Changes in the Distribution Range of the Genus Cardiocrinum in China Under Climate Change and Human Activities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
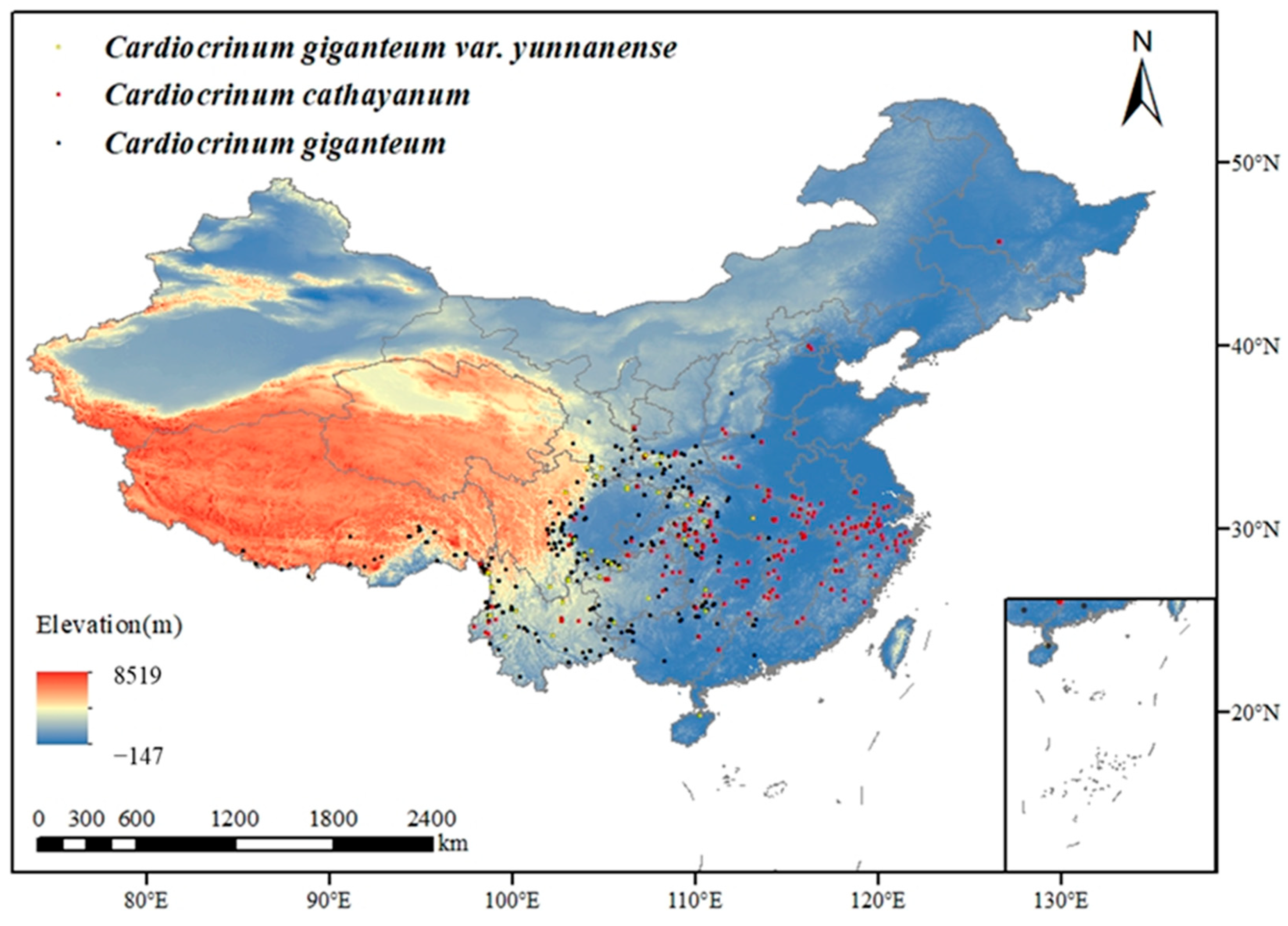
2.1. The Source and Acquisition of Cardiocrinum Data
2.2. Environmental Parameters
2.3. Maximum Entropy Model (MaxEnt) Simulation
3. Results
3.1. Model Accuracy Assessment
3.2. Key Environmental Variables
3.3. The Distribution of the Suitable Area of Cardiocrinum Under the Current Climate
3.4. Potential Habitat Change for Cardiocrinum in the Future
3.5. Migration of Centroid of Cardiocrinum-Suitable Area Under Different Climate Scenarios
3.6. Ecological Niche Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Z.Y.; Zhang, L.; Zhang, H.; Wei, Y. Study on Phenotypic Diversity of Fruitsand Seeds of Wild Cardiocrinum giganteum in 5 Regions. Seed 2024, 43, 93–102. [Google Scholar]
- Zhang, C.B.; Chen, F. Cardiocrinum giganteum var. yunnanense (Elwes) Stearn, A newly Recorded Vascular Plants of Liliaceae from Chongqing. Till. Cult. 2023, 43, 112–113. [Google Scholar]
- Cardiocrinum giganteum var. yunnanense. Informatin System of Chinese Rare and Endangered Plant. Available online: https://www.iplant.cn/rep/prot/Cardiocrinum%20giganteum%20var.%20yunnanense (accessed on 13 May 2024).
- National Forestry and Grassland Administration; Ministry of Agriculture and Rural Affairs of the People’s Republic of China. List of National Key Protected Wild Plants; National Forestry and Grassland Administration: Beijing, China, 2021. [Google Scholar]
- Wang, Z.Y.; Li, R.C.; Huang, X.L.; Liao, J.M.; Zhao, Z.H. Distribution Characteristics and Network Pharmacological Analysis of Regalosides in Cardiocrinum giganteum Bulbs. Guangxi For. Sci. 2024, 53, 554–566. [Google Scholar]
- Shou, J.W.; Zhang, R.R.; Wu, H.Y.; Xia, X.; Nie, H.; Jiang, R.W.; Shaw, P.C. Isolation of novel biflavonoids from Cardiocrinum giganteum seeds and characterization of their antitussive activities. J. Ethnopharmacol. 2018, 222, 171–176. [Google Scholar] [CrossRef]
- Guan, W.L. Mountain wildflowers-Cardiocrinum giganteum var. yunnanense. Landsc. Archit. Acad. J. 2007, 2, 37. [Google Scholar]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Gliottone, I.; Pham, M.P. Current and future predicting habitat suitability map of Cunninghamia konishii Hayata using MaxEnt model under climate change in Northern Vietnam. Eur. J. Ecol. 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Graham, E.M.; Reside, A.E.; Atkinson, I.; Baird, D.; Hodgson, L.; James, C.S.; Vanderwal, J.J. Climate change and biodiversity in Australia: A systematic modelling approach to nationwide species distributions. Australas. J. Environ. Manag. 2019, 26, 112–123. [Google Scholar] [CrossRef]
- Wang, G.H.; Xie, C.P.; Wei, L.J.; Gao, Z.Q.; Yang, H.L.; Jim, C.Y. Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder. Mol. Divers. 2023, 15, 1033. [Google Scholar] [CrossRef]
- Wei, L.; Wang, G.; Xie, C.; Gao, Z.; Huang, Q.; Jim, C.Y. Predicting suitable habitat for the endangered tree Ormosia microphylla in China. Sci. Rep. 2024, 14, 10330. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosennzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Lu, Z.; Rohani, E.R.; Ou, J.; Tong, X.; Han, R. Current and future distribution of Forsythia suspensa in China under climate change adopting the MaxEnt model. Front. Plant Sci. 2024, 15, 1394799. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Conf. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, G.; Bu, W.; Gao, Y. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar]
- Rahmanian, S.; Nasiri, V.; Amindin, A.; Karami, S.; Maleki, S.; Pouyan, S.; Borz, S.A. Prediction of plant diversity using multi-seasonal remotely sensed and geodiversity data in a mountainous area. Remote Sens. 2023, 15, 387. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM: A bioclimate analysis and prediction system. Plant Prot. Q. 1991, 6, 8–9. [Google Scholar]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compure habitat-suitability maps without absence sata? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Yee, T.W.; Mackenzie, M. Vector generalized additive models in plant ecology. Ecol. Modell. 2002, 157, 141–156. [Google Scholar] [CrossRef]
- Lehmann, A.; Overton, J.M.; Leathwick, J.R. GRASP: Generalized regression analysis and spatial prediction. Ecol. Modell. 2002, 160, 165–183. [Google Scholar] [CrossRef]
- Harte, J.; Newman, E.A. Maximum information entropy: A foundation for ecological theory. Trends Ecol. Evol. 2014, 29, 384–389. [Google Scholar] [CrossRef]
- Vayssières, M.P.; Plant, R.E.; Allen-Diaz, B.H. Classification trees: An alternative non-parametric approach for predicting species distributions. J. Veg. Sci. 2000, 11, 679–694. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Amindin, A.; Pourghasemi, H.R.; Safaeian, R.; Rahmanian, S.; Tiefenbacher, J.P.; Naimi, B. Predicting Current and Future Habitat Suitability of an Endemic Species Using Data-Fusion Approach: Responses to Climate Change. Rangel. Ecol. Manag. 2024, 94, 149–162. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Wieprecht, S.; Zhang, S.H.; Wu, Y.J. Evaluating the ecological influence of hydraulic projects: A review of aquatic habitat suitability models. Renew. Sustain. Energy Rev. 2017, 68, 748–762. [Google Scholar] [CrossRef]
- Liu, D.; Xie, C.; Jim, C.Y.; Liu, Y.; Hou, S. Predicting the Potential Distribution of the Alien Invasive Alligator Gar Atractosteus spatula in China. Sustainability. 2023, 15, 6419. [Google Scholar] [CrossRef]
- Gastón, A.; García-Vinas, J.I. Modelling species distributions with penalised logistic regressions: A comparison with maximum entropy models. Ecol. Modell. 2011, 222, 2037–2041. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xie, L.Y.; Zhou, X.Y.; Chen, R.F.; Zhao, G.H.; Zhang, F.G. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Zhang, L.; Wang, C.; Xu, Y. Predicting Possible Distribution of Tea (Camellia sinensis L.) under Climate Change Scenarios Using MaxEnt Model in China. Agriculture 2021, 11, 1122. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 33, 607–611. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 11, 2868–2883. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Lu, Z.Q.; Zhao, J.L.; Li, Q.J. Genomic data reveal two distinct species from the widespread alpine ginger Roscoea tibetica Batalin (Zingiberaceae). Acta Phytotaxon. Sin. 2020, 59, 1232–1243. [Google Scholar] [CrossRef]
- Zhao, J.L.; Gugger, P.F.; Xia, Y.M.; Li, Q.J. Ecological divergence of two closely related Roscoea species associated with late Quaternary climate change. J. Biogeog. 2016, 43, 1990–2001. [Google Scholar] [CrossRef]
- Dong, R.; Hua, L.M.; Hua, R.; Ye, G.H.; Bao, D.; Cai, X.C.; Cai, B.; Zhao, X.C.; Chu, B.; Tang, Z.S. Prediction of the potentially suitable areas of Ligularia virgaurea and Ligularia sagitta on the Qinghai–Tibet Plateau based on future climate change using the MaxEnt model. Front. Plant Sci. 2023, 14, 1193690. [Google Scholar] [CrossRef]
- Sander, J.; Wardell-Johnson, G. Impacts of soil fertility on species and phylogenetic turnover in the high-rainfall zone of the southwest Australian global biodiversity hotspot. Plant Soil 2011, 345, 103–124. [Google Scholar] [CrossRef]
- Li, J.; Yin, Z.; Cheng, F.; Gadow, K.V.; Hao, M.; Fan, C.; Zhao, X.; Zhang, C. Effects of stand structure, individual dominant species and environment on herb diversity in a temperate forest region. Ecol. Indic. 2025, 172, 113262. [Google Scholar] [CrossRef]
- Murphy, S.J.; Salpeter, K.; Comita, L.S. Highe beta-diversity observed for herbs over woody plants is driven by stronger habitat filtering in a tropical understory. Ecology 2016, 97, 2074–2084. [Google Scholar] [CrossRef]
- Beck, J.J.; Givnish, T.J. Fine: Cale environmental heterogeneity and spatial niche partitioning among spring-flowering forest herbs. Am. J. Bot. 2021, 108, 63–73. [Google Scholar] [CrossRef]
- Ni, M.; Vellend, M. Soil properties constrain forest understory plant distributions along an elevation gradient. Philos. Trans. R. Soc. B 2024, 379, 20230373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, Z.Y.; Zhang, H.; Chi, M.; Deng, J.Y.; Wei, Y. Study on Introduction and Culture of Cardiocrinum in Beijing. In Proceedings of the 2020 China Botanical Garden Academic Annual Conference, Shenyang, China, 10–13 October 2020; pp. 65–70. [Google Scholar]
- Li, Y.F.; Song, J.; Guan, W.L.; Li, F.R. Seed dormancy and germination in Cardiocrinum giganteum var. yunnanense, a perennial herb in China with post-dispersal embryo growth. Seed Sci. Technol. 2020, 48, 303–314. [Google Scholar]
- Guan, W.L.; Li, S.F.; Chen, X.; Li, Y.F.; He, F.M. Dormancy Characteristics and Dormancy Break of Cardiocrinum giganteum Seed. Acta Bot. Boreali-Occident. Sin. 2010, 30, 2479–2483. [Google Scholar]
- Zhu, P.Z.; Zhang, G.H.; Wang, H.X.; Zhang, B.J.; Liu, Y.N. Soil moisture variations in response to precipitation properties and plant communities on steep gully slope on the Loess Plateau. Agric. Manage. Water Qual. 2021, 256, 107086. [Google Scholar] [CrossRef]
- Li, M.; Ling, K.H.; Lam, H.; Shaw, P.C.; Cheng, L.; Techen, N.; Khan, I.A.; Chang, Y.S.; But, P.P.H. Cardiocrinum seeds as a replacement for Aristolochia fruits in treating cough. J. Ethnopharmacol. 2010, 130, 429–432. [Google Scholar] [CrossRef]
- Wang, B.; Qian, X.M.; Zhou, J.; Li, L.; Wang, J.L.; Feng, S.L. Investigation and Application of Wild Plant Resources of Cardiocrinum in Northern Foot of Qinling Mountains. Shanxi Agric. Sci. 2023, 69, 34–38. [Google Scholar]
- Wan, Z.Z.; Long, C.L.; Chen, Z.Y.; Yang, D.; Jin, Z.H. Advances in Study of Cardiocrinum. J. Yunnan Agric. Univ. Nat. Sci. 2007, 22, 30–34. [Google Scholar]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2021, 29, 1073–1086. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, X.; Mao, K.; Wang, M.; Wang, K.; Xi, Z.; Liu, J. Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. J. Biogeogr. 2018, 45, 1334–1344. [Google Scholar] [CrossRef]
- Su, Q.T.; Du, Z.X.; Xue, Y.X.; Li, H.; Zhang, Y.X.; Zhang, S.J.; Huang, X.Y.; Zhou, B.; Qian, H.; Xiao, Y.A.; et al. Habitat Suitability Modeling of Endemic Genus Chimonanthus in China under Climate Change. Forests 2024, 15, 1625. [Google Scholar] [CrossRef]
- Thomas, Z.A.; Mooney, S.; Cadd, H.; Baker, A.; Turney, C.; Schneider, L.; Hogg, A.; Haberle, S.; Green, K.; Weyrich, L.S. Late Holocene climate anomaly concurrent with fire activity and ecosystem shifts in the eastern Australian Highlands. Sci. Total Environ. 2022, 802, 149542. [Google Scholar] [CrossRef]
- Su, Q.T.; Du, Z.X.; Luo, Y.; Zhou, B.; Xiao, Y.A.; Zhou, Z.R. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios. Biology 2024, 13, 745. [Google Scholar] [CrossRef]
- Yang, S.; Ding, Z.; Li, Y.; Wang, X.; Jiang, W.; Huang, X. Warming-induced northwestward migration of the East Asian monsoon rain belt from the Last Glacial Maximum to the mid-Holocene. Proc. Natl. Acad. Sci. USA 2015, 112, 13178–13183. [Google Scholar] [CrossRef]
- Jiao, K.W.; Gao, J.B.; Wu, S.H.; Hou, W.J. Research progress on the response processes of vegetation activity to climate change. Acta Ecol. Sin. 2018, 38, 2229–2238. [Google Scholar]
- Gu, C.J.; Tu, Y.L.; Liu, L.S.; Bo, W.; Zhang, Y.L.; Yu, H.B.; Wang, X.L.; Zhuoga, Y.J.; Zhang, B.H.; Cui, B.H. Predicting the potential global distribution of Ageratina adenophora under current and future climate change scenarios. Ecol. Evol. 2021, 11, 12092–12113. [Google Scholar]
- Lu, R.S.; Chen, Y.; Tamaki, I.; Sakaguchi, S.; Ding, Y.Q.; Takahashi, D.; Li, P.; Isaji, Y.; Chen, J.; Qiu, Y.X. Pre-quaternary diversification and glacial demographic expansions of Cardiocrinum (Liliaceae) in temperate forest biomes of Sino-Japanese Floristic Region. Mol. Phylogenet. Evol. 2020, 143, 106693. [Google Scholar] [CrossRef]
- Yang, L.Q.; Hu, H.Y.; Xie, C.; Lai, C.X.; Yang, M.; He, X.J.; Zhou, S.D. Molecular phylogeny, biogeography and ecological niche modelling of Cardiocrinum (Liliaceae): Insights into the evolutionary history of endemic genera distributed across the Sino-Japanese floristic region. Ann. Bot. 2017, 119, 59–72. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, Q.; Jia, S.W. Genomic Phylogeography of Gymnocarpos przewalskii (Caryophyllaceae): Insights into Habitat Fragmentation in Arid Northwestern China. Mol. Divers. 2020, 12, 335. [Google Scholar] [CrossRef]
- Carretero, M.A.; Sillero, N. Evaluating how species niche modelling is affected by partial distributions with an empirical case. Acta Oecologica 2016, 77, 207–216. [Google Scholar] [CrossRef]





| Variables | Abbreviations |
|---|---|
| Annual mean temperature | bio1 |
| Mean diurnal range | bio2 |
| Isothermality | bio3 |
| Temperature seasonality | bio4 |
| Max. temperature of warmest month | bio5 |
| Min. temperature of coldest month | bio6 |
| Temperature annual range | bio7 |
| Mean temperature of wettest quarter | bio8 |
| Mean temperature of driest quarter | bio9 |
| Mean temperature of warmest quarter | bio10 |
| Mean temperature of coldest quarter | bio11 |
| Annual precipitation | bio12 |
| Precipitation of wettest month | bio13 |
| Precipitation of driest month | bio14 |
| Precipitation seasonality | bio15 |
| Precipitation of wettest quarter | bio16 |
| Precipitation of driest quarter | bio17 |
| Precipitation of warmest quarter | bio18 |
| Precipitation of coldest quarter | bio19 |
| Elevation | elev |
| Human activity | ha |
| Slope | slo |
| Aspect | asp |
| Species | AUC Training | AUC Test | TSS |
|---|---|---|---|
| C. cathayanum | 0.989 | 0.983 | 0.913 |
| C. giganteum | 0.982 | 0.980 | 0.936 |
| C. giganteum var. yunnanense | 0.995 | 0.996 | 0.989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, S.; Xiao, H.; Li, H.; Liao, D.; Xue, Y.; Huang, X.; Su, Q.; Xiao, Y. Changes in the Distribution Range of the Genus Cardiocrinum in China Under Climate Change and Human Activities. Biology 2025, 14, 581. https://doi.org/10.3390/biology14050581
Zhang Y, Zhang S, Xiao H, Li H, Liao D, Xue Y, Huang X, Su Q, Xiao Y. Changes in the Distribution Range of the Genus Cardiocrinum in China Under Climate Change and Human Activities. Biology. 2025; 14(5):581. https://doi.org/10.3390/biology14050581
Chicago/Turabian StyleZhang, Yuxin, Shujian Zhang, Haiyan Xiao, Heng Li, Da Liao, Yuxi Xue, Xinyi Huang, Qitao Su, and Yian Xiao. 2025. "Changes in the Distribution Range of the Genus Cardiocrinum in China Under Climate Change and Human Activities" Biology 14, no. 5: 581. https://doi.org/10.3390/biology14050581
APA StyleZhang, Y., Zhang, S., Xiao, H., Li, H., Liao, D., Xue, Y., Huang, X., Su, Q., & Xiao, Y. (2025). Changes in the Distribution Range of the Genus Cardiocrinum in China Under Climate Change and Human Activities. Biology, 14(5), 581. https://doi.org/10.3390/biology14050581





