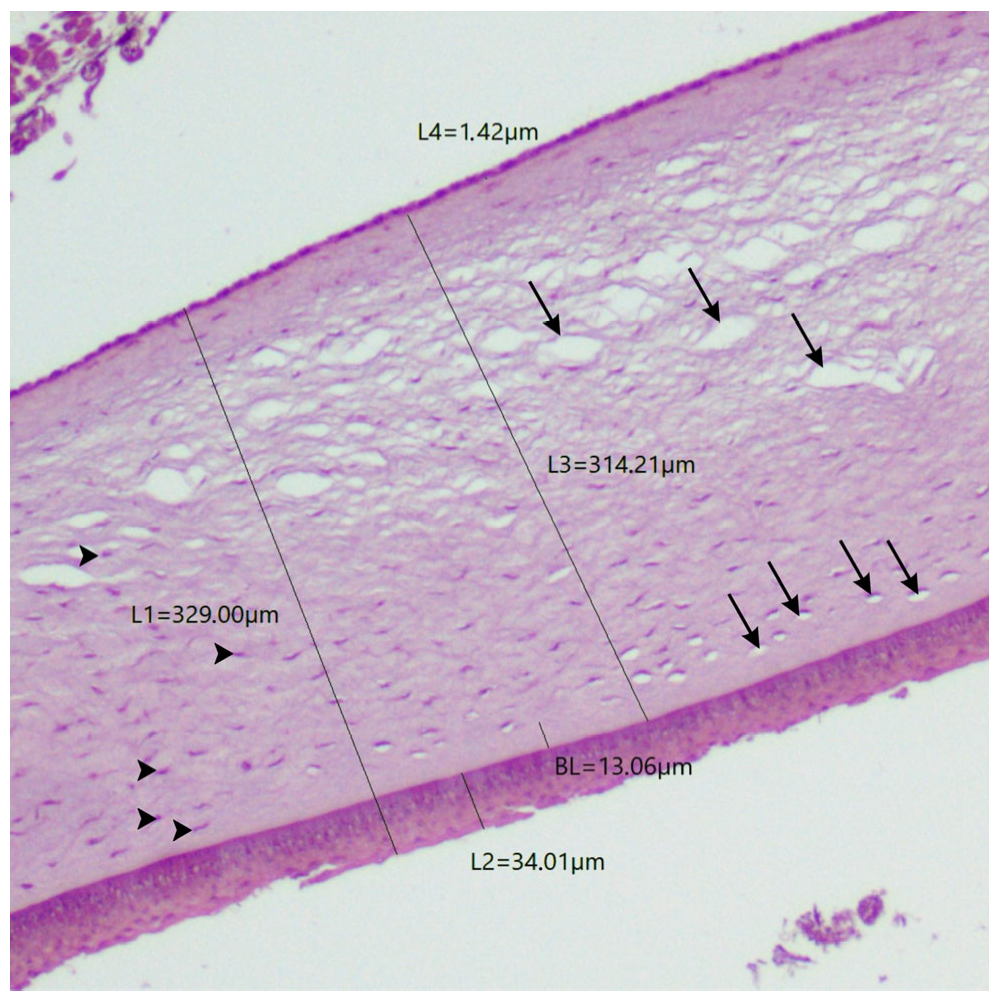
| Pitangus sulphuratus | 341.45 | 329 | 25.58 | 34 | 10.16 | 13.06 | 311.64 | 314.21 | 2.01 | 1.42 |
| Ramphastos toco | 374.36 | 721.38 | 18.65 | 16.31 | 6.27 | 9.3 | 344.2 | 694.87 | 3.06 | 3.27 |
| Turdus rufiventris | 179.33 | 192.25 | 19 | 21.09 | 3 | 3.34 | 140.45 | 143.45 | 3.14 | 2.11 |
| Rhea americana | 327.76 | 439 | 39.95 | 39.03 | 3.62 | 4.32 | 299.71 | 399.14 | 4.61 | 3.17 |
| Ara macao | 282.88 | 266.97 | 21.08 | 23.43 | 3.52 | 7.84 | 232.06 | 200.07 | 19.72 | 13.94 |
| Nyctibius griseus | 224.5 | 352.5 | 8.5 | 10 | 2.81 | 4.48 | 206.6 | 329.44 | 3.37 | 1.55 |
| Epithelial Layers | ||
|---|---|---|
| Species | Central | Peripheral |
| Asio stygius | 3 to 4 layers (1 basal, 1 to 2 polyhedral squamous, and 1 flat squamous) | 3 to 4 layers (1 basal, 1 to 2 polyhedral squamous, and 1 flat squamous) |
| Crotophaga ani | 5 to 6 layers (1 basal, 2 to 3 polyhedral squamous, and 2 flat squamous) | 4 to 5 layers (1 basal, 1 to 2 polyhedral squamous, and 2 flat squamous) |
| Pitangus sulphuratus | 3 to 4 layers (1 basal, 1 to 2 polyhedral squamous, and 1 flat squamous) | 4 to 5 layers (1 basal, 1 to 2 polyhedral squamous, and 2 flat squamous) |
| Ramphastos toco | 3 to 5 layers (1 basal, 1 to 3 polyhedral squamous, and 1 flat squamous) | 3 to 4 layers (1 basal, 1 to 2 polyhedral squamous, and 1 flat squamous) |
| Turdus rufiventris | 3 to 5 layers (1 basal, 1 to 3 polyhedral squamous, and 1 flat squamous) | 3 to 5 layers (1 basal, 1 to 3 polyhedral squamous, and 1 flat squamous) |
| Rhea americana | 3 to 6 layers (1 basal, 1 to 3 polyhedral squamous, and 1 to 2 flat squamous) | 3 to 6 layers (1 basal, 1 to 3 polyhedral squamous, and 1 to 2 flat squamous) |
| Ara macao | 3 to 5 layers (1 basal, 1 to 3 polyhedral squamous, and 1 flat squamous) | 3 to 5 layers (1 basal, 1 to 3 polyhedral squamous, and 1 flat squamous) |
| Nyctibius griseus | 3 to 4 layers (1 basal, 1 to 2 polyhedral squamous, and 1 flat squamous) | 3 to 4 layers (1 basal, 1 to 2 polyhedral squamous, and 1 flat squamous) |
| Species (Popular Name) | Size and Weight of the Bird 1 | Habits 1,2 | Feeding 1,2 | Total Corneal Thickness | Source |
|---|---|---|---|---|---|
| Eudyptula minor (Little Penguin) | 30 cm 1.1–1.2 kg | Diurnal, amphibious, flightless | Piscivore | 380 ± 54 µm (central region) | (Collin & Collin, 2021) [36] |
| Spheniscus demersus (African Penguin) | 45 cm 3.1 kg | Diurnal, amphibious, flightless | Piscivore | 450 µm (region not specified) | (Sokolenko et al., 2021) [35] |
| Spheniscus demersus (African Penguin) | 45 cm 3.1 kg | Diurnal, amphibious, flightless | Piscivore | 384 ± 30 µm (central region) | (Gonzalez-Alonso-Alegre et al., 2015) [11] |
| Spheniscus humboldti (Humboldt Penguin) | 66–70 cm 4–5 kg | Diurnal, amphibious, flightless | Piscivore | 636 µm 3 (region not specified) | (Popova et al., 2022) [1] |
| Gallus gallus domesticus (Domestic chickens) | 40–60 cm 2580.2 g | Diurnal, terrestrial, domestic | Granivore and insectivore | 242 µm (central region) | (Montiani-Ferreira et al., 2004) [16] |
| Gallus gallus domesticus (Domestic chickens) | 40–60 cm 2.6–4.5 kg | Diurnal, terrestrial, domestic | Granivore and insectivore | 225.3 ± 30 µm (region not specified) | (Gonçalves et al., 2016) [45] |
| Coturnix coturnix (Common Quail) | 17.5 cm 70–155 g | Diurnal, terrestrial, grassland | Granivore | 154 ± 17.7 µm (region not specified) | (Gonçalves et al., 2016) [45] |
| Coturnix japonica (Japanese Quail) | 16–18 cm 90–115 g | Diurnal, terrestrial, grassland | Granivore | 138.64 µm (region not specified) | (Mayakkannan et al., 2018) [46] |
| Ostrich (species not described by the author) | 180–270 cm 90–130 kg | Diurnal, terrestrial, flightless | Omnivore | 550 ± 35 µm (central region) | (Liu et al., 2016) [12] |
| Rhea americana (Greater Rhea) | 1.34–1.70m 26–36 kg | Diurnal, terrestrial, flightless | Omnivore | 327.76 µm (central region) 439 µm (peripheral region) | This study |
| Harpia harpyja (Harpy Eagle) | 89–102 cm 6–9 kg | Diurnal, raptor, rainforests | Carnivore | 563 µm (region not specified) | (Grego et al., 2025) [47] |
| Aquila chrysaetos (Golden Eagle) | 70–84 cm 3–6.125 kg | Diurnal, raptor, open or semi-open areas | Carnivore | 640 µm (central region) 1200 µm (peripheral region) | (Murphy & Dubielzig, 1993) [42] |
| Falcon tinnunculus (Common Kestrel) | 36–58 cm 907 g | Diurnal, raptor, open or semi-open areas | Carnivore | 129 µm (central region) Varies from 197 to 210.8 µm (peripheral region) | (Werther et al., 2017) [18] |
| Asio stygius (Stygian Owl) | 38–46 cm 400–675 g | Nocturnal, raptor, open or semi-open areas | Carnivore | 254.76 µm (central region) 642.2 µm (peripheral region) | This study |
| Nyctibius albicollis (Common Pauraque) | 20–30 cm 50–70 g | Crepuscular to nocturnal, open or semi-open areas | Insectivore | 146.2 ± 34.5 µm (central region) (149.2 ± 35.8 μm (peripheral region) | (Tozetti et al., 2024) [48] |
| Nyctibius griseus (Common Potoo) | 34–38 cm 160–190 g | Nocturnal, open or semi-open areas | Insectivore | 224.5 µm (central region) 352.5 µm (peripheral region) | This study |
| Columba livia (Domestic Pigeon) | 29–35 cm 315–410 g | Diurnal, domestic, urban areas | Granivore | 157 µm (central region) 188 µm and 169 µm (peripheral nasal and temporal regions) | (Chard & Gundlach, 1938) [14] |
| Calypte anna (Anna’s Hummingbird) | 10 cm 4–4.5 g | Diurnal, scrub forest | Nectarivore | 59 µm (central region) 48 µm (peripheral region) | (Moore et al., 2019) [49] |
| Pitangus sulphuratus (Great Kiskadee) | 21–26 cm 52–68 g | Diurnal, rainforests, urban areas | Omnivore | 341.45 µm (central region) 329 µm (peripheral region) | This study |
| Turdus rufiventris (Rufous-bellied Thrush) | 25 cm 68 g | Diurnal, rainforests, urban areas | Omnivore | 179.33 µm (central region) 192.25 µm (peripheral region) | This study |
| Lonchura oryzivora (Java Sparrow) | 15–17 cm 24.5 g | Diurnal, open grassland | Granivore | 166 ± 5 µm (region not specified) | (Popova et al., 2022) [1] |
| Crotophaga ani (Smooth-billed Ani) | 35 cm 115 g | Diurnal, rainforests, urban areas | Omnivore | 172.27 µm (central region) 220.75 µm (peripheral region) | This study |
| Ramphastos toco (Toco Toucan) | 61 cm 592–760 g | Diurnal, scrub forests | Omnivore | 374.36 µm (central region) 721.38 µm (peripheral region) | This study |
| Ara macao (Scarlet Macaw) | 89 cm 1.2 kg | Diurnal, rainforests | Frugivore | 282.88 µm (central region) 266.97 µm (peripheral region) | This study |
| Anodorhynchus hyacinthinus (Hyacinth Macaw) | 1 m 1.2–1.7 kg | Diurnal, rainforests | Frugivore | 472 µm 3 (region not specified) | (Popova et al., 2022) [1] |
| Platalea leucorodia (Eurasian Spoonbill) | 80–90 cm 1.7–2 kg | Diurnal, wetlands | Piscivore | 436 µm 3 (region not specified) | (Popova et al., 2022) [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozetti, R.A.R.; Moreira, M.V.L.; Araújo, R.L.S.; Hirano, L.Q.L.; Moore, B.A.; Galera, P.D. Comparative Corneal Histomorphometry Between Birds of Different Species. Biology 2025, 14, 603. https://doi.org/10.3390/biology14060603
Tozetti RAR, Moreira MVL, Araújo RLS, Hirano LQL, Moore BA, Galera PD. Comparative Corneal Histomorphometry Between Birds of Different Species. Biology. 2025; 14(6):603. https://doi.org/10.3390/biology14060603
Chicago/Turabian StyleTozetti, Rafaela A. R., Matheus V. L. Moreira, Rosélia L. S. Araújo, Liria Q. L. Hirano, Bret A. Moore, and Paula D. Galera. 2025. "Comparative Corneal Histomorphometry Between Birds of Different Species" Biology 14, no. 6: 603. https://doi.org/10.3390/biology14060603
APA StyleTozetti, R. A. R., Moreira, M. V. L., Araújo, R. L. S., Hirano, L. Q. L., Moore, B. A., & Galera, P. D. (2025). Comparative Corneal Histomorphometry Between Birds of Different Species. Biology, 14(6), 603. https://doi.org/10.3390/biology14060603





