Osthole: A Coumarin with Dual Roles in Biology and Chemistry
Simple Summary
Abstract
1. Introduction
2. Bioactivities of Osthole and Its Derivatives
2.1. Anticancer Activity
2.2. Immunomodulatory and Anti-Inflammatory Activities
2.3. Neuroprotective Effects
2.4. Osteogenic Effects and Cardiovascular Benefits
2.5. Hepatoprotective Effects and Therapeutic Potential in Metabolic Disease
2.6. Antimicrobial and Antiparasitic Activities
2.7. Insecticidal Activity
2.8. Other Activities
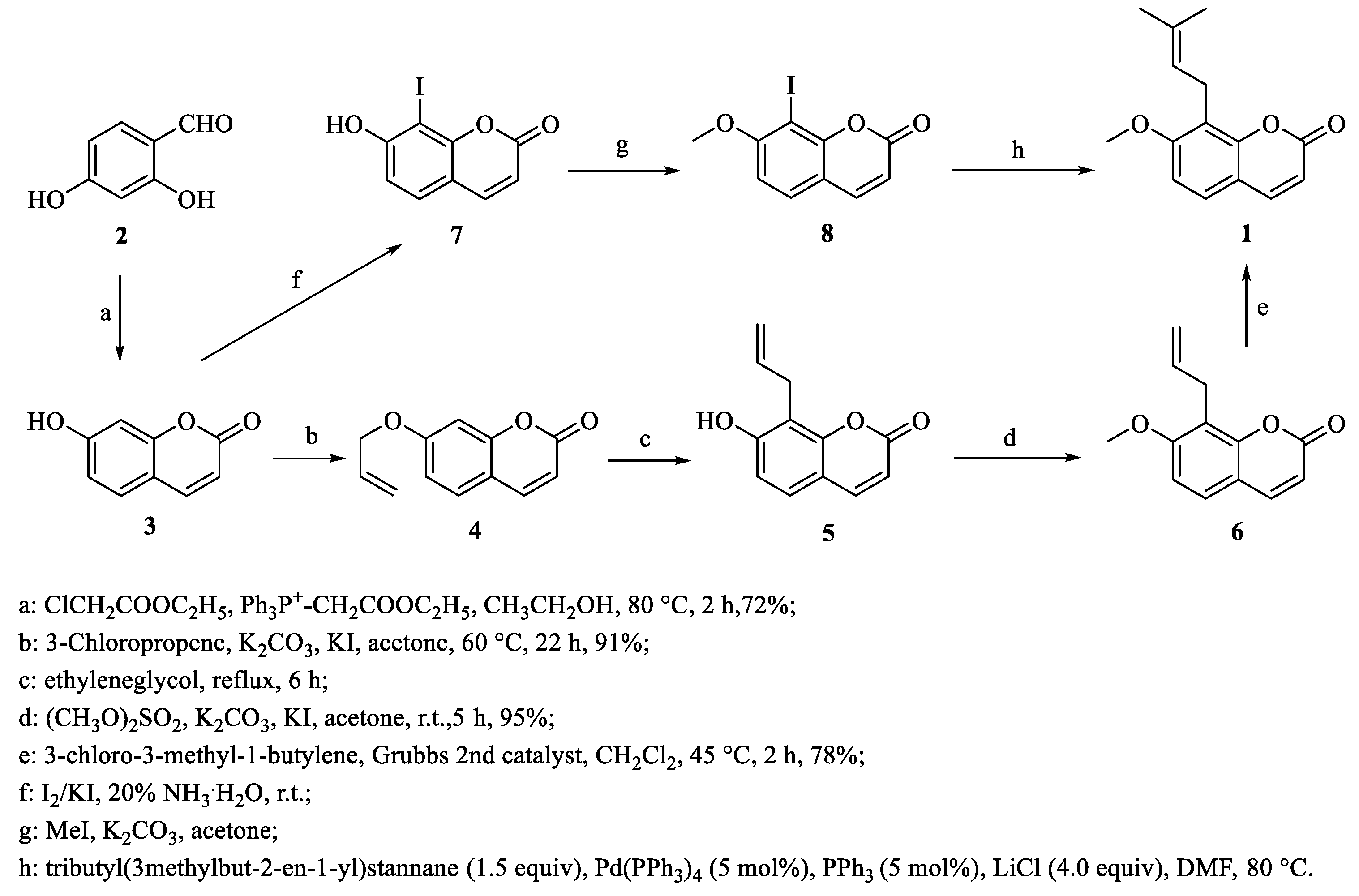
3. Total Synthesis of Osthole and Its Derivatives
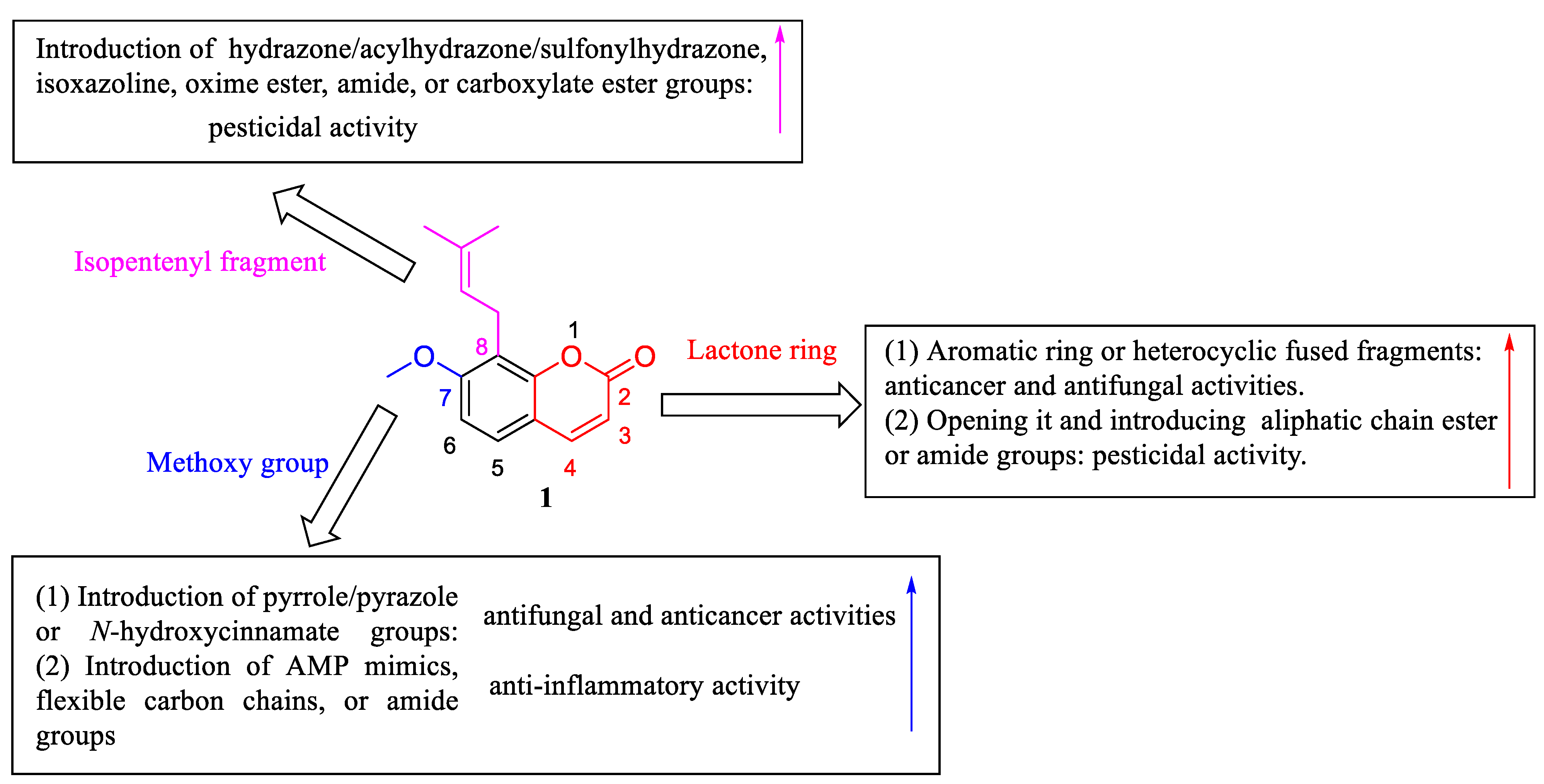
4. Structural Modifications of Osthole and Its Derivatives
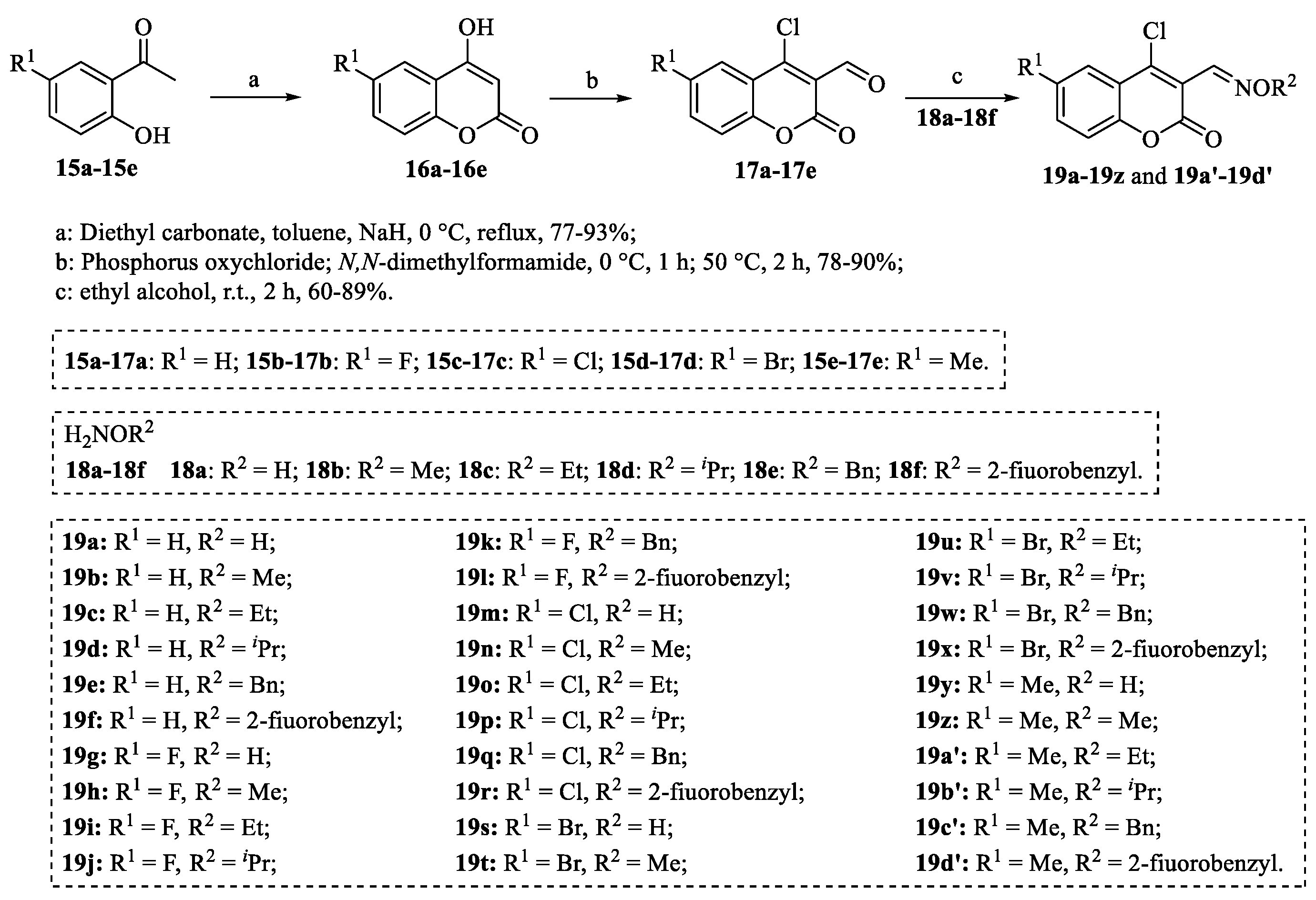
4.1. Structural Modifications on the Lactone Ring of Osthole
4.2. Structural Modifications at the C-7 Position of Osthole
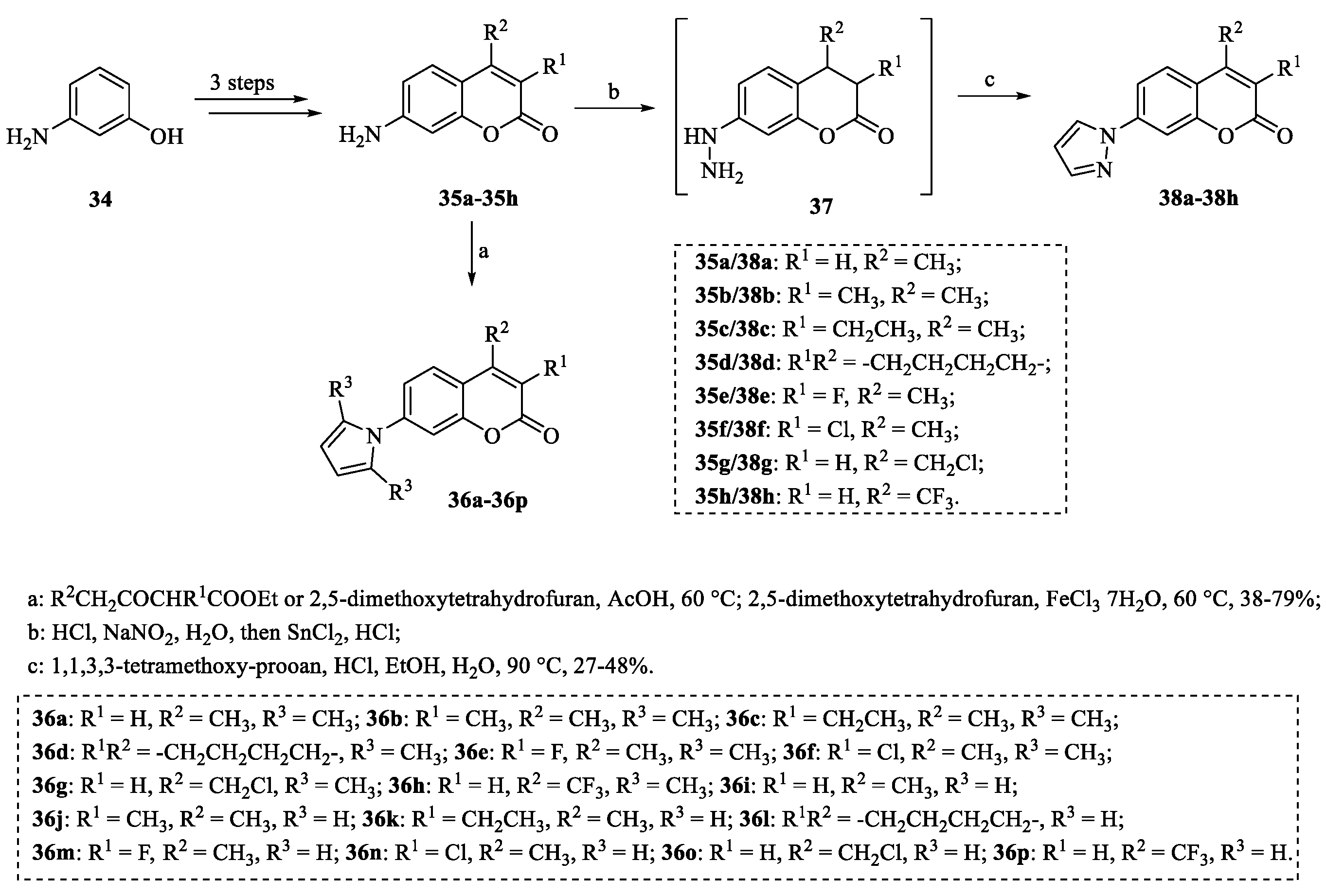
4.3. Structural Modifications at the C-8 Position of Osthole
4.4. Structural Modifications at the C-7 and C-8 Positions and on the Lactone Ring of Osthole
5. Structure–Activity Relationships of Osthole and Its Derivatives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AMP | Adenosine 5′-monophosphate |
| AMPK | Adenosine 5′-monophosphate-activated protein kinase |
| ASD | Autistic spectrum disorders |
| COX-2 | Cyclooxygenase 2 |
| CREB | cAMP-response element binding protein |
| ERK1/2 | Phosphor-extracellular signalregulated kinase 1/2 |
| FASN | Fatty acid synthase |
| HDAC | Histone deacetylase |
| HER2 | Human epidermal growth factor receptor 2 |
| MAPK | Mitogen-activated protein kinase |
| NOS | Nitric oxide synthase |
| JAK | Janus kinase |
| NF-κB | Nuclear factor kappa-B |
| PI3K | Phosphatidylinositol 3 kinase |
| PTEN | Phosphate and tension homology deleted on chromosome ten |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducer and activator of transcription 3 |
References
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Dai, W.L.; Wang, J.N.; Zhang, X.L.; Zheng, Y.T.; Bi, S.Y.; Pang, L.W.; Ren, T.Q.; Yang, Y.; Sun, Y.; et al. Osthole: An up-to-date review of its anticancer potential and mechanisms of action. Front. Pharmacol. 2022, 13, 945627. [Google Scholar] [CrossRef]
- Li, S.H.; Li, M.Y.; Yuan, T.T.; Wang, G.W.; Zeng, J.B.; Shi, Z.M.; Liu, J.H.; Su, J.C. Osthole activates the cholinergic anti-inflammatory pathway via α7nAChR upregulation to alleviate inflammatory responses. Chem. Biodivers. 2024, 21, e202400290. [Google Scholar] [CrossRef]
- Hu, H.Y.; Chen, M.; Dai, G.Z.; Du, G.Q.; Wang, X.Z.; He, J.; Zhao, Y.F.; Han, D.P.; Cao, Y.L.; Zheng, Y.X.; et al. An inhibitory role of osthole in rat MSCs osteogenic differentiation and proliferation via Wnt/β-catenin and Erk1/2-MAPK pathways. Cell. Physiol. Biochem. 2016, 38, 2375–2388. [Google Scholar] [CrossRef]
- Li, S.H.; Yan, Y.H.; Jiao, Y.A.; Gao, Z.; Xia, Y.; Kong, L.; Yao, Y.J.; Tao, Z.Y.; Song, J.; Yan, Y.P.; et al. Neuroprotective effect of osthole on neuron synapses in an Alzheimer’s disease cell model via upregulation of microRNA-9. J. Mol. Neurosci. 2016, 60, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.; Hua, X.W.; Wu, C.C.; Zhou, S.; Wang, B.L.; Li, Z.M. Synthesis of osthole derivatives with grignard reagents and their larvicidal activities on mosquitoes. Chin. J. Chem. 2015, 33, 1353–1358. [Google Scholar] [CrossRef]
- Liang, L.; Yang, B.; Wu, Y.Y.; Sun, L. Osthole suppresses the proliferation and induces apoptosis via inhibiting the PI3K/AKT signaling pathway of endometrial cancer JEC cells. Exp. Ther. Med. 2021, 22, 10605. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Song, H.Y.; Zhou, Z.Y.; Ma, J.; Luo, Z.Y.; Zhou, Y.; Wang, J.Y.; Liu, S.; Han, X.H. Osthole inhibits the migration and invasion of highly metastatic breast cancer cells by suppressing ITGα3/ITGβ5 signaling. Acta Pharmacol. Sin. 2022, 43, 1544–1555. [Google Scholar] [CrossRef]
- Dai, X.X.; Yin, C.T.; Zhang, Y.; Guo, G.L.; Zhao, C.G.; Wang, O.C.; Xiang, Y.Q.; Zhang, X.H.; Liang, G. Osthole inhibits triple negative breast cancer cells by suppressing STAT3. J. Exp. Clin. Cancer Res. 2018, 37, 322. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, C.L.; Wu, E.H.; Han, L.; Deng, X.L.; Shi, Z.F. UPLC-Q-TOF/MS-based metabolomics approach reveals osthole intervention in breast cancer 4T1 cells. Int. J. Mol. Sci. 2023, 24, 1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tian, H.; Zhang, G.D.; Sun, N.; Wang, S.M. Effect of combined treatment with lobaplatin and osthole on inducing apoptosis and inhibiting proliferation in human breast cancer MDA-MB-231 cells. Med. Oncol. 2022, 39, 103579. [Google Scholar] [CrossRef]
- Park, W.; Park, S.; Song, G.; Lim, W. Inhibitory effects of osthole on human breast cancer cell progression via induction of cell cycle arrest, mitochondrial dysfunction, and ER stress. Nutrients 2019, 11, 2777. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Lee, J.Y.; Song, J.; Song, G.; Lim, W. Osthole interacts with an ER-mitochondria axis and facilitates tumor suppression in ovarian cancer. J. Cell. Physiol. 2021, 236, 1025–1042. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhou, J.L.; Xu, Y.Q.; Huang, X.F.; Wang, X.F.; Huang, W.H.; Li, H. Osthole inhibits ovarian carcinoma cells through LC3-mediated autophagy and GSDME-dependent pyroptosis except for apoptosis. Eur. J. Pharmacol. 2020, 874, 172990. [Google Scholar] [CrossRef]
- Arslan, A.K.K.; Uzunhisarcikli, E.; Ökçesiz, A.; Eken, A.; Yerer, M.B. Anticaner effects of coumarin compounds osthole and imperatorin, alone and in combination with 5-fluorouacil in colon carcinoma cells. Acta Pol. Pharm. 2021, 78, 243–251. [Google Scholar]
- Song, J.; Ham, J.; Park, W.; Song, G.; Lim, W. Osthole impairs mitochondrial metabolism and the autophagic flux in colorectal cancer. Phytomedicine 2024, 125, 115383. [Google Scholar] [CrossRef]
- Torshizi, G.H.; Tabrizi, M.H.; Karimi, E.; Younesi, A.; Larian, Z. Designing nanostructured lipid carriers modified with folate-conjugated chitosan for targeted delivery of osthole to HT-29 colon cancer cells: Investigation of anticancer, antioxidant, and antibacterial activities. Cancer Nanotechnol. 2024, 15, 7. [Google Scholar] [CrossRef]
- Zhou, X.H.; Kang, J.; Zhang, L.L.; Cheng, Y. Osthole inhibits malignant phenotypes and induces ferroptosis in KRAS-mutant colorectal cancer cells via suppressing AMPK/Akt signaling. Cancer Chemother. Pharmacol. 2023, 92, 119–134. [Google Scholar] [CrossRef]
- Zhou, X.H.; Kang, J.; Zhong, Z.D.; Cheng, Y. Osthole induces apoptosis of the HT-29 cells via endoplasmic reticulum stress and autophagy. Oncol. Lett. 2021, 22, 12987. [Google Scholar] [CrossRef]
- Huang, H.; Xue, J.; Xie, M.L.; Xie, T. Osthole inhibits GSK-3β/AMPK/mTOR pathway-controlled glycolysis and increases radiosensitivity of subcutaneous transplanted hepatocellular carcinoma in nude mice. Strahlenther. Onkol. 2024, 200, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.S.; Wu, Y.; Li, X.; Rao, H.; Tian, X.X.; Wu, D.N.; Qiu, Z.P.; Zheng, G.H.; Hu, J.J. Osthole delays hepatocarcinogenesis in mice by suppressing AKT/FASN axis and ERK phosphorylation. Eur. J. Pharmacol. 2020, 867, 172788. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, L.R.; Jiang, G.R.; Liu, M.; Liang, G.Q.; Yuan, Q. Osthole attenuates angiogenesis in an orthotopic mouse model of hepatocellular carcinoma via the downregulation of nuclear factor-κB and vascular endothelial growth factor. Oncol. Lett. 2018, 16, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.F.; Sun, D.; Yu, Y.; Yu, J.H. Osthole resensitizes CD133+ hepatocellular carcinoma cells to cisplatin treatment via PTEN/AKT pathway. Aging 2020, 12, 14406–14417. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Zhang, M.Z.; Wang, L.; Zhang, L.; Ma, M.H.; Jing, M.X.; Li, J.P.; Song, R.D.; Zhang, Y.Q.; Yang, Z.Z.; et al. Potential mechanisms of osthole against bladder cancer cells based on network pharmacology, molecular docking, and experimental validation. BMC Complement. Med. Ther. 2023, 23, 122. [Google Scholar] [CrossRef]
- Lv, X.F.; Yang, H.J.; Zhong, H.; He, L.; Wang, L. Osthole exhibits an antitumor effect in retinoblastoma through inhibiting the PI3K/AKT/mTOR pathway via regulating the hsa_circ_0007534/miR-214-3p axis. Pharm. Biol. 2022, 60, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.L.; Li, J.; Li, Z.J.; Li, J.; Wang, S.; Yan, Y.; Zou, K.; Zou, L.J. Osthole enhances antitumor activity and irradiation sensitivity of cervical cancer cells by suppressing ATM/NF-κB signaling. Oncol. Rep. 2018, 40, 737–747. [Google Scholar] [CrossRef]
- Jakubowicz-Gil, J.; Paduch, R.; Skalicka-Wozniak, K.; Sumorek-Wiadro, J.; Zajac, A.; Gawron, A. Hsps responsible for apoptosis induction failure in cervical cancer cells upon osthole and tamoxifen treatment. Postępy Hig. Med. Doświadczalnej 2019, 73, 563–571. [Google Scholar] [CrossRef]
- Su, J.; Zhang, F.; Li, X.; Liu, Z. Osthole promotes the suppressive effects of cisplatin on NRF2 expression to prevent drug-resistant cervical cancer progression. Biochem. Biophys. Res. Commun. 2019, 514, 510–517. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.J.; Jin, M.Z.; Cao, Z.W.; Ren, Y.Y.; Gu, Z.W. Osthole induces cell cycle arrest and apoptosis in head and neck squamous cell carcinoma by suppressing the PI3K/AKT signaling pathway. Chem.-Biol. Interact. 2020, 316, 108934. [Google Scholar] [CrossRef]
- Huangfu, M.J.; Wei, R.M.; Wang, J.; Qin, J.L.; Yu, D.; Guan, X.; Li, X.M.; Fu, M.L.; Liu, H.P.; Chen, X. Osthole induces necroptosis via ROS overproduction in glioma cells. FEBS Open Bio 2021, 11, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Sumorek-Wiadro, J.; Zajac, A.; Badziul, D.; Langner, E.; Skalicka-Wozniak, K.; Maciejczyk, A.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. Coumarins modulate the anti-glioma properties of temozolomide. Eur. J. Pharmacol. 2020, 881, 173207. [Google Scholar] [CrossRef] [PubMed]
- Sumorek-Wiadro, J.; Zajac, A.; Langner, E.; Skalicka-Wozniak, K.; Maciejczyk, A.; Rzeski, W.; Jakubowicz-Gil, J. Antiglioma potential of coumarins combined with sorafenib. Molecules 2020, 25, 5192. [Google Scholar] [CrossRef]
- Zou, T.L.; Wang, H.F.; Ren, T.; Shao, Z.Y.; Yuan, R.Y.; Gao, Y.; Zhang, Y.J.; Wang, X.A.; Liu, Y.B. Osthole inhibits the progression of human gallbladder cancer cells through JAK/STAT3 signal pathway both in vitro and in vivo. Anti-Cancer Drugs 2019, 30, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Abosharaf, H.A.; Diab, T.; Atlam, F.M.; Mohamed, T.M. Osthole extracted from a citrus fruit that affects apoptosis on A549 cell line by histone deacetylasese inhibition (HDACs). Biotechnol. Rep. 2020, 28, e00531. [Google Scholar] [CrossRef]
- Xu, X.J.; Liu, X.Y.; Zhang, Y. Osthole inhibits gastric cancer cell proliferation through regulation of PI3K/AKT. PLoS ONE 2018, 13, e0193449. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, F.; Tian, Z.Y.; Song, W.; Cheng, B.F.; Feng, Z.W. Osthole synergizes with HER2 inhibitor, trastuzumab in HER2-overexpressed N87 gastric cancer by inducing apoptosis and inhibition of AKT-MAPK pathway. Front. Pharmacol. 2018, 9, 1392. [Google Scholar] [CrossRef]
- Zhu, X.B.; Li, Z.Z.; Li, T.T.; Long, F.; Lv, Y.S.; Liu, L.; Liu, X.F.; Zhan, Q.M. Osthole inhibits the PI3K/AKT signaling pathway via activation of PTEN and induces cell cycle arrest and apoptosis in esophageal squamous cell carcinoma. Biomed. Pharmacother. 2018, 102, 502–509. [Google Scholar] [CrossRef]
- Jarzab, A.; Luszczki, J.; Guz, M.; Skalicka-Wozniak, K.; Halasa, M.; Smok-Kalwat, J.; Polberg, K.; Stepulak, A. Combination of osthole and cisplatin against rhabdomyosarcoma TE671 cells yielded additive pharmacologic interaction by means of isobolographic analysis. Anticancer Res. 2018, 38, 205–210. [Google Scholar]
- Wang, B.T.; Zheng, X.; Liu, J.; Zhang, Z.; Qiu, C.Y.; Yang, L.; Zhang, L.Q.; Zhang, Q.; Gao, H.W.; Wang, X.M. Osthole inhibits pancreatic cancer progression by directly exerting negative effects on cancer cells and attenuating tumor-infiltrating M2 macrophages. J. Pharmacol. Sci. 2018, 137, 290–298. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chang, D.C.; Lo, Y.S.; Hsi, Y.T.; Lin, C.C.; Chuang, Y.C.; Lin, S.H.; Hsieh, M.J.; Chen, M.K. Osthole induces human nasopharyngeal cancer cells apoptosis through Fas-Fas ligand and mitochondrial pathway. Environ. Toxicol. 2018, 33, 446–453. [Google Scholar] [CrossRef]
- Zhou, Y.; Du, Z.T.; Wu, Q.Q.; Guo, M.; Chen, Z.C.; Sun, C.H.; Li, X.B.; Zou, Y.; Zheng, Z.W.; Chen, P.; et al. Discovery of novel osthole derivatives exerting anti-inflammatory effect on DSS-induced ulcerative colitis and LPS-induced acute lung injury in mice. Eur. J. Med. Chem. 2024, 268, 152864. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Wang, Y.S.; Huang, J.C.; Wang, T.T.; Li, B.Y.; Guo, B.; Yue, Z.P. Osthole exhibits the remedial potential for polycystic ovary syndrome mice through Nrf2-Foxo1-GSH-NF-κB pathway. Cell Biol. Int. 2024, 48, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Gan, Z.F.; Ji, K.; Li, X.; Wu, J.B.; Liu, Y.; Wang, X.K.; Liang, H.Y.; Liu, Y.N.; Li, X.T.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine 2019, 58, 116252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, F.; Zhou, R.J.; Zhu, Z.Y.; Xie, M.L. PPARα/γ antagonists reverse the ameliorative effects of osthole on hepatic lipid metabolism and inflammatory response in steatohepatitic rats. Inflammopharmacology 2018, 26, 425–433. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, J.; Xie, M.L. Osthole inhibits oleic acid/lipopolysaccharide-induced lipid accumulation and inflammatory response through activating PPARα signaling pathway in cultured hepatocytes. Exp. Gerontol. 2019, 119, 7–13. [Google Scholar] [CrossRef]
- Jiang, X.L.; Lu, Z.J.; Zhang, Q.; Yu, J.L.; Han, D.; Liu, J.H.; Li, P.; Li, F. Osthole: A potential AMPK agonist that inhibits NLRP3 inflammasome activation by regulating mitochondrial homeostasis for combating rheumatoid arthritis. Phytomedicine 2023, 110, 154640. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Tao, C.; Luo, L.; He, J.; Wang, Q. Osthole regulates N6-methyladenosine-modified TGM2 to inhibit the progression of rheumatoid arthritis and associated interstitial lung disease. MedComm 2023, 4, e219. [Google Scholar] [CrossRef]
- Zhang, W.L.; Li, Z.C.; Dai, Z.W.; Chen, S.Y.; Guo, W.X.; Wang, Z.W.; Wei, J.S. GelMA hydrogel as a promising delivery system for osthole in the treatment of rheumatoid arthritis: Targeting the miR-1224-3p/AGO1 axis. Int. J. Mol. Sci. 2023, 24, 13210. [Google Scholar] [CrossRef]
- Ma, T.; Wang, X.P.; Qu, W.J.; Yang, L.S.; Jing, C.; Zhu, B.R.; Zhang, Y.K.; Xie, W.P. Osthole suppresses knee osteoarthritis development by enhancing autophagy activated via the AMPK/ULK1 pathway. Molecules 2022, 27, 8624. [Google Scholar] [CrossRef]
- Fu, X.P.; Hong, C.H. Osthole attenuates mouse atopic dermatitis by inhibiting thymic stromal lymphopoietin production from keratinocytes. Exp. Dermatol. 2019, 28, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Kordulewska, N.K.; Król-Grzymala, A. The effect of osthole on transient receptor potential channels: A possible alternative therapy for atopic dermatitis. J. Inflamm. Res. 2024, 17, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Kordulewska, N.K.; Topa, J.; Stryinski, R.; Jarmolowska, B. Osthole inhibits expression of genes associated with toll-like receptor 2 signaling pathway in an organotypic 3D skin model of human epidermis with atopic dermatitis. Cells 2022, 11, 88. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, J.X.; Qi, M.X.; Li, X.; Yang, Y.; Zhu, C.; Wang, C.M.; Tang, Z.X.; Ma, Y.X.; Yu, G. Osthole relieves skin damage and inhibits chronic itch through modulation of Akt/ZO-3 pathway in atopic dermatitis. Eur. J. Pharmacol. 2023, 947, 175649. [Google Scholar]
- Bao, Y.X.; Meng, X.L.; Liu, F.N.; Wang, F.; Yang, J.H.; Wang, H.Y.; Xie, G.H. Protective effects of osthole against inflammation induced by lipopolysaccharide in BV2 cells. Mol. Med. Rep. 2018, 17, 4561–4566. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, M.Y.; Kuo, Y.H.; Cheng, J.C.; Chang, L.Z.; Chang, M.S.; Chuang, T.N.; Hsieh, W.T.; Xiao, Y.R.; Wu, B.T.; et al. Osthole antagonizes microglial activation in an NRF2-dependent manner. Molecules 2023, 28, 28020507. [Google Scholar] [CrossRef]
- Singh, G.; Bhatti, R.; Mannan, R.; Singh, D.; Kesavan, A.; Singh, P. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacology 2019, 27, 949–960. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Kostyra, E.; Chwala, B.; Moszynska, M.; Cieslinska, A.; Fiedorowicz, E.; Jarmolowska, B. A novel concept of immunological and allergy interactions in autism spectrum disorders: Molecular, anti-inflammatory effect of osthole. Int. Immunopharmacol. 2019, 72, 1–11. [Google Scholar] [CrossRef]
- Chu, Q.B.; Zhu, Y.F.; Cao, T.J.; Zhang, Y.; Chang, Z.C.; Liu, Y.; Lu, J.H.; Zhang, Y.Z. Studies on the neuroprotection of osthole on glutamate-induced apoptotic cells and an Alzheimer’s disease mouse model via modulation oxidative stress. Appl. Biochem. Biotechnol. 2020, 190, 634–644. [Google Scholar] [CrossRef]
- Liu, J.M.; Wu, Q.H.; Wu, Q.Q.; Zhong, G.C.; Liang, Y.; Gu, Y.; Hu, Y.H.; Wang, W.J.; Hao, N.; Fang, S.H.; et al. Modulating endoplasmic reticulum stress in APP/PS1 mice by Gomisin B and osthole in Bushen-Yizhi formula: Synergistic effects and therapeutic implications for Alzheimer’s disease. Phytomedicine 2023, 119, 155023. [Google Scholar] [CrossRef]
- Wang, X.; Fu, X.M.; Luo, X.R.; Lai, Y.Y.; Cai, C.P.; Liao, Y.F.; Dai, Z.; Fang, S.H.; Fang, J.S. Network proximity analysis deciphers the pharmacological mechanism of osthole against D-galactose induced cognitive disorder in rats. Molecules 2024, 29, 21. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Y.; Zhang, M.J.; Chen, Y.F.; Yang, W.D. Natural products as sources of acetylcholinesterase inhibitors: Synthesis, biological activities, and molecular docking studies of osthole-based ester derivatives. Front. Plant Sci. 2022, 13, 1054650. [Google Scholar] [CrossRef] [PubMed]
- Barangi, S.; Hosseinzadeh, P.; Karimi, G.; Tayarani-Najaran, Z.; Mehri, S. Osthole attenuated cytotoxicity induced by 6-OHDA in SH-SY5Y cells through inhibition of JAK/STAT and MAPK pathways. Iran. J. Basic Med. Sci. 2023, 26, 953–959. [Google Scholar]
- Wang, S.G.; Ding, S.L.; Wang, Y.L.; He, C.Y.; Yao, R.P.; Jin, H. Osthole exerts neuroprotection in a mouse model of Parkinson’s disease. Curr. Top. Nutraceutical Res. 2023, 21, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Wang, X.; Zhen, F.; Chen, R.; Geng, D.Q.; Yao, R.Q. Osthole alleviates MPTP-induced Parkinson’s disease mice by suppressing Notch signaling pathway. Int. J. Neurosci. 2019, 129, 833–841. [Google Scholar] [CrossRef]
- Yao, Y.J.; Liang, X.C.; Shi, Y.; Lin, Y.; Yang, J.X. Osthole delays tert-butyl hydroperoxide-induced premature senescence in neural stem cells. Cell. Reprogramm. 2018, 20, 268–274. [Google Scholar] [CrossRef]
- Yan, Y.H.; Kong, L.; Xia, Y.; Liang, W.B.; Wang, L.T.; Song, J.; Yao, Y.J.; Lin, Y.; Yang, J.X. Osthole promotes endogenous neural stem cell proliferation and improved neurological function through Notch signaling pathway in mice acute mechanical brain injury. Brain Behav. Immun. 2018, 67, 118–129. [Google Scholar] [CrossRef]
- Zhao, C.; Rollo, B.; Shahid Javaid, M.; Huang, Z.; He, W.; Xu, H.; Kwan, P.; Zhang, C. An integrated in vitro human iPSCs-derived neuron and in vivo animal approach for preclinical screening of anti-seizure compounds. J. Adv. Res. 2024, 64, 249–262. [Google Scholar] [CrossRef]
- Singh, L.; Kaur, A.; Garg, S.; Bhatti, R. Skimmetin/osthole mitigates pain-depression dyad via inhibiting inflammatory and oxidative stress-mediated neurotransmitter dysregulation. Metab. Brain Dis. 2021, 36, 111–121. [Google Scholar] [CrossRef]
- Jin, Z.X.; Liao, X.Y.; Da, W.W.; Zhao, Y.J.; Li, X.F.; Tang, D.Z. Osthole enhances the bone mass of senile osteoporosis and stimulates the expression of osteoprotegerin by activating β-catenin signaling. Stem Cell Res. Ther. 2021, 12, 154. [Google Scholar] [CrossRef]
- Chen, J.C.; Liao, X.F.; Gan, J.W. Review on the protective activity of osthole against the pathogenesis of osteoporosis. Front. Pharmacol. 2023, 14, 1236892. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, L.N.; Zheng, S.Y.; Xu, J.K.; Pan, Y.L.; Tu, P.C.; Sun, J.; Guo, Y. Osthole inhibits osteoclasts formation and bone resorption by regulating NF-κB signaling and NFATc1 activations stimulated by RANKL. J. Cell. Biochem. 2019, 120, 16052–16061. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.S.; Yin, J.Y.; Dai, X.; Liu, T.Y.; Shi, H.F.; Zhang, Y.Y.; Wang, S.; Yue, G.Y.; Zhang, Y.F.; Zhao, D.D.; et al. Cnidii Fructus: A traditional Chinese medicine herb and source of antiosteoporotic drugs. Phytomedicine 2024, 128, 155375. [Google Scholar] [CrossRef] [PubMed]
- García-Arroyo, F.E.; Gonzaga-Sánchez, G.; Silva-Palacios, A.; Roldán, F.J.; Loredo-Mendoza, M.L.; Alvarez-Alvarez, Y.Q.; Coyotl, J.A.D.; Orozco, K.A.V.; Tapia, E.; Osorio-Alonso, H.; et al. Osthole prevents heart damage induced by diet-induced metabolic syndrome: Role of fructokinase (KHK). Antioxidants 2023, 12, 1023. [Google Scholar] [CrossRef]
- Li, Y.L.; Li, Y.Q.; Shi, F.G.; Wang, L.N.; Li, L.S.; Yang, D.L. Osthole attenuates right ventricular remodeling via decreased myocardial apoptosis and inflammation in monocrotaline-induced rats. Eur. J. Pharmacol. 2018, 818, 525–533. [Google Scholar] [CrossRef]
- Hou, H.; Yang, Y.P.; Chen, R.; Guo, Z.P. Osthole protects H9c2 cardiomyocytes against trastuzumab-induced damage by enhancing autophagy through the p38MAPK/mTOR signaling pathway. Toxicol. In Vitro 2023, 93, 105704. [Google Scholar] [CrossRef]
- Lu, W.F.; He, Y.; Yu, T.; Huang, K.L.; Liu, S.Z. Osthole down-regulates miR-30a and promotes autophagy to protect rats against myocardial ischemia/reperfusion injury. Turk Gogus Kalp Damar. 2019, 27, 178–184. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, W.; Zhang, X.X.; Lin, Y.D.; Chen, H.; Li, H. Osthole prevents acetaminophen-induced liver injury in mice. Acta Pharmacol. Sin. 2018, 39, 74–84. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, L.; Chu, W.H.; Wang, P.; Fu, Y.Q. Osthole alleviates D-galactose-induced liver injury in vivo via the TLR4/MAPK/NF-κB pathways. Molecules 2023, 28, 443. [Google Scholar] [CrossRef]
- Zhou, W.B.; Zhang, X.X.; Cai, Y.; Sun, W.; Li, H. Osthole prevents tamoxifen-induced liver injury in mice. Acta Pharmacol. Sin. 2019, 40, 608–619. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, Y.; Shao, Q.Q.; Fang, K.; Dong, R.L.; Jiang, S.J.; Lu, F.E.; Luo, J.L.; Chen, G. Ameliorative effects of osthole on experimental renal fibrosis in vivo and in vitro by inhibiting IL-11/ERK1/2 signaling. Front. Pharmacol. 2021, 12, 646331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Huang, Q.; Cai, X.X.; Jiang, S.; Xu, N.; Zhou, Q.; Cao, X.Y.; Hultström, M.; Tian, J.; Lai, E.Y. Osthole ameliorates renal fibrosis in mice by suppressing fibroblast activation and epithelial-mesenchymal transition. Front. Physiol. 2018, 9, 1650. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Wang, Y.F.; Yan, J.; Yuan, R.Y.; Zhang, J.M.; Guo, X.H.; Zhao, M.M.; Li, F.F.; Li, X.T. Osthole ameliorates early diabetic kidney damage by suppressing oxidative stress, inflammation and inhibiting TGF-81/Smads signaling pathway. Int. Immunopharmacol. 2024, 133, 112131. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Chen, J.B.; Ren, D.; Du, B.; Wu, L.; Zhang, Y.Y.; Wang, Z.Y.; Qian, S. Synthesis of osthol-based botanical fungicides and their antifungal application in crop protection. Bioorg. Med. Chem. 2021, 40, 116184. [Google Scholar] [CrossRef]
- Kong, Y.; Yan, H.; Hu, J.J.; Dang, Y.X.; Han, Z.H.; Tian, B.; Wang, P.X. Antibacterial activity and mechanism of action of osthole against Listeria monocytogenes. J. Agric. Food Chem. 2024, 72, 10853–10861. [Google Scholar] [CrossRef]
- Zheng, H.L.; Chen, Y.H.; Guo, Q.L.; Wei, H.; Yue, J.Y.; Zhou, H.Y.; Zhao, M.M. Inhibitory effect of osthole from Cnidium monnieri (L.) Cusson on Fusarium oxysporum, a common fungal pathogen of potato. Molecules 2021, 26, 3818. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.Y.; Mo, F.X.; Ding, Y.; Zhou, A.A.; Guo, X.; Li, R.T.; Li, M.; Ou, M.G.; Li, M. Natural product osthole can significantly disrupt cell wall integrity and dynamic balance of Fusarium oxysporum. Pestic. Biochem. Physiol. 2023, 196, 105623. [Google Scholar] [CrossRef]
- Hu, X.F.; Wang, J.; Zhang, Y.B.; Li, R.Y.; Li, M. Molecular mechanism of osthole against chitin synthesis of Ustilaginoidea virens based on combined transcriptome and metabolome analyses. Pestic. Biochem. Physiol. 2023, 196, 105612. [Google Scholar] [CrossRef]
- Lai, D.; Wang, D.L.; Shao, X.H.; Qin, J.; Zhuang, Q.L.; Xu, H.H.; Xiao, W.Q. Comparative physiological and transcriptome analysis provide insights into the inhibitory effect of osthole on Penicillium choerospondiatis. Pestic. Biochem. Physiol. 2024, 198, 105749. [Google Scholar] [CrossRef]
- Sun, Y.B.; Chen, Y.; Liu, T.; Wang, Y.Y.; Wang, Y.; Han, L.R.; Ma, Z.Q.; Feng, J.T. Evaluating the efficacy of osthole and matrine for control of Sorghum purple spot. J. Plant Dis. Prot. 2021, 128, 1263–1268. [Google Scholar] [CrossRef]
- Yu, C.Q.; Xie, T.T.; Liu, S.H.; Bai, L.G. Fabrication of a biochar-doped monolithic adsorbent and its application for the extraction and determination of coumarins from Angelicae Pubescentis Radix. J. Chromatogr. A 2024, 1714, 115940. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Guo, D.S.; Lu, M.H.; Yue, J.Y.; Liu, Y.; Shang, C.M.; An, D.R.; Zhao, M.M. Inhibitory effect of osthole from Cnidium monnieri on tobacco mosaic virus (TMV) infection in Nicotiana glutinosa. Molecules 2020, 25, 65. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Ren, Q.Y.; Luo, J.; Chen, Z.; Xu, X.F.; Guo, J.H.; Tan, Y.C.; Liu, W.G.; Qu, Z.Q.; Wu, Z.G.; et al. Ultrasound-assisted extraction extracts from Stemona japonica (Blume) Miq. and Cnidium monnieri (L.) Cuss. could be used as potential Rhipicephalus sanguineus control agents. Exp. Parasitol. 2020, 217, 107955. [Google Scholar] [CrossRef]
- Ren, Z.L.; Lv, M.; Yang, Y.L.; Gu, S.Y.; Li, L.L.; Liu, H.Q.; Xu, H. Structural optimization of natural plant products: Construction, pesticidal activities, and toxicology study of new 2-isopropanol-4-methoxy-7-alkyl/aryloxycarbonyl-(E)-vinyl-2,3-dihydrobenzofurans. J. Agric. Food Chem. 2025, 73, 1804–1812. [Google Scholar] [CrossRef]
- Xu, J.W.; Lv, M.; Li, T.Z.; Wen, H.P.; Xu, H. Optimization of osthole in the lactone ring as an agrochemical candidate: Synthesis, characterization, and pesticidal activities of osthole amide/ester derivatives and their effects on morphological changes of mite epidermis. J. Agric. Food Chem. 2023, 71, 6570–6583. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Lv, M.; Liu, H.Q.; Wen, H.P.; Zhang, Y.L.; Xu, H. Optimization of osthole as a pesticide candidate: Synthesis, crystal structures, and agrochemical properties of acrylate derivatives of isopropenyl 2,3-dihydrobenzofurans. J. Agric. Food Chem. 2023, 71, 18301–18311. [Google Scholar] [CrossRef]
- Dong, F.; Chen, X.; Men, X.Y.; Li, Z.; Kong, Y.J.; Yuan, Y.Y.; Ge, F. Contact toxicity, antifeedant activity, and oviposition preference of osthole against agricultural pests. Insects 2023, 14, 725. [Google Scholar] [CrossRef]
- Idrees, A.; Afzal, A.; Chohan, T.A.; Hayat, S.; Qadir, Z.A.; Gaafar, A.R.Z.; Zuan, A.T.K.; Li, J. Laboratory evaluation of selected botanicals and insecticides against invasive Spodoptera frugiperda (Lepidoptera: Noctuidae). J. King Saud Univ. Sci. 2023, 35, 102811. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Hu, Q.; Du, X.G. Acaricidal of activity of matrine and osthole mixture against Tetranychus urticae Koch on Strawberry. Chin. J. Biol. Control 2022, 38, 1037–1042. [Google Scholar]
- Zeng, X.H.; Chen, S.H.; Wang, S.Z.; Zhang, L.; Xu, P.; Guo, Y.H. Study on the contact toxicity and stomach poisoning of four botanical pesticide against Coptotermes formosanus. Chin. J. Hyg. Insectic. Equip. 2019, 25, 370–373. [Google Scholar]
- Xia, C.X.; Li, S.Q.; Cai, W.L.; Yan, X.; Zhang, H.Y. Insecticidal activity of osthole powder and its effect on enzyme activity of Sitophilus zeamais. Chin. Bull. Entomol. 2009, 46, 740–744. [Google Scholar]
- Meng, L.L.; Wang, J.Z.; Zhang, Z.Y.; Sun, S.L.; Zhang, M.Z.; Zhang, P.F.; Ding, B.L.; Pu, Y.Y. Stomach action of osthole and its effect on two enzyme activities of Plutella xylostella larvae. J. Beijing Univ. Agric. 2010, 25, 24–27. [Google Scholar]
- Wang, Z.Q.; Kim, J.R.; Wang, M.; Shu, S.H.; Ahn, Y.J. Larvicidal activity of Cnidium monnieri fruit coumarins and structurally related compounds against insecticide-susceptible and insecticide-resistant Culex pipiens pallens and Aedes aegypti. Pest Manag. Sci. 2012, 68, 1041–1047. [Google Scholar] [CrossRef]
- Sun, X.Y.; Sun, L.L.; Qi, H.; Gao, Q.; Wang, G.X.; Wei, N.N.; Wang, K. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol. Pharmacol. 2018, 94, 1164–1173. [Google Scholar] [CrossRef]
- Torres, K.V.; Pantke, S.; Rudolf, D.; Eberhardt, M.M.; Leffler, A. The coumarin osthole is a non-electrophilic agonist of TRPA1. Neurosci. Lett. 2022, 789, 136878. [Google Scholar] [CrossRef]
- Wang, P.P.; Fan, Z.J.; Wei, W.P.; Yang, C.S.; Wang, Y.; Shen, X.; Yan, X.; Zhou, Z.H. Biosynthesis of the plant coumarin osthole by engineered Saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, Y.; Huo, Y.W.; Li, Y.; Abel, P.W.; Jiang, H.H.; Zou, X.H.; Jiao, H.Z.; Kuang, X.L.; Wolff, D.W.; et al. Airway relaxation mechanisms and structural basis of osthole for improving lung function in asthma. Sci. Signal. 2020, 13, eaax0273. [Google Scholar] [CrossRef]
- Park, J.; Shin, S.; Bu, Y.; Choi, H.Y.; Lee, K. Vasorelaxant and blood pressure-lowering effects of Cnidium monnieri fruit ethanol extract in sprague dawley and spontaneously hypertensive rats. Int. J. Mol. Sci. 2024, 25, 4223. [Google Scholar] [CrossRef]
- Liu, G.L.; Hao, B.; Liu, S.P.; Wang, G.X. Synthesis and anthelmintic activity of osthol analogs against Dactylogyrus intermedius in goldfish. Eur. J. Med. Chem. 2012, 54, 582–590. [Google Scholar] [CrossRef]
- Yin, Q.; Yan, H.; Zhang, Y.Q.; Wang, Y.G.; Zhang, G.J.; He, Y.X.; Zhang, W.H. Palladium-catalyzed synthesis of 8-allyl or 8-prenylcoumarins by using organotin reagents as multicoupling nucleophiles. Appl. Organomet. Chem. 2013, 27, 85–88. [Google Scholar] [CrossRef]
- Konrádová, D.; Kozubíková, H.; Dolezal, K.; Pospísil, J. Microwave-assisted synthesis of phenylpropanoids and coumarins: Total synthesis of osthol. Eur. J. Org. Chem. 2017, 2017, 5204–5213. [Google Scholar] [CrossRef]
- Schmidt, B.; Riemer, M. Synthesis of allyl- and prenylcoumarins via microwave-promoted tandem claisen rearrangement/wittig olefination. Synthesis 2016, 48, 141–149. [Google Scholar] [CrossRef]
- Gulías, M.; Marcos-Atanes, D.; Mascareñas, J.L.; Font, M. Practical, large-scale preparation of benzoxepines and coumarins through Rhodium(III)-catalyzed C-H activation/annulation reactions. Org. Process Res. Dev. 2019, 23, 1669–1673. [Google Scholar] [CrossRef]
- Dai, P.; Wang, Q.Q.; Teng, P.; Jiao, J.; Li, Y.F.; Xia, Q.; Zhang, W.H. Design, synthesis, antifungal activity, and 3D-QASR of novel oxime ether-containing coumarin derivatives as potential fungicides. J. Agric. Food Chem. 2024, 72, 5983–5992. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.N.; Wang, K.H.; Wang, B.B.; Wang, Z.W.; Liu, Y.X.; Wang, Q.M. Design, synthesis, and biological activities of novel coumarin derivatives as pesticide candidates. J. Agric. Food Chem. 2024, 72, 4658–4668. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Zhang, R.R.; Wang, J.Q.; Yu, X.; Zhang, Y.L.; Wang, Q.Q.; Zhang, W.H. Microwave-promoted synthesis of novel fused osthole analogues. Chin. J. Chem. 2016, 34, 1344–1352. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Zhang, R.R.; Wang, J.Q.; Yu, X.; Zhang, Y.L.; Wang, Q.Q.; Zhang, W.H. Microwave-assisted synthesis and antifungal activity of novel fused Osthole derivatives. Eur. J. Med. Chem. 2016, 124, 10–16. [Google Scholar] [CrossRef]
- Zhang, S.G.; Liang, C.G.; Sun, Y.Q.; Teng, P.; Wang, J.Q.; Zhang, W.H. Design, synthesis and antifungal activities of novel pyrrole- and pyrazole-substituted coumarin derivatives. Mol. Divers. 2019, 23, 915–925. [Google Scholar] [CrossRef]
- Li, S.C.; Lv, M.; Sun, Z.Q.; Hao, M.; Xu, H. Optimization of osthole in the lactone ring: Structural elucidation, pesticidal activities, and control efficiency of osthole ester derivatives. J. Agric. Food Chem. 2021, 69, 6465–6474. [Google Scholar] [CrossRef]
- Yang, H.Y.; Hsu, Y.F.; Chiu, P.T.; Ho, S.J.; Wang, C.H.; Chi, C.C.; Huang, Y.H.; Lee, C.F.; Li, Y.S.; Ou, G.; et al. Anti-cancer activity of an osthole derivative, NBM-T-BMX-OS01: Targeting vascular endothelial growth factor receptor signaling and angiogenesis. PLoS ONE 2013, 8, e81592. [Google Scholar] [CrossRef]
- Pai, J.T.; Hsu, C.Y.; Hua, K.T.; Yu, S.Y.; Huang, C.Y.; Chen, C.N.; Liao, C.H.; Weng, M.S. NBM-T-BBX-OS01, semisynthesized from osthole, induced G1 growth arrest through HDAC6 inhibition in lung cancer cells. Molecules 2015, 20, 8000–8019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Y.H.; Yang, G.X.; Gan, H.X.; Sang, D.Y.; Zhou, J.Y.; Su, L.; Wang, R.; Ma, L. Design, synthesis and biological evaluation of novel osthole-based derivatives as potential neuroprotective agents. Bioorg. Med. Chem. Lett. 2020, 30, 127633. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Lv, M.; Xu, H. Osthole: Synthesis, structural modifications, and biological properties. Mini-Rev. Med. Chem. 2022, 22, 2124–2137. [Google Scholar]
- Hao, M.; Jiang, L.L.; Lv, M.; Ding, H.X.; Zhou, Y.M.; Xu, H. Plant natural product-based pesticides in crop protection: Semi-synthesis, mono-crystal structures and agrochemical activities of osthole ester derivatives, and study of their toxicology against Tetranychus cinnabarinus (Boisduval). Pest Manag. Sci. 2024, 80, 6356–6365. [Google Scholar] [CrossRef]
- Hao, M.; Lv, M.; Zhou, L.; Li, H.J.; Xu, J.W.; Xu, H. Construction, pesticidal activities, control effects, and detoxification enzyme activities of osthole ester/amide derivatives. J. Agric. Food Chem. 2022, 70, 9337–9345. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Lv, M.; Sun, Z.Q.; Li, T.Z.; Zhang, S.Y.; Xu, H. Regioselective hemisynthesis and insecticidal activity of C8-hydrazones/acylhydrazones/sulfonylhydrazones coumarin-type derivatives of osthole. Bioorg. Med. Chem. Lett. 2021, 40, 127962. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, C.C.; Chao, S.W.; Lee, S.S.; Hsu, F.L.; Lu, Y.L.; Hung, M.F.; Chang, C.I. Synthesis of N-hydroxycinnamides capped with a naturally occurring moiety as inhibitors of histone deacetylase. Chemmedchem 2010, 5, 598–607. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, C.C.; Chao, S.W.; Yu, C.C.; Yang, C.Y.; Guh, J.H.; Lin, Y.C.; Kuo, C.I.; Yang, P.; Chang, C.I. Synthesis and evaluation of aliphatic-chain hydroxamates capped with osthole derivatives as histone deacetylase inhibitors. Eur. J. Med. Chem. 2011, 46, 4042–4049. [Google Scholar] [CrossRef]
- Ren, Z.L.; Lv, M.; Li, T.Z.; Hao, M.; Li, S.C.; Xu, H. Construction of oxime ester derivatives of osthole from Cnidium monnieri, and evaluation of their agricultural activities and control efficiency. Pest Manag. Sci. 2020, 76, 3560–3567. [Google Scholar] [CrossRef]
- Shan, X.J.; Lv, M.; Wang, J.R.; Qin, Y.J.; Xu, H. Acaricidal and insecticidal efficacy of new esters derivatives of a natural coumarin osthole. Ind. Crops Prod. 2022, 182, 114855. [Google Scholar] [CrossRef]
- Farooq, S.; Alharthi, F.A.; Alsalme, A.; Hussain, A.; Dar, B.A.; Hamid, A.; Koul, S. Dihydropyrimidinones: Efficient one-pot green synthesis using Montmorillonite-KSF and evaluation of their cytotoxic activity. RSC Adv. 2020, 10, 42221. [Google Scholar] [CrossRef] [PubMed]
- You, L.S.; An, R.; Wang, X.H.; Li, Y.M. Discovery of novel osthole derivatives as potential anti-breast cancer treatment. Bioorg. Med. Chem. Lett. 2010, 20, 7426–7428. [Google Scholar] [CrossRef] [PubMed]





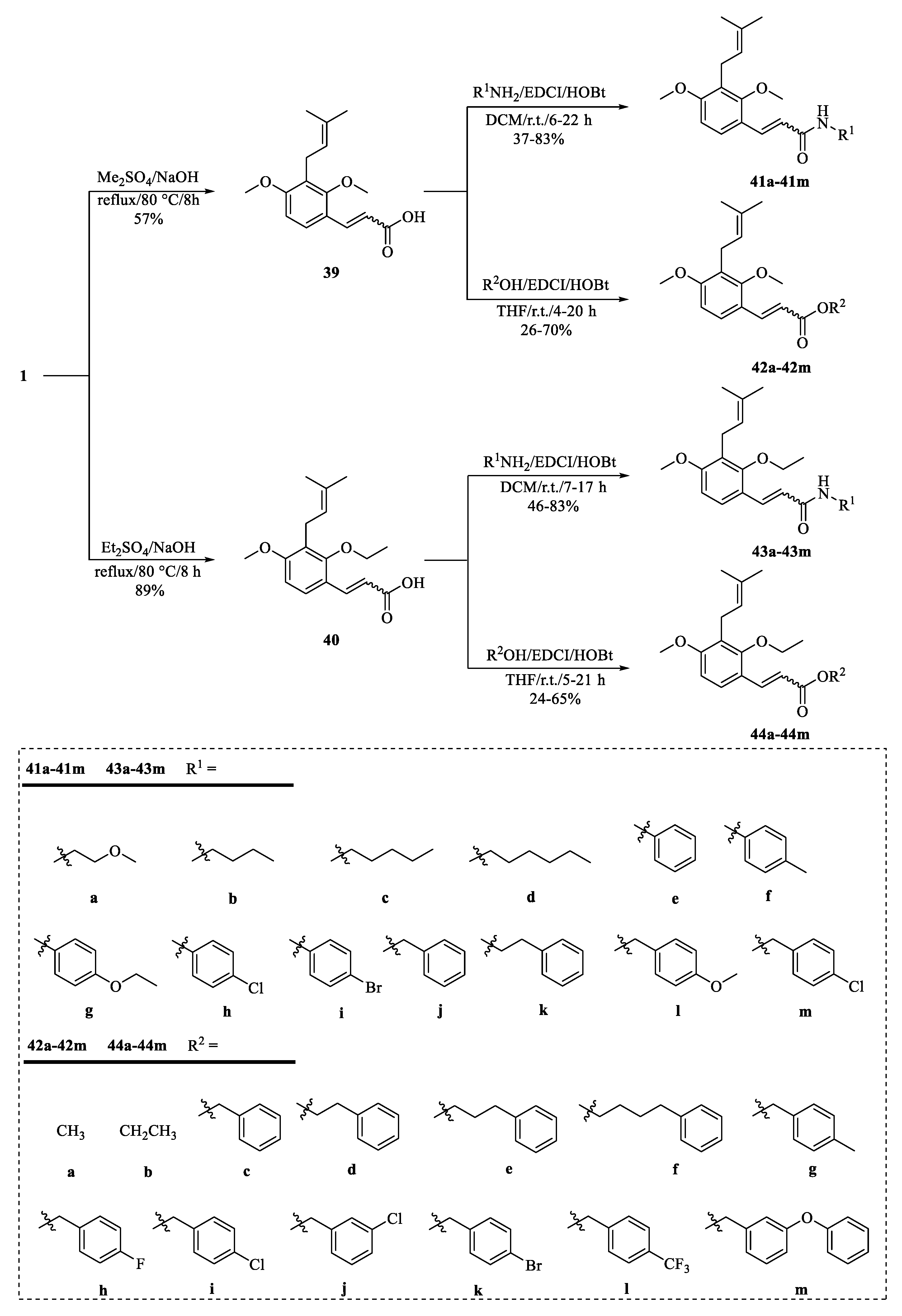
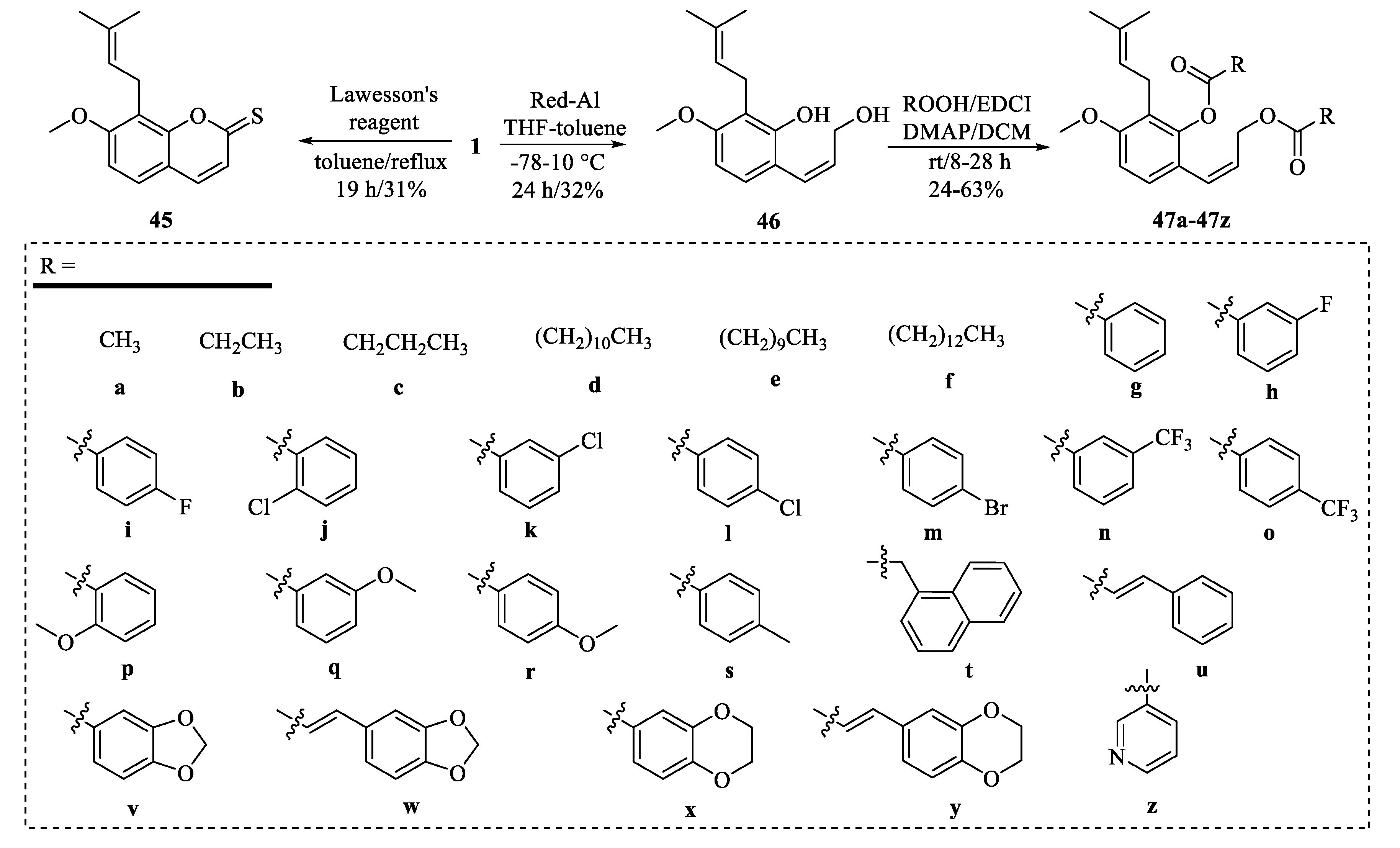


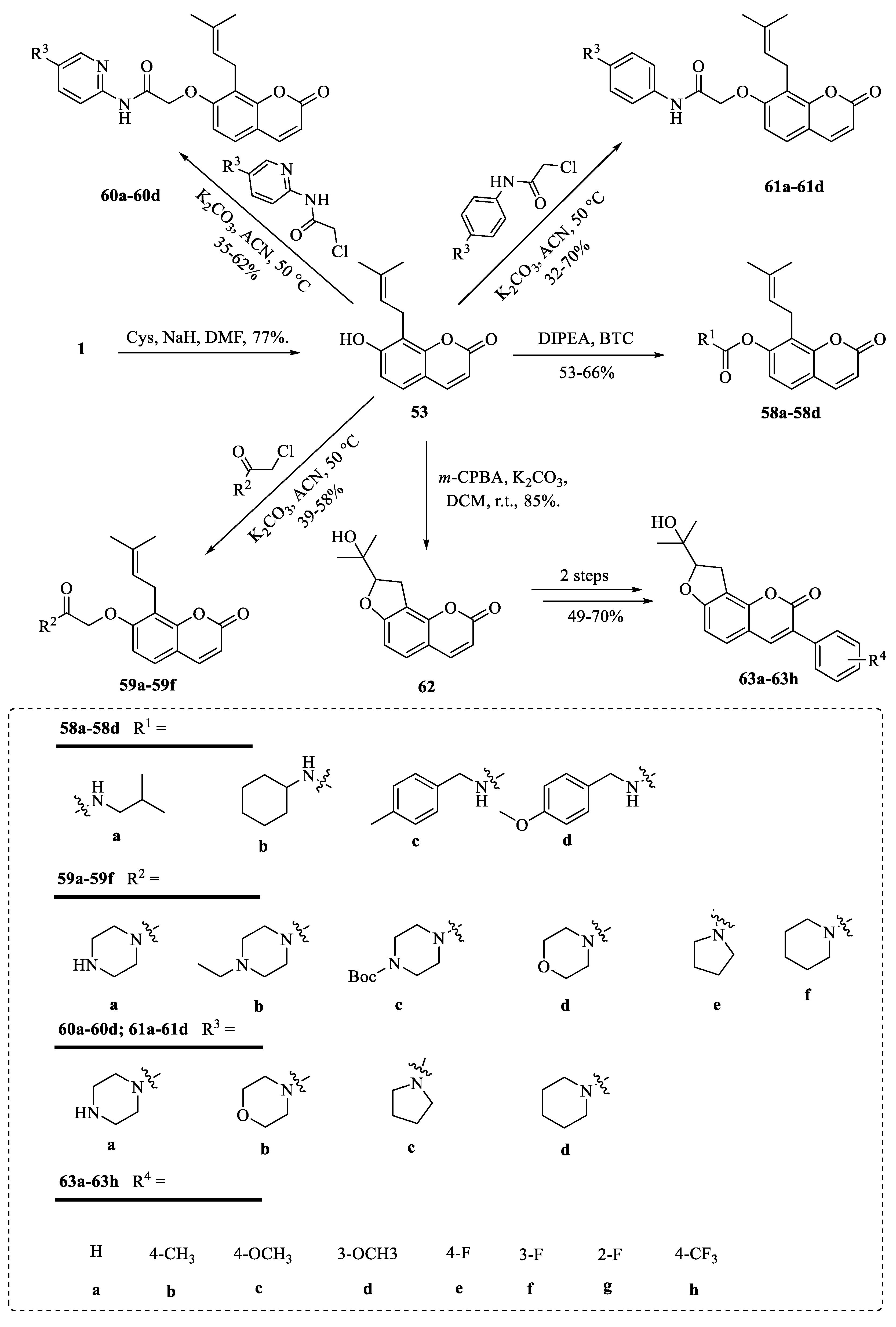
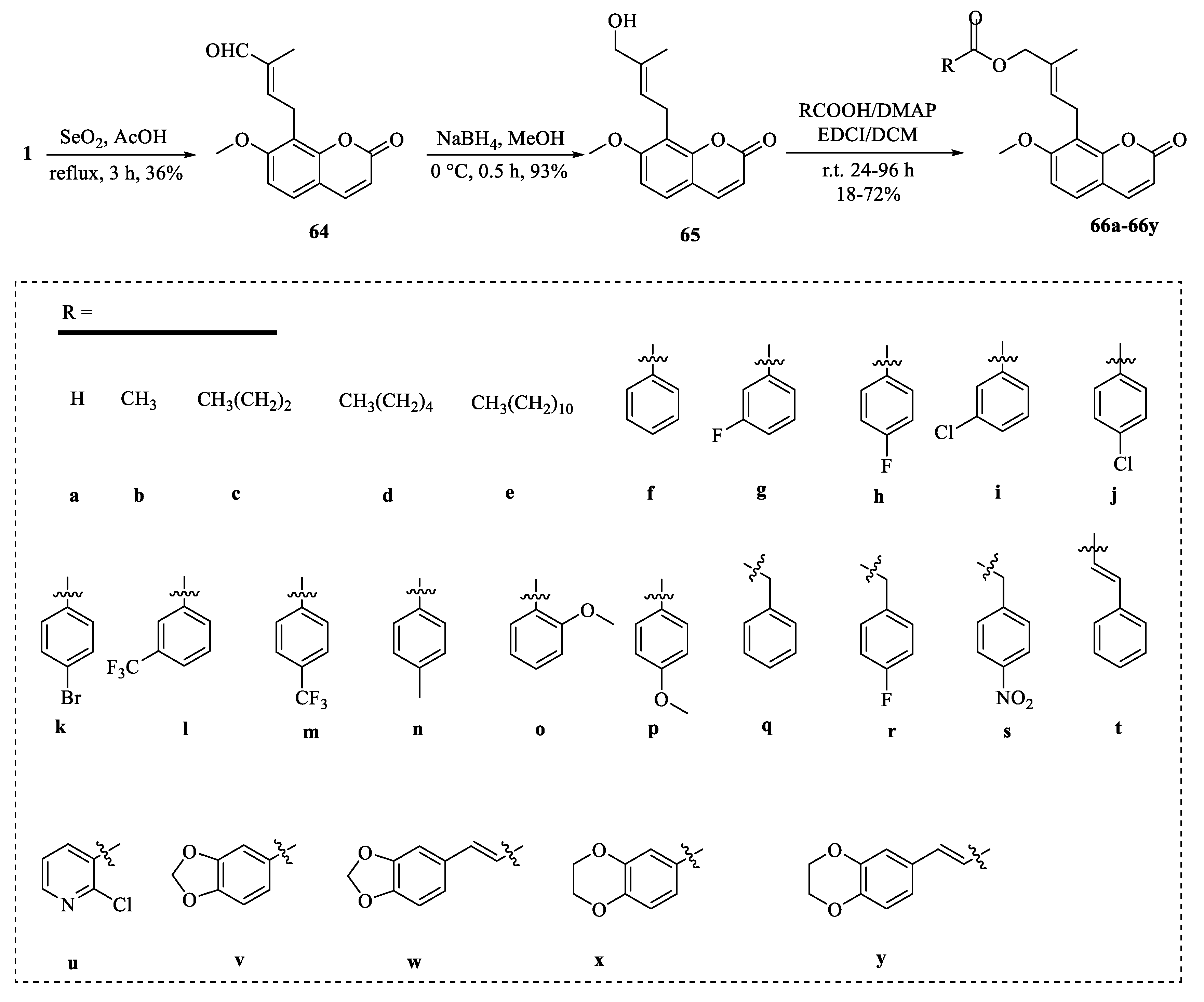
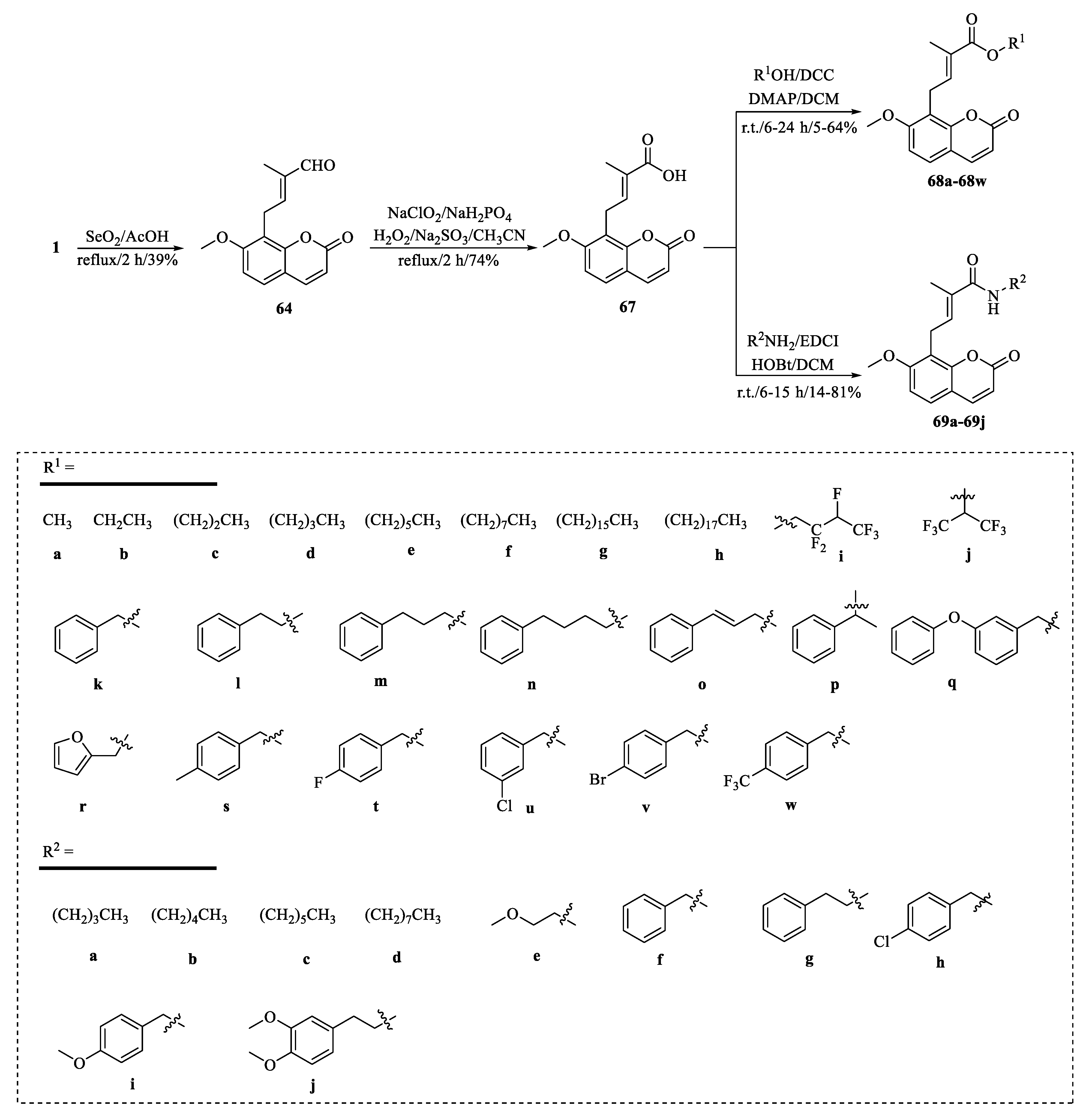


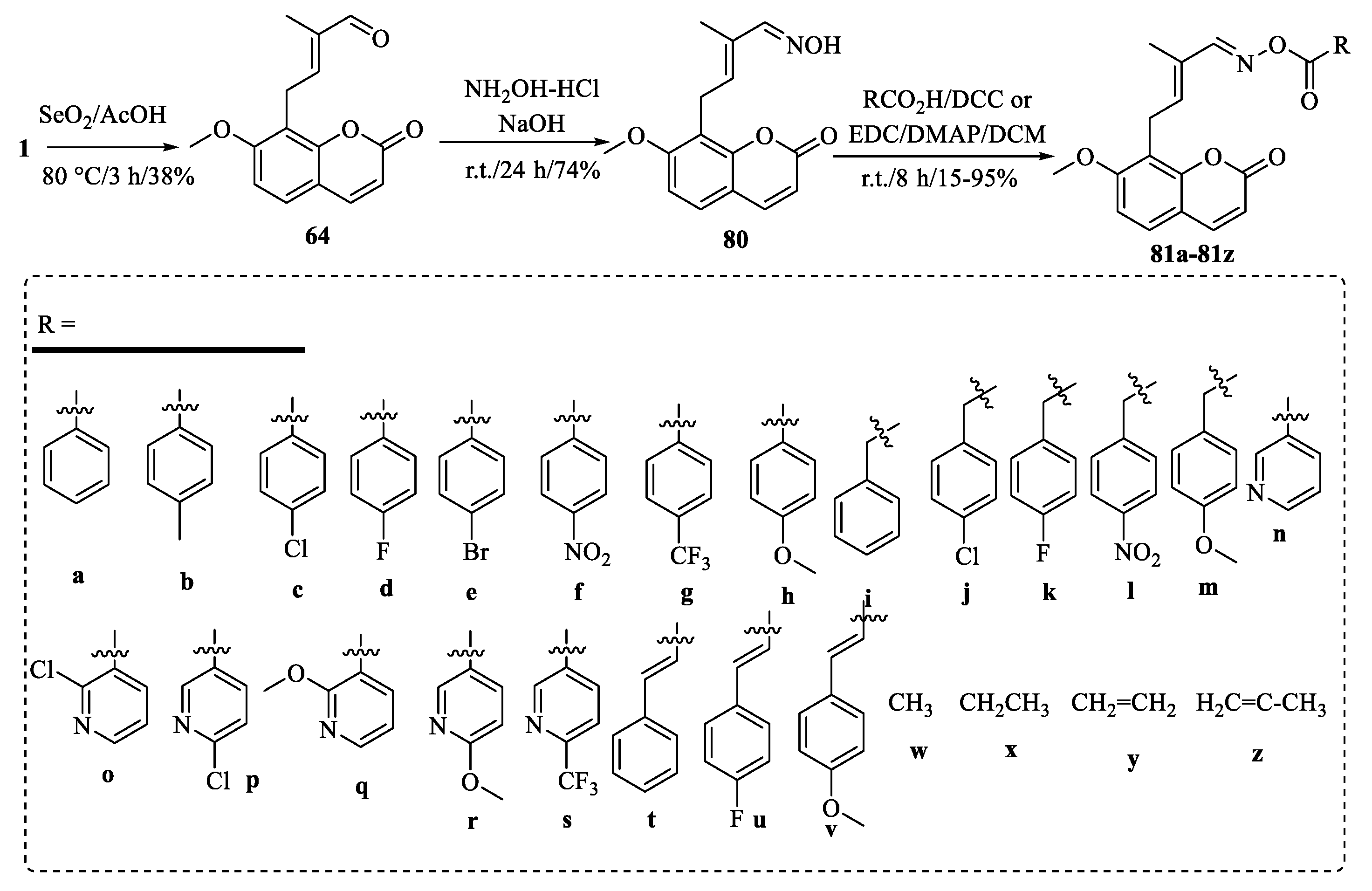
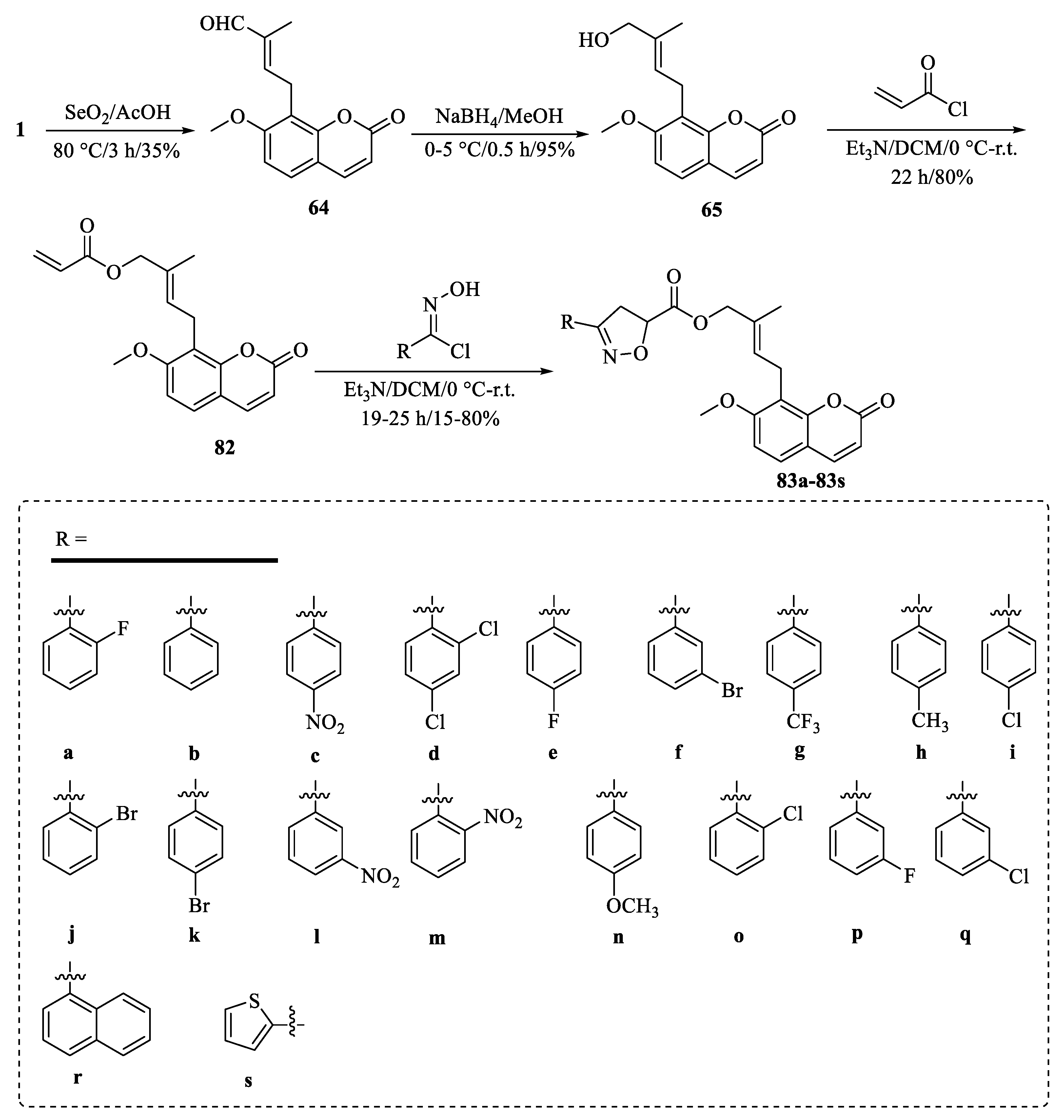
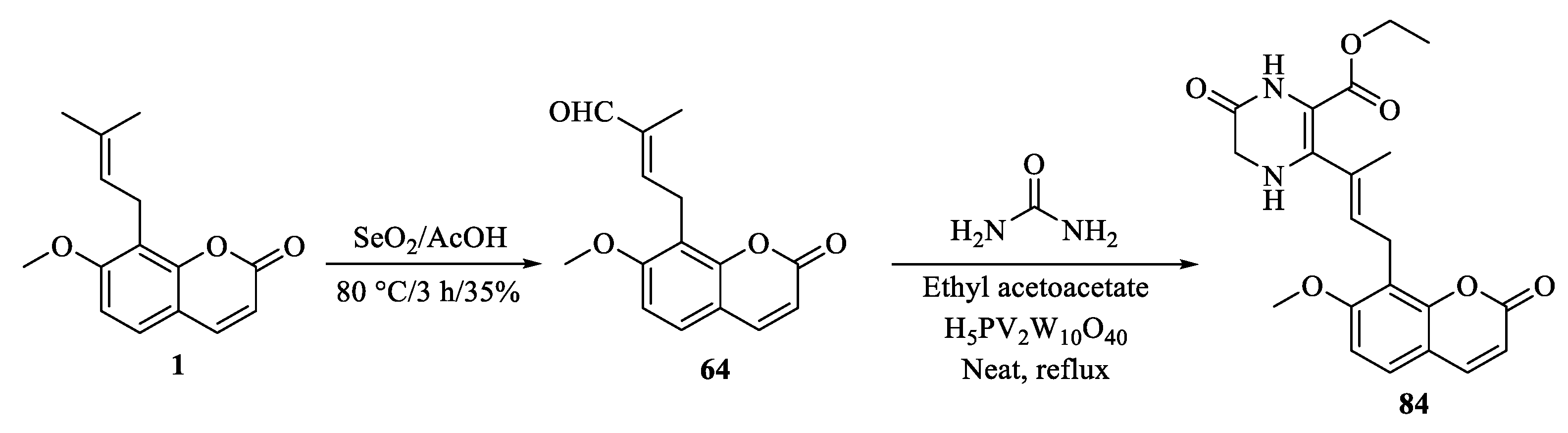
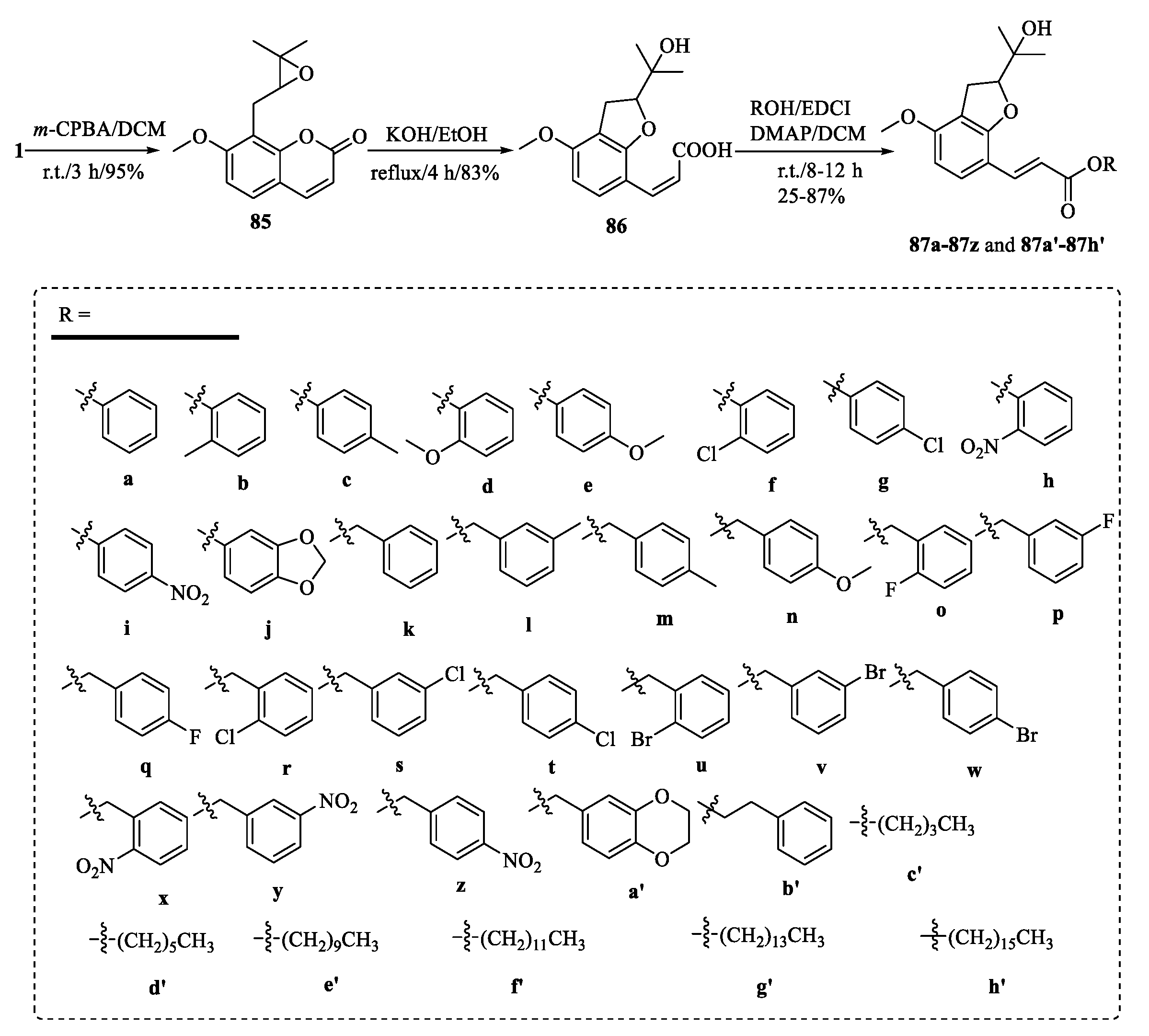
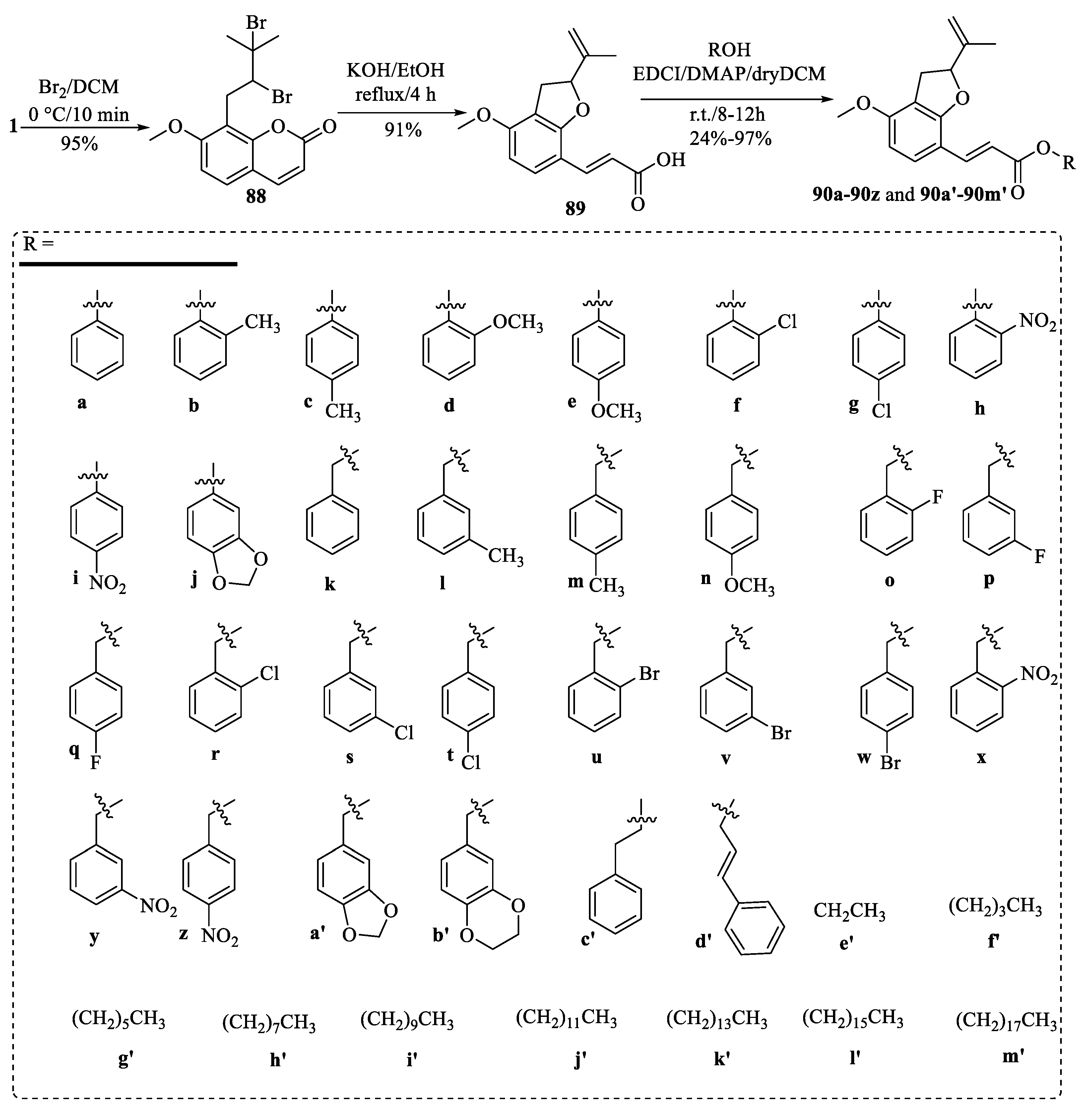

| Tumor | Cell Lines/Model | Involved Mechanism | Period (h) | IC50 (µM) |
|---|---|---|---|---|
| Endometrial carcinoma | JEC, Ishikawa and KLE cells | Suppressing the proliferation and inducing apoptosis via inhibiting the PI3K/AKT signaling pathway | \ | \ [8] |
| Breast cancer | 4T1 cells | Inhibiting cell proliferation, blocking the cells from remaining in S-phase, and inducing apoptosis | 48 | 178.40 [11] |
| MDA-MB-231 cells | Inducing apoptosis arrest by elevation in the translation level of p53/Bax/caspase-3 p17 and downregulating of the Bcl-2 protein | \ | \ [12] | |
| MDA-MB-231 cells BT-549 cells MDA-MB-468 cells MCF-7 cells | Inducing apoptosis and cell cycle arrest in TNBC cells through inhibition of STAT3 phosphorylation and nuclear translocation | 24 | 129.40 [10] | |
| 106.20 [10] | ||||
| 105.40 [10] | ||||
| 168.00 [10] | ||||
| MDA-MB-231 MDA-MB-231BO MCF-7 cells | Inhibiting cell migration and invasion via suppressing of ITGα3 and ITGβ5 signaling | 24 | 29.8 [9] | |
| 111.4 [9] | ||||
| 260.6 [9] | ||||
| BT-474 and MCF-7 cells | Inducing cell cycle arrest, mitochondrial dysfunction, and ER stress | \ | \ [13] | |
| Ovarian carcinoma | A2780 cell OVCAR3 cell | Inducing LC3-mediated autophagy and GSDME-dependent pyroptosis | 24 | 75.24 [15] |
| 73.58 [15] | ||||
| ES2 and OV90 cells | Targeting PI3K/MAPK signaling pathway | \ | \ [14] | |
| Colorectal cancer | HCT116 and HT29 cells | Regulating autophagy- and mitochondria-mediated signal transduction. | \ | \ [17] |
| HT-29 cells | Inducing apoptosis via cycle arrest and activating the intrinsic apoptotic pathway | 24 | 115.08 [18] | |
| HCT116 and SW480 cells | Inhibits malignant phenotypes and inducing ferroptosis in KRAS mutant cells via suppressing AMPK/Akt signaling | \ | \ [19] | |
| CCL-222 cells | Reducing the Akt mRNA expression and the activity p38 MAPK-α | 24 | 23.00 [16] | |
| Colorectal cancer | HT-29 cells | Inducing apoptosis of the cells via endoplasmic reticulum stress and autophagy | \ | \ [20] |
| Hepatocellular carcinoma | HCC-LM3 cells | Inhibiting GSK-3β/AMPK/mTOR pathway-controlled glycolysis | \ | [21] |
| AKT/c-Met-driven HCC mouse model | Delaying hepatocarcinogenesis by suppressing AKT/FASN axis and ERK phosphorylation | \ | [22] | |
| Huh7 and HepG2 cells | Resensitizing CD133+ hepatocellular carcinoma cells to cisplatin treatment via PTEN/AKT pathway | \ | [24] | |
| Hepa1–6 cells | Inhibiting angiogenesis in an orthotopic mouse model of HCC via the NF-κB/VEGF signaling pathway. | \ | [23] | |
| Bladder cancer | 5637 cells 253 J cells | Inhibiting invasion, migration, and epithelial–mesenchymal transition by inhibiting PI3K-AKT and JAK/STAT3 pathways | 24 | 146.40 [25] 160.80 [25] |
| Retinoblastoma | Y-79 cells | Inhibiting the PI3K/AKT/mTOR pathway via regulating the hsa_circ_0007534/miR-214-3p axis | 24 | 200.00 [26] |
| Cervical cancer | Hela B cells | Targeting DCLK1 mechanistically via interacting with Val468. | \ | \ [28] |
| CDDP-resistant cervical cancer cells | Reversing chemoresistance of CDDP-resistant cervical cancer to CDDP through repressing NRF2 expression partly associated with PI3K/AKT blockage | \ | \ [29] | |
| HeLa, SiHa, C-33A and CaSki cells | Suppressing ATM/NF-κB signaling | \ | \ [27] | |
| Head and neck squamous cell carcinoma | FaDu cells Cal27 cells SCC25 cells HN4 cells | Inducing cell cycle arrest and apoptosis via suppressing the PI3K/AKT signaling pathway | 24 | 122.35 [30] 183.32 [30] 189.26 [30] 237.42 [30] |
| Glioma | U87 and C6 cells | Triggering glioma cell necroptosis via ROS overproduction | \ | \ [31] |
| MOGGCCM and T98G cells | Inhibiting of anti-apoptotic Bcl-2 expression, decreasing mitochondrial membrane potential, and activation of caspase 3 via the mitochondrial (internal) pathway | \ | \ [32] | |
| AA and GBM cells | Increasing the level of apoptosis via blocking of the expression of Bcl-2 and PI3K | \ | \ [33] | |
| Gallbladder cancer | NOZ and SGC-996 cells | Inhibiting the progression of human gallbladder cancer cells via the JAK/STAT3 signal pathway | \ | \ [34] |
| Lung cancer | A549 cells | Inducing the apoptosis in A549 cells by inhibiting the histone deacetylase | 24 | 188.50 [35] |
| Gastric cancer | HGC-27 and SGC-7901 cells | Inducting cell cycle arrest at G2/M phase by the regulation of PI3K/AKT | \ | \ [36] |
| N87 and SK-BR-3 cells | Inducing apoptosis and inhibiting AKT/MAPK pathway | \ | \ [37] | |
| Esophageal squamous cell carcinoma | KYSE150 cells KYSE410 cells | Inhibiting the PI3K/AKT signaling pathway via activation of PTEN | 24 | 198.45 [38] 235.67 [38] |
| Rhabdomyosarcoma | TE671 cells | Inhibiting of cell cycle progression and inducing of apoptosis via the AKT and ERK kinase signaling pathways | 96 | 4.05 * [39] |
| Pancreatic cancer | Panc 02 cells and RAW 264.7 cells | Attenuating tumor-infiltrating M2 macrophages | \ | \ [40] |
| Nasopharyngeal cancer | NPC-039 and NPC-BM cells | Inducing apoptosis mediated by the Fas-Fas ligand and mitochondrial pathway | \ | \ [41] |
| Object | Period (h) | Values (µg/mL) |
|---|---|---|
| Listeria monocytogenes ATCC19115 | 6 | MIC = 62.5 [85] |
| Staphylococcus aureus ATCC25923 | 20–24 | MIC = 128 [84] |
| Escherichia coli ATCC25922 | MIC = 128 [84] | |
| Staphylococcus aureus (MRSA) ATCC33591 | MIC = 128 [84] | |
| Staphylococcus aureus (Methicillin-resistant isolate) ATCC43300 | MIC = 128 [84] | |
| Erwinia carotorora | 48 | 98.00% 1 [84] |
| Ralstonia solanacearum | 25.00% 1 [84] | |
| Pseudomonas syringae pv. actinidiae | 68.00% 1 [84] | |
| Fusarium oxysporum | 72 | 57.40% 2 [86] |
| Fusarium moniliforme J. Sheld | 70.66% 2 [86] | |
| Thanatephorus cucumeris Donk | 75.90% 2 [86] | |
| Penicillium choerospondiatis | 144 | EC50 = 9.86 [89] |
| Ustilaginoidea virens | 240 | EC50 = 5.04 [88] |
| Fusarium oxysporum | 168 | EC50 = 6.40 [87] |
| Cercospora sorghi | 72 | EC50 = 1.46 [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, M.; Ding, H.; Xu, H. Osthole: A Coumarin with Dual Roles in Biology and Chemistry. Biology 2025, 14, 588. https://doi.org/10.3390/biology14060588
Lv M, Ding H, Xu H. Osthole: A Coumarin with Dual Roles in Biology and Chemistry. Biology. 2025; 14(6):588. https://doi.org/10.3390/biology14060588
Chicago/Turabian StyleLv, Min, Haixia Ding, and Hui Xu. 2025. "Osthole: A Coumarin with Dual Roles in Biology and Chemistry" Biology 14, no. 6: 588. https://doi.org/10.3390/biology14060588
APA StyleLv, M., Ding, H., & Xu, H. (2025). Osthole: A Coumarin with Dual Roles in Biology and Chemistry. Biology, 14(6), 588. https://doi.org/10.3390/biology14060588